2017
UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE FÍSICA
A multiplexed organ-on-chip device for the study of the
Blood-Brain Barrier
Marciano Palma do Carmo
Mestrado Integrado em Engenharia Biomédica e Biofísica
Perfil em Radiações em Diagnóstico e Terapia
Dissertação orientada por:
2017
UNIVERSITY OF LISBON
FACULTY OF SCIENCES
DEPARTMENT OF PHYSICS
A multiplexed organ-on-chip device for the study of the
Blood-Brain Barrier
Marciano Palma do Carmo
Integrated Master in Biomedical Engineering and Biophysics
Profile in Radiations in Diagnosis and Therapy
Dissertation guided by Marinke van der Helm, MSc. and Dr. Hugo Ferreira, and co-supervised
by Dr. Loes Segerink
“The important thing is not to stop questioning.” Albert Einstein
vii
Acknowledgments
My deepest and sincere gratitude is to my daily supervisor, Marinke van der Helm, not only for her excellent supervision and for teaching me everything, but also for all her help, patience, and especially friendship. You did your best to make me feel at home and you definitely succeeded with it, so thank you very much! Furthermore, I thank Loes Segerink for her keen eye on science and all her enthusiasm for the project, from which I benefited a lot, and also for never saying no every time I came into her office with a new crazy idea. I also want to thank Albert van der Berg for giving me the opportunity to perform my Master’s assignment in such an amazing group. In addition, I want to thank Andries van der Meer for all his guidance, expertise in organs-on-chips and for all the valuable ideas, discussions and recommendations he gave me during the course of my work. Moreover, I want to thank my internal supervisor, Hugo Ferreira, for all his supervision throughout this project and for his enthusiasm and passion about biomedical engineering, in particular for micro and nanotechnologies, which contributed greatly for my desire in doing my Master’s assignment in such a field.
Next, I want to thank the entire BIOS staff for all the provided help. In particular, I thank Johan Bomer for teaching me everything I know about microfabrication, for letting me follow him inside the cleanroom for days, and also for always being willing to try any idea I came up with, even though he knew from the start that most of them would fail terribly. I also want to thank Paul ter Braak for the course on cell culturing, all his help with cell-related issues, and for making the BioLab such a fun place to be. Furthermore, I want to thank Hans de Boer for fabricating both my chip holder and the mold I used for PDMS membrane fabrication. I also want to thank Anne Leferink for inviting me to be her teaching assistant, from which I learned a lot on membrane fabrication, and for giving and letting me use several of her materials. Next, I want to thank Hai Le The for all his help with my abstract submission, cleanroom-related issues, for all his enthusiasm in membrane fabrication, and for being such a nice guy to be with. You are the person who made the cleanroom and the cycling back home fun! Furthermore, I want to thank Hugo Albers for teaching me everything he knew on PDMS chip fabrication, for his help with CleWin and COMSOL, for being such a nice roommate in the student corner, and for all the fun times we had around Enschede! I also want to thank Josh Loessberg-Zahl for all the fruitful discussions and fun times in the lab. In addition, I thank Mathijs Bronkhorst, my other student corner roomie, for all his help with COMSOL and for teaching and letting me use his setup. I also owe thanks to Martijn Tibbe for letting me use his setup, Wesley van den Beld for growing the nitride layer for my membranes and Andrea Minuto for all the help with fabricating my devices in the DesignLab. Furthermore, I want to thank all the BIOS members for being such an amazing group of people who made me feel at home for ten months and helped me out whenever I needed. The group activities were a lot of fun, in particular the barbecue, the mountain biking, and the weekly football games.
viii
Last, but not least, I am thankful for all the support my parents, family and friends gave me not only during these ten months but since I started the journey of becoming a biomedical engineer. In particular, I thank my girlfriend Margarida for all her support, for putting up with me and not turning off our skype chats every time I started talking about work, and especially for her uplifting personality. Your love has made all the difference.
ix
Abstract
The blood-brain barrier (BBB) constitutes a complex interface between blood and the central nervous system (CNS), playing a vital role in maintaining brain homeostasis and protecting it from most toxic substances and pathogens. However, due to the extremely low permeability that arises from the tight junctions formed by the endothelial cells, the BBB also inhibits the brain uptake of many pharmaceuticals, therefore posing a major obstacle for drug development studies. Furthermore, the disturbance of the function of this unique structure can lead to many neurodegenerative disorders that are not yet fully understood, such as brain tumors or Alzheimer’s disease.
