O R I G I N A L P A P E R
Antidepressant, antioxidant and neurotrophic properties
of the standardized extract of
Cocos nucifera
husk fiber in mice
Eliane Brito Cortez Lima1•Caren Na´dia Soares de Sousa1•Germana Silva Vasconcelos1• Lucas Nascimento Meneses1•Yuri Freitas e Silva Pereira1•Naiara Coelho Ximenes1• Manuel Alves Santos Ju´nior1•Nata´lia Castelo Branco Matos1•Rayanne Brito2• Diogo Miron2• Luzia Kalyne Almeida Moreira Leal2•Danielle Maceˆdo1• Silvaˆnia Maria Mendes Vasconcelos1
Received: 5 October 2015 / Accepted: 18 January 2016 / Published online: 8 February 2016
ÓThe Japanese Society of Pharmacognosy and Springer Japan 2016
Abstract The plant Cocos nucifera and its derivatives have shown antidepressant-like effects, although its hydroalcoholic extract has not been studied with this end in mind. Therefore, we decided to determine the antidepres-sant-like effects of the standardized hydroalcoholic extract
ofCocos nuciferahusk fiber (HECN) as well as oxidative
alterations in the prefrontal cortex (PFC), hippocampus (HC) and striatum (ST), and the levels of brain-derived neurotrophic factor (BDNF) in the HC of mice. The extract was characterized based on the content of total polyphenols as well as two phenol compounds—catechin and chloro-genic acid—by HPLC-PDA. Male animals were treated per os (p.o.) for 7 days with distilled water or HECN (50, 100 or 200 mg/kg), or intraperitoneally with vitamin E (Vit E 400 mg/kg). One hour after the last drug administration,
the animals were submitted to the open field test, forced swimming test (FST), tail suspension test (TST) and, immediately after the behavioral tests, had their brain removed for neurochemical determinations. The results showed that HECN100 decreased the immobility time in the FST and TST presenting, thus demonstrating an antidepressant-like effect. The administration of HECN decreased malondialdehyde levels in all doses and brain areas studied with the exception of HECN50 in the HC. The administration of HECN also decreased nitrite levels in all doses and brain regions studied. HECN100 also increased the levels of BDNF in HC of mice. In conclusion, we demonstrated that HECN has antidepressant-like properties, probably based on its antioxidant and
& Silvaˆnia Maria Mendes Vasconcelos
silvania_vasconcelos@yahoo.com.br; silvania@pq.cnpq.br
Eliane Brito Cortez Lima elianebcortez@yahoo.com.br
Caren Na´dia Soares de Sousa carensoarez@yahoo.com.br
Germana Silva Vasconcelos germana_vasconcelos@yahoo.com.br
Lucas Nascimento Meneses lucasmeneses07@gmail.com
Yuri Freitas e Silva Pereira yuriffreitas@hotmail.com
Naiara Coelho Ximenes naiaracx@gmail.com
Manuel Alves Santos Ju´nior manuelalvesjr08@gmail.com
Nata´lia Castelo Branco Matos nataliacbmatos@gmail.com
Rayanne Brito rayanne.bf@gmail.com
Diogo Miron
diogomiron@hotmail.com
Luzia Kalyne Almeida Moreira Leal kalyneleal@gmail.com
Danielle Maceˆdo
daniellesilmacedo@gmail.com
1 Neuropharmacology Laboratory, Department of Physiology
and Pharmacology, Federal University of Ceara´, Rua Cel. Nunes de Melo 1127, Fortaleza, Ceara´ 60431-270, Brazil
2 Center of Pharmaceutical and Cosmetic Studies, Department
of Pharmacy, Federal University of Ceara´, Fortaleza, Ceara´, Brazil
neurotrophic effects, and is thus relevant for the treatment of depression.
Keywords Cocos nucifera Behavioral testOxidative stressPhenolsBDNF
Introduction
Major depressive disorder (MDD) is one of the most common and debilitating psychiatric conditions. Current predictions from The World Health Organization indicate that by 2030 MDD will be the leading cause of disease burden [1]. Depression is associated with a high risk of suicide and medical comorbidities [2,3]. Although medi-cation treatments can be effective for MDD, only 30–40 % of MDD patients respond to the initial therapy with con-ventional antidepressants [4]. The pathophysiology of depression is currently related to various theories such as monoaminergic, neurotrophic and oxidative causes.
Evidence indicates that disruption of antioxidant defenses and the presence of oxidative imbalance may play a role in a wide range of neuropsychiatric disorders, including schizophrenia, bipolar disorder and MDD [5,6]. Several studies have reported that oxidative stress is related to changes in reactive oxygen species (ROS) as well as in the activity of antioxidant enzymes in brain regions [7,8], including the prefrontal cortex (PFC), hippocampus (HC) and striatum (ST), which are structures related to the pathophysiology of depression [9, 10]. Studies have con-sistently reported increased ROS in the plasma of MDD patients, especially when it is associated with melancholy [11].
Furthermore, studies concerning stress and depression have also postulated the ‘‘neurotrophin hypothesis of depression’’, which suggests an association of brain-derived neurotrophic factor (BDNF) deficiencies with the occurrence of depression, concluding that antidepressants act restoring central BDNF levels [12–15]. BDNF is involved in a wide range of neuropsychiatric and neu-rodegenerative diseases [12]. A number of individual studies and later meta-analyses have demonstrated that BDNF levels are decreased in mood disorders [16, 17]. Substances that are capable of increasing BDNF levels have been proposed as possible alternative treatment for these central nervous system diseases.
Several medicinal plants [18, 19] and derivatives [20,
21] have shown antidepressant effects in classic depression animal testing such as the forced swimming test (FST) and tail suspension test (TST). Cocos nucifera is a common plant on the coast of Brazil, and its fruit is reversed by a rich bark fiber. Studies performed with C. nucifera husk fiber extract have indicated that this plant is rich in
flavonoids, catechins and condensed tannins [22–24]. This plant has exhibited many biological effects, such as anti-inflammatory, analgesic, anthelminthic, anti-trichomonas, antibacterial, antiviral, antioxidant and antidepressant properties [22, 24–29]. Given the importance of studies focusing on the development of antidepressants from nat-ural sources, we decided to analyze the action of C. nuci-feraextract on behavioral and oxidative parameters in the PFC, HC and ST in mice, as well as the levels of BDNF in the mouse HC.
Materials and methods
Plant materials and extract preparation
Cocos nucifera (Arecaceae) typical variety, commonly
known as ‘‘coconut’’, was collected in Ceara´, Brazil (Irri-gation Project Curu Paraipaba). A specimen was registered (number 53862) and deposited at the Federal University of Ceara´ Herbarium.
