REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
PublicaçãoOficialdaSociedadeBrasileiradeAnestesiologiawww.sba.com.br
SCIENTIFIC
ARTICLE
Postoperative
surveillance
in
neurosurgical
patients
---usefulness
of
neurological
assessment
scores
and
bispectral
index
Silvia
Herrero
a,∗,
Enrique
Carrero
b,
Ricard
Valero
b,
Jose
Rios
c,d,
Neus
Fábregas
baUniversidaddeBarcelona,HospitalClínic,SaladeRecuperaciónPós-Anestésicos,Villarroel,Barcelona,Spain bUniversidaddeBarcelona,HospitalClínic,ServiciodeAnestesiología,Villarroel,Barcelona,Spain
cUniversitatAutònomadeBarcelona,LaboratóriodeBioestatísticaeEpidemiologia,Barcelona,Spain dHospitalClínic,IDIBAPS,BioestadísticayPlataformadeGestióndeDatos,Barcelona,Spain
Received25July2015;accepted22September2015 Availableonline12April2016
KEYWORDS
Bispectralindex monitor;
Craniotomyelective; Neurologic
examination; Neurosurgical procedures; Postoperativecare; Postoperative complications
Abstract
Backgroundandobjectives: WeexaminedtheadditiveeffectoftheRamsayscale,Canadian Neurological Scale(CNS), NursingDelirium Screening Scale(Nu-DESC), andBispectral Index (BIS) tosee whetheralongwith theassessmentofpupilsandGlasgowComaScale (GCS)it improvedearlydetectionofpostoperativeneurologicalcomplications.
Methods:Wedesignedaprospectiveobservationalstudyoftwoelectiveneurosurgerygroupsof patients:craniotomies(CG)andnon-craniotomies(NCG).Weanalyzetheconcordanceandthe oddsratio(OR)ofalteredneurologicalscalesandBISinthePost-AnesthesiaCareUnit(PACU) forpostoperativeneurologicalcomplications.Wecomparedtheisolatedassessmentofpupils andGCS(pupils-GCS)withalltheneurologicassessmentscalesandBIS(scales-BIS).
Results:In theCG(n=70),16 patients(22.9%)hadneurologicalcomplicationsinPACU.The scales-BISregisteredmorealterationsthanthepupils-GCS(31.4%vs.20%;p<0.001),weremore sensitive(94%vs. 50%)andallowed amorepreciseestimate for neurologicalcomplications inPACU(p=0.002;OR=7.15, 95%CI=2.1---24.7 vs.p=0.002;OR=9.5,95%CI=2.3---39.4).In the NCG(n=46),therewere noneurologicalcomplicationsinPACU.The scales-BIS showed alterationsin 18cases (39.1%)versus 1(2.2%) withthe pupils-GCS(p<0.001).Altered CNS on PACU admissionincreased therisk of neurologicalcomplications inthe ward (p=0.048; OR=7.28,95%CI=1.021---52.006).
Conclusions: Appliedtogether,theassessmentofpupils,GCS,Ramsayscale,CNS,Nu-DESCand BISimprovedearlydetectionofpostoperativeneurologicalcomplicationsinPACUafterelective craniotomies.
©2016SociedadeBrasileiradeAnestesiologia.Publishedby ElsevierEditoraLtda.Thisisan openaccessarticleundertheCCBY-NC-NDlicense( http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗Correspondingauthor.
E-mails:sherrern@clinic.ub.es,silviahn1505@gmail.com(S.Herrero). http://dx.doi.org/10.1016/j.bjane.2015.09.003
PALAVRAS-CHAVE
MonitorBIS;
Craniotomiaeletiva; Exameneurológico; Procedimentos neurocirúrgicos; Cuidadosno pós-operatório; Complicac¸õesno pós-operatório
Monitoramentodepacientesneurocirúrgicosnopós-operatório---utilidade dosescoresdeavaliac¸ãoneurológicaedoíndicebispectral
Resumo
Justificativaeobjetivos: AvaliamosoefeitoaditivodaescaladeRamsay,EscalaNeurológica Canadense(CNS),EscaladaEnfermagemdeTriagemdeDelírio(Nu-DESC)eÍndiceBispectral (BIS)paraobservarse,juntamentecomaavaliac¸ãodaspupilasedaEscaladeComadeGlasgow (GCS),melhoravaadetecc¸ãoprecocedecomplicac¸õesneurológicasnopós-operatório.
Métodos: Projetamos um estudo observacional, prospectivo, de dois grupos de pacientes submetidosàneurocirurgiaeletiva:craniotomia(GrupoC)enão-craniotomia(GrupoNC). Anal-isamosaconcordânciaearazãodechance(OR)dealterac¸õesnasescalasneurológicasenoBIS nasaladerecuperac¸ãopós-anestesia(SRPA)paracomplicac¸õesneurológicasnopós-operatório. Comparamosaavaliac¸ãoisoladadaspupilasedaGCS(pupilas-GCS)comtodasasescalasde avaliac¸ãoneurológicaeoBIS(escalas-BIS).
Resultados: NoGrupoC(n=70),16pacientes(22,9%)apresentaramcomplicac¸ões neurológ-icasnaSRPA.Asescalas-BISregistrarammaisalterac¸ões queaspupilas-GCS(31,4%vs.20%;
p<0,001), foram mais sensíveis (94% vs. 50%) e permitiram uma estimativa mais precisa dascomplicac¸ões neurológicasnaSRPA(p=0,002;OR=7,15, IC95%=2,1---24.7 vs.p=0,002; OR=9,5,IC 95%=2,3---39,4).No grupo NC(n=46),não houve complicac¸ões neurológicas na SRPA.Asescalas-BISmostraramalterac¸õesem18casos(39,1%)versusumcaso(2,2%)comas pupilas-GCS(p<0,001).Alterac¸ãonaCNSnaadmissãoàSRPAaumentouoriscodecomplicac¸ões neurológicasnaenfermaria(p=0,048;OR=7,28,IC95%=1,021-52,006).
Conclusões:Aplicadosemconjunto,aavaliac¸ãodaspupilas,GCS,escaladeRamsay,CNS, Nu-DESCeBISmelhoraramadetecc¸ãoprecocedecomplicac¸õesneurológicasnopós-operatóriona SRPAapóscraniotomiaseletivas.
©2016SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.Este ´eum artigoOpen Accesssobumalicenc¸aCCBY-NC-ND( http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Neurosurgical patients have a high risk of neurologi-cal complications in the immediate postoperative period increasingbothmorbidityandmortality1andrequiring
spe-cializedpostoperativecare.TheNeurologicalIntensiveCare Evaluationscoreisasimpleassessmentdevelopedand val-idated specifically to assess the postoperative neurologic statusofpostoperativecardiacpatients.2,3Itisnota
com-pleteneurologicassessment evaluation,andwouldnotbe enoughfor patientswhohaveundergonebrain surgery.To thebestofourknowledgethereisnotsuchaneurological score validated for the postoperative neurosurgical pop-ulation in the Post Anesthesia Care Unit (PACU). In our institution,wetypicallyusetheassessmentofthepupillary sizeandreactivityandGlasgowComaScale(GCS)4
evaluat-ingthemobilityofallfourlimbs.
Thereareseveralvalidatedclinicalneurological assess-ment scales, as the National Institute of Health Stroke Scale,5 theMini-Mental StateExamination,6 andthe
Diag-nosticandStatisticalManualofMentalDisorders.7Amajor
drawback of these assessment scales is that they are long and complex, and therefore not easily applicable fortheimmediatepostoperativeperiod.8Otherneurologic
assessment scales, such asthe Ramsay scale,9 the
Cana-dian Neurological Scale (CNS)10 and the Nursing Delirium
ScreeningScale(Nu-DESC)11(AppendixA)arebettersuited
tothe assessment ofextubated postoperative neurosurgi-calpatientsinthe PACU.Themain limitations ofperiodic
evaluationswithclinicalscalesinclude:inter-observer sub-jectivityandhighinter-observervariability,11discontinuous
records,observerdifficultyindifferentiatingamonglevels ofdeepsedation12andtheoverworkednursingcareresulting
fromtheirsystematicapplication.
