Universidade Federal de Ouro Preto
Programa de Pós Graduação em Ecologia de
Biomas Tropicais
Se reconstruirmos elas virão?
Abelhas e vespas solitárias que nidificam em
cavidades preexistentes em matas ciliares
restauradas no cerrado do Sudeste do Brasil
Gustavo Júnior de Araújo
Ouro Preto
Universidade Federal de Ouro Preto
Programa de Pós Graduação em Ecologia de
Biomas Tropicais
Se reconstruirmos elas virão?
Abelhas e vespas solitárias que nidificam em
cavidades preexistentes em matas ciliares
restauradas no cerrado do Sudeste do Brasil
Orientadora: Prof. ª Dr. ª Yasmine Antonini
Ouro Preto
2015
Agradecimentos:
À Universidade Federal de Ouro Preto, Programa de Pós-Graduação em Ecologia de Biomas Tropicais pela oportunidade de realização do mestrado.
À CEMIG pela concessão da bolsa e financiamento do projeto sem os quais não seria possível e realização.
À Prof. Dra
Yasmine Antonini por me dar a oportunidade de ser seu aluno, por abrir as portas do seu laboratório, pelos ensinamentos, pela paciência³, pela amizade e por se tonar uma referência tanto como pessoal quanto profissional para mim .
Ao Roberth Fagundes, o grande responsável na minha escolha em me especializar em Ecologia e responsável por minha vinda para Ouro Preto.
Ao Vinícius Albano de Araújo por aceitar o convite em participar da minha banca de defesa.
Ao Marcel Hermes e José Eustáquio identificação das espécies.
Aos meus pais, Ademir e Zeliana, e à minha irmã Bárbara, por me apoiarem em todos os momentos ao longo da minha vida. A vocês devo a pessoa que sou hoje, por isto, vos amo, admiro e respeito.
Aos meus amigos do laboratório de Biodiversidade, onde constitui uma família e toda turma do Biomas.
Aos meus amigos Hermano, Tiago, Wellington, André Damas, André Amâncio, Ana Laura, Nathália, Rodrigo, Brehna, Ana Clara, Laís (alma de gata) e Ana Caroline pelas risadas e momentos de descontração.
À Graziella França, peça fundamental para o desenvolvimento das minhas análises estatísticas.
À professora Maria Cristina por nos disponibilizar os dados de Botânica.
Sumário
Lista de figuras da introdução geral ... VIII Lista de tabelas Capítulo 1... IX Lista de figuras Capítulo 1 ... X Lista de tabelas Capítulo 2... XII Lista de figuras Capítulo 2 ... XIII
Resumo ... 14
Introdução geral ... 15
Referências bibliográficas: ... 22
Capítulo 1: Efficiency of restored riparian forests on the conservation of trap-nesting solitary bees and wasps communities in a Cerrado area, on the Southeastern BrazilErro! Indicador não d Abstract ... 26
Introduction ... 27
Material and methods ... 29
Results ... 33
Discussion ... 42
References ... 52
Capítulo 2: Field of Dreams: if you restore it, will they come? Trap nesting bees and wasps on recovered riparian forests... 59
Abstract ... 59
Introduction ... 60
Material and methods ... 63
Results ... 68
Discussion ... 74
Acknowledgments: ... 79
References ... 80
Considerações finais ... 86
VIII Lista de figuras da introdução geral
IX Lista de tabelas Capítulo 1
X Lista de figuras Capítulo 1
Figure 1: Location of the five areas of restored riparian forest around the Hydroelectric Power Plant of Volta Grande reservoir, between Minas Gerais and São Paulo states………...……….28 Figure 2: Distribution of treatments in the study area with five treatments, each replicated four times……….………….………. 30
Figure 3: Expected richness of trap-nesting bee and wasps recorded from September 2013 to November 2014 on the five sampling units of the Volta Grande reservoir, Brazil……….……….………….…..…..34
XI Figure 7: Frequency of occupation for the most abundant species of solitary bees that nest in pre-existing cavities in restored riparian forests at the Hydroelectric Power Plant of Volta Grande reservoir – MG, Brasil………..………...….38 Figure 8: Network of interactions between parasites and their hosts. The squares represent the hosts and the circles represent parasites, forms sizes is related to the number of interactions that each species and thickness of the lines represents the number of times that these interactions have been carried out. Parasites: Anthrax sp1, Anthrax sp2, Coelioxys sp., Chrysis sp., Macrosiagon sp., Mellitobia sp., Mesocheira sp., Mesoplia sp., Mutillidae sp. Hots: Ancistroceroides sp., C. analis, C. tarsata, E. melanotricha, M. melanosaurus, Megachile sp1, P. grandis, P. guadulpensis,
Penepodium sp1, Penepodium sp2, T. nitidum, T.
XII Lista de tabelas Capítulo 2
Table 1: Geographic location of the sampling areas by Municipality – State and UTM georeference, beyond the age of accomplished restoration process, width of the riparian
forest and main matrix……….………....65
XIII Lista de figuras Capítulo 2
Figure 1: Sampling units on the Riparian Forest of Volta Grande Reservoir, Southwest Cerrado Domain, Brazil. Sampling areas are represented as black
dots…………..………...….64
Figure 2: Analysis of non-metric multidimensional scaling (NMDS) for sorting and graphical visualization of the PERMANOVA and PERMDISP analyses of bees and
wasps among the sampling units related to age (A) and size (B). The Bray-Curtis
dissimilarity index was used………..……..69
Figure 3: Abundance of wasps in areas with different ages (A) and richness of bees in areas with different widths (B) in the restored riparian forests of Volta Grande
Reservoir, Brazil……….……….………70
Figure 4: Total richness (A) and abundance (B) of trap-nesting bees and wasps, in the sampling units based on sorrounding matrix of the restored riparian forests of the Volta
Grande Reservoir, Brazil……….………..…..71
Figure 5: Regression plots for the significant relationship between (A) variance of tree richness and total species abundance (B) variance of tree richness and wasps species
abundance, (C) variance of tree abundance and total abundance, (D) variance of tree
abundance and wasps abundance, (E) variance of tree height and total abundance, (F)
variance of tree height and wasps abundance and (G) variance of tree richness and
wasps richness……….……..………..72
Figure 6: Diagram ordination of structural variables produced by canonical correspondence analysis based on the distribution of species of bees and solitary wasps
that nest in pre-existing cavities present in at least three sample units of the reforested
14 Resumo
15 Introdução geral
O desenvolvimento econômico criado pela nossa civilização segue um modelo no qual não foram consideradas as fragilidades e a importância dos ambientes terrestres (Sporl & Ross 2004) o que levou a extinção de muitas espécies e/ou colocando outras milhares em perigo. Isso em detrimento aos impactos ambientais causados pelas ações antrópicas, como por exemplo, o desmatamento que ocorre nos mais diferentes tipos de ambientes, o que acaba comprometendo os seus recursos naturais e afetando diretamente a fauna local (Primo & Váz 2006, Uezu & Cullen 2012).
Dentre os mais diversos tipos de formações vegetais afetadas pelas ações antrópicas, destacam-se as matas ciliares. Características das margens dos cursos d’água destas formações protegem os recursos hídricos impedindo os processos erosivos que levam ao assoreamento dos seus leitos, filtram a água no processo de absorção evitando a contaminação dos mananciais com resíduos químicos oriundos das atividades agrícolas. O sombreamento destas matas contribui para a estabilidade térmica dos ambientes aquáticos circundados por elas evitando mudanças bruscas em suas temperaturas, o que poderia levar a morte de várias espécies aquáticas (Naiman & Décamps 1997, Barrela et al 2000, Fonseca et al 2001). No entanto as suas funções vão além, pois, as formações ciliares são utilizadas como refúgio para os mais diferentes tipos de animais, onde eles encontram abrigo e alimento além de atuarem como corredores ecológicos no processo de deslocamento da fauna, o que acaba contribuindo para o fluxo gênico de diversas populações (Duringan & Silveira 1999, Lacerda & Figueiredo 2009, Metzger 2010). No Cerrado as matas ciliares apresentam 33% das plantas fanerógamas registradas para este bioma (Felfilli et al 2001).
16 maior em detrimento das ações humanas, como a sua derrubada para utilização da madeira, crescimento urbano, construção de hidrelétricas e implantação de pastagens (Primo & Váz 2006, Martins 2001) o que torna as ações de recuperação e a conservação destas matas cada vez mais necessário (Lacerda & Figueiredo 2009, Metzger 2010).