The extract was prepared from fiber from the bark ofC.
nucifera distilled through a Soxhlet extractor system for
6 h. An ethanol:water solution (2:1) was used as solvent extractor. The hydroalcoholic extract ofC. nucifra(HECN) was concentrated to a 1:1 ratio between drug and solvent in a rotary evaporator.
Phytochemicals analysis
The total polyphenol content in the extracts was deter-mined by the Folin-Ciocalteu colorimetric method [30,31]. An aliquot (100 lL) of samples was mixed with 250 lL Folin-Ciocalteu reagent (1N). The mixture was shaken before adding 1250 lL Na2CO3(20 %) and adjusted with
purified water to a final volume of 10 mL. After 40 min standing in darkness, the color was measured in a spec-trophotometer (Beckman Coulter DU 640, Krefeld, Ger-many) at 715 nm. The amount of total polyphenols was calculated from the regression equation of the calibration curve of the standard gallic acid (dissolved in Milli-Q water), at concentrations ranging from 2lg/mL to 16lg/ mL. The analyses were performed in triplicate.
Analysis of polyphenols by high performance liquid chromatography
The analysis of catechin and phenolic acids (chlorogenic, gallic and caffeic) was performed in a Waters (Milford, MA) HPLC consisting of an Alliance 2695 system and a photo-diode-array detector (model 2996). The volume injected was 20lL, flow rate 1.1 mL min-1, the oven was set at 32°C,
Plus C18 column (4.69100 mm, 3.5lm; Agilent
Tech-nologies, Santa Clara, CA), a Phenomenex C18 (4.694 mm, 5lm) as pre-column, and isocratic mode consisting of fosforic acid 0.1 % pH 2.3: acetonitrile: n -propanol (983: 13: 4) as mobile phase. Peak purity analysis was performed using Empower 2 software (Waters) in the UV spectrum in the range of 200–400 nm recorded at a frequency of 1 Hz. The threshold was calculated employing noise and the solvent angle in default mode. TheC. nucifera
extract was diluted (1:10) in ultrapure water and filtered through a PTFE filter (0.45 mm, Millipore) before injection into high performance liquid chromatography (HPLC). Calibration curves (324 and 278 nm, respectively) ranging from 5lg mL-1 to 30lg mL-1 were prepared for the determination of these compounds in the extract.
Animals
Adult male Swiss mice (8 weeks old) weighing 20–30 g were housed eight per cage in standard polycarbonate cages (20942920.5 cm) at normal ambient conditions
(22±1°C; humidity 60 ±5 %; 12 h light–dark cycle) with food and water ad libitum. The experimental proce-dures were performed between 14:00 and 16:00 p.m. All experiments were performed in accordance with theGuide for the Care and Use of Laboratory Animals, Department
of Health and Human Services Resources, 1996 US and
adhered to Brazilian legislation on animal experimentation. The project was approved by the Ethics in Animal Research Committee (CEPA) UFC (protocol 32/2011). All efforts were made to minimize animal suffering and to reduce the number of animals used.
Experimental design
Animals were treated for 7 days with distilled water (control), HECN (50, 100 or 200 mg/kg, p.o.) or Vitamin E (Vit E, 400 mg/kg, i.p.) which was diluted in 2 % Tween 80 in saline. Diazepam (DZP, 2 mg/kg), diluted in saline, was used as a control in the evaluation of locomotor activity. One hour after the last drug administration, the animals were submitted to behavioral tests. Afterwards they were sacrificed and the PFC, HC and ST were dis-sected on ice. All biological material was immediately stored at-70°C until assay.
Behavioral assessment
Open field test
The open field area was made of acrylic with transparent walls and a black floor (30930915 cm), and was
divided into nine squares of equal area. The open field test
(OFT) was used to evaluate the exploratory activity of the mouse [32]. Each mouse was placed in the center of the arena, and the number of squares crossed with all four paws (i.e., locomotor activity) over a 6-min period was recorded. Before introducing each animal to the area, the arena was cleaned with 5 % alcohol to eliminate possible bias due to odors that could remain on surfaces from previous animals.
Forced swimming test
The FST was performed 1 h after the last drug adminis-tration. Mice were individually forced to swim in an open cylindrical container (diameter, 22 cm; height, 40 cm) that contained a water column of 20 cm held at 25 ±1 °C. The
time that each animal remained immobile during a 5 min period was recorded. Immobility was defined as the animal floating in the water without struggling and making only very minimal movements necessary to keep its head above the water. An increase in the duration of immobility is indicative of depressed-like behavior [33]. Any mouse appearing to have difficulty keeping its head above water was removed from the cylinder and excluded from the analysis.
Tail suspension test
For the TST, each mouse was suspended by the tail on the edge of a shelf placed 58 cm above a table-top. The mouse was secured in place via adhesive tape placed approxi-mately 1 cm from the tip of the tail. The time that each mouse remained immobile over a 6-min period was recorded. As previously described, each animal was sub-mitted to this test only once [34].
Determination of oxidative stress parameters
Nitrite/nitrate assay
The assay was based on the Griess reaction to determine the production of NO [35]. Homogenates were prepared to a concentration of 10 %, and samples were centrifuged (15 min; 11,000 rpm; 4 °C). Briefly, 100lL of the
supernatant was incubated at room temperature for 10 min with 100lL of Griess reagent, which consisted of equal parts (1:1:1:1) of 1 % sulfanilamide dissolved in 1 % of phosphoric acid (H3PO4), 0.1 %N
-(1-naphthyl)-ethylene-diaminedihydrochloride, and distilled water. Absorbance was measured at 540 nm in a microplate reader (ASYS model UVM 340; Biochrom, Holliston, MA). Nitrite/ni-trate content was determined based on a standard nitrite curve, which was generated using sodium nitrite (NaNO2)
Lipid peroxidation assay
The rate of lipid peroxidation was estimated by determi-nation of malondialdehyde (MDA) using the thiobarbituric acid reactive substances (TBARS) test [36]. Brain areas were homogenized to 10 % tissue with 50 mM potassium phosphate monobasic buffer, pH 7.4. Then, 63lL homo-genate were mixed with 100lL 35 % perchloric acid. Samples were centrifuged (5000 rpm;10 min) and 150lL supernatant was removed, mixed with 50lL thiobarbituric acid at 1.2 %, and then heated in a boiling water bath for 30 min. After cooling, lipid peroxidation was determined by measuring absorbance at 535 nm and expressing as nmol MDA/mg protein.