Thebispectralindex(BIS)(BISTMbrainfunction
monitor-ingsystem,Covidien,Boulder,USA)isanindexderivedfrom theanalysisofelectroencephalographyscaledtocorrelate withthedepthofhypnosis.Todayithasbecomestandard monitoring during generalanesthesia. BIS hasalso proven useful in predicting states of excessive sedation.13 While
somestudiesshowapoorcorrelationbetweenBISandthe clinical sedationscales,12,14---16 other studies showthat BIS
correlates withclinical scales ifBIS recordingsassociated withelevatedelectromyography(EMG)areexcluded.14,17
BecauseBISrecordsanddisplayscontinuously,ithasthe potential advantage of acting as an early warning signal of any of the neurological complications that are asso-ciated with a decreased level of consciousness. On the other hand, the clinical neurologic assessment scales are alsousefultodetectneurologicalalterationsnotassociated withdecreases inlevel ofconsciousness(motoror speech impairment,forinstance).Todate,wehavenotfoundany publishedstudies thathavecomparedBISwithother neu-rological assessment scales inpostoperative neurosurgical patients.
complicatedcasesaccordingtothecriteriaof neuroanesthe-siaandneurosurgery.ThePACUisequippedfor apatient’s overnightmonitoring ifnecessary andthe patient---nursing ratiocan varyfrom2:1 to4:1depending onthepatient’s clinicalstatus.
Theaimofourstudywastoassesstheusefulnessof neu-rologicalassessmentscalesandBISfortheearlydetection ofpostoperativeneurologicalcomplicationsinthe neurosur-gicalpatient.Ourhypothesiswasthattheadditiveeffectof theRamsayscale,theCNS,theNu-DESCandBIStotheusual assessmentofpupilsandGCSwouldimproveearlydetection ofpostoperativeneurologicalcomplications.
Methods
This wasan observational prospective study for 6 months (October2011toApril2012).Thestudywasapprovedbythe EthicsandResearchCommitteeatourInstitution(Number 2011/6854,dated22-9-2011,Chairman:GomisR).Weasked permissionandobtainedwritteninformedconsentfromthe patients.
Studypopulation
After obtaining informed consent, all patients scheduled forelectiveneurosurgeryandhavingrecoveredinthePACU duringthestudyperiodweredividedintotwogroups, the Craniotomy Group (CG), and the Non-Craniotomy Group (NCG).Weexcludedpatientswhorefusedtoparticipatein thestudy,thosewhodonot speakor understand the lan-guage, patients who remained intubated postoperatively, andthosewhoweretransferredtotheSICUforpostoperatve care.IntheCG wealsoexcludedpatientswhounderwent ventriculoperitonealshuntorcranioplastyand,intheNCG, thosewhounderwentsubcutaneousinternalpulsegenerator implant,cerebrospinalfluid(CSF)infusiontestunder seda-tionorpatientswhohadpreviouslyundergonecraniotomy.
Designanddatacollection
Wecollectedthefollowingdemographicdata:age,sex,the AmericanSocietyofAnesthesiologists(ASA)physicalstatus classificationsystem,diagnosis,typeofsurgery, and anes-thetictechnique.Wecollectedintraoperativedataincluding blood loss greater than 1000mL,hemodynamic instability requiringtheadministrationofvasoactivedrugs(repeated
dosesoraninfusion),thepresenceofrelativehypoxemiafor agivenFiO2administered(PaO2/FiO2ratio<250),anisocoria
uponemergence,anddeath.
Themainparametersregisteredwere:
(a) Neurological assessment scales: size andreactivity of thepupils,GCS,Ramsayscale, CNS, andtheNu-DESC (AppendixA).
(b) BISrecording:BISvalue,signalqualityindex(SQI),EMG andsuppressionratio(SR). The BISsensorwasplaced onthecontralateral sideof thecraniotomy. In poste-riorfossacraniotomysurgery andinthe NCG,theBIS wereplacedinthedominanthemispheretostandardize themeasurement.Ineachpatient,weusedthesameBIS sensorforallBISrecordings.Incasesofpoorsignal qual-ity,thesensorwasrepositionedonthesamesideuntil anadequatesignalwasobtained.AllBISmeasurements wererecordedbeforeanyneurologicalevaluation. (c) Postoperativeneurologicalcomplications.Neurological
complicationsweredefinedasanyclinicallysignificant neurological alteration documented in the patient’s medicalrecordduringtheirtimeinthePACU,inthe neu-rosurgicalward,andanycomputedtomographyand/or magneticresonanceimaging(CT-MRI)findingconsidered asacomplicationaccordingtotheneurosurgeon crite-ria.
(d) Follow-up:afterdischarge,anyvisittotheemergency departmentorreadmissionwasrecordedduringthefirst postoperativemonth.
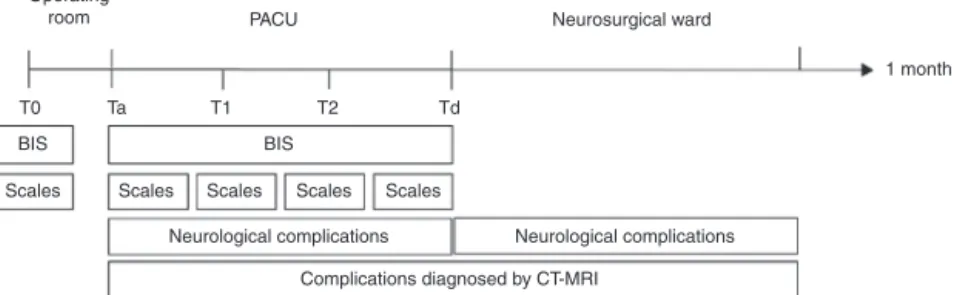
The time data recorded were: T0 --- baseline, patient arrivalat thepre-anesthesiabeforeadministrationof pre-medication; Ta --- patient admission to the PACU after surgery,oncestabilized; T1,T2 ---recordsin each nursing shift,Td---PACUdischargetotheneurosurgicalward(Fig.1). WealsorecordedanyepisodeswithBIS<70maintainedfor 1min after admission to the PACU, regardless of time of study.
We defined as altered neurologic scales the follow-ingcriteria: anisocoriaand/or loss of pupillaryreactivity; GCS<15except GCS=14 withocular response=3; Ramsay scale1or≥4;CNS<10,exceptCNS=8.5withlevelof con-sciousness=1.5; Nu-DESC>0. We excluded those patients withGCS=14 (ocular response=3) and CNS=8.5 (level of consciousness=1.5)becausethesescores might bedue to residualeffects of anesthesia or analgesic effects of opi-oids. We defined as altered BIS a BIS value below 70 for
Operating
room PACU
T0
BIS BIS
Neurological complications Neurological complications
Complications diagnosed by CT-MRI
Scales Scales Scales Scales Scales
Ta T1 T2 Td
Neurosurgical ward
1 month
1min. It wasnot considered an alteration ifthe value of theneurologicalscale or theBISwasalreadyabnormalat baseline.
Wedefinedasalteredpupils-GCSoralteredscales-BISif atleastoneoftheassessments(pupils,GCSorpupils,GCS, Ramsayscale,CNS,Nu-DESC,BIS)wasalteredaccordingto ourdefinition.
Whenanabnormalityinaneurologicalassessmentscales wasobserved,theanesthesiologistinchargeofthepatient’s carewould proceedto treatthe patientaccording tothe establishedprotocolsineachcase.
WecomparedBISamongpatientswithandwithout post-operativeneurologicalcomplicationsandwedidananalysis toseeiftherewasaBISthresholdabletopredictthe pres-enceofpostoperativeneurologicalcomplications.
We analyzed the concordance between altered neuro-logicscalesandalteredBISandtheconcordancebetween alteredneurologicscalesoralteredBISandtheoccurrence of neurological complications in PACU, in the ward, and abnormalities diagnosed onthe CT-MRI.We also analyzed theabilityofthealteredpupils-GCSandalteredscales-BIS assessments to identify neurological complications in the PACU.Finallywestudiedestimatesofriskfortheoccurrence ofpostoperativeneurologicalcomplications.