There have been several attempts to establish reliable in vivo and in vitro models of the BBB that can increase the knowledge on such pathological states of the brain and provide useful insights on drug delivery across this barrier. However, as in vivo models require expensive and specific equipment, are labor intensive, and give rise to many ethical and moral concerns, much effort is being put into developing an in vitro model that truthfully resembles the BBB and is easy to analyze, reproducible, and allows higher throughput screening than the in vivo models. Therefore, the main goal of the present work was to design, fabricate and validate a microfluidic chip that could serve as a tool for the study of the blood-brain barrier and allowed the creation of several different experimental conditions at the same time in the same chip, thus increasing the throughput of the system. Two different models, a two-dimensional and a three-two-dimensional, were fabricated for this purpose.
The first model that was built, which consisted of two poly(dimethylsiloxane) (PDMS) parts with a membrane in between, allowed the monitoring of the tightness of the endothelial cell layers by integrating on-chip transendothelial electrical resistance (TEER) and permeability analysis. Even though preliminary, the results obtained for both these assays were quite encouraging and TEER and permeability were found to be as high as 27.5 Ω·cm2
and as low as 3.6·10-5 cm/s, respectively. Moreover, polystyrene (PS), silicon-rich nitride (SiRN) and PDMS membranes were fabricated in order to improve the permeable supports on which cells were seeded inside this device. The second model, on the other hand, was made in plexiglas and allowed the creation of lumen-shaped three-dimensional structures within collagen on which cells were seeded. No experiments regarding TEER or permeability were performed in the second model, although permeability studies could be done with the appropriate protocol.
The capability of individually addressing the microfluidic chambers without any mixing occurring between them was proven for both devices in experiments using dyes, trypsin and ethanol. Furthermore, confluent monolayers of endothelial cells were observed in the two models with both phase contrast and fluorescence microscopy, making it possible for us to conclude that two different yet equally physiologically relevant multiplexed devices that allow the individual addressing of their microfluidic chambers had been developed.
However, further experimenting is required in order to fully characterize the devices, especially concerning TEER measurements, permeability assays and dynamic cell culturing. Moreover, finding a coating agent that would allow the use of lower concentrations of collagen to fabricate the hollow channels would make the second model more advantageous. Furthermore, the co-culture of endothelial cells with other cell types that are known to enhance the tightness of the BBB, such as astrocytes or
x
pericytes, should also be looked into for both devices. Performing on-chip drug screening studies would also be of interest.
xi
Sumário em Português
A barreira hemato-encefálica representa a mais complexa interface entre o sangue e o sistema nervoso central, sendo bastante importante na manutenção da homeostasia do cérebro e em proteger o mesmo de diversas substâncias tóxicas e patogénicas. Contudo, devido à baixa permeabilidade que advém das ligações formadas entre as diversas células endoteliais, esta barreira inibe também a entrada de vários agentes farmacêuticos no cérebro, constituindo assim um grande obstáculo ao desenvolvimento de novas terapias. Para além disto, qualquer pertubação no funcionamento desta estrutura pode originar diversas doenças neurodegenerativas.