Determination of catalase activity
Catalase activity was determined by the method that uses H2O2 to generate H2O and O2 [37, 38]. The activity was
measured by the degree of this reaction. The standard assay substrate mixture contained 0.30 mL H2O2 in 50 mL of
0.05 M sodium phosphate buffer, pH 7.0. The sample aliquot (20lL) was added to 980 lL substrate mixture. Initial absorbance was recorded after 1 min, and final absorbance after 6 min. The reaction was followed at 230 nm. A standard curve was established using purified catalase (Sigma, St Louis, MO) under identical conditions. All samples were diluted with 0.1 mmol/L sodium phosphate buffer (pH 7.0) to induce a 50 % inhibition of the diluent rate (i.e., the unin-hibited reaction). Results are expressed in mmol min-1(mg protein)-1. Protein concentration used for calculations was measured using the Lowry method [39].
Determination of BDNF levels
The HC were homogenized in 20 volumes of phosphate-buffered saline (PBS) buffer with protease (EMD Bio-sciences) and phosphatase (Sigma-Aldrich) inhibitors and centrifuged (10,000 rpm; 5 min). BDNF level in each sample was quantified by immunoenzymatic determination according to the manufacturer’s instructions (EMD Milli-pore, Bedford, MA). The results are expressed as pico-grams of BDNF/g tissue.
Statistical analysis
The results were analyzed by analysis of variance (ANOVA) followed by Turkey test (post hoc). Differences were considered statistically significant at P\0.05. GraphPad Prism 5.0 for Windows (GraphPad Software, San Diego, CA) was used for statistical analysis and gra-phic preparation.
Results
The chemical analysis of HECN by spectrophotometry allowed quantification of total polyphenols (5.8±0.6 g %)
through a calibration curve, which determined the linearity of the analytical method (y=12.954x-0.5773; r=0.9985). HPLC analysis of HECN detected the presence
of catechin and chlorogenic acid, while gallic and caffeic acids were not identified in the extract. Peak purity analysis of catechin and chlorogenic acid did not detect impurities (Fig.1), improving the reliability regarding the identifica-tion and quantificaidentifica-tion of these compounds. The concentra-tions (mean ±standard deviation forn=3) of catechin and chlorogenic acid in the C. nucifera extract were 26.58±0.70 and 9.56 ±0.27lg mL-1, respectively.
Catechin was the major compound found in the extract, at a concentration 2.5 times greater than that of chlorogenic acid. In the OFT (Fig.2), HECN (50, 100 and 200 mg/kg) did not cause any change in crossing behavior in comparison to the control group, while administration of DZP (2 mg/kg) decreased all these parameters significantly when compared to the control group [F(4,50)=3.6758;P\0.0100].
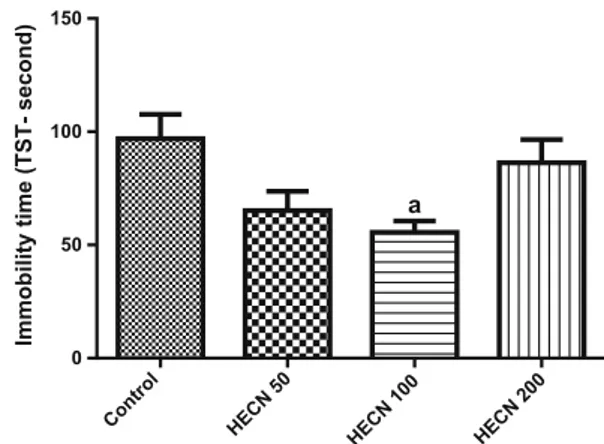
In FST (Fig.3) and TST (Fig.4), HECN (100 mg/kg) decreased the immobility time as compared to the control {FST [F(3, 41)=3.297; P=0.0297] and TST [F(3, 37)=4.461;P=0.0090]}.
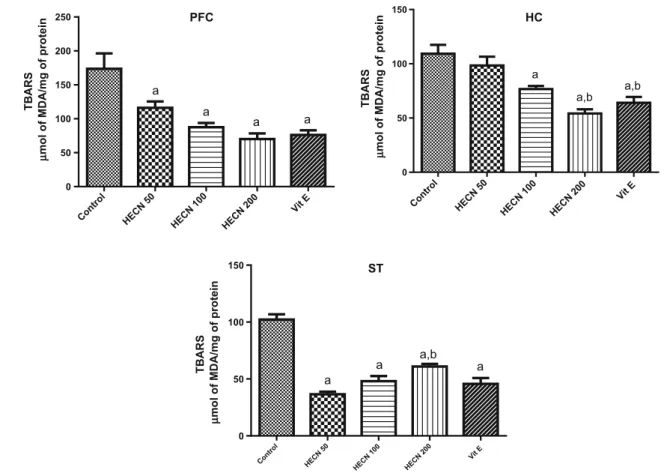
The effects of HECN in the levels of lipid peroxidation are present in Fig.5. We observed that after the adminis-tration of HECN in all doses studied there was a decrease in the levels of MDA in the PFC [F(4, 42)=11.05; P\0.0001] and ST [F(4, 40)=43.99;P\0.0001], when compared to control. A similar effect was observed in the HC [F(4, 40) =13.85;P\0.0001] only with HECN (100 or 200) when compared to control. Vitamin E, used as a positive control, also decreased the levels of MDA.
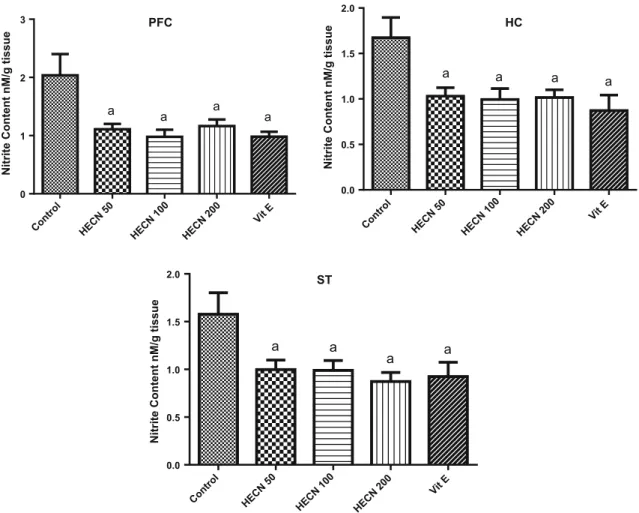
The administration of HECN also decreased the levels of nitrite (Fig.6) in all doses in the three brain regions {PFC [F(4,50)=5.177; P=0.0014]; HC [F(4, 50)=4.703;
P=0.0027]; ST [F(4, 46)=4.053; P =0.0067]} when
compared to control groups. As expected, vitamin E also decreased this parameter in all brain areas investigated.