O processo de recuperação de áreas degradadas não pode ser representado apenas pelo plantio de mudas, buscando apenas a reintrodução de espécies arbóreas, e sim assumir a função de reconstruir as interações que foram perdidas na comunidade como um todo, fazendo com que este ambiente seja capaz de se auto-sustentar (Rodrigues & Gandolfi 2004). A este processo é dado o nome de restauração ambiental (Wismar & Beschta 1998). As técnicas utilizadas para a restauração ambiental dependem da presença das espécies nativas da região, envolvendo não só as árvores, mas os diferentes grupos da fauna e suas interações com a flora (Pickett 1983). Este processo também deve levar em consideração a dinâmica pelo qual a comunidade vegetal passa, chamada sucessão ecológica, que com o tempo tende a se tornar mais diversificada, complexa e estável. Com isto plantios heterogêneos com espécies de diferentes estágios sucessionais vem sendo utilizadas na recuperação de áreas degradadas (Durigan & Nogueira 1990, Glufke 1999). Este processo permite o aparecimento de clareiras levando o reestabelecimento de novas espécies ao longo do tempo, assim como em comunidades naturais (Whitmore 1990).
17 da dispersão das espécies de árvores nativas tropicais. Desta forma o reestabelecimento
das florestas sem os animais seria impossível (Ferretti 2006).
Dentre os polinizadores as abelhas são consideradas os mais importantes (Faegri
& Van der Pijl 1976), pois ao se alimentam dos recursos florais (néctar e pólen) acabam
visitando diversas espécies de plantas floridas, contribuindo para sua reprodução
cruzada através da dispersão dos grãos de pólen. Além de aumentar o sucesso da
polinização, estes insetos também contribuem para a o aumento da variabilidade
genética das espécies as quais visitam (Camillo 2003, Vilhena & Augusto 2007,
Oliveira & Schlindwein 2009).
Embora as abelhas apresentem uma estreita relação com as vespas, mostrando
semelhanças quanto aos seus hábitos de alimentação, as vespas, ao contrário das abelhas
que alimentam suas crias com pólen e néctar, alimentam os jovens com presas do grupo
dos Arthropoda (Evans & Eberhard 1970, Krombein 1967). Isto faz com que além da
sua importância como polinizadores as vespas sejam também de importância
fundamental na regulação da população desses invertebrados, muitos considerados
pragas para em cultivos agrícolas (Fernandes et al 2009, O’Neill 2002).
Abelhas e vespas apresentam diferentes níveis de organização social, começando
pelas espécies solitárias onde a fêmea escolhe o local, constrói seu ninho e fornece
todos os recursos necessários para o desenvolvimento da prole, sem que haja cuidado
parental e culminando em espécies com colônias formadas por milhares de indivíduos,
onde há a sobreposição de gerações, cuidado parental e o monopólio reprodutivo pela
rainha (comportamento eussocial) (Michener 2000).
Várias são as técnicas utilizadas para a amostragem de abelhas e vespas, como
coleta ativa (rede entomológica), isca atrativa e a utilização e ninhos artificiais
18 ou ninhos-armadilhas é uma técnica de amostragem bastante eficaz, pois ela amostra somente as espécies que realmente ocorrem na região, excluindo aquelas que estejam transitando pela área, evitando assim a superestimativa dos dados, como pode ocorrer na metodologia de rede entomológica (Camillo et al 1995). Além disso, possibilita um estudo mais detalhado sobre a biologia das espécies coletadas, como material utilizado na construção dos ninhos, tipo de alimento ofertado para a prole, parasitas associados, razão dos indivíduos do ninho e até mesmo a taxa de mortalidade (Garófalo 2000, Aguiar et al 2005)
Desta forma a utilização de ninhos-armadilha nos permite inventariar a fauna de abelhas e vespas solitárias que nidificam em cavidades, insetos estes de grande importância ambiental e que vem desaparecendo em várias partes do globo devido ao desmatamento, com isto, esta técnica nos permite avaliar qualidade ambiental destas áreas (Pires et at 2012, Loyola & Martins 2006).
Grande parte da vegetação ciliar da área do reservatório da Usina Hidrelétrica (UHE) Volta Grande, área alvo desse estudo e atualmente sob concessão da Companhia Energética de Minas Gerais S.A (CEMIG), foi impactada pela supressão vegetal e alagamento durante as fases de barramento do rio, construção e enchimento do reservatório da usina. No cumprimento de dispositivos legais, diferentes estratégias de recuperação das áreas degradadas foram implementadas pela CEMIG ao longo dos anos.
19 entorno, ocasionando um mosaico de paisagens que apresentaram diferentes respostas ao processo de recuperação e, consequentemente, fragmentos de vegetação ciliar em diferentes estágios sucessionais (Durigan & Nogueira, 1990).
Ainda atendendo a uma demanda legal, a CEMIG, em parceria com a Universidade Federal de Ouro Preto (UFOP) - MG, implementou na área do reservatório de Volta Grande o projeto denominado "Prociliar - P&D 484". Em síntese, as atividades desenvolvidas visam avaliar a efetividade e sustentabilidade das matas ciliares do reservatório da UHE Volta Grande na manutenção dos processos ecológicos e da biodiversidade. O projeto "Prociliar - P&D 484", ao qual este trabalho está inserido, conta com equipe multidisciplinar composta por professores e alunos desta instituição de ensino superior.
Então, o presente estudo teve como objetivo avaliar diferentes estratégias e processos de recuperação de áreas degradadas estabelecidos em fragmentos de vegetação ciliar no entorno do reservatório Volta Grande, situado no rio Grande, na divisa dos estados de Minas Gerais e São Paulo, região Sudeste do Brasil.
20
largura, período de recuperação, matriz adjacente e as variáveis estruturais da vegetação das matas ciliares restauradas sobre a riqueza e abundância destes insetos.
Figura 1: Construção do Reservatório da Usina Hidrelétrica de Volta Grande pela Companhia Energética de Minas Gerais (CEMIG), sobre o Rio Grande, entre os estados de Minas Gerais e São Paulo, no fim dos anos 70.
22 Referências bibliográficas:
Aguiar, C.M.L.; Garófalo, C.A.; Almeida, G.F. Abelhas (Hymenoptera, Apoidea) que nidificam em ninhos-armadilha em áreas de floresta semi-decídua e caatinga, Bahia, Brasil. Revista Brasileira de Zoologia, Curitiba, v.22, p.1030-1038, 2005.
Barrella, W.; Petrere Jr.; Smith, W.S.; Montag, L.F.A. (2000) As relações entre as matas ciliares, os rios e os peixes. In: Rodrigues RR, Leitão Filho HF (Eds) Matas ciliares: conservação e recuperação. São Paulo, EDUSP FAPESP, 2000. 320 p.
Brasil, Código Florestal. Lei n° 12.651 de 25 de maio de 2012. 2012.
Camillo, E. Polinização do Maracujá. Ribeirão Preto, Holos Editora, 2003. 44 p.
Camillo, E.; Garófalo, C.A.; Serrano, J.C.; Mucillo, G. Diversidade e abundância sazonal de abelhas e vespas solitárias em ninhos-armadilhas (Hymenoptera: Apocrita: Aculeata). Revista Brasileira de Entomologia, Curitiba v.39, p. 459-470, 1995.
Durigan, G.; Nogueira, J.C.B. Recomposição de matas ciliares: orientações básicas. São Paulo v.4, p. 14, 1990.
Durigan, G.; Silveira, E.R. 1999) Recomposição de mata ciliar em domínio de cerrado, Assis, SP. Scientia Florestalis, v. 56, p. 135-144, 1999.
Evans, H.E.; Eberhard, M.J.W. The Wasps. Ann Arbor: University of Michigan Press, 1970. 265 p.
Faegri, K.; Van der Pijl, L. The principles of pollination ecology. 2ed. Oxford: Pergamon Press, 1976.
Felfili, J.M.; Silva, J.M.C. Biogeografia do Bioma Cerrado. Estudo fitofisionômico na Chapada do Espigão Mestre do São Francisco. UnB. Brasília, DF, 2001. 152 p. Fernandes, F.L.; Mantovani, E.C.; Neto, H.B.; Nunes, V.V. Efeitos de variáveis
ambientais, irrigação e vespas predadoras sobre Leucoptera coffeella(Guérin-Méneville) (Lepidoptera: Lyonetiidae) no cafeeiro. Neotropical Entomology v.38, p. 410-417, 2009.
Ferretti, A.R. Recomposição florestal com essências nativas do Estado de São Paulo. In: Crestana MSM Florestas. Sistemas de recuperação com essências nativas, produção de mudas e legislações. Cati p. 1-22, 2006.
23
Ribeiro JF, Fonseca CEL, Silva JCS (Org). Cerrado: caracterização e recuperação
de matas de galeria. Planaltina: Embrapa Cerrados, 2001. p 815-870.