Várias têm sido as tentativas de desenvolver um modelo fidedigno da barreira hemato-encefálica, quer in vivo, ex-vivo, in silico e in vitro, que ajude a melhor compreender estes estados patológicos do cérebro e que possa fornecer novas perspectivas acerca do transporte de agentes terapêuticos através desta barreira. No que diz respeito aos modelos in vivo, estes não só requerem equipamento bastante específico e caro, como também são bastante morosos e dão origem a diversas questões éticas e morais. Os modelos ex-vivo, por outro lado, permitem analisar tecidos vivos, os quais podem ser fatias de um órgão ou o órgão completo, fora do seu contexto biológico, permitindo desta forma um maior controlo de todas as condições experimentais do que os modelos in vivo. No entanto, garantir que o ambiente artificial em que o tecido é analisado tenha exactamente as mesmas condições que o seu ambiente biológico pode tornar-se complicado, o que pode por sua vez causar a morte do tecido. No caso dos modelos in silico, estes são construídos com recurso a modelos computacionais baseados em dados obtidos em experiências realizadas in vivo, o que os torna bastante fidedignos em prever, por exemplo, a permeabilidade da barreira hemato-encefálica a um determinado medicamento. O facto destes modelos muitas vezes não terem em conta toda a complexidade da barreira hemato-encefálica é uma das suas limitações. No caso dos modelos in vitro, estes permitem o estudo das mais variadas estruturas biológicas fora do seu contexto natural. Para tal, células derivadas de tecidos cerebrais são cultivadas em modelos construídos propositadamente para o estudo em questão, o que permite um maior controlo sobre todas as condições experimentais. Deste modo, é perceptível que muitos esforços têm sido feitos para desenvolver um modelo in vitro que simule correctamente a barreira hemato-encefálica e que seja de fácil análise, reprodutível, e permita um maior rastreio (de agentes terapêuticos, por exemplo) que os modelos in vivo.
O objectivo principal deste trabalho foi desenvolver e validar um chip microfluídico que pudesse ser usado como uma ferramenta para o estudo da barreira hemato-encefálica e que permitisse a criação de diferentes condições experimentais ao mesmo tempo, no mesmo chip. Para atingir este objectivo, foram construídos dois modelos diferentes: um bi-dimenisonal e um tri-dimensional.
O primeiro modelo construído, o qual consistia em duas partes de dimetil polissiloxano com uma membrana entre elas, foi replicado a partir de um molde feito de silício, o qual por sua vez foi fabricado numa sala estéril com recurso a técnicas de microfabricação. Células endoteliais foram cultivadas neste modelo e as barreiras formadas pelas mesmas mantiveram-se viáveis, em todos os casos, durante pelo menos cinco dias de cultura celular, período após o qual o núcleo e o citoesqueleto das células foram coloridos de modo a verificar a integridade das barreiras formadas. Este modelo possibilitou ainda a
xii
monitorização da complexidade das barreiras de células endoteliais ao integrar funcionalidades que permitiam a análise da resistência eléctrica transendotelial e da permeabilidade das mesmas. Embora preliminares, os resultados obtidos em ambos os testes foram bastante encorajadores e os valores obtidos para a resistência eléctrica e para a permeabilidade das barreiras foram de 27.5 Ω·cm2 e 3.6·10-5 cm/s, respectivamente. Para além disto, foram também fabricadas membranas feitas de poliestireno, nitrato rico em silício e dimetil polissiloxano, com o intuito de substituir os suportes permeáveis feitos em policarbonato nos quais as células eram cultivadas.
O segundo modelo, por outro lado, foi feito em acrílico. Para tal, um bloco de acrílico foi cortado a laser e as diferentes peças foram coladas umas às outras com recurso a um adesivo biocompatível. Depois de montado o chip, um gel de colagénio foi inserido em cada um dos compartimentos do mesmo e micro-agulhas foram posicionadas por entre os buracos das tampas do chip. Depois de solidificar o colagénio, as micro-agulhas foram cuidadosamente retiradas. Isto permitiu a criação, em colagénio, de estruturas tridimensionais em forma de lúmen nas quais as células foram cultivadas. Tal como no modelo bi-dimensional, as células endoteliais cultivadas no colagénio mantiveram-se viáveis durante pelo menos cinco dias de cultura celular. Não foram realizadas neste modelo quaisquer experiências que tivessem como intuito determinar a resistência eléctrica e a permeabilidade das barreiras de células endoteliais, embora estudos de permeabilidade pudessem ser feitos com o protocolo adequado.