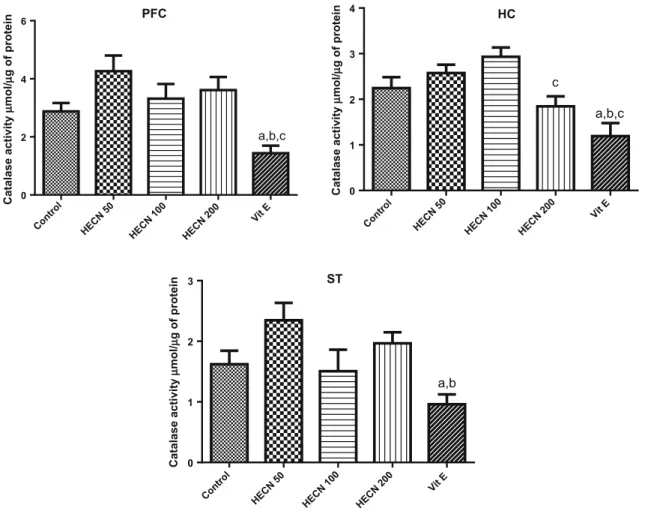
On the other hand, no effect was observed in the activity of catalase after the administration of HECN (50, 100 or 200) in the PFC [F(4, 42)=6.646; P=0.0003], HC [F(4, 41)=8.161; P\0.0105] or ST [F(4, 40)=4.968;
P=0.0024] when compared to the control group (Fig.7).
Decreased activity of catalase in the HC was observed when compared HECN200 to HECN100. Vitamin E, similarly as observed in the levels of MDA and nitrite, showed a decrease in the activity of catalase in the three brain regions.
levels of BDNF [F(3, 37)=6.337;P=0.0016] at dose of
100 mg/kg when compared to control. On the other hand, HECN200 showed lower levels of BDNF when compared with HECN100 (Fig.8).
Discussion
In recent decades, oxidative stress has been implicated in the pathogenesis of various neurodegenerative and neu-ropsychiatric disorders, including depression. Furthermore, many natural products have shown antidepressant proper-ties [40–42]. Based on these findings, the behavioral effects of HECN were investigated through classic animal tests of depressant-like activity, such as FST and TST. We also
observed that the antidepressant-like effects of HECN were accompanied by decreases in oxidative stress and increases in BDNF levels, supporting the hypothesis that its effects are neurochemical.
The spontaneous locomotor activity (SLA) test is used as a general parameter in investigating the central action of a drug. A decrease in locomotor activity means that the drug has a depressing effect on the CNS in the study ani-mal, i.e., there is a motor change that may interfere with other behavioral tests conducted in the same animal. Our results showed that HECN did not interfere with SLA tests, suggesting that other tests performed would not be com-promised by the animal’s locomotor activity.
that the antidepressant action of HECN is achieved best at this dose.
The antidepressant action of HECN determined in the present study may be due to the presence of several chemical compounds in the extract, including phenols such as catechin (flavonol) and chlorogenic acid. Catechin, epicatechin, quercetin and resveratrol are polyphenolic inhibitors of MAO A and B, presenting inhibitory activities identified by an in vitro score [43–45], and are able to cross the blood–brain barrier and provide neuroprotective effects [46,47]. MAO-A oxidates preferentially serotonin (5-hy-droxytryptamine) and noradrenaline, while MAO-B oxi-dates preferentially phenylethylamine [48]. It is believed that the pathophysiology of depression involves impair-ment of 5-hydroxytryptamine and noradrenaline, therefore
selective MAO-A inhibitors are prescribed for the treat-ment of depression. As well as catechin and chlorogenic acid, other phenol compounds present in the HECN may be also related with the antidepressant activity because one of its metabolites, caffeic acid, is known to exhibit antide-pressant and/or anxiolytic activity by indirect modulation of the alpha1A-adrenoceptor system in rodents [49,50].
Several studies have shown that reactive oxygen species (ROS) play an important role in the neurobiology of many diseases, especially neurological and psychiatric diseases [7,
51, 52]. Oxidative stress may be a common pathogenic mechanism underlying many major psychiatric disorders, as the brain has comparatively greater vulnerability to oxida-tive damage [53]. Evidence indicates that oxidative stress can stimulate numerous pathways, leading to increased production of oxygen radicals that can attack proteins, nucleic acids and lipid membranes, thereby disrupting neu-rons function and integrity [54]. One of the most plausible causes for these neuronal alterations is elevated oxidative stress due to increased production of free radicals.
In this context, it has already been reported that free radicals are elevated in MDD [5]. Recently, Cumurcu et al. [55] showed that total serum oxidant status is associated with MDD, since there was an elevation of this parameter in depressed patients when compared to healthy volunteers, and antidepressant treatment was able to reverse this alteration. Within the last decade, a growing body of pre-clinical [56] and clinical [57] literature, support this ‘‘ox-idative stress hypothesis of MDD’’.
Given the evidence suggesting the involvement of oxidative stress in the pathophysiology of depression, natural antioxidants (including polyphenols) obtained from fruits, nuts, leaves and roots of plants have been examined. It is possible that bioactive compounds are effective in prevent brain neurodegeneration [58].
Control
HECN 50mg/kgHECN 100mg/kgHECN 200mg/kg
DZP
0 10 20 30 40 50
a
Number of squares crossed
Fig. 2 Effects of hydroalcoholic extract ofCocos nuciferahusk fiber (HECN) on the number of squares crossed in open field test (OFT). Barsrepresent mean±standard error of the means (SEM) of 10–12 animals/group.a P\0.05 vs. control according to ANOVA followed
by the Tukey’s multiple comparison test
Control
HECN 50 HECN 100 HECN 200 0
20 40 60 80
Immobility time (FST- second)
a
Fig. 3 Effects of HECN on the immobility time in the forced swimming test (FST). Bars represent mean±SEM of 10–12 animals/group.a P\0.05 vs. control according to ANOVA followed
by the Tukey’s multiple comparison test
Control HECN 50
HECN 100
HECN 200
0 50 100 150
Immobility time (TST- second)
a
Fig. 4 Effects of HECN on the immobility time in the tail suspension test (TST). Bars represent mean±SEM of 10 animals/group. a P\0.05 vs. control according to ANOVA followed by the Tukey’s
Based on our findings for an antidepressant-like effect of HECN, we decided to investigate oxidative alterations in the PFC, HC and ST of mice treated with HECN. We observed decreased levels of MDA in the brain areas of mice administered HECN and vitamin E, indicating that there was a decrease of lipid peroxidation in the three brain areas. Oxidizing substances, such as ketamine [59, 60], cause increases in MDA levels, while substances with antioxidant properties such as vitamin E, tend to decrease this parameter [61]. For this reason vitamin E was used here as a standard antioxidant.