Garófalo, C.A. Comunidades de abelhas (Hymenoptera, Apoidea) que utilizam
ninhos-armadilhas em fragmentos de matas do Cerrado de São Paulo. An. Encontro
Abelhas, Ribeirão Preto, v.4, p. 121-128, 2000.
Glufke, C. Espécies florestais para recuperação de áreas degradadas. Porto Alegre:
Fundação de Zoobotânica do Rio Grande do Sul, 1999. 48 p.
Krombein, K.V. Trap - nesting wasps and bees. Life histories and associates.
Washington :Smithsonian Inst. Press, 1967. 570 p.
Krug, C.; Alves-dos-Santos, I. O Uso de Diferentes Métodos para Amostragem da
Fauna de Abelhas (Hymenoptera, Apoidea), um Estudo em Floresta Ombrófila
Mista em Santa Catarina. Neotropical Entomology v. 37, p. 265-278, 2008.
Lacerda, D.M.A.; Figueiredo, P.S. Restauração de matas ciliares do rio Mearim no
Município de Barra do Corda-MA: seleção de espécies e comparação de
metodologias de reflorestamento. Acta Amazônica, v. 39, p. 295-304, 2009.
Loyola, R.D.; Martins, R.P. Trap-nest occupation by solitary wasps and bees
(Hymenoptera: Aculeata) in a forest urban remanent. Neotropical Entomology, v.
35, p. 41-48, 2006.
Loyola, R.D.; Martins, R.P. Habitat structure components are effective predictors of
trap-nesting Hymenoptera diversity. Basic and Applied Ecology, v.9, p. 735-742,
2008.
Martins, S.V. Recuperação de matas ciliares. Viçosa: Aprenda fácil, 2001. 143 p.
Metzger, J.P. O Código Florestal tem base científica? Revista Natureza & Conservação,
v. 8, p.1-5, 2010.
Michener, C.D. The bees of the World. Baltimore: The Johns Hopkins University Press,
2000. 913 p.
Morato, F.E.; Campos, L.A.O. Efeitos da fragmentação florestal sobre vespas e abelhas
solitárias em uma área da Amazônia Central. Revista brasileira de Zoologia, v. 17,
p. 429-444, 2000.
Naiman, R.J.; Anderson, E.C. Streams and rivers: their physical and biological
variability. The rain forests of home: profile of a North American bioregion. Island
24
Oliveira, R.; Schilindwein, C. Searching for a manageable pollinator for acerola Orchards: the solitary oil-collecting bee Centris analis (Hymenoptera: Apidae: Centridini). J Economic Entomology, v.102, p. 265-273, 2009.
O’Neill, K.M.; Larson, D.P.; Kemp, W.P. Sweep sampling technique affects estimates to the relative abundance and community composition of grasshoppers (Orthoptera: Acrididae). J Agriculture Urban Entomology, v.19, p. 125-131, 2002.
Peruquetti, R.C.; Campos, L.A.O.; Coelho, C.D.P.; Abrantes, C.V.M.; Lisboa, L.C.O. Abelhas Euglossini (Apiade) de áreas de Mata Atlântica: abundância, riqueza e aspectos biológicos. Revista Brasileira de Zoologia, v. 16, p. 101-118, 1999.
Pickett, S.T. Differential adaptation of tropical tree species to canopy gaps and its role in community dynamics. Tropical Ecology, v. 24, p. 68-84, 1983.
Pires, E.P.; Pompeu, D.C.; Souza-Silva, M. Nesting of solitary wasps and bees (Hymenoptera: Aculeata) in the Biological Reserve Boqueirão, Ingaí, MG. Bioscience, v. 28, p. 302-311, 2012.
Primo, D.C.; Vaz, L.M.S. Degradação e perturbação ambiental em matas ciliares: estudo de caso do rio Itapicuru-açu em Ponto Novo e Filadélfia Bahia. Revista Eletrônica da Faculdade de Tecnologia e Ciências, v. 7, p. 1-11, 2006.
Rodrigues, R.R.; Gandolfi, S. Conceitos, tendências e ações para recuperação de florestas ciliares. In: Rodrigues RR, Leitão Filho HF Matas ciliares: conservação e recuperação. 3(ed) São Paulo. Edusp/Fapesp. 2004. 248 p.
Sporl, C.; Ross, J.L.S. Análise comparativa da fragilidade ambiental com aplicação de três modelos. GEOUSP - Espaço e Tempo, v. 15, p. 39-49, 2004.
Uezo, A.; Cullen, J.L. Da fragmentação florestal à restauração da paisagem: aliando conhecimento científico e oportunidades legais para a conservação. In: Paes A, Uezu A, Lorini ML, Cunha A. Conservação da biodiversidade com SIG. São Paulo. Oficina de Textos. 2012. 239 p.
Vilhena, A.M.G.F.; Augusto, S.C. Polinizadores da aceroleira Malpighia emarginata DC (Malpighiaceae) em área de cerrado no Triângulo Mineiro. Bioscience, v. 23, p. 14-23, 2007.
Wissmar, R.C.; Beschta, R.L. Restoration and management of riparian ecosystems: A catchment perspective. Freshwater Biology, v. 40, p. 571-585, 1998.
25
Capítulo 1
Efficiency of restored riparian forests on the conservation of trap-nesting solitary bees
and wasps communities in a Cerrado area, on the Southeastern Brazil
26 Abstract
Riparian forests play an important role in the preservation of water bodies and, in
maintaining biodiversity, act as refuges for many species or can be used as ecological
corridors. The restoration of these environments is critical to the recovery of pollinator
communities. In this work we study the role of restored riparian forest on the riverbanks
of the Volta Grande Reservoir (MG and SP) in maintaining bees and wasps
communities that nest in preexisting cavities so as to verify if there is difference in
richness, abundance and composition throughout the seasons (dry and wet), if there is a
relationship between richness and abundance of these individuals and their parasites and
the degree of specialization of parasites in relation to the hosts. Were recorded 12
species of wasps, eight of bees and nine species of parasites of the orders Coleoptera,
Diptera, Hymenoptera in addition to mites and fungi. The wasps Trypoxylon nitidum
(Smith, 1856), Pachodynerus grandis Willink & Roig-Alsina, 1998 and the bee Centris (Heterocentris) analis (Fabricius, 1804) were the most abundant. Areas with longer time of restoration showed higher species richness. However the abundance was higher
in most recent areas. The composition of bees and wasps assembly has not changed
between the four seasonal periods evaluated, although it has changed between sampling
areas. The richness and abundance were higher in warmer and rainy periods. The rate of
bee mortality was 45.68% and 48.62% for the wasps. Richness parasites correlated
positively with the richness and abundance of bees and wasps. The network of
host-parasite interaction has a modular configuration with generalists and specialists.
Recovered riparian forests are providing environmental conditions necessary for the
maintenance of bees and wasps communities that nest in preexisting cavities.
27 Introduction
Riparian forests have a high variation in structure, composition and distribution
of their species, contributing to different ecological formations, because their
physiognomy, floristic and structural features (Kageyama et al 2001, Rodrigues 2000).
From its association with adjacent vegetation formations are formed mixed forests with
species characteristic of the two physiognomies (Ivanauskas et al 1997). However,
riparian forests are different from adjacent formations due to the larger size and density
of the trees by the proximity to water courses (Andrade et al 2002). This vegetation
greatly influences the preservation of biodiversity by providing refuge and food for
wildlife, act as ecological corridor, maintain the microclimate and water quality, and
contain the erosion processes, thus preventing silting of bodies of water (Kageyama et al 2001).
However riparian forests have been extensively affected by human activities,
mainly by the construction of dams to generate electricity. The dammed water inundates
riparian forests and exterminates the whole community associated with it (Ferreira &
Dias 2004). We know very little about how this kind of human impact affects
interspecific interactions, such as: plant-pollinator, predator-prey and parasite host
(Cane et al 2006, Loyola & Martins 2006).
Bees and wasps respond to habitats disturbances in different ways. Studies have
show a decline in species richness and abundance of bee communities in more disturbed
habitat or smaller fragments (Aizen & Feinsinger 1994; Gathmann et al 1994) are often
difficult to be interpreted (Cane 2001).
Solitary bees and wasps are pollinators and predators on several species of plants
28
resources for predators and parasites (Roubik 1989, Krombein 1967, Barbieri Júnior et
al 2012).
Data on communities of trap-nesting bees and wasps and their natural enemies
have been used in research on habitat quality (Frankie et al 1988, Tscharntke et al
1998), the effects of habitat fragmentation and of landscape complexity on community
composition and predator-prey interactions (Morato & Campos 2000, Steffan-Dewenter
2002, Kruess & Tscharntke 2002, Klein et al 2006) and how urban environments can
support such insects (Tommasi et al 2004, Zanette et al 2005).