Statisticalanalysis
Forthe analysis of demographicdata, age wasexpressed asmean±standarddeviation(SD)andanalyzedwithttest for independentgroups. Categorical variables such assex andASA,wereexpressedinabsolutevalues(valid percent-age)andwereanalyzedusingChi-squareortheFisherexact testandMann---WhitneyUtestforordinalvariables,asASA. The BIS, SQI and EMG values were expressed as median andinterquartilerange(IQR). TheanalysisofBIS,SQIand EMGbetweeneach recording timefrombaselinetest was performedusingtheWilcoxonsignedranks.Foranalysisof differencesbetweengroupsateachtimeofregistration,we appliedtheMann---WhitneyU.Theanalysisofdifferencesin BISvaluesbetweenpostoperativeneurologicalcomplicated anduncomplicatedpatientswasdone withMann---Whitney
Utest. The receiveroperating characteristic(ROC) curve wasusedtodeterminateif,globally, theBIShada predic-tivevaluefortheoccurrenceofpostoperativeneurological complications andwe calculated the likelihood ratio pos-itive (LR+),18 defined as ratio sensitivity/(1-especificity),
for possible candidates of a BIS cut-off value. We per-formedabinarylogisticregressionanalysisinordertoobtain estimatesofrisk fortheoccurrenceofpostoperative neu-rologicalcomplicationsbycalculatingoddsratios(OR)and 95%confidenceintervals(95%CI)ofthealteredneurological scalesandBIS.
TheCochranQtestwasusedfortheintragroupanalysis ofthedifferencesbetweenthetimesofrecordingofaltered neurologicscalesandalteredBIS.TheMcNemar’stestwas appliedforcomparisonbetweendata recordingtimesand baselinedata.TheFisher exacttest wasusedtocompare theproportionsofalterationsintheneurologicscalesand theBISbetweenCGandNCG.Comparisonsbetweenaltered GCS-pupilsandalteredscales-BISwereperformedusingthe McNemartest.
Analysisofconcordancebetweenalterationsinthe neu-rological assessment scales or BIS and the occurrence of postoperativeneurologiccomplicationswasperformedusing KappaindexaccordingtoLandis&Kochcriteria.19We
con-sideredapoorconcordanceifkappavalueswerelessthan0, slightbetween0and0.20,fairbetween0.21and0.40, mod-eratebetween0.41and0.60,substantialbetween0.61and 0.80andalmostperfectbetween0.81and1.Wecalculated sensitivity,specificity,positiveandnegativepredictive val-uesofalteredpupils-GCSandalteredscales-BIStoidentify neurologicalcomplicationsinthePACU.Weconsidered sig-nificantp-values≤0.05andBonferronicorrectionwasused whenindicated. Analyseswere performedwiththeuse of SPSSsoftware(version18.0,SPSSInc.,Chicago,IL,USA).
Results
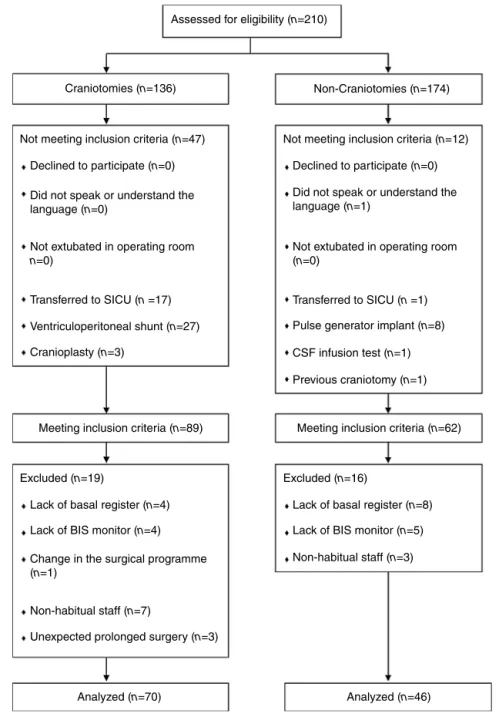
Of the151 patients whomet inclusioncriteriaduring the studyperiod,weanalyzedatotalof116patients,70inthe CGand46intheNCG.Nineteenpatientswereexcludedin theCGand16patientsintheNCG(Fig.2).
Subjectsdemographicandclinicalcharacteristics
CGpatients wereathigherpreoperative riskandtheNCG patients were older. In both groups, intravenous anesthe-sia wasthetechniquemost commonlyused:67(95.7%) in CGand45(97.8%)inNCG.In3cases(4.3%)theanesthetic techniquewasconscioussedation(CG)andin1case(2.2%) inhalation anesthesia(NCG). Intraoperative complications occurred in 16 cases (22.9%) in CG and 4 cases (8.7%) in NCG(Table1).
CG patients remained in the PACU an average of 16.5±5.6h,57ofthem(81.4%)spentthefirstpostoperative nightinthePACU(19.1±1.6h)while13(18.6%)were trans-ferred tothe ward thesame day of surgery(5.6±1.1h). In the NCG group, the average PACU stay was 5±5.4h. Four patients(8.7%), 3spinal cancer tumor resectionand 1 cervical microdiscectomy, spent the first postoperative nightinthePACU(20.1±2.8h),42(91.3%)weretransferred fromthePACUtothewardafterrecoveringfromanesthesia (3.6±2.6h).InNCGonly4patientswhostayedovernightin thePACUhaddatarecordedattimesT1andT2.
BISrecordings
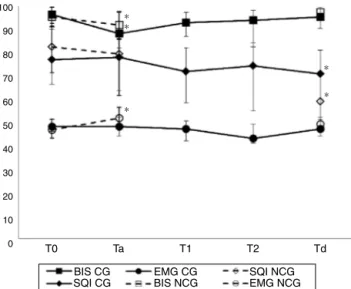
BISvaluesweresignificantlydecreaseduponPACUadmission whencomparedtobaselineintheCG (p<0.001)andNCG (p=0.002).TheSQIremainedabove70,exceptatdischarge on NCG (SQI=57.5). In both groups, the SQI at discharge decreased significantly compared to baseline (p<0.001). EMGwasbelow50ateveryrecordedtimeinbothgroups. In the NCG, EMG value increasedsignificantly upon PACU admissionwhencomparedtobaseline(p=0.001)(Fig.3).SR was0inallpatients.In16CGpatientsandonlyonepatient intheNCGtherewererecordedepisodeswithBISvalues<70 maintainedforoneminuteduringadmissioninthePACU.
Assessed for eligibility (n=210)
Craniotomies (n=136)
Not meeting inclusion criteria (n=47) Not meeting inclusion criteria (n=12)
Declined to participate (n=0) Declined to participate (n=0)
Did not speak or understand the language (n=0)
Did not speak or understand the language (n=1)
Not extubated in operating room
n=0)
Not extubated in operating room (n=0)
Ventriculoperitoneal shunt (n=27) Pulse generator implant (n=8)
CSF infusion test (n=1)
Previous craniotomy (n=1)
Transferred to SICU (n =17) Transferred to SICU (n =1)
Cranioplasty (n=3)
Lack of basal register (n=4) Lack of basal register (n=8)
Lack of BIS monitor (n=4) Lack of BIS monitor (n=5)
Change in the surgical programme (n=1)
Non-habitual staff (n=7)
Non-habitual staff (n=3)
Unexpected prolonged surgery (n=3)
Analyzed (n=70) Analyzed (n=46) Excluded (n=19) Excluded (n=16)
Meeting inclusion criteria (n=89) Meeting inclusion criteria (n=62) Non-Craniotomies (n=174)
Figure2 Studyflowchart(SICU,surgicalintensivecareunit;CSF,cerebrospinalfluid;BIS,bispectralindex).
patients who did not develop complications in the ward (median=93, IQR=12) and those who did (median=82, IQR=16)(p=0.019).TheROCcurveshowedthatthe mea-sured values of T2 BIS could predict the occurrence of neurological complications in the ward (p=0.019; area underthecurve0.743,95%CI=0.566---0.920),withtheBISof 90resultinginaLR+forcomplicationof3.8fromasensitivity valueof69%andspecificityof81.8%.