HECN administration in all doses also decreased the levels of nitrite in all brain areas studied. As expected, vitamin E, a natural brain antioxidant [62] also decreased the concentration of nitrite in PCF, HC and ST. Studies have focused on the biochemical and molecular actions of nitric oxide (NO) in normal conditions, as well as its potential alteration in pathological conditions like depres-sion [63, 64]. Furthermore, the relationship between NO and depression is not conclusive [65, 66]. In previous studies, plasma NO levels in depressed patients were found to be either increased [67] or decreased [68], possibly due
to the different subtypes of depression or the different drug treatments applied to the patients.
Catalase is an enzyme that reacts very effectively with hydrogen peroxide (H2O2) to form water and ROS.
Cata-lase protects cells against H2O2 generated intracellularly.
Studies have shown increased blood catalase activity is related to central nervous system (CNS) diseases [69,70]. A previous study [70] showed an increase in catalase activity (cerebellum, HC, ST, cortex) in mice with depression-like behavior, suggesting that an alteration in catalase activity may contribute to depression phys-iopathology. Recently, studies have demonstrated that major depression is related to elevated catalase and superoxide dismutase-SOD (antioxidative enzyme) activi-ties in plasma, and that there is a significant positive cor-relation between SOD activity and a Hamilton Depression Rating Scale score [11].
Our results showed that HECN administration did not modify the concentration of catalase in PFC, HC and ST. However, vitamin E decreased catalase concentration. The absence of effects on catalase activity may suggest that the antioxidant action of the extract is independent of its action PFC
Control HECN 50
HECN 100 HECN 200
Vit E
0 50 100 150 200 250
a
a
a a
HC
0 50 100 150
a
a,b a,b
ST
0 50 100 150
a
a a,b a
Control HECN 50
HECN 100 HECN 200 Vit E
Control HECN 50
HECN 100 HEC
N 200 Vit E
TBARS
µµ
mol of MDA/mg of protein
TBARS
µ
mol of MDA/mg of protein
TBARS
µ
mol of MDA/mg of protein
Fig. 5 Levels of malondialdehyde in prefrontal cortex (PFC), hippocampus (HC) and striatum (ST) of adult mice treated with vehicle, HECN (50, 100 or 200 mg/kg) or Vitamin E (400 mg/kg). Barsrepresent mean±SEM of 7–11 animals/group.a P\0.05 vs.
control andb P\0.05 vs. HECN 50 according to ANOVA followed
in this specific enzyme. According to Mate´s and Sa´nchez-Jime´nez [71], catalase is not essential to some cell types under normal conditions, but plays an important role in the acquisition of tolerance to oxidative and nitrosative stress (O&NS) in the cellular adaptive response.
Besides oxidative stress, other hypotheses for the pathophysiology of depression are related to the neurobi-ology of the disease. Among them, BDNF—a member of the nerve growth factor family that is highly expressed in hippocampus and cortex [72]—is gaining prominence. BDNF modulates neuronal plasticity, inhibits cell death cascades, and increases the cell survival proteins that are responsible for the proliferation and maintenance of CNS neurons [73]; hence, substances that increase the concen-tration of BDNF may be an important factor in the treat-ment of CNS disorders such as depression [73–77].
Recent evidence suggests that neurotrophic factors play critical roles in the pathogenesis of depression [75, 78,
79]. Postmortem analyses have revealed lower levels of BDNF in patients with major depression [80], and clinical studies have shown that the blood levels of BDNF in
patients with major depression is lower than normal [81], while antidepressant treatment seems to be able to nor-malize them [82].
Interestingly, our results showed that BDNF levels in the hippocampus were increased by HECN (100 mg/kg), sug-gesting an antidepressant-like effect. This effect is proba-bly related to the presence of phenols in the extract, such as catechin (flavonol) and chlorogenic acid. Flavonoids, a large part of polyphenols obtained from plants, are spec-ulated to be effective in the treatment of several CNS disorders [83, 84] such as depression [85, 86]. Evidence suggests that natural products rich in flavonoids, which increase the BDNF levels, are promising therapeutic targets for the treatment of depression [87,88].
Corroborating the biological potential of phenols as a therapeutic tool for the treatment of depression, the ability of caffeic acid—a major metabolite of chlorogenic acid— to attenuate the decrease in the expression levels of BDNF mRNA in the frontal cortex of mice following forced swimming has been shown [89]. In addition, both phenolic acids have proven antioxidant potential [90].
PFC
0 1 2 3
Nitrite Content nM/g tissue
a a a a
HC
0.0 0.5 1.0 1.5 2.0
a a a
a
ST
0.0 0.5 1.0 1.5 2.0
a a
a a
Control HECN 50
HECN 100 HECN 200
Vit E Control
HECN 50 HECN 100 HECN 200
Vit E
Control HECN 50
HECN 100 HECN 200
Vit E
Nitrite Content nM/g tissue
Nitrite Content nM/g tissue
In conclusion, this study has shown HECN has an antidepressant-like and neuroprotective effect, suggesting its relevance in the treatment of depression.
Acknowledgments The authors would like to thank the National Counsel of Technological and Scientific Development (CNPq), the Coordination of Capacitation of Graduate Personnel (CAPES), and the Ceara Foundation for the Support of Scientific and Technological Development (FUNCAP).