Although Brazil has a very rich fauna of solitary bees and wasps and many
studies with such bees have been made in the last years (Aguiar & Martins 2002,
Alves-dos-Santos 2003, Buschini & Woiski 2008, Loyola & Martins 2006, Morato & Campos
2000, Nascimento & Garófalo 2010, Pires et al 2012, Viana et al 2001), there is still
very little biological information available about the role of recovered riparian forest on
the assemblage of cavity-nesting bees and wasps.
In this study we examined species richness, abundance and composition of
cavity-nesting bees and wasps and its relations with their parasites in five areas of
recovered riparian forest of Volta Grande Reservoir, Southeastern Brazil. We assume
that there is difference on richness, abundance and composition of the bees and wasps
communities along the seasons (wet and dry) and that is a positive relation to host
richness and abundance and their nests parasites. Also we quantify the degree of
29 Material and methods
Study area
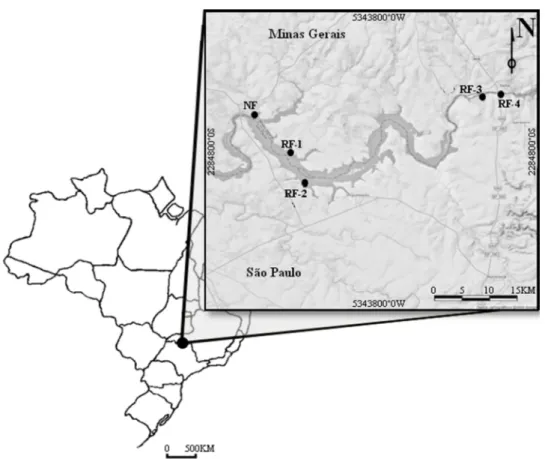
The study was conducted in five patches of reforested riparian forest (hereafter
referred to as RF-1, RF-2, RF-3, RF-4 and RF-5) in the region of Volta Grande
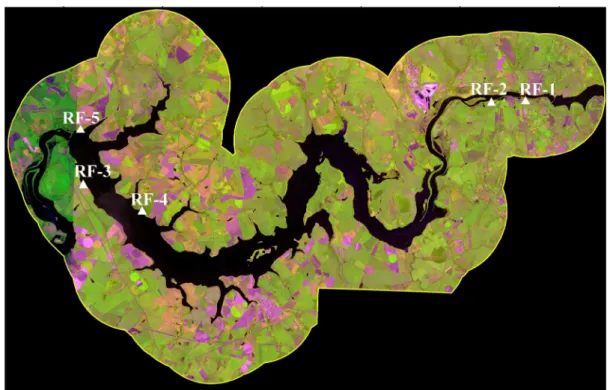
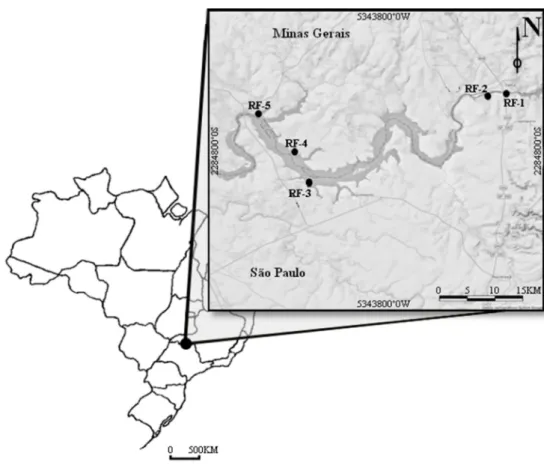
Reservoir located on the Rio Grande river (20°01'54" S / 48°13'17" W) (Figure 1).
Figure 1: Location of the five areas of restored riparian forest around the Hydroelectric Power Plant of Volta Grande reservoir, between Minas Gerais and São Paulo states.
Most of original riparian vegetation of the sampling areas were removed and/or
flooded in the construction of the reservoir. Sampling units were replanted in a single
replanting event that included between 30 and 40 species of trees. The landscape is a
mosaic of monoculture (sugar-cane, rubber-tree plantation) and fragments of Cerrado
30
as of 20 years. In the area RF5 have been occurring a natural secondary succession since
30 years ago.
Table 1: Geographic location of the sampling areas by Municipality – State and UTM georeference, beyond the age of accomplished restoration process and width of the riparian forest.
Sampling Unit Municipality Location (UTM) Age Width RF1 Igarapava, SP 23k-208838/7787209 10 100 RF2 Igarapava, SP 23k-205429/7786874 20 100 RF3 Miguelópolis, MG 22k-800294/7786874 10 30 RF4 Água Comprida, MG 22k-798082/7775015 20 30 RF5 Conc. Alagoas, MG 22k-791531/7783262 30 400
The average rainfall over the last 11 years indicates that the region has a
well-defined dry season between the months of May and October and a rainy season from
November to April, with the average annual precipitation reaching 1,506 millimeters
(Roldão & Assunção 2012).
Sampling Design
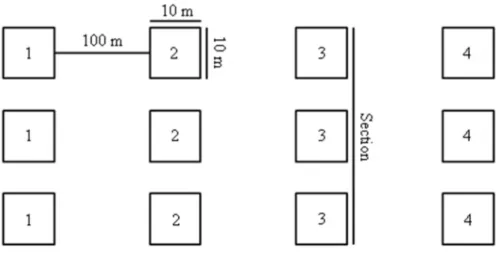
In each of the five sampling units, 12 plots of 100 m2 were installed (Figure 2).
Trap-nests were made using woodblock which measured 07x25x12cm. The woodblocks
had 45 tubular cavities distributed equally between 6, 9 and 12 mm diameters and
10cm-deep, the cavities were made randomly, and in each cavity was placed a black
cardboard tube with the respective diameters. In each plot were installed two
woodblocks placed 1.5 meters high in the most central tree, forming a sampling unit
(hereafter “SU”) totaling 90 trap-nests, making a total of 5.400 nesting sites in the
31
Figure 2: Distribution of treatments in the study area with five treatments, each replicated four times. Sampling was performed between November 2013 and September 2014. Sampling events were separated into the start of the dry season (Ds; May to July), end of the dry season (De: August to October), start of the rainy season (Rs; November to
January), and end of the rainy season (Re; February to April). The subdivision of the rainy and dry seasons into start and end periods provided a more reliable scale for
temporal analysis.
Trap-nests were inspected each 20 days; those occupied by bees or wasps were
collected; the occupied cardboard tube was replaced with a empty one of the same
measure, to keep the number of empty trap constant; and taken to laboratory. In the
laboratory, nests were individually placed in small capped glass tubes with mesh, kept
in chambers with temperature of 25° C degrees until adults hatched. Adults were pinned
and identified to species or genus (morphospecies), using taxonomic keys. Voucher
species are deposited at the Coleção Taxonômica do Laboratório de Biodiversidade,
32
Analyses
Following Klein et al. (2006), we calculated the ratio of sampled to expected species richness for trap-nesting species at each SU. Expected values were obtained from the number of occupied trap nests found in each of the five collecting points inside the SUs, using 100 randomizations with the Jacknife estimator of first order.
A Generalized Linear Model (GLM) was used to verify the relationship of the number of cells built by bees and wasps with temperature and precipitation, richness and abundance among the four seasonal periods and also if the rate of mortality changed between the sampling units using the R software (R Development Core Team, 2013). To detect differences in species composition among the dry and rainy seasons was used multivariate analysis of variance permutation (PERMANOVA) (Anderson 2001). GLM was also used to compare the values of Shannon-Wiener Index (H') and Evennes (J') calculated for each sampling unit. Differences in species composition were evaluated by quantitative similarity (Bray-Curtis) using the Past 3.06.
Host-parasite network
33
there were groups of parasite species strongly associated with a particular set of host, as
expected in a modular network. For this we used the modularity index (M) based on
Simulated Annealing (SA) (range 0–1) (Guimera et al 2004) using the software MODULAR (Marquiti et al 2014). To characterize the degree of specialization or partitioning, among two parties in the network, we used the H 2' index (Blüthgen et al
2006) that range from 0 (highly generalist) to 1 (highly specialized). The significance
was tested using Monte Carlo. Generalized Linear Model (GLM) was used to test
whether the number of parasite species has a positive relationship with richness and
abundance of hosts.
Results
Trap-nesting bees and wasps assemblage
Were recorded 12 species of wasps and eight species of bees that occupied 368
trap-nests (Table 2). Of these, 299 (81.5%) were built by wasps, being 30.68% of
Vespidae with six species, 35.58% Crabronidae with two species, 13.85% Sphecidae
with two species and one species of Pompilidae (1.4%). Of the 69 nests built by bees,
34 Table 2: Solitary wasps and bees captured in trap-nests at the Riparian forest of Volta Grande Reservoir
from November 2013 to February 2014 and from May to September 2014.