Alterationsintheneurologicalevaluationscales
andBIS
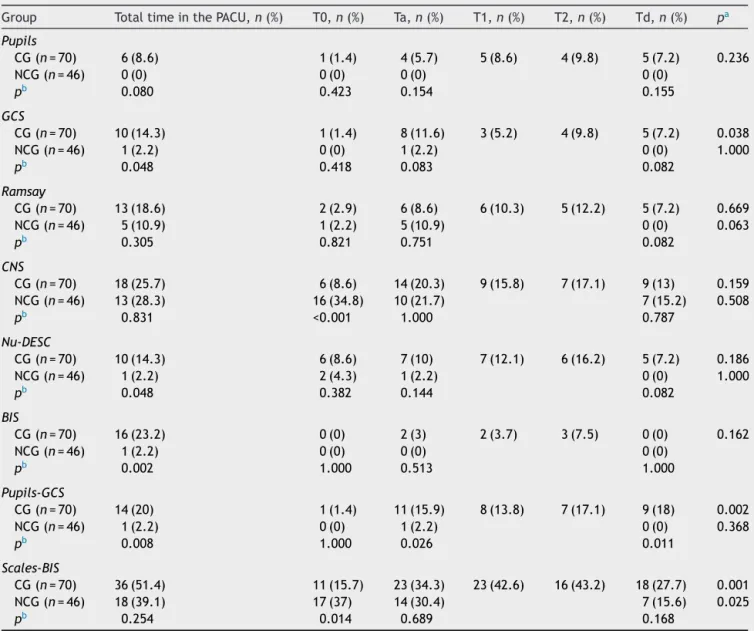
Thepupils-GCSassessmentwasalteredintheCGin14cases (20%)andthescales-BISin36cases(51.4%)(p<0.001).Inthe NCGwe registeredalteredpupils-GCSin1case(2.2%)and alteredscales-BISin18cases(39.1%)(p<0.001)(Table2).In
theCG,wefoundamoderateconcordancebetweenaltered BISandalteredGCS(=0.438;p=0.001)andafair
concord-ancebetween alteredBIS andalteredNu-DESC (=0.345; p=0.008) and altered BIS and altered Ramsay (=0.260; p=0.029).
Postoperativeneurologicalcomplications
Table1 Demographicandclinicalcharacteristicsofthesubjects.
Variable Total
(n=116)
CG (n=70)
NCG (n=46)
p
Age,years,mean±SD 54±16.5 51.1±16.5 58.5±15.7 0.018
Sex,women,n(%) 69(59.5) 43(61.4) 26(56.5) 0.6
ASA,n(%) <0.001
ASAI 10(8.6) 0(0.0) 10(21.7) <0.001
ASAII 68(58.6) 39(56.7) 29(63) 0.435
ASAIII 38(32.8) 31(44.3) 7(15.2) 0.001
Intervention,n(%)
Supratentorial 24(34.3)
Transsphenoidal 20(28.6)
Posteriorfossa 12(17.1)
Functionalsurgery 11(15.7)
Othercraniotomies 3(4.3)
Lumbarmicrodiscectomy 16(34.8)
Lumbarlaminectomy 12(26)
Spinaltumor 5(10.9)
Cervicalmicrodiscectomy 4(8.7)
Brachialplexussurgery 4(8.7)
Cervicallaminectomy 3(6.5)
Other 2(4.4)
Intraop.Complications,n(%) 20(17.2) 16(22.9) 4(8.7) 0.074
Hemodynamicinstability 11(9.5) 9(12.9) 2(4.3) 0.196
Bleeding 7(6) 5(7.1) 2(4.3) 0.704
Anisocoria 2(1.7) 2(2.9) 0(0) 0.522
Hypoxemia 1(0.9) 1(1.4) 0(0) 1.000
Mortality 0(0) 0(0) 0(0)
---CG,CraniotomyGroup;NCG,Non-CraniotomyGroup;ASA,AmericanSocietyofAnesthesiologists;SD,standarddeviation.Thesame
patientcoulddevelopseveralcomplications.
100
90
80
70
60
50
40
30
20
10
0 T0 Ta
BIS CG SQI CG
EMG CG BIS NCG
SQI NCG EMG NCG
T1 T2 Td
∗ ∗ ∗
∗
∗
Figure3 Changesinbispectralindexrecordsduringthe post-operativeperiod(Dataexpressedasmedianandinterquartile range(bars).T0, basal;Ta, admissiontothe post-anesthesia careunit(PACU);T1andT2,recordsineachnursingshift(only inCG);Td, dischargetotheneurosurgical ward;BIS, Bispec-tralIndex;SQI,signalqualityindex;EMG,electromyogram;CG, craniotomygroup(closedsymbols);NCG,non-craniotomygroup (opensymbols).*SignificantdifferencescomparedtoT0value ineachgroup).
were registered. In the second case, a CT wasrequested forexpressiveaphasiaandareducedGCS,showingalarge pneumocephalus.Therewerealterationsinthepupils,the Ramsayscale,theCNS,theNu-DESCandtheBIS.Anurgent CTwasrequestedinthethirdcaseafteraworseningaphasia wasnotedanditalsoshowedapneumocephalus.The neu-rological examination showedalterations in GCS,Ramsay, CNS,Nu-DESCandBISvaluesbetween33and55.Threeof the5patientswhobledintraoperativelymorethan1000mL showed neurological complications in the PACU (aphasia, agitation, anisocoria). In all three cases the neurological scaleswerealtered.
Table2 AlterationsintheneurologicalevaluationscalesandBISinthepostanesthesiacareunit.
Group TotaltimeinthePACU,n(%) T0,n(%) Ta,n(%) T1,n(%) T2,n(%) Td,n(%) pa
Pupils
CG(n=70) 6(8.6) 1(1.4) 4(5.7) 5(8.6) 4(9.8) 5(7.2) 0.236
NCG(n=46) 0(0) 0(0) 0(0) 0(0)
pb 0.080 0.423 0.154 0.155
GCS
CG(n=70) 10(14.3) 1(1.4) 8(11.6) 3(5.2) 4(9.8) 5(7.2) 0.038
NCG(n=46) 1(2.2) 0(0) 1(2.2) 0(0) 1.000
pb 0.048 0.418 0.083 0.082
Ramsay
CG(n=70) 13(18.6) 2(2.9) 6(8.6) 6(10.3) 5(12.2) 5(7.2) 0.669
NCG(n=46) 5(10.9) 1(2.2) 5(10.9) 0(0) 0.063
pb 0.305 0.821 0.751 0.082
CNS
CG(n=70) 18(25.7) 6(8.6) 14(20.3) 9(15.8) 7(17.1) 9(13) 0.159 NCG(n=46) 13(28.3) 16(34.8) 10(21.7) 7(15.2) 0.508
pb 0.831 <0.001 1.000 0.787
Nu-DESC
CG(n=70) 10(14.3) 6(8.6) 7(10) 7(12.1) 6(16.2) 5(7.2) 0.186
NCG(n=46) 1(2.2) 2(4.3) 1(2.2) 0(0) 1.000
pb 0.048 0.382 0.144 0.082
BIS
CG(n=70) 16(23.2) 0(0) 2(3) 2(3.7) 3(7.5) 0(0) 0.162
NCG(n=46) 1(2.2) 0(0) 0(0) 0(0)
pb 0.002 1.000 0.513 1.000
Pupils-GCS
CG(n=70) 14(20) 1(1.4) 11(15.9) 8(13.8) 7(17.1) 9(18) 0.002
NCG(n=46) 1(2.2) 0(0) 1(2.2) 0(0) 0.368
pb 0.008 1.000 0.026 0.011
Scales-BIS
CG(n=70) 36(51.4) 11(15.7) 23(34.3) 23(42.6) 16(43.2) 18(27.7) 0.001 NCG(n=46) 18(39.1) 17(37) 14(30.4) 7(15.6) 0.025
pb 0.254 0.014 0.689 0.168
BIS,BispectralIndex;PACU,PostAnesthesiaCareUnit;GCS,GlasgowComaScale;CNS,CanadianNeurologicalScale;Nu-DESC,Nursing DeliriumScreeningScale;Pupils-GCS,alterationinpupiland/orGCS;Scales-BIS,alterationinanyoftheneurologicalassessmentscales and/orBIS;CG,CraniotomyGroup;NCG,Non-CraniotomyGroup;T0,basal;Ta,admissioninthePACU;T1andT2,recordsineachnursing shift;Td,dischargefromthePACU.