A capacidade de utilizar individualmente os diferentes compartimentos microfluídicos sem que ocorresse qualquer mistura entre os mesmos foi provada em ambas as plataformas. Em relação ao modelo bi-dimenional, foram realizadas, numa primeira fase, experiências com corantes, enquanto numa segunda fase tripsina, álcool e meio de cultura foram inseridos nos diferentes compartimentos microfluídicos para confirmar se ocorria alguma mistura das soluções que afectasse as diversas barreiras celulares. Para tal, os chips foram ligados a uma bomba microfluídica que puxou as diversas soluções em todos os compartimentos microfluídicos. No caso das experiências em que foram utilizados trisipsina, álcool e meio de cultura, foi realizada uma coloração para verificar a viabilidade das barreiras celulares no final da experiência, a qual revelou que os diferentes compartimentos podiam ser utilizados sem que houvesse qualquer contaminação entre os diferentes compartimentos que pudesse pôr em causa a integridade das barreiras celulares. No que diz respeito ao modelo tri-dimensional, a capacidade de utilizar individualmente os diversos compartimentos foi apenas provada ao introduzir os diferentes corantes nos mesmos, o que revelou que o método de fabricação do chip assegurava uma plataforma robusta na qual diversas experiências podiam ser realizadas sem qualquer risco de contaminação. Para além disto, foi também possível cultivar células endoteliais neste chip durante pelo menos cinco dias, embora a coloração das mesmas não tenha sido bem sucedida uma vez que os agentes fluorescentes se difundiram pelo colagénio. Isto fez com que fosse possível concluir que, embora diferentes, dois modelos igualmente relevantes em termos fisiológicos tinham sido desenvolvidos.
Contudo, ambos os modelos necessitam de uma caracterização mais profunda. No caso do primeiro modelo, este beneficiaria caso melhorias fossem feitas no que diz respeito a medições de resistência eléctrica, de permeabilidade, e também de cultura dinâmica de células. Para tal, experiências futuras e protocolos adequados são necessários. No caso do segundo modelo, encontrar um agente que permita revestir as estruturas em acrílico de modo a que seja possível utilizar concentrações mais baixas de colagénio seria bastante benéfico. Para além disto, a criação de co-culturas de células endoteliais com células cerebrais que são conhecidas por aumentar a complexidade e a impermeabilidade da barreira hemato-encefálica, tais como astrócitos ou perócitos, devia também ser realizada em ambos os modelos de modo a verificar se as mesmas aumentariam a complexidade da barreira hemato-encefálica formada, como descrito na literatura. Por último, seria também de elevado interesse realizar testes de rastreio de diversos agentes farmacêuticos utilizados no tratamento de várias patologias, como por exemplo para as doenças de Alzheimer e de Parkinson, de modo a obter novas perspecivas no que à permeabilidade da barreira hemato-encefálica a estas substâncias diz respeito.
Palavras-chave:
BHE-em-chip, barreira hemato-encefálica, microfluídica, microfabricação, órgãos-em-chips.xiii
Contents
Acknowledgments ... vii
Abstract ... ix
Sumário em Português ... xi
List of figures ... xvii
List of tables ... xxi
List of abbreviations ... xxiii
1 Introduction ... 1
1.1 Project description ...1
1.2 Preview ...1
2 Background & theory ... 3
2.1 Anatomy and physiology of the blood-brain barrier ...3
2.2 Ways to study the BBB ...5
2.3 In vitro BBB model evaluation...7
2.3.1 Cells ... 8
2.3.2 Shear stress ... 8
2.3.3 Permeability ... 9
2.3.4 Transendothelial electrical resistance ... 10