Family Species
Sampling units
Total RF1 RF2 RF3 RF4 RF5
Vespidae
Ancistroceroides sp. 3 0 13 0 0 16
Hypancistrocerus sp. 1 1 2 0 0 2
Minixi brasilianum (de Saussure,
1875) 0 2 1 0 1 4
Pachodynerus anodontus Willink
& Roig-Alsina, 1998 0 1 0 0 1 2
Pachodynerus guadulpensis (de
Saussure, 1853) 0 0 0 3 0 3
Pachodynerus grandis Willink &
Roig-Alsina, 1998 17 36 21 4 5 82
Pachodynerus pannus Willink &
Roig-Alsina 1998 1 0 1 0 0 2
Crabronidae Trypoxylon nitidum (Smith,
1856) 51 30 26 0 2 109
Trypoxylon (Trypargilum)
lactitarse Saussure 13 8 0 1 0 22
Sphecidae Penepodium sp1 11 29 0 0 4 44
Penepodium sp2 3 0 0 0 4 7
Pompilidae Pepsis sp1 0 0 1 4 0 5
Apidae Centris (Heterocentris) analis
(Fabricius, 1804) 8 12 0 3 3 26
Centris (Heterocentris) tarsata
(Smith, 1874) 0 1 0 0 6 7
Centris (Heterocentris) terminata
(Smith, 1874) 4 1 0 0 3 8
Euglossa(Euglossa)
melanotricha Moure, 1967 0 1 1 0 1 3
Tetrapediini sp. 0 0 0 0 2 2
Megachilidae Antidium sp. 0 0 0 0 1 1
Megachile (Melanosaurus) sp. 9 1 6 0 2 18
Megachile sp. 3 0 0 0 0 3
Total: 123 123 72 15 35 368
Three nests were occupied simultaneously by Centris (Heterocentris) tarsata
35 The species accumulation curve indicated that the sampled richness is still
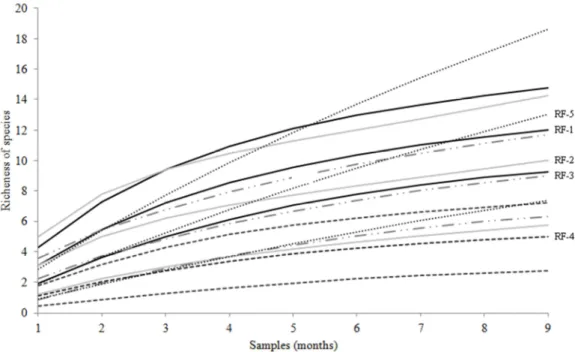
somewhat lower than expected with the effort employed (Figure 3) and there may be a
higher species diversity of trap-nesting bee and wasp on reference site. The lower
estimated richness was recorded in RF5 (s = 22) with 59.38% of total species. However
RF2 registered 83.10% of the expected species (Table 3).
Figure 3: Expected richness of trap-nesting bee and wasps recorded from September 2013 to November
2014 on the five sampling units of the Volta Grande reservoir, Brazil.
Table 3: Values of Jacknife for trap-nesting bees and wasps for the five sampling units of riparian forests
of Volta Grande Reservoir, Brazil.
Sampling units Jacknife 1 (± SD) %
RF1 15.56±1.41 77,12
RF2 14.44±2.35 83,10
RF3 12.56±1.94 71,65
RF4 6.78±1.18 73,74
RF5 21.89±3.64 59,38
In the sampling units RF5 (S = 13), RF1 and RF2 and (S = 12 each) were
registered the highest richness of bees and wasps (Table 4). In the RF1 and RF2
36 was higher in RF4 (J '= 0.9502) and RF5 (J' = 0.9375). The values of diversity index did
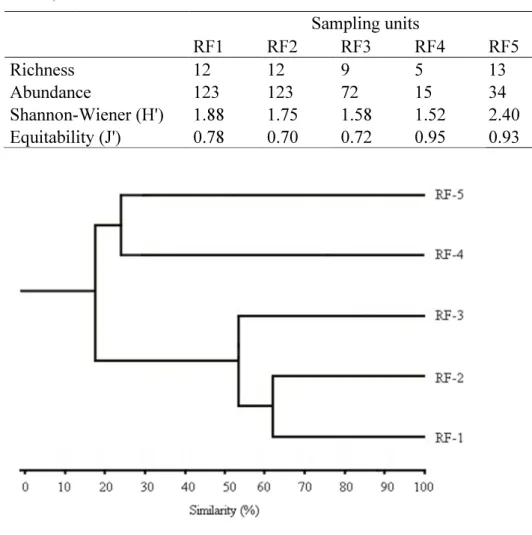
not vary significantly between the sampling units (F = 2.804 p = 0.06392). RF-1 and
RF-2 shared 62% of the species and RF-3 shared with RF-1 and RF-2 53%. RF-4 and
RF-5 shared only 25% of species (Figure 4).
Table 4: Values of Diversity Index and Eveness for Five sampling units of riparian forests of the Volta Grande Reservoir, Brazil.
Sampling units
RF1 RF2 RF3 RF4 RF5
Richness 12 12 9 5 13
Abundance 123 123 72 15 34
Shannon-Wiener (H') 1.88 1.75 1.58 1.52 2.40
Equitability (J') 0.78 0.70 0.72 0.95 0.93
Figure 4: Cluster of sampling units based on composition of trap-nesting bees and wasps at the Riparian Forest of Volta Grande Reservoir, Brazil.
37 1853), Megachile, Tetrapediini sp. and Antidium sp., they were found in only one sampling unit.
Seasonality
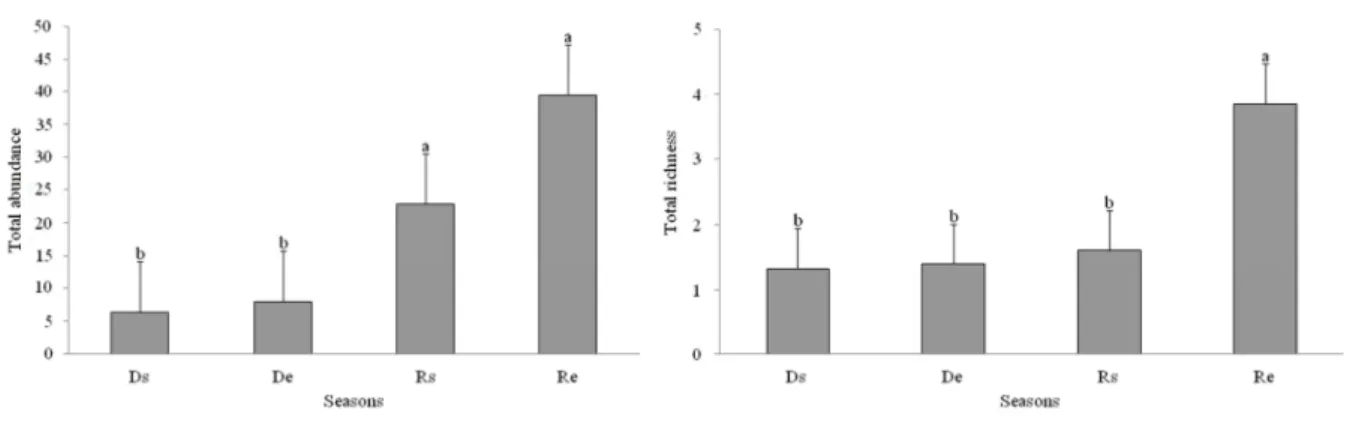
There was no difference in species composition among the dry and rainy seasons
(F = 1.5732; R² = 0.08037; p = 0.172). However, abundance of bees and wasps was
higher during the rainy season (Rs and Re) (F = 10,331, p <0.0001), with increasing
occupancy in Rs and Re (Figure 5A), the highest value for total richness was found at
the end of the rainy season (Re), (F = 9.4704, p <0.0001) (Figure 5B). The number of
cells founded by wasps was positively related with temperature (F = 7.6827, P = 0.012)
and precipitation (F = 5,879; P = 0.026).
Figure 5: Total abundance (A) and richness (B) of trap-nesting bee and wasps at the four season periods
sampled in the riparian forests of the Volta Grande Reservoir, Brazil. Ds: start of Dry Season, De: end of
Dry Season, Rs: start of Rainy Season and Re: end of Rainy Season. Bars are mean and different letters
means statistical difference.