a pwithingroup(time)comparison. b pbetweengroupcomparison.
valueupto38(EMG28,SQI96,SR0).Prodromalsymptoms includednauseaandhyperventilation.AnEEGrecorded24h later, whenthe patientwas asymptomatic, showeda left fronto-temporalcontinuousdelta-thetaslowingwithoutany associatedclinicalchanges.Thisneurologicalalterationwas reported as‘‘absenceseizure with an altered respiratory pattern’’ in the patient’s medical record. In the PACU, besidesalterationsinBIS,healsoshowedalteredGCS, Ram-say scoreand Nu-DESC. Onepatient (cerebral lymphoma) died from septic shock. In the CG, 5 urgent CT’s were requestedontheward:4forsevereheadache(3with pneu-mocephalus),and1forseizures(pneumocephalus).In4of the 5 urgent CT’s cases, impaired neurologic assessment scaleswereregisteredinthePACU.IntheNCG,onepatient developed paraplegia. The CT scan showed spinal cord
compressionfromamassivehematoma.Thepatient under-wentsurgeryagain,butremainedwithresidualparaparesis andrequiredapermanentindwellingurinarycatheter. Dur-ing his stay in the PACU, neurological assessment scales and BIS were not altered. A patient with Neuro-Behc¸et disease was scheduled for decompression of the sciatic nerveandglutealtumorexcision(lymphoma).Afterstarting chemotherapy,hewastransferredtotheICUwherehedied ofmultipleorganfailuretwoweeksaftersurgery.
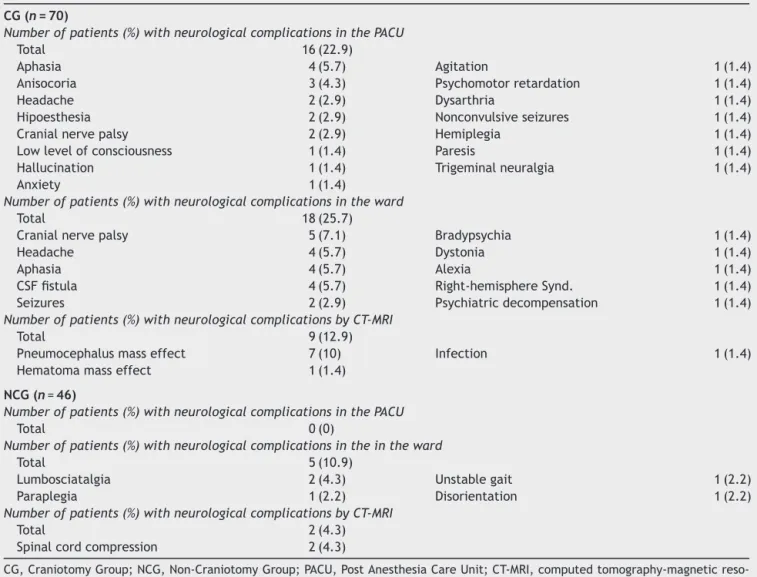
Table3 Postoperativeneurologicalcomplications.
CG(n=70)
Numberofpatients(%)withneurologicalcomplicationsinthePACU
Total 16(22.9)
Aphasia 4(5.7) Agitation 1(1.4)
Anisocoria 3(4.3) Psychomotorretardation 1(1.4)
Headache 2(2.9) Dysarthria 1(1.4)
Hipoesthesia 2(2.9) Nonconvulsiveseizures 1(1.4)
Cranialnervepalsy 2(2.9) Hemiplegia 1(1.4)
Lowlevelofconsciousness 1(1.4) Paresis 1(1.4)
Hallucination 1(1.4) Trigeminalneuralgia 1(1.4)
Anxiety 1(1.4)
Numberofpatients(%)withneurologicalcomplicationsintheward
Total 18(25.7)
Cranialnervepalsy 5(7.1) Bradypsychia 1(1.4)
Headache 4(5.7) Dystonia 1(1.4)
Aphasia 4(5.7) Alexia 1(1.4)
CSFfistula 4(5.7) Right-hemisphereSynd. 1(1.4)
Seizures 2(2.9) Psychiatricdecompensation 1(1.4)
Numberofpatients(%)withneurologicalcomplicationsbyCT-MRI
Total 9(12.9)
Pneumocephalusmasseffect 7(10) Infection 1(1.4)
Hematomamasseffect 1(1.4)
NCG(n=46)
Numberofpatients(%)withneurologicalcomplicationsinthePACU
Total 0(0)
Numberofpatients(%)withneurologicalcomplicationsintheintheward
Total 5(10.9)
Lumbosciatalgia 2(4.3) Unstablegait 1(2.2)
Paraplegia 1(2.2) Disorientation 1(2.2)
Numberofpatients(%)withneurologicalcomplicationsbyCT-MRI
Total 2(4.3)
Spinalcordcompression 2(4.3)
CG,CraniotomyGroup;NCG,Non-CraniotomyGroup;PACU,PostAnesthesiaCareUnit;CT-MRI,computedtomography-magnetic
reso-nanceimaging;CSF,cerebrospinalfluid.Thesamepatientcoulddevelopmorethanonecomplication.ComplicationsinthePACU,inthe
wardandbyCT-MRIwerenotadditive.
requiredanadditionaloperation:onefromaresidualtumor andthe other from a hematoma(patient withparaplegia detectedintheward).
Duringthefirstmonthaftersurgery20patients(17.2%) wenttotheemergencyroomorwerereadmittedtothe hos-pital:17(24.3%)intheCGand3(6.5%)inNCG(p=0.014). IntheCGgroupthemostcommoncauseofreadmissionwas aCSFfistula-5cases(7.1%).InCGgroup8patients(11.4%) underwentsecondorrepeatsurgery:5(7.1%)forCSFleaks, 2(2.9%)forsurgicalwoundinfectionand1(1.4%)for epis-taxis.IntheNCGonepatientwastreated forneuropathic pain,anotherforlumbosciatalgiaandthethirdforparalytic ileus.Nonerequiredfurthersurgery.
Concordancebetweenalterationsinneurological assessmentscalesorBISandpostoperative neurologicalcomplications
IntheCGgroup,wefoundmoderateconcordancebetween alterations of GCS and the occurrence of neurological
complications in the PACUand fair concordance between alterations of theRamsay scale, CNS,Nu-DESC or BISand theoccurrenceof neurologicalcomplicationsinthePACU. Alterations in CNS scores showed moderate concordance withabnormalities seenontheCT-MRI,alterations inGCS showed fair concordance. Equally,altered pupils-GCS and scales-BISshowedmoderateandfairconcordance, respec-tively,withneurologicalcomplicationsinthePACU(Table4). In the NCG, we could not analyze concordance in most cases, because at least one of the variables remained constant.
Predictivevalues
Table4 Analysisofconcordancebetweenalterationsinneurologicalassessmentscalesorbisandoccurrenceofpostoperative neurologicalcomplicationsincraniotomygroup.