2.4 Existing microfluidic BBB models ... 14
2.5 Summary ... 17
3Microfluidic devices design ... 19
3.1 Single BBB chip ... 19
3.1.1 Design and fabrication ... 19
3.1.2 Drawbacks & requirements ... 20
3.2 2D Multiplexed BBB chip ... 20
3.2.1 Chip and photomask design ... 20
3.2.2 Mold fabrication ... 22
3.3 3D BBB Device ... 23
3.3.1 Design and Fabrication ... 23
xiv
4.1 Methods ... 25
4.1.1 Polystyrene membrane ... 25
4.1.2 Silicon-rich nitride membrane ... 25
4.1.3 Polydimethylsiloxane membrane ... 27
4.2 Results & discussion ... 30
4.2.1 Polystyrene membrane ... 30
4.2.2 Silicon-rich nitride membrane ... 31
4.2.3 Polydimethylsiloxane membrane ... 32
5Microfluidic devices validation ... 35
5.1 Methods ... 35
5.1.1 Device fabrication ... 35
5.1.2 Fluidic characterization of two-dimensional devices ... 37
5.1.3 Characterization of lumen robustness in the 3D devices ... 39
5.1.4 On-chip cell culture ... 39
5.1.5 Cell observation ... 41
5.1.6 Mechanical modulation ... 41
5.1.7 Individually addressable channels ... 42
5.1.8 Dextran permeability assay ... 43
5.1.9 TEER assay ... 44
5.2 Results ... 46
5.2.1 Device fabrication ... 46
5.2.2 Fluidic characterization ... 51
5.2.3 Characterization of lumen robustness in the 3D devices ... 54
5.2.4 On-chip cell culture ... 55
5.2.5 Mechanical modulation ... 60
5.2.6 Individually addressable channels ... 60
5.2.7 Dextran permeability analysis ... 64
5.2.8 TEER analysis ... 66
6Conclusion ... 69
7Recommendations & future perspectives ... 71
8Bibliography ... 73
Appendices ... 79
xv
BMATLAB scripts ... 85
B.1 MATLAB script for the calculation of flow profile ... 85
B.2 MATLAB script for tracking particles ... 87
B.3 MATLAB script for computing the positions of the tracked particles ... 95
B.4 MATLAB script for calculating the velocities of the tracked particles from the saved tracks .... 96
CSupplementary information ... 99
C.1 Fluidic characterization ... 99
C.2 Mechanical modulation ... 100
C.3 Channels individually addressed using trypsin and EGM-2 ... 100
C.4 Dextran permeability assay ... 101
C.5 TEER assay ... 101
C.6 3D device ... 103
xvii
List of figures
Figure 2.1 – Schematic representation of the BBB ... 3
Figure 2.2 – Structure of BBB tight and adherens junctions ... 4
Figure 2.3 – Main routes of molecular transport across the BBB ... 5
Figure 2.4 – Equivalent circuit diagram describing the contribution of the trans- and paracellular pathway to the total impedance, Z, of the cellular system ... 12
Figure 2.5 – Impedance simulation of a cell monolayer on a chip ... 13
Figure 2.6 - Models of the BBB reported in literature ... 14
Figure 3.1 - Design of the current BBB chip ... 19
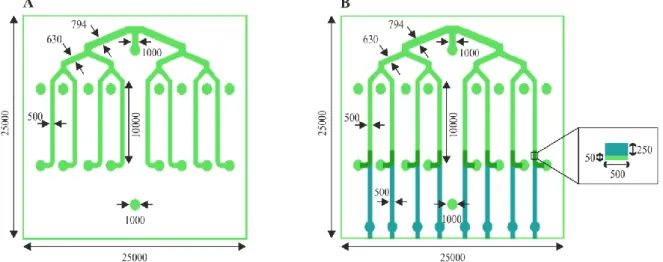
Figure 3.2 – Fully assembled (A) and exploded view (B) of the 2D BBB multiplex device ... 21
Figure 3.3 – Photomasks design ... 21
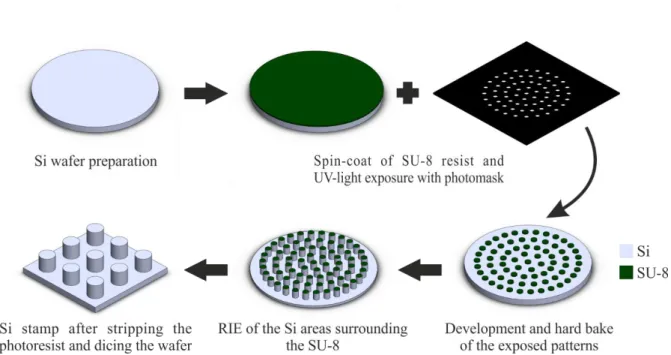
Figure 3.4 – Fabrication process of the molds ... 22
Figure 3.5 – Fully assembled (A) and exploded view (B) of the 3D BBB multiplex device ... 23
Figure 4.1 - Schematic representation of the SiRN membrane fabrication process ... 26
Figure 4.2 - Schematic representation of the Si stamp fabrication process ... 27
Figure 4.3 – Fabrication process of porous PDMS membranes with the aid of a Si stamp ... 28
Figure 4.4 - Fabrication process of PDMS membranes ... 29
Figure 4.5 - Fabricated PS membrane ... 30
Figure 4.6 - Fabricated porous SiRN membrane ... 31
Figure 4.7 – SEM image of the pillars in the fabricated Si stamp ... 32
Figure 4.8 – Broken Si pillars on the stamp (left) and on the PS sheet (right) ... 32