References
1. World Health Organization (2008) The global burden of disease: 2004 update. World Health Organization, Geneva, p 146 2. Kessler R, Berglund P, Demler O, Jin R, Koretz D, Merikangas
K, Rush A, Walters E, Wang P (2003) The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). J Am Med Assoc 289:3095–3105 3. Kessler R (2012) The costs of depression. Psychiatr Clin North
Am 35:1–14 PFC
Control HECN 50
HECN 100 HECN 200
Vit E
0 2 4 6
a,b,c
HC
0 1 2 3 4
a,b,c c
ST
0 1 2 3
a,b
Catalase activity
µµ
mol/
µ
g of protein
Control HECN 50
HECN 100 HECN 200
Vit E
Control HECN
50
HECN 100 HECN
200 Vit E
Catalase activity
µ
mol/
µ
g of protein
Catalase activity
µ
mol/
µ
g of protein
Fig. 7 Catalase activity in PFC, HC and ST of adult mice treated with vehicle, HECN (50, 100 or 200 mg/kg) or Vitamin E (400 mg/ kg).Barsrepresent mean±SEM of 7–10 animals/group.a P\0.05
vs. control, andb P\0.05 vs. HECN 50,c P\0.05 vs. HECN 100
according to ANOVA followed by the Tukey’s multiple comparison test
Control HECN 50
HECN 10 0
HECN 200
0 5000 10000 15000
a
BDNF (pg/g tissue)
b,c
Fig. 8 Levels of brain-derived neurotrophic factor (BDNF) in the hippocampus of animals treated during 7 days with saline (control) or HECN (50, 100 or 200 mg/kg).Barsrepresent mean±SEM of 9–10 animals/group.a P\0.05 vs. control,b P\0.05 vs. HECN 50,c
P\0.05 vs. HECN 100 according to ANOVA followed by the
4. Rush A, Trivedi M, Wisniewski S, Nierenberg A, Stewart J, Warden D, Niederehe G, Thase M, Lavori P, Lebowitz B, McGrath P, Rosenbaum J, Sackeim H, Kupfer D, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpa-tients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917
5. Bajpai A, Verma A, Srivastava M, Srivastava R (2014) Oxidative stress and major depression. J Clin Diagn Res 8:CC04–CC07 6. Palta P, Samuel L, Miller E, Szanton S (2014) Depression and
oxidative stress: results from a meta-analysis of observational studies. Psychosom Med 76:12–19
7. Kumar B, Kuhad A, Chopra K (2011) Neuropsychopharmaco-logical effect of sesamol in unpredictable chronic mild stress model of depression: behavioral and biochemical evidences. Psychopharmacology 214:819–828
8. Zhang X, Yao J (2013) Oxidative stress and therapeutic impli-cations in psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 46:197–199
9. Duman R, Voleti B (2012) Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci 35:47–56
10. Che Y, Zhou Z, Shu Y, Zhai C, Zhu Y, Gong S, Cui Y, Wang J (2015) Chronic unpredictable stress impairs endogenous antiox-idant defense in rat brain. Neurosci Lett 584:208–213
11. Bilici M, Efe H, Koroglu M, Uydu H, Bekaroglu M, Deger O (2001) Antioxidative enzyme activities and lipid peroxidation in major depression: alterations by antidepressant treatments. J Af-fect Disord 64:43–51
12. Autry A, Monteggia L (2012) Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 64:238–258 13. Wolkowitz O, Wolf J, Shelly W, Rosser R, Burke H, Lerner G,
Reus V, Nelson J, Epel E, Mellon S (2011) Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuropsychopharmacol Biol Psychiatry 35:1623–1630
14. Mao Q, Huang Z, Zhong X, Xian X, Ip S (2014) Brain-derived neurotrophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav Brain Res 26:140–145
15. Liu Y, Jia G, Gou L, Sun L, Fu X, Lan N, Li S, Yin X (2013) Antidepressant-like effects of tea polyphenols on mouse model of chronic unpredictable mild stress. Pharmacol Biochem Behav 104:27–32
16. Bocchio-Chiavetto L, Bagnardi V, Zanardini R, Molteni R, Nielsen M, Placentino A, Giovannini C, Rillosi L, Ventriglia M, Riva MA, Gennarelli M (2010) Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry 11:763–773
17. Fernandes B, Berk M, Turck C, Steiner J, Gonc¸alves C (2014) Decreased peripheral brain-derived neurotrophic factor levels are a biomarker of disease activity in major psychiatric disorders: a comparative meta-analysis. Mol Psychiatry 19:750–751 18. Cito´ M, Silva M, Santos L, Fernandes M, Melo F, Aguiar J,
Lopes I, Sousa P, Vasconcelos S, Maceˆdo D, Sousa F (2015) Antidepressant-like effect of Hoodia gordonii in a forced swim-ming test in mice: evidence for involvement of the monoamin-ergic system. Braz J Med Biol Res 48:57–64
19. Gaire B, Lim D (2014) Antidepressant effects of Radix et Caulis Acanthopanacis Santicosi extracts on rat models with depression in terms of immobile behavior. J Tradit Chin Med 34:317–323 20. De Sousa F, Oliveira I, Silva M, de Melo C, Santiago V, de
Castro Chaves R, Fernandes M, Gutierrez S, Vasconcelos S, Maceˆdo D, Barbosa Filho J (2014) Involvement of monoamin-ergic system in the antidepressant-like effect of riparin I from Aniba riparia (Nees) Mez (Lauraceae) in mice. Fundam Clin Pharmacol 28:95–103
21. Do Amaral J, Silva M, de Aquino Neto M, Moura B, de Carvalho A, Vasconcelos P, Barbosa Filho J, Gutierrez S, Vasconcelos S, Maceˆdo D, de Sousa F (2013) Antidepressant-like effect of bis-eugenol in the mice forced swimming test: evidence for the involvement of the monoaminergic system. Fundam Clin Phar-macol 27:471–482
22. Esquenazi D, Wigg M, Miranda M, Rodrigues H, Tostes J, Rozental S, Alviano C (2002) Antimicrobial and antiviral activ-ities of polyphenolics fromCocos nuciferaLinn. (Palmae) husk fiber extract. Res in Microbiol 153:647–652
23. Costa C, Bevilaqua C, Morais S, Camurc¸a-Vasconcelos A, Maciel M, Braga R, Oliveira L (2010) Anthelmintic activity of Cocos nuciferaL. on intestinal nematodes of mice. Res in Vet Sci 88:101–103
24. Mendonc¸a-Filho R, Rodrigues I, Alviano D, Santos A, Soares R, Alviano C, Lopes A, Rosa Mdo S (2004) Leishmanicidal activity of polyphenolic-rich extract from husk fiber of Cocos nucifera Linn. (Palmae). Res Microbiol 155:136–143
25. Koschek P, Alviano D, Alviano C, Gattass C (2007) The husk fiber ofCocos nuciferaL. (Palmae) is a source of anti-neoplastic activity. Braz J Med Biol Res 40:1339–1343
26. Calzada F, Mulia Y, Contrera T (2007) Effect of Mexican medicinal plant used to treat trichomoniasis on Trichomonas vaginalistrophozoites. J Ethnopharmacol 113:248–251 27. Akinyele T, Okoh O, Akinpelu D, Okoh A (2011) In-vitro
antibacterial properties of crude aqueous andn-hexane extracts of the husk ofCocos nucifera. Molecules 16:2135–2145
28. Silva R, Oliveira e Silva D, Fontes H, Alviano C, Fernandes P, Alviano D (2013) Anti-inflammatory, antioxidant, and antimi-crobial activities ofCocos nucifera var. typica. BMC Comple-ment Altern Med 13:107
29. Lima E, Sousa C, Meneses L, Ximenes N, Santos Ju´nior M, Vasconcelos G, Lima N, Patrocı´nio M, Macedo D, Vasconcelos S (2015) Cocos nucifera (L.) (Arecaceae): a phytochemical and pharmacological review. Braz J Med Biol Res 48:953–964. doi:10.1590/1414-431X20154773
30. Folin O, Ciocalteu V (1927) On tyrosine and tryptophane deter-minations in proteins. J Biol Chem 73:627–650
31. Wootton-Beard PC, Moran A, Ryan L (2011) Stability of the total antioxidant capacity and total polyphenol content of 23 com-mercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Intern 44:217–224
32. Archer J (1973) Tests for emotionality in rats and mice: a review. Anim Behav 21:205–235
33. Porsolt R, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treat-ments. Eur J Pharmacol 47:379–391
34. Steru L, Chermat R, Thierry B, Simon P (1985) The tail sus-pension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
35. Green L, Tannenbaum S, Goldman P (1981) Nitrate synthesis in the germfree and conventional rat. Science 212:56–58
36. Huong N, Matsumoto K, Kasai R, Yamasaki K, Watanabe H (1998) In vitro antioxidant activity of Vietnamese ginseng saponin and its components. Biol Pharm Bull 21:978–981 37. Maehly A, Chance B (1954) The assay catalases and peroxidases.