Complicationsinthe PACU
Complicationsinthe ward
Complicationsdiagnosed byCT-MRI
Kappa p Kappa p Kappa p
Pupils 0.169 0.098 0.044 0.655 0.000 0.250
GCS 0.440 <0.001 0.213 0.058 0.399 0.006
Ramsay 0.263 0.027 0.136 0.244 0.143 0.326
CNS 0.379 0.001 0.177 0.138 0.530 <0.001
Nu-DESC 0.347 0.003 0.213 0.058 0.175 0.229
BIS 0.349 0.004 0.220 0.066 0.108 0.460
Pupils-GCS 0.407 0.001 0.274 0.020 0.240 0.097
Scales-BIS 0.381 <0.001 0.155 0.133 0.161 0.132
CraniotomyGroup(n=70).BIS,BispectralIndex;PACU,PostAnesthesiaCareUnit;CT-MRI,computedtomography-magneticresonance
imaging;GCS,GlasgowComaScale;CNS,CanadianNeurologicalScale,Nu-DESC,NursingDeliriumScreeningScale,Pupils-GCS,alteration
inpupiland/orGCS;Scales-BIS,alterationinanyoftheneurologicalassessmentscalesand/orBIS.
OddsratioofalteredneurologicalscalesandBIS forpostoperativeneurologicalcomplications
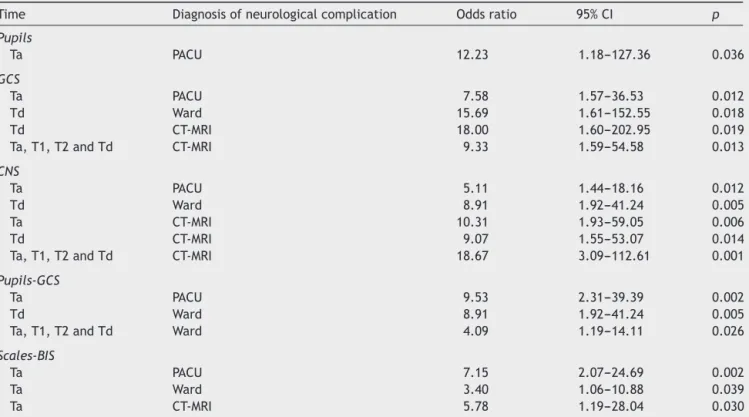
IntheCG,alteredpupils,GCS,CNS,pupils-GCSand scales-BIS were estimates of risk for postoperative neurological complications. On admission to the PACU, the scales-BIS
assessmentallowedamorepreciseestimateforthe occur-renceof neurologicalcomplications inthePACU(Table5). In the NCG, the probability of developing neurological complicationsinthewardamongpatientswithalteredCNS onadmissiontothePACUwas7.28timesofthosewith non-alteredCNS(p=0.048;95%CI=1.021---52.006).
Table 5 Significant odds ratio of altered neurological scales and BIS for the occurrence of postoperative neurological complicationsincraniotomygroup.
Time Diagnosisofneurologicalcomplication Oddsratio 95%CI p
Pupils
Ta PACU 12.23 1.18---127.36 0.036
GCS
Ta PACU 7.58 1.57---36.53 0.012
Td Ward 15.69 1.61---152.55 0.018
Td CT-MRI 18.00 1.60---202.95 0.019
Ta,T1,T2andTd CT-MRI 9.33 1.59---54.58 0.013
CNS
Ta PACU 5.11 1.44---18.16 0.012
Td Ward 8.91 1.92---41.24 0.005
Ta CT-MRI 10.31 1.93---59.05 0.006
Td CT-MRI 9.07 1.55---53.07 0.014
Ta,T1,T2andTd CT-MRI 18.67 3.09---112.61 0.001
Pupils-GCS
Ta PACU 9.53 2.31---39.39 0.002
Td Ward 8.91 1.92---41.24 0.005
Ta,T1,T2andTd Ward 4.09 1.19---14.11 0.026
Scales-BIS
Ta PACU 7.15 2.07---24.69 0.002
Ta Ward 3.40 1.06---10.88 0.039
Ta CT-MRI 5.78 1.19---28.04 0.030
CraniotomyGroup(n=70);BIS,BispectralIndex;GCS,GlasgowComaScale;CNS,CanadianNeurologicalScale;Pupils-GCS,alterationin
pupiland/orGCS;Scales-BIS,alterationinanyoftheneurologicalassessmentscalesand/orBIS;PACU,post-anesthesiacareunit;T0,
basal;Ta,admissioninthePACU;T1andT2,recordsineachnursingshift;Td,dischargefromthePACU;CT-MRI,computed
Discussion
The scales-BIS assessment let us detect a high percent-age of neurological alterations that were not detected using the traditional evaluation pupils-GCS. In the CG, thatfactconfirmsthataftercraniotomy,patientshave an increasedrisk of neurological complications in the imme-diate postoperative period. Detecting these disturbances allows early diagnostic or therapeutic interventions that can decrease postoperative neurologic complications and improveprognosis.8 Inour study,allpatients whoneeded
anurgent CTinthePACUhadalteredneurological assess-mentscales.Webelievethatourresultssupporttheuseof amore completeneurological assessment ratherthanthe simpleanalysisofpupilsandGCS.
BenefitsandlimitationsofBIS
BIS could be useful as an additional tool to predict the occurrenceofneurologicalpostoperativecomplications,but probablynotbeyondotherwell know methods.The study demonstratesthat the standard perioperative assessment tools of GCS and careful neurologic examination proved effective to identify clinically significant complications, regardlessofBISrecordings. TheBISisacontinuous moni-toringthathastoalertusforamoreexhaustiveneurological assessment.ClinicalexaminationandBISdonothavetobe mutuallyexclusivebut complementarytools. BIS monitor-ingwasfeasible inthe postoperative periodin extubated patients. Highvalues of SQI, although the EMG couldnot be less than 40, support the fact that these were reli-ablerecordings.Theresidualanestheticeffectcouldexplain thedecreasein BISvalueswhenthepatientwasadmitted tothe PACU, as BIS began recovering shortly afterwards. Ourneurologicassessmentscalesincludeddirectorindirect assessment of the level of consciousness, although, each scaleassigningadifferentspecificvalue.Thatcouldexplain theagreementbetween alteredBISandaltered neurolog-icalassessment scales. However,we found no association betweenBISalterationsandalterationsoftheRamsayscale. Otherstudies have found similarresults whenEMG recor-dingswereabove30.20 On theother hand, we alsofound
thatalteredBISwasassociatedwiththeoccurrenceof neu-rologicalcomplicationsinthePACU.Interestingly,wefound significant differences in basal BIS values (T0) between patients who did and who did not develop neurological complicationsbyCT-MRI.Thisresultmaysuggestthatbasal BIScouldbeapotentialriskfactorofpostoperative neuro-logicalcomplicationsassociatedwithCT-MRIabnormalities in patients scheduled for craniotomy. However, this is an isolatedresultinasmallsampleofpatientsanditdoesnot allow us to make any valid conclusion. In a recent study inpatients with delayed awakeningafter craniotomy,the cerebral state index monitoring, a monitor that analyses electroencephalogramtracing similarly tothe BIS,proved tohave ahighpredictionprobabilityforlong-term uncon-sciousness (>24h) when the measured values in the first 6hpostoperativelywasbelow54---63.21 Wecouldnot
ana-lyzewhethertheBISwasabetterpredictorofneurological complications associated with a decreased level of con-sciousness,becausethenumberoftheseeventswastoolow.
Inaddition,ourcriteriatodefinetheBISalteration(BIS<70 for1min)mightbetoodemanding,accordingtotheresults oftheROCcurve.ItremainstobestudiedwhethertheBIS betterpredictstheimpairedconsciousnessinpatientsafter craniotomyandwhatcutoffvalueintheBISmightprovide thehighestpredictiveprobability.Nevertheless,the intro-ductionofaBISscaleissubjecttolimitationsasEMGactivity orfilteringeffectofairinthesubarachnoidspace.BIShas risksofraisinganunwarrantedalarmbellsbyvirtueofits poor degreeof specificityandcouldsuggest nursesnotto observesimplefacts,suchaslevelofconsciousnessandfocal deficitsthatareevaluatedinaclinicalexamination.