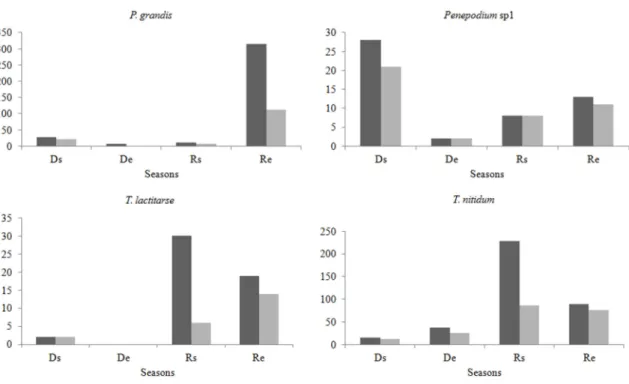
Some wasp species (P. grandis, T. nitidum and Penepodium sp.1) were recorded
throughout the sampling period, but the number of founded nests varied throughout the
seasons (Figure 6). Some species such as P. anodontus and P. pannus nested only in Re,
38
Despite the low abundance of Pepsis sp., this species has been recorded only during the
Re period.
Figure 6: Total number of cells built by the four more abundant trap-nesting bees and wasps at the Riparian Forest of Volta Grande Reservoir, throughout the four sampling season period. Ds: start of Dry Season, De: end of Dry Season, Rs: start of Rainy Season and Re: End of Rainy Season.
C. analis was the bee species that built the largest number of cells (n = 76) and
also the only registered during the entire sampling period (Figure 7). The other bee
species showed a pattern of more seasonal occupation. C. tarsata for example was
recorded only on De period, Megachile sp. only on Re, C. terminata in Ds and Re,
Tetrapediini sp. on Rs and Anthidium sp. on Ds. E. melanotricha built their nests in
three of four sampled seasonal periods, only being absent on Re, although it has
39
Figure 7: Frequency of occupation for the most abundant species of solitary bees that nest in pre-existing cavities in restored riparian forests at the Hydroelectric Power Plant of Volta Grande reservoir – MG, Brasil.
Mortality
Of 1.271 cells built by bees and wasps, 618 (48.62%) did not eclode adults. In
regard to mortality, 44.6% occurred in the egg stage, 18.8% larva, 31.5% pupal stage
and 0.7% adult stage. The highest mortality was recorded in Ds and De (57.14% and
56.28%, respectively), the seasonal periods Rs and Re mortality was lower (40.11% and
49.44%, respectively). The parasitic insects have accounted for 14.72% of total
mortality. There was no difference of mortality rate among bees and wasps on sampling
units (F = 2.2964, p = 0.1187). Although, the mortality rate for wasps was a little higher
than bees (49.14% and 46.78% respectively).
The mortality rate was higher among most the abundant species, like T. nitidum
and P. grandis, at the beginning of the dry season (78.57% and 86.66%, respectively)
(Figure 6). Some less abundant species also showed high mortality rate, for example,
Ancistroceroides sp. with 70.83% and Hypancistrocerus sp. with 85.71%.
Of the 232 cells built by bees, 45.68% did not eclode adults and 16.3% of the
40 (representing 61.63% of the bees), and only 8.82% were parasitized. For C. analis the highest mortality occurred in Ds (76.47% of cells built). For E. melanotricha no
individual emerged from the cells built in Rs. Interaction host X parasite
Nine parasite species were recorded and in seven trap-nests there were more
than one parasite species. Mites (Pyemotes sp) and fungi were responsible for the mortality of 10 individuals of Pachodynerus. The wasps P. grandis (n=35), T. nitidum
(n=22) and Penepodium sp1 (n=10) had their nests heavily parasitized. However, in Penepodium sp1 we found only one parasite species different from P. grandis and T. nitidum.
We found 25 (23% of the total expected) host-parasite interactions among and the conectance was of 0.231. The average number of interactions (medium degree) was
1.2, and each parasite species shared 72% of the hosts (overlapping niche = 23%). Each host species shared 56% of parasites on average (niche overlap = 22%).
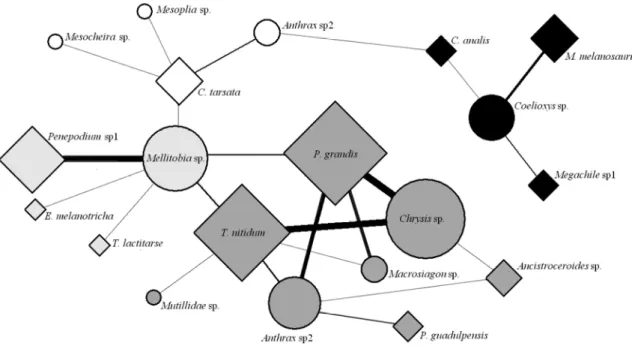
Four subgroups were identified in the host-parasite network (Figure 8); two
groups formed by one parasite species (Mellitobia sp. and Coelioxys sp.), each with three interacting host species, a group formed by one host species (C. tarsata)
interacting with three species of unique parasites, and a group formed by several species of parasite sharing various hosts. The greatest number of cells parasited was recorded among the most abundant host species (T. nittidum and P. grandis) and the most
abundant parasite was Chrysis sp.. Although the most abundant parasites (Chysis sp., Anthrax sp1. and Mellitobia sp.) have shared most abundant hosts (P. grandis, T.
nittidum, Penepodium sp1.) there was a greater preference of Mellitobia sp. to
Penepodium sp1 and Macrosiagon sp. P. grandis. The parasite Coelioxys sp formed,
41 specialization (H2 '= 0.52), with lower rates than expected by chance (H2obs = 2.71;
H2ran = 3.2 ± 0.05; p <0.001) due to the high number of hosts shared by the parasites
inside the modules.
Figure 8: Network of interactions between parasites and their hosts. The squares represent the hosts and
the circles represent parasites, forms sizes is related to the number of interactions that each species and
thickness of the lines represents the number of times that these interactions have been carried out.
Parasites: Anthrax sp1, Anthrax sp2, Coelioxys sp., Chrysis sp., Macrosiagon sp., Mellitobia sp., Mesocheira sp., Mesoplia sp., Mutillidae sp. Hots: Ancistroceroides sp., C. analis, C. tarsata, E. melanotricha, M. melanosaurus, Megachile sp1, P. grandis, P. guadulpensis, Penepodium sp1, Penepodium sp2, T. nitidum, T. lactitarse.
The sample unit RF4 showed higher connectance (C = 50%), followed by RF5
(C = 44.44%) and RF1 (C= 40%), RF3 and RF2 showed the lowest values (C = 26.66%
and C = 22.24%, respectively). On the sampling units RF2 and RF4 we registered more
specialized parasites to hosts (H2= 1.000 and 0.75 respectively). For sampling unit RF3
42
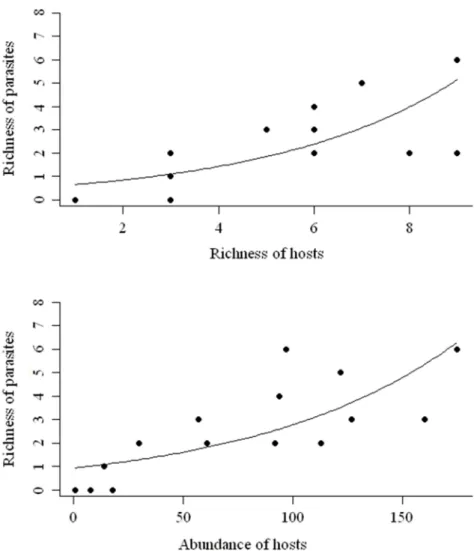
a positive relationship to richness (F= 19,703; p = 0.0008) and abundance (F= 5.207; p=
0.041) of hosts (Figure 9A and B).
Figure 9: Relationship between richness of parasites with richness (A) and abundance (B) of hosts
(trap-nesting bees and wasps) recorded on the riparian forests of Volta Grande Reservoir, Brazil.
Discussion
Bees and wasps assemblage
The number of trap-nests occupied by solitary bees and wasps, as found in our
study, was relatively high compared with data obtained in other studies conducted in
43 riparian forest fragments (Aguiar & Martins 2002, Alves-dos-Santos 2003, Loyola &
Martins 2006, Pires et al. 2012, Melo & Zanella 2012).
The high nest-occupancy reveals the importance of these recovered remnants in
sheltering species with narrow ecological tolerance like E. melanotricha, some
Megachile and Antidium typical from Cerrado environments (Jesus & Garófalo 2000,
Morato & Campos 2000). Therefore, recovering riparian fragments is very important for
the maintenance of pollinators, like bees and wasps. On these riparian forest fragments,
we found a species richness higher than that found for other Cerrado environments
(Pires et al 2012, Carvalho 2011) with nine and 16 species, respectively.