Methods Biochem Anal 1:357–359
38. Chance B, Maehly A (1955) Assay catalases and peroxidases. Methods Enzymol 2:764–768
induces antidepressive-like effects and increases BDNF levels in the mouse hippocampus. Fundam Clin Pharmacol 29:394–403 41. Cito´ M, Silva M, Santos L, Fernandes M, Melo F, Aguiar J,
Lopes I, Sousa P, Vasconcelos S, Maceˆdo D, Sousa F (2015) Antidepressant-like effect of Hoodia gordonii in a forced swim-ming test in mice: evidence for involvement of the monoamin-ergic system. Braz J Med Biol 48:57–64
42. Herrera-Ruiz M, Garcı´a-Beltra´n Y, Mora S, Dı´az-Ve´liz G, Viana G, Tortoriello J, Ramı´rez G (2006) Antidepressant and anxiolytic effects of hydroalcoholic extract from Salvia elegans. J Ethnophar-macol 107:53–58
43. De Boer A, Gaillard P (2007) Drug targeting to the brain. Annu Rev Pharmacol Toxicol 47:323–355
44. Ne´meth K, Plumb G, Berrin J, Juge N, Jacob R, Naim H, Wil-liamson G, Swallow D, Kroon P (2003) Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr 4:29–42
45. Youdim K, Dobbie M, Kuhnle G, Proteggente A, Abbott N, Rice-Evans C (2003) Interaction between flavonoids and the blood-brain barrier: in vitro studies. J Neurochem 85:180–192 46. Youdim K, Shukitt-Hale B, Joseph J (2004) Flavonoids and the
brain: interactions at the blood–brain barrier and their physio-logical effects on the central nervous system. Free Radic Biol Med 37:1683–1693
47. Wu L, Zhang Q, Zhang X, Lv C, Li J, Yuan Y, Yin F (2012) Pharmacokinetics and blood-brain barrier penetration of (? )-catechin and (-)-epicatechin in rats by microdialysis sampling coupled to high-performance liquid chromatography with chemiluminescence detection. J Agric Food Chem 60:9377–9383 48. Waldmeier P (1987) Amine oxidases and their endogenous sub-strates (with special reference to monoamine oxidase and the brain). J Neural Transm Suppl 23:55–72
49. Takeda H, Tsuji M, Miyamoto J, Masuya J, Iimori M, Matsumiya T (2003) Caffeic acid produces antidepressive- and/or anxiolytic-like effects through indirect modulation of the alpha 1A-adrenoceptor system in mice. NeuroReport 14:1067–1070 50. Gonthier M, Verny M, Besson C, Re´me´sy C, Scalbert A (2003)
Chlorogenic acid bioavailability largely depends on its metabo-lism by the gut microflora in rats. J Nutr 133:1853–1859 51. Takuma K, Baba A, Matsuda T (2004) Astrocyte apoptosis:
implications for neuroprotection. Prog Neurobiol 72:111–127 52. Maes M, Galecki P, Chang Y, Berk M (2011) A review on the
oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the(neuro)degener-ative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry 35:676–692
53. Ng F, Berk M, Dean O, Bush A (2008) Oxidative stress in psy-chiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol 11:851–876
54. Kova´cs P, Jura´nek I, Stankovicova´ T, Svec P (1996) Lipid per-oxidation during acute stress. Pharmazie 51:51–53
55. Cumurcu B, Ozyurt H, Etikan I, Demir S, Karlidag R (2009) Total antioxidant capacity and total oxidant status in patients with major depression: impact of antidepressant treatment. Psychiatry Clin Neurosci 63:639–645
56. Silva M, de Sousa C, Gomes P, de Oliveira G, Arau´jo F, Ximenes N, da Silva J, Silva Vasconcelos G, Leal L, Maceˆdo D, Vasconcelos S (2016) Evidence for protective effect of lipoic acid and desvenlafaxine on oxidative stress in a model depres-sion in mice. Prog Neuropsychopharmacol Biol Psychiatry 64:142–148
57. Michel T, Camara S, Tatschner T (2010) Increased xanthine oxidase in the thalamus and putamen in depression. World J Biol Psychiatry 11:314–320
58. Essa M, Vijayan R, Castellano-Gonzalez G, Memon M, Braidy N, Guillemin G (2012) Neuroprotective effect of natural products against Alzheimer’s disease. Neurochem Res 37:1829–1842 59. Monte A, de Souza G, McIntyre R, Soczynska J, dos Santos J,
Cordeiro R, Ribeiro BM, de Lucena D, Vasconcelos S, de Sousa F, Carvalho A, Maceˆdo D (2013) Prevention and reversal of ketamine-induced schizophrenia related behavior by minocycline in mice: possible involvement of antioxidant and nitrergic path-ways. J Psychopharmacol 27:1032–1043
60. da Silva F, do Carmo de Oliveira Cito´ M, da Silva M, Moura B, de Aquino Neto M, Feitosa M, de Castro Chaves R, Macedo D, de Vasconcelos S, de Franc¸a Fonteles M, Desousa F (2010) Behavioral alterations and pro-oxidant effect of a single ketamine administration to mice. Brain Res Bull 83:9–15
61. Celikbilek A, Gocmen A, Tanik N, Yaras N, Yargicoglu P, Gumuslu S (2014) Serum lipid peroxidation markers are corre-lated with those in brain samples in different stress models. Acta Neuropsychiatr 26:51–57
62. Gu¨mu¨s¸tas¸ K, Meta Gu¨zeyli F, Atu¨keren P, Sanus G, Kemerdere R, Tanriverdi T, Kaynar M (2007) The effects of vitamin E on lipid peroxidation, nitric oxide production and superoxide dis-mutase expression in hyperglycemic rats with cerebral ischemia-reperfusion injury. Turk Neurosurg 17:78–82