Associationbetweenalteredneurological
assessmentscalesorBISanddevelopmentof
postoperativeneurologicalcomplications
---estimatesofrisk
Altered neurological assessment scales were associated with the development of postoperative neurological complications. A previous study showed that the level of awarenessinpostoperativepatientswithsubarachnoid hem-orrhage who underwent surgery was a good predictor of neurological outcome.22 Other studies have found
addi-tionalpredictorsofpostoperativeneurologiccomplications, including the duration of surgery, the surgical position, intraoperative bleeding,or the appearanceofnew neuro-logical deficit after surgery.23,24 We also found that most
craniotomypatientswithsignificantintraoperativebleeding showedpostoperativeneurologiccomplicationsinthePACU. We could findno previous studies comparing neurological assessmentscalesinpatientsaftercraniotomytobest pre-dicttheoccurrenceofneurologiccomplications.Wefound that,independently,theGCSandCNSwerethemostprecise estimates ofrisk.GCSis well-knowntopredict neurologic outcome in head injury and subarachnoid hemorrhage.25
TheCNSinturnallowsmoresubtleabnormalitiesofmotor function,which probablyexplains whythisscale detected themost alterationsinboth groups.Ourresultsalsoshow that traditional examination of the pupilsand the GCS is useful to estimate the occurrence of postoperative neu-rologicalcomplications,but,probably,notenough. Iacono et al.,26 introduced The Basic Neurological Check for use
onanypatientwithaconfirmedorsuspectedneuroscience diagnosis, exceptstroke. Thistoolincluded assessment of orientation,abilitytofollowcommands,motorstrengthand assessmentforfacialdroop.Althoughnoformalprocesswas conducted to evaluate its effectiveness, nurses verbalize moreconfidenceintheirabilitytoidentifyclinicalsymptoms promotingearlydiagnosisandtreatment.Complete neuro-logical examination withassessment ofthe Ramsayscale, CNS,Nu-DESCandBIShelpstoimprovethisestimate.The highsensitivityofthescales-BISassessmentsuggeststhatif apreliminaryscreening ofthepopulationat riskof devel-opingpostoperativeneurologicalcomplicationswasmade, probably,thepredictiveaccuracyoftheassessmentwould increase.
inappropriatebehavior,inappropriatecommunication, illu-sions/hallucinations or psychomotor retardation in the postoperative period. The main mechanism seems to be associatedwithalteredsynapticneurotransmissioninduced by anesthesia or brain damage due to surgical trauma, ischemia,edema,etc.Probably,theuseofaspecificscale forassessingthesealterationswouldhelptodetect them. Ontheotherhand,weconsideredanyalterationinthescale asanalertsignalalthoughdidnotexceedthecutoff diag-nosisofdeliriumaccordingtotheoriginalscale.11
An interesting finding was the ability of neurological assessments at admission and discharge from the PACU to predict the appearance of postoperative neurological complications.Basedonourresults,thepresenceof abnor-mal scales-BIS on admissionto the PACU or the presence ofalteredpupils-GCSoralteredCNSatdischargefromthe PACU would be good indicators for recommending longer surveillanceandpatientmonitoring.Eightyonepercentof our patients underwent overnight monitoring after elec-tive craniotomy but only 34% had altered scales-BIS at admissiontothe PACUand31% alteredpupils-GCS or CNS at discharge. The level of care after an elective cran-iotomy is a matter of concern. Identifying predictors for postoperative neurological complications could be useful to select patients who require a more intensive level of observation and monitoring. Several publications show some preoperative and intraoperative predictors: Hanak et al.27 found diabetes and older age to be predictive
forpostoperativeICUadmission.Inaneurosurgicalpatient population, the application of the surgical Apgar score, based onthe estimated blood loss, the lowest intraoper-ative mean arterial blood pressure and the lowest heart rate predicted 30 day postoperative mortality, complica-tion rate, prolonged ICU and hospital stay. The authors statedthisscoremaybeuseful toefficiently plan postop-erativecare.28 Wandereretal.29 developedandvalidated
an intraoperative predictive model for unplanned postop-erativeintensivecareunitadmissionthatmayimprovethe process of allocating intensive care unit beds postopera-tively.Inthisregard,ourstudyaddsthepotentialusefulness of applying neurological assessment scales and BISin the PACU.
Limitationsofthestudy
We were aware of some limitations of the study. First, both thepatients and thenursing staff inthe PACUknew about thisstudy.Second, we excluded the moreseriously illpatients (patientsintubatedor dischargedtothe SICU) becausethepresentstudy focusedonpatients’extubated in the operating room and who remained in the PACU postoperatively.Third,itwasimpossibletoobtainBIS val-ues with EMG activity <30. The extubated neurosurgical patient in the PACU, unlike the intubated patient, does not requirepharmacological sedation making it more dif-ficulttodecreasetheEMGactivity.However,thequalityof thesignobtainedwasappropriate.Fourth,ourcriteriafor definingneurologicalcomplicationswerebasedonmedical reportsandtheneurosurgeon’sassessmentofCT-MRI.While someauthorshaveusedstrictercriteria(well-defined neuro-logicimpairment,preciseimagingabnormalities,orspecific
neurologicalinterventions),24 inother casesthecriteriais
broaderwhendefiningneurologicalcomplications (postop-erativenewdeficits).23 Finally,patientsintheCGandNCG
werenotcomparable.Thedemographicandclinical differ-encesareexplainedbythedifferentnatureoftheirsurgical pathologies.WhileintheCG,themainpathologywas neo-plastic with great general status affectation, patients in theNCGhadosteoarticularpathology,typicalofolderages, resultinginmoremotorimpairment.The higherincidence ofintraoperativecomplicationsintheCGwasdue,probably, toincreasedpreoperativesurgicalrisk.
Insummary,ourstudysuggeststhatneurological assess-ment scales and BIS, independently and pooled, could be useful to predict the occurrence of neurological complicationspostoperatively.By addingassessments,the numberofeventsdetectedwashigher,theconfidence inter-valdecreased, andthe estimatesbecame more accurate. BISdoesnotsubstituteneurologicalclinicalexaminationto identify clinically significant complications. We could not determine the scale or combination of scales that best estimates the occurrence of neurological complications. To find the best predictive model and the cut offs stud-ies with a large number of patients and more complex logistic regression analysis is needed. It would also be interesting to investigate what neurological assessment scales are best for each different neurological compli-cation, and whether these neurologic assessment scales and BIS are applicable to predict outcome in other pro-cedures with a high risk of postoperative neurological complications,likecardiacororthopedicsurgery,orin neu-rocriticalpatients(subarachnoidhemorrhage,head injury, etc.).
Appliedtogether, the assessment of pupils,GCS, Ram-sayscale,CNS,Nu-DESCandBIS,improvedearlydetection ofpostoperative neurological complications in PACUafter electivecraniotomies.
Conflicts
interests
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorsthanktheNeurosurgeryServiceinourfacilities aswellasDr.VictorObach(NeurologyDepartment)andDr. JordiRumia(NeurosurgeryService)fortheirhelp determin-ingwhichneurologicalscalesweremostappropriateforthis study,theothermembersofthePostNeuroanesthesiaCare Groupaandtheanesthesiaresidentsfortheirhelpwithdata
collection,Dr.JosepMaríaNicolasforhissupport,andtoall theothersinourinstitutionthatcontributedandmadethis projectpossible.
aPostNeuroanesthesiaCareGroup:MartaCarmeRN;Enrique
Appendix
A.
Neurological
assessment
scales
applied
in
the
study
Pupilexamination
Assessment Score
Pupilsize(left/right) 1mm 1
2mm 2
3mm 3
4mm 4
5mm 5
Pupilreaction(left/right) Noreaction 0
Reacts 1
Pupilanisocoria,pupilsofdifferentsize;pupilnoreaction,apupil thatdoesnotcontractwhenexposedtoabrightlight.