Figure 4.9 – Continuous PDMS membrane ... 33
Figure 4.10 – Porous PDMS membrane ... 34
Figure 4.11 - (Semi-)Porous PDMS membrane, fabricated by curing the first layer of resist at 120ºC for 10 min, before (A) and after being peeled off the wafer (B and C) ... 34
Figure 5.1 – Schematic illustration of the two-layer PC membrane-based devices ... 36
Figure 5.2 - Schematic illustration of the two-layer PDMS membrane-based devices ... 37
Figure 5.3 – Dynamic culture setup. ... 42
Figure 5.4 - Schematic representation of the experiments with food dye, trypsin and ethanol in every other outlet of the 2D BBB multiplex device ... 43
Figure 5.5 - Schematic representation of the on-chip dextran permeability assay ... 44
Figure 5.6 - Schematic illustration of the top view of the BBB multiplex device with Pt electrodes (A) and of the electrical circuit of a cell layer on one of the channels of the BBB multiplex device (B) ... 45
Figure 5.7 – Fabrication of single-layer PDMS on glass devices ... 46
Figure 5.8 – Problems in the fabrication of two-layer PC membrane-based PDMS devices using the toluene/PDMS mortar... 47
Figure 5.9 – A) Fully assembled two-layer device with a PC membrane and B) Highlight of a fully enclosed channel after hCMEC/D3 cells were loaded into the device ... 48
xviii
Figure 5.10 – A), B) Fabrication and C) schematic illustration, of two-layer PDMS membrane-based
devices ... 48
Figure 5.11 – A), B) Single BBB device with a PDMS membrane and C) respective schematic illustration ... 49
Figure 5.12 – Fully assembled two-layer PDMS membrane-based device with Pt electrodes ... 49
Figure 5.13 – 3D multiplex BBB device both fully assembled and separated in pieces ... 50
Figure 5.14 – Beads and fluorescein distribution throughout the channels of the 2D BBB multiplex devices ... 51
Figure 5.15 – Flow inside the 2D BBB multiplex device... 52
Figure 5.16 – Flow rates inside the microfluidic devices obtained empirically ... 53
Figure 5.17 – Assessing the integrity of the three hollow lumens inside Collagen I ... 54
Figure 5.18 – Stained cells inside a single-layer PDMS on glass device: blue – nuclei; green – f-actin ... 55
Figure 5.19 – Cells inside two of the channels of a two-layer PC membrane-based device ... 56
Figure 5.20 – hCMEC/D3 viability on the PC membrane: green (Calcein – AM) – live cells ... 56
Figure 5.21 – Staining of hCMEC/D3 cell layer on the PC membrane: blue – nuclei, green – f-actin 57 Figure 5.22 – Cells inside two of the channels of a two-layer PDMS membrane-based device ... 58
Figure 5.23 – Staining of the hCMEC/D3 cells on a two-layer device with a PDMS membrane. Blue – nuclei, green – f-actin ... 59
Figure 5.24 – hCMEC/D3 cells seeded in one the A) top and B) bottom parts of one of the Collagen I lumens fabricated inside the 3D BBB multiplex chip ... 59
Figure 5.25 – hCMEC/D3 cells on one of the microfluidic channel before and after dynamic culture 60 Figure 5.26 – Individually addressable channels of the BBB multiplex device with blue, yellow, red and green food dyes ... 61
Figure 5.27 – Food dyes inside the three-dimensional BBB multiplex chip ... 61
Figure 5.28 – Trypsin experiment: Cells inside two channels of the BBB device before, during and after (live/dead staining) 0.05% trypsin and normal EGM-2 culture medium were flushed in every other channel ... 62
Figure 5.29 – Live/dead staining of the cells inside the microfluidic device after normal EGM-2 and 5% ethanol were pulled from every other outlet. Blue – nuclei, green – calcein AM (live cells), red – ethidium homodimer-1 (dead cells) ... 63