63. Garg R, Kumar A (2008) Possible role of citalopram and desipra-mine against sleep deprivation-induced anxiety like-behavior alter-ations and oxidative damage in mice. Indian J Exp Biol 46:770–776 64. Freudenberg F, Alttoa A, Reif A (2015) Neuronal nitric oxide synthase (NOS1) and its adaptor, NOS1AP, as a genetic risk factors for psychiatric disorders. Genes Brain Behav 14:46–63 65. Okumura T, Kishi T, Okochi T, Ikeda M, Kitajima T,
Yama-nouchi Y, Kinoshita Y, Kawashima K, Tsunoka T, Inada T, Ozaki N, Iwata N (2010) Genetic association analysis of func-tional polymorphisms in neuronal nitric oxide synthase 1 gene (NOS1) and mood disorders and fluvoxamine response in major depressive disorder in the Japanese population. Neuropsychobi-ology 61:57–63
66. Galecki P, Maes M, Florkowski A, Lewinski A, Galecka E, Bienkiewicz M, Szemraj J (2011) Association between inducible and neuronal nitric oxide synthase polymorphisms and recurrent depressive disorder. J Affect Disord 129:175–182
67. Kim Y, Paik J, Lee S, Yoon D, Han C, Lee B (2006) Increased plasma nitric oxide level associated with suicide attempt in depressive patients. Prog Neuropsychopharmacol Biol Psychiatry 30:1091–1096
68. Chrapko W, Jurasz P, Radomski M, Lara N, Archer S, Le Mel-le´do J (2004) Decreased platelet nitric oxide synthase activity and plasma nitric oxide metabolites in major depressive disorder. Biol Psychiatry 56:129–134
69. Rachkauskas G (1998) The level of lipid peroxidation and the function of the antioxidant system in different forms of schizophrenia. Lik Sprava 5:92–93
70. Wang C, Wu H, Jing X, Meng Q, Liu B, Zhang H, Gao G (2012) Oxidative parameters in the rat brain of chronic mild stress model for depression: relation to anhedonia-like responses. J Membr Biol 245:675–681
71. Mate´s J, Sa´nchez-Jime´nez F (1999) Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci 4:339–345
72. Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E (2005) Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res 53:129–139
74. Van der Kolk N, Speelman A, van Nimwegen M, Kessels R, IntHout J, Hakobjan M, Munneke M, Bloem B, van de Warren-burg B (2015) BDNF polymorphism associates with decline in set shifting in Parkinson’s disease. Neurobiol Aging 36:1605–1606 75. Duman RS (2004) Role of neurotrophic factors in the etiology
and treatment of mood disorders. NeuroMol Med 5:11–25 76. Mello B, Monte A, McIntyre R, Soczynska J, Custo´dio C,
Cor-deiro R, Chaves J, Vasconcelos S, Nobre H Jr, Florenc¸o de Sousa F, Hyphantis T, Carvalho A, Maceˆdo D (2013) Effects of doxy-cycline on depressive-like behavior in mice after lipopolysac-charide (LPS) administration. J Psychiatr Res 47:1521–1529 77. Cai S, Huang S, Hao W (2015) New hypothesis and treatment
targets of depression: an integrated view of key findings. Neu-rosci Bull 31:61–74
78. Cryan J, Valentino R, Lucki I (2005) Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev 29:547–569
79. Krishnan V, Nestler E (2008) The molecular neurobiology of depression. Nature 455:894–902
80. Castre´n E, Vo˜ikar V, Rantama¨ki T (2007) Role of neurotrophic factors in depression. Curr Opin Pharmacol 7:18–21
81. Dwivedi Y (2013) Involvement of brain-derived neurotrophic factor in late-life depression. Am J Geriatr Psychiatr 21:433–449 82. Hellweg R, Ziegenhorn A, Heuser I, Deuschle M (2008) Serum concentrations of nerve growth factor and brain-derived neu-rotrophic factor in depressed patients before and after antide-pressant treatment. Pharmacopsychiatry 41:66–71
83. Numakawa T (2014) Possible protective action of neurotrophic factors and natural compounds against common neurodegenera-tive diseases. Neural Regen Res 9:1506–1508
84. Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M (2014) Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—a mini-re-view. Life Sci 113:1–6
85. Donato F, de Gomes M, Goes A, Filho C, Del Fabbro L, Antunes M, Souza L, Boeira S, Jesse C (2014) Hesperidin exerts antide-pressant-like effects in acute and chronic treatments in mice: possible role ofL-arginine-NO-cGMP pathway and BDNF levels.
Brain Res Bull 104:19–26
86. Souza L, de Gomes M, Goes A, Del Fabbro L, Filho C, Boeira S, Jesse C (2013) Evidence for the involvement of the serotonergic 5-HT1A receptors in the antidepressant-like effect caused by hesperidin in mice. Prog Neuropsychopharmacol 40:103–109 87. Stringer T, Guerrieri D, Vivar C, Van Praag H (2015)
Plant-derived flavanol (-) epicatechin mitigates anxiety in association with elevated hippocampal monoamine and BDNF levels, but does not influence pattern separation in mice. Transl Psychiatry 6:493
88. Zheng M, Fan Y, Shi D, Liu C (2013) Antidepressant-like effect of flavonoids extracted fromApocynum venetumleaves on brain monoamine levels and dopaminergic system. J Ethnopharmacol 147:108–113
89. Takeda H, Tsuji M, Inazu M, Egashira T, Matsumiya T (2002) Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur J Pharmacol 449:261–267