Effort base curves of species accumulation indicate that bees and wasps species
richness may actually be higher in all of five sampling units. However, for equal
sampling effort, we trapped greater species richness in the more “complex” area (RF5),
possibly reflecting resource availability (Lassau et al 2005). The heterogeneity of
structure RF5 may support more potential niches for a functionally diverse set of fauna,
and is likely to support a greater range of food webs than less complex habitats (Klopfer
& MacArthur 1960). However, on RF5 was recorded lower abundance of bees and
wasps, which might also be explained by the higher availability of habitat. Since
preexisting cavities constitute one of the dimensions of the niche of these organisms, in
the areas with greater availability of niches, such as RF5, the rate of trap-nests
occupancy tends to be lower (Pires et al 2012, Morato & Campos 2000). This may be
occurring in RF1 and RF2, which recorded higher values of abundance. The riparian
RF1 is younger (10 years since the recovery) and in RF2 the disturbances take places
frequently, mainly in the vegetation, generated by factors associated with edge effect
and anthropic impacts (Gustavo Junior, pers obs). The large number of singletons and
44 with trap-nests (Buschini & Woiski 2008 Gazola & Garofalo 2009, Pires et al 2012).
The type of landscape around the sampling units was a factor that determined the structure of the assembly of trap-nesting bees and wasps, as observed in the Deikumah
et al (2013) studies. The species composition on sampling units inserted into matrix with sugar-cane monoculture was the most similar, with common species found in open environments as T. nitidum, T. lactitarse, P. grandis and C. analis (Pires et al 2012). However, the sampling units embedded in a matrix predominantly with fragments of Cerrado showed a richer community, probably due to greater availability of sites for foraging and greater number of niches, allowing the occurrence of species most sensitive to disturbances in the environment such as Antidium sp. and Tetrapediini sp. (Morato 2001, Teixeira et al 2011). The lower richness and abundance of RF4 compared with other sampling units probably occurred by the use of agrochemicals and pesticides in the surrounding forest monocultures (Malaspina et al 2008) leading to the predominance of species of generalist habits.
Although most recorded species shows wide distribution, we found some peculiarities relating the occurrence on sampling units. The most abundant species of wasps occurred in almost all sampling areas, especially the species Trypoxylon and P. grandis species. In previous studies conducted in different biomes (Cerrado, Pires et al
45 communities can be a result of greater availability of prey, which is raised according to the increase in the intensity of land use. Thus, in most anthropogenic environments there is an increase of the prey Eumeninae (lepidopteran larvae) for the supply of cells. This could then explain the high abundance P. grandis in RF1 and RF2 sampling unit suffering more intensely with anthropic impacts. The lower abundance of C. tarsata was not expected. In other studies this species tends to present higher abundance compared with other bee species (Pires et al 2012, Aguiar & Martins 2002, Viana et al 2001). According to Aguiar et al (1998), having the availability of sites for nesting, C. tarsata is able to successfully nest, even in areas of open vegetation. However, the
number of nests founded by this species was much lower compared to C. analis. On sampling units studied, C. analis seems to be more efficient using "cavity", as well as having built more nests in four of the five areas and nests during the entire sampling period. As recorded by Aguiar & Martins (2002) C. tarsata occurred only in dry periods, which shows a more marked seasonality for this species, which may have contributed to a small number of built nests, resulting in a lower abundance. Although we have not measured food resource in the sampling unit, plant species are preferably used by this group (collecting bees oil) helping to explain the high abundance (Machado 2004).
46 & Campos (2000).
Although records of bees within the Euglossa genus are relatively common in
trap-nests, their occupancy rate is always lower compared to other species (Morato &
Campos 2000, Melo & Zanella 2012, Gazola & Garófalo 2009, Aguiar et al 2005).
Species of this genus are restricted to forests, apparently being more sensitive and
vulnerable to fragmentation and habitat loss (Giangarelli et al 2009). This could explain
the lower number of nests founded by E. melanotricha since part of the areas studied
shows intermediate stages of succession and are more susceptible to disturbances from
outside the fragments. The lower occupancy by species of this genus in fragmented
environments has also been reported by Morato & Campos (2000). Sofia & Suzuki
(2004) suggested that the presence of qualifying them as bioindicators.
There was no difference in species diversity among the studied areas. All
sampling areas are in secondary stages of regeneration, and according to Michener
(1979) the species richness of bees recorded for a given area may be influenced by
temporal variation of species composition and high proportion of rare species. Once
these areas have been modified and have not returned to its original state, areas with
intermediate stage of recovery may have similarities regarding the diversity of species.
Seasonality
More important nest occupation in Rs and Re seem to be indirectly associated
with the climate. We know that low temperatures and low humidity have a negative
effect on the activities performed by solitary bees and wasps, which have lower
thermoregulatory capacity (Camillo et al 1995, Loyola & Martins 2006).
Indeed, of all species collected during this study, those belonging to the genus
Trypoxylon and Pachodynerus occupied fewer trap-nests when the temperature and
47 temperature are key climate variables for bees and wasps as they affect the availability
of resources in the environment, mainly prey for the supply of the cells, thus directly or
indirectly influence the nesting activities. Both species of wasps mentioned above,
provide different food resources for their immature; the species of the Crabronidae
family include species Trypoxylon, that hunt spiders to feed their immature; the species
of Vespidae, which includes the species of the genus Pachodynerus, hunt immature of
Lepidoptera. In this case the availability of prey may have influenced the nesting
activity. Penepodium sp1 did not present any preference for rainfall, since we detected
nests been founding all over year and mostly on RF2. Garcia & Adis (1993) also found
the same for this genus. According to Junk et al (1989) for Penepodium species the
availability of prey (cockroach of Eunyctibora genus) all over year could explain its
high abundance.
Other species of wasps were most influenced by the seasonality and nested in a
single seasonal period, for example Ancistroceroides sp., Hypancistrocerus and P.
guadulpensis. These Eumeninae species share the same trophic niche (lepidopteran
larva) so they may compete for prey to provision their nests. P. grandis was the first
species to occupy the nests, in the rainy season and occurred in less abundance in the
dry season. The other species also colonized nests during the dry season when the
abundance of P. grandis was smaller, perhaps in an attempt to avoid competition for
food. A similar result was observed by Teixeira (2011). The spatial and temporal
separation may be one way to minimize the overlap of species with similar niches
(Frankie & Newstrom 1993, Frankie et al 1988, Morato et al 1999).
In general, although no significant correlation was found between temperature,
rainfall, and nesting frequency of bee species in our study, the climate directly
48 1996) and the resources availability for nest construction and maintenance (mud, preys
and pollen). Consequently, climate factors indirectly affected solitary bee and wasp
nesting (Aguiar & Martins 2002, Camillo et al 1995, Pereira et al 1999). However the
abundance of Centris was higher in the rainy season and this seems to be a tendency for
species of this genus. Aguiar & Garófalo (2004) also found significant correlations
between number of nests occupied by C. tarsata and monthly precipitation in the State
of Bahia.
Composition
Different assemblage of trap-nesting bees and wasps occupied the restored
riparian forest fragments. The older and more complex sites shared less species with the
less complex sites. Two species of wasps (T. nitidum and P. grandis) were the most
responsible for the compositional differences, preferring sites more disturbed, with
greater availability of the clearings, as reported by Fye (1972).
Wasps established more nests than bees. For wasps, the availability of prey
(spiders, lepidopteran larvae, cockroaches) is always higher and is available all year.
Bees however, rely on a single resource (flowers) that is not always available all year.
The largest number of nests occupied by wasps seems then be common as in other
studies was also recorded the same pattern (Pires 2012, Melo & Zanella 2012, Loyola &
Martins 2006, Klein et al 2006, Krobein 1967).
According to Holzschuh et al (2010), besides the availability of prey, habitat
characteristics may also explain how the amount of nests occupied by bees tends to be
higher in more heterogeneous and preserved habitat. For the wasps, areas more open
facilitate the encounter and capture of prey as recorded by Pires et al (2012) and
Buschini (2005). In fact, the highest richness of bees was found in RF5, area control in
49 and RF2, areas in intermediary stage of succession and that undergoes frequent
anthropogenic disturbances (Gustavo Araújo, pers obs).
In their study of wasps of the genus Pachodynerus, Buschini & Buss (2010)
reported preference of this group by open areas with occurrence of herbaceous
flowering plants in high abundance. We also found a similar result as we recorded
higher species richness of Pachodynerus in RF1, RF2 and RF3, areas with constant
external disturbances due to the planting and harvesting of sugar-cane. This could
enable the development of herbaceous plants, which serve as a food source for adult
wasps.