Figure 5.30 – Live/dead staining of the hCMEC/D3 cells in the areas where the channels merge with each other in the experiments with A) trypsin and B) ethanol. Blue – nuclei, green – calcein AM (live cells), red – ethidium homodimer-1 (dead cells) ... 64
Figure 5.31 – Permeability coefficients of the eight BBBs of device 1 to the 40 kDa dextran ... 64
Figure 5.32 – Average TEER values obtained for the eight cell barriers inside the BBB multiplex device at different time points after seeding the cells (at t = 0 h) ... 66
Figure C.1 – Plot of the velocities inside the microfluidic channels of the BBB device obtained with COMSOL ... 99
Figure C.2 – Air trapped inside the microfluidic channels when investigating the influence of fluidic shear stress in cell growth, morphology and proliferation ... 100
Figure C.3 – Permeability coefficients of the eight BBB’s of device 2 to the 40 kDa dextran... 100
Figure C.4 – Channels of the 2D BBB multiplex device individually addressed using trypsin and EGM-2. Blue – nuclei; green – Calcein AM (live cells); red – Ethidium homodimer-1 (dead cells) ... 101
Figure C.5 – Impedance spectra obtained in obtained for channel 1 of device 2 when A) there were no cells in the device and when B) hCMEC/D3 cells had been seeded ... 102
Figure C.6 – Nuclei (blue) and f-actin (green) staining of the hCMEC/D3 cells on collagen I inside the 3D BBB-on-chip device ... 103
Figure D.1 - Si mold with SU-8 structures for the fabrication of simple BBB multiplex devices ... 105
Figure D.2 - Si mold with SU-8 structures for the fabrication of BBB multiplex devices with Pt electrodes ... 105
xix
Figure D.3 – Chip holder used in fabrication of 2D devices ... 106 Figure D.4 – Mold for the fabrication of the PDMS rings A) dismounted and B) fully assembled ... 106 Figure D.5 – Diced Si stamp (left) and SiRN membrane (right) ... 106
xxi
List of tables
Table 5.1 – Measured average flow rates, and corresponding calculated shear rates, inside the
microfluidic channels ... 53
Table C.1 – TEER values obtained for the cell layers seeded in device 1 ... 101
Table C.2 – TEER values obtained for the cell layers seeded in device 2 ... 102
xxiii
List of abbreviations
AC Alternating currentACM Astrocyte-conditioned medium AJs Adherens junctions
BBB Blood-brain barrier
BL Basal lamina
BSA Bovine serum albumin CCL Cell layer capacitance (F)
CDL Double-layer capacitance (F)
CEL Electrode capacitance (F)
Cmem Cell membrane capacitance (F)
CNS Central nervous system
DC Direct current
DI Deionized water
DMSO Dimethylsulfoxide
EAE Experimental allergic encephalomyelitis EBM-2 Endothelial basal medium-2
EGM-2 Endothelial growth medium-2 EVOM Epithelial Voltohmmeter FDTS Perfluorodecylthrichlorosilane
FN Fibronectin
fTEER Frequency at which TEER is read (Hz) GBM Glioblastoma multiform
hBMVEC Human brain-derived microvascular endothelial cells hCMEC/D3 Human cerebral microvascular endothelial cells hiPSC Human induced pluripotent stem cells
HMDS Hexamethyldisilazane HNO3 Nitric acid
ID Inner diameter
JAMs Junctional adhesion molecules KOH Potassium hydroxide
LPCVD Low-pressure chemical vapor deposition
N2 Nitrogen
NaOH Sodium hydroxide NVU Neurovascular unit
OD Outer diameter
xxiv PB Permeabilization buffer
PBS Phosphate-buffered saline
PC Polycarbonate
PCTE Polycarbonate track-etched PDMS Poly (dimethylsiloxane)
PE Polyester
PET Polyethylene terephthalate PMMA Poly (methylmethacrylate)
PS Polystyrene
Pt Platinum
PT790 Plasma term 790 PTFE Polytetrafluoroethylene PVP Polyvinylpyrrolidone QDR Quick dump rinse RIE Reactive ion etching Rmed Medium resistance (Ω)
Rmembrane Membrane resistance (Ω)
Rpores Resistance of medium inside membrane pores (Ω)