GlasgowComaScale(GCS)4
Category Responses Score
Eyeopening Spontaneously 4
Tospeech 3
Topain 2
None 1
Verbalresponse Orientated 5
Confused 4
Inappropriate 3 Incomprehensible 2
None 1
Motorresponse Obeyscommands 6 Localizestopain 5 Withdrawsfrompain 4 Flexiontopain 3 Extensiontopain 2
None 1
Maximumscore 15
RamsayScale9
Levelofactivity Score
Patientanxiousandagitatedorrestlessorboth 1 Patientco-operative,orientated,andtranquil 2 Patientrespondstocommandsonly 3 Patientexhibitsbriskresponsetolight
glabellartaporloudauditorystimulus
4
Patientexhibitsasluggishresponsetolight glabellartaporloudauditorystimulus
5
Patientexhibitsnoresponse 6
CanadianNeurologicalScale(CNS)10
Mentation Score
Levelofconsciousness Alert 3.0
Drowsy 1.5
Orientation Oriented 1.0
Disoriented/NA 0.0
Speech Normal 1.0
Expressivedeficit 0.5 Receptivedeficit 0.0
Total:
SectionA1 Motorfunctions Weakness Score
Nocomprehension deficit
Face None 0.5
Present 0.0 Arm,proximal None 1.5 Mild 1.0 Significant 0.5 Total 0.0 Arm,distal None 1.5 Mild 1.0 Significant 0.5 Total 0.0 Leg,proximal None 1.5 Mild 1.0 Significant 0.5 Total 0.0 Leg,distal None 1.5 Mild 1.0 Significant 0.5 Total 0.0 Total:
SectionA2 Motorresponse Weakness Score
Comprehension deficit
Face Symmetrical 0.5 Asymmetrical 0.0
Arms Equal 1.5
Unequal 0.0
Legs Equal 1.5
Unequal 0.0 Total:
TheNursingDeliriumScreeningScale(Nu-DESC)11
Symptom Score(0---2)
I.Disorientation
Verbalorbehavioralmanifestationofnotbeing orientedtotimeorplaceormisperceiving personsintheenvironment
II.Inappropriatebehavior
Behaviorinappropriatetoplaceand/orforthe person;e.g.,pullingattubesordressings, attemptingtogetoutofbedwhenthatis contraindicated,andthelike
III.Inappropriatecommunication
Communicationinappropriatetoplaceand/or fortheperson;e.g.,incoherence,
noncommunicativeness,nonsensicalor unintelligiblespeech
IV.Illusions/hallucinations
Seeingorhearingthingsthatarenotthere; distortionsofvisualobjects
V.Psychomotorretardation
Delayedresponsiveness,fewornospontaneous actions/words;e.g.,whenthepatientis prodded,reactionisdeferredand/orthe patientisunarousable
Total:
References
1.FàbregasN. ComplicacionesNeurológicasPerioperatorias.In: GomarC,VillalongaA,CastilloJ,CarreroE,TerceroFJ,editors. FormaciónContinuadaenAnestesiología yReanimación [Con-tinuingmedicaleducationinanesthesiologyandresuscitation], vol.2.Madrid:Ergon;2013.p.739---47.
2.BeauchampK,BakerS,McDanielC,etal.Reliabilityofnurses’ neurologicalassessmentsinthecardiothoracicsurgical inten-sivecareunit.AmJCritCare.2001;10:298---305.
3.Bickert AT, Gallagher C, Reiner A, et al. Nursing neuro-logicassessmentsaftercardiacoperations.AnnThoracSurg. 2008;85:554---60.
4.TeasdaleG,JennettB.Assessmentofcomaandimpaired con-sciousness.Apracticalscale.Lancet.1974;2:81---4.
5.Brott T, Adams HP Jr, Olinger CP, et al. Measurements of acutecerebralinfarction:aclinicalexaminationscale.Stroke. 1989;20:864---70.
6.Folstein MF, Folstein S, Mchugh PR. ‘‘Mini-Mental State’’. A practicalmethodforgradingthecognitivestateofpatientsfor theclinicians.JPsychiatrRes.1975;12:189---98.
7.American Psychiatric Association. Diagnostic and statistical manualofmentaldisorders.Textrevision(DSM-IV-TR),4thed. WashingtonDC:AmericanPsychiatricAssociation;2000. 8.FàbregasN,BruderN.Recovery andneurologicalevaluation.
BestPractResClinAnaesthesiol.2007;21:431---47.
9.Ramsay MA, Savege TM, Simpson BR, et al. Controlled sedation with alphaxalone---alphadolone. Br Med J. 1974;2: 656---9.
10.CôtéR, BattistaRN, WolfsonC,et al. The Canadian Neuro-logicalScale:validationandreliabilityassessment.Neurology. 1989;39:638---43.
11.GaudreauJD,GagnonP,HarelF,etal.Fast,systematic,and continuousdeliriumassessment in hospitalizedpatients: the Nursing Delirium Screening Scale. J Pain Symptom Manag. 2005;29:368---75.
12.Hodgate A, Ching N, Angonese L. Variability in agreement betweenphysicians and nurseswhen measuringthe Glasgow ComaScale in the emergency department limits its clinical usefulness.EmergMedAustralas.2006;18:379---84.
13.DeDeyneC,StruysM,DecruyenaereJ,etal.Useof continu-ousbispectralEEGtoassessdepthofsedationinICUpatients. IntensiveCareMed.1998;24:1294---8.
14.AdesanyaAO,RoseroE,WyrickC,etal.Assessingthe predic-tivevalueofthebispectralindexvspatientstateonclinical
assessment of sedation in postoperative cardiac surgery patients.JCritCare.2009;24:322---8.
15.Bruhn J, Bouillon TW, Shafer SL. Electromyographic activ-ity falsely elevates the bispectral index. Anesthesiology. 2000;92:1485---7.
16.RiessML,GraefeUA,GoetersC,etal.Sedationassessmentin criticallyillpatientswithbispectralindex.EurJAnaesthesiol. 2002;19:18---22.
17.MondelloE,SiliottiR,NotoG,et al.BispectralindexinICU: correlationwithRamsayscoreonassessmentofsedationlevel. JClinMonitComput.2002;17:271---7.
18.DeeksJJ,AltmanDG.Diagnostictests4:likelihoodratios.BMJ. 2004;329:168---9.
19.LandisJR,KochGG.Themeasurementofobserveragreement forcategoricaldata.Biometrics.1977;33:159---74.
20.Tonner PH,WeiC,BeinB, et al.Comparison oftwo bispec-tralindex algorithmsinmonitoringsedationinpostoperative intensivecarepatients.CritCareMed.2005;33:580---4. 21.XuM,LeiYN,ZhouJX.Useofcerebralstateindextopredict
long-termunconsciousnessinpatientsafterelectivecraniotomy withdelayrecovery.BMCNeurol.2011;11:15.
22.Fàbregas N,Valero R, CarreroE, etal. Outcomeofpatients whounderwentsurgicalrepairofaneurysmaftersubarachnoid haemorrhage.MedClin(Barc).1998;111:81---7.
23.KaakajiW,BarnettGH,BernhardD,etal.Clinicalandeconomic consequencesofearlydischargeofpatientsfollowing supraten-torialstereotacticbrainbiopsy.JNeurosurg.2001;94:892---8. 24.RhondaliO, GentyC,HalleC,etal.Dopatientsstillrequire
admissiontoanintensivecareunitafterelectivecraniotomy forbrainsurgery?JNeurosurgAnesthesiol.2011;23:118---23. 25.GiraldoEA,MandrekarJN,RubinMN,etal.Timingofclinical
gradeassessmentandpooroutcomeinpatientswithaneurysmal subarachnoidhemorrhage.JNeurosurg.2012;117:15---9. 26.IaconoLA,WellsC,Mann-FinnertyK.Standardizingneurological
assessments.JNeurosciNurs.2014;46:125---32.
27.HanakBW, WalcottBP, NahedBV, etal. Postoperative inten-sivecare unitrequirements afterelectivecraniotomy.World Neurosurg.2014;81:165---72.
28.ZiewaczJE,DavisMC,LauD,etal.Validationofthesurgical Apgarscoreinaneurosurgicalpatientpopulation.JNeurosurg. 2013;118:270---9.