Mortality
The high mortality of bees and wasps seems to be common and was recorded in
different studies of physiognomies, ranging from 42% to 55% (Aguiar et al 2005, Jesus
& Garófalo 2000 Couto & Camilo 2007). In this study 47.15% of cells founded suffered
some kind of damage, either by parasites or fungi. According Frankie & Newstrom
(1993) elevated temperatures may result in the early death of the immature stages of
life, however in our work, we could not find any relationship between mortality of
immature and temperature variation.
Mortality in wasps was higher than in bees and this happened because the wasps
were more parasitized than bees. It was also recorded by Loyola & Martins (2006) who
attribute it to greater species richness of parasites associated with wasps than bees.
Host-Parasite Interaction
The positive relationship found between the richness of parasites and the
richness and abundance of bees and wasps, has been recorded by Vizentin-Bugoni et al
(2014) who attributed it to an increase in vacant niches (more hosts) which could be
50
of knowledge about the parasites of trap-nesting bees and wasps makes difficult to
compare the network of interactions between different regions.
Bees and wasps formed, together with its associated parasites, an interactive
networking, with some species-specific interactions previously reported besides some
new associations. More specialist species as Mesocheira sp. only parasitized nests of C.
tarsata (Aguiar & Martins 2002, Aguiar et al 2005, Gazola & Garófalo 2009). Anthrax
and Coelioxys, are generalists and parasitized nests of C. analis, M. melanosauros and
several species of wasps (Gazola & Garófalo 2009). Macrosiagon sp. parasitized only
nests of wasps. According Krombein (1967) e Callan (1981) this genus usually
parasitize the nests of Eumeninae, being rarer in Sphecidae, which confirms our results.
This preference may be related to the type of resource used by the host to feed the
immature.
The most abundant parasites (Mellitobia, Anthrax sp1 and Chrysis sp1) also
seem to be more generalists and also parasitize the most abundant hosts, as T.nitidum
and P. grandis. Although Anthrax sp1, had presented preference for parasitize P.
grandis and Mellitobia sp. for Penepodium sp1. The parasite-host associations found in
this study have already been reported in other studies such as Garcia & Adis (1993).
In this study, the interaction network formed by the parasites and their hosts
seems to follow the hypothesis of asymmetric abundance proposed by Vázquez et al
(2007). According to this hypothesis the abundance of species in the community
determinates the frequency and the power of interaction networks, resulting in
asymmetric structures.
The formation of sub-groups (modules) on the network of host-parasite
interaction observed in this study is expected in communities where there is strong
51 modular presentation in a network of interactions may be related to the evolution history
of the community, and the pressures suffered by these species, as the most abundant
hosts are also the most parasitized. This may be connected to the dispersal ability of
species of parasites (Poulin 2010, Lewinsohn et al 2006).
The connectance between network components in each sampling unit varied.
Higher values were found between RF4 and RF5 (50% and 44.44%, respectively),
although we have not considered the abundance with which each parasite species
occurred in each host species.
A higher number of parasitized nests were found in RF1 units, RF2 and RF3
where were the most abundant species were also recorded. These sampling units are
surrounded by an extensive matrix of sugar-cane, which may be adding for the greater
competitive pressure on the parasites, due to the degree of isolation.
An increased specialization of the parasites to their hosts was observed in the
RF4 area (H2 = 1.000), but this may not be representing greater specialization as both
the richness and abundance in this area were low, compared with the other sample units.
However, in RF2 we found a level of specialization slightly lower than in RF4 but the
richness and abundance were higher. According to Vázquez et al (2007) the abundance
of species within the community could be a major factor mediating this specialization.
Restored riparian forests constitute important areas of refuge for bees and wasps
that nest in preexisting cavities. Seasonality strongly influences the richness and
abundance of these insects and their associated parasites, probably due to differences in
the amount and types of features offered. The community establishment of these
Aculeata was also linked to the vegetation structure (type of surrounding matrix, width
52 References
Aguiar AJC, Martins CF (2002) Abelhas e vespas solitárias em ninhos-armadilha na
Reserva Biológica Guaribas (Mamanguape, Paraíba, Brasil). Revista Brasileira de
Zoologia 19: 101-116.
Aguiar CML, Garófalo, CA (2004) Nesting biology of Centris (Hemisiella)
tarsata (Hymenoptera, Apidae, Centridini). Revista Brasileira Zoologia 21: 477-486.
Aguiar CML, Garófalo CA, Almeida G F (2005) Trap-nesting bees (Hymenoptera,
Apoidea) in áreas of dry semideciduous Forest and caatinga, Bahia, Brasil. Rev Brasil
Zool 22: 1030-1038.
Aguiar LMS, Machado RB, Zortéa M, Mendes SL, Rylands AB (1998) Working with the
Iucn red list categories: The experience of the workshop on the conservation of brazilian
bats. Boletim do Museu de Biologia Mello Leitão, Nova Série, Santa Teresa 9: 3-11.
Aizen MA, Feinsinger P (1994) Forest fragmentation, pollination, and plant reproduction in
a Chaco dry forest, Argentina. Ecology 75:330–351.
Almeida-Neto M, Guimarães P, Guimarães PR, Loyola RD, Ulrich W (2008) A consistent
metric for nestedness analysis in ecological systems: reconciling concept and
measurement. Oikos 117: 1227–1239.
Alves-dos-Santos I (2003) Trap-nesting bees and wasps on the university campus in Sao
Paulo, southeastern Brazil (Hymenoptera: Aculeata). Journal of the Kansas
Entomological Society 76:328-334.
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance.
Austral Ecology 26:32-46.
Andrade LA, Felfili JM, Violatti L (2002) Phytosociology of an area of" cerrado denso" at
the RECOR-IBGE. Acta botânica brasílica 16:225-240.
Barbieri Junior CA, Dias AMP (2012) Braconidae (Hymenoptera) fauna in native,
degraded and restoration áreas of the Vale do Paraíba, São Paulo state, Brasil. Brazilian
Journal of Biology 72: 305-310.
Bluthgen N, Menzel F, Bluthgen N (2006) Measuring specialization in species interactions
networks, Ecology 6:9.Begon M, Townsend CR, Harper JL (2007) Ecologia: De
Indivíduos a Ecossistemas. Porto Alegre, Artmed, 752p.
Buschini MLT (2005) Species diversity and community structure in trap-nesting bee in
53 Buschini MLT, Buss CE (2010) Biological aspects of different species of Pachodynerus (Hymenoptera; Vespidae; Eumeninae). Brazilian Journal of Biology 70: 623-629.
Buschini MLT, Niesing F, Wolff LL (2006) Nesting biology of Trypoxylon (Trypargilum) lactitarse Saussure (Hymenoptera, Crabronidae) in trap-nests in southern Brazil. Brazilian Journal of Biology 66:919–929.
Buschini MLB, Woiski TD (2008) Alpha–beta diversity in trap-nesting wasps (Hymenoptera: Aculeata) in Southern Brazil. Acta Zoologica (Stockholm) 89: 351–358. Callan EMC (1981) Further records of Macrosiagon (Coleoptera: Rhipiphoridae) reared
from eumenid and sphecid wasps in Australia. Australian Entomol Mag 7:81-83.
Camillo E, Garófalo CA, Serrano JC, Muccilo G (1995) Diversidade e abundância sazonal de abelhas e vespas solitárias em ninhos armadilhas (Hymenoptera, ApocIita, Aculeata). Revista Brasileira de Entomologia 39: 459-470.
Cane JH (2001) Habitat fragmentation and native bees: a premature verdict? Conservation Ecology 5: 3.
Cane JH, Minckley R, Roulston T, Kervin L, Williams NM (2006) Multiple response of desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecological Applications 16:632–644.
Carvalho SM (2011) Diversidade de abelhas e vespas solitárias (Hymenoptera, Apoidea) que nidificam em ninhos-armadilhas disponibilidados em áreas de Cerrado em fragmentos próximos de Mata Estacional Semidecidual – MG. 63f. Dissertação (Mestrado em ecologia) Programa de Ecologia e Conservação dos Recursos Naturais da Universidade Federal de Uberlância. Uberlândia
Couto RM, Camilo E (2007) Influence of temperature on the immatures mortality of Centris (Heterocentris) analis (Hymenoptera, Apidea, Centridini). Rev Zool 97: 51-55. Deikumah JP, McAlpine AM, Maron M (2013) Matrix Intensification Alters Avian
Functional Group Composition in Adjacent Rainforest Fragments. Plos one 8:1-10. Ferreira DA, Dias HC (2004) Situação atual da Mata Ciliar do Ribeirão São Bartolomeu em
Viçosa, MG. Rev Árvore 28: 617-623.
Frankie GW, Newstrom LE (1993) Nesting-habitat preferences of selected Centris bees species in Costa Rican dry forest. Biotropica 25: 322-333.