RTEER TEER resistance (Ω)
RPM Rotations per minute
RT Room temperature
SCCM Standard cubic centimeter per minute SEM Scanning electron microscope / microscopy SF6 Sulfur hexafluoride
Si Silicon
SiRN Silicon rich nitride
TEER Transendothelial electrical resistance
TJs Tight junction
TNF-α Tumor necrosis factor alpha
UV Ultraviolet
Zmem Membrane impedance
1
Chapter 1
Introduction
1.1 Project description
The final goal of the present work is to develop a multiplexed organ-on-chip platform for the study of the blood-brain barrier (BBB) that allows the creation of different experimental conditions at the same time (e.g., for the screening of different drugs), thus reducing the elapsed time between experiments and enhancing the throughput of the system. In order to do this, the current microfluidic model of the BBB used at BIOS Lab on a Chip group was redesigned, with the main goal of fabricating a new device that could allow several parallel and compartmentalized cultures of brain endothelial cells. Moreover, improvements were also made to the supports on which cells are grown. After thorough characterization, the validity of the multiplex organ-on-chip device as a reliable model of the BBB was investigated.
1.2 Preview
In the next chapter the anatomy and physiology of the BBB, as well as the standard ways to mimic it, will be described. Furthermore, the most common ways to evaluate the functionality of a BBB model will also be explained. Subsequently, the procedures that were used to design the microfluidic devices and the molds from which these were replicated are detailed. In chapter 4, the methods and corresponding results for the fabrication of different types of membranes to integrate in the BBB device are described. The methods and results regarding device validation, specifically the fabrication of the different devices, the on-chip culturing of cells, and the different experiments performed on the devices are displayed and discussed in chapter 5. After this, conclusions and future prospects about this work are depicted.
Finally, in appendix A the paper ‘Individually addressable channels in a multiplexed organ-on-chip device’, and the corresponding poster, that was accepted at the MicroNano Conference (13-14 December 2016, Amsterdam, the Netherlands) is included and in appendices B, C and D the MATLAB scripts, supplementary information concerning some of the experiments and figures of important parts and tools that were used throughout this project are shown, respectively.
3
Chapter 2
Background & theory
2.1 Anatomy and physiology of the blood-brain barrier
The Blood-Brain Barrier (BBB) is a highly selective structure in the central nervous system that separates the circulating blood from the extracellular brain fluids. This structure is formed by endothelial cells, which constitute the walls of the brain capillaries with a combined surface area that makes it the largest interface for blood-brain exchange.1-4 At the apical region of these endothelial cells, a complex set of specific proteins, such as occludins, claudins, and junctional adhesion molecules (JAMs), are linked to regulatory proteins (Zonula Occludens (ZO) 1, 2 and 3, and cingulin) and together form tight junctions (TJs) with high electrical resistivity. TJs are the main structures responsible for the BBB properties and not only regulate diffusion between the apical and basolateral domains of the cell membrane but also limit the paracellular permeability of polar solutes through paracellular diffusional pathways from the blood plasma to the brain extracellular fluid.7,8,9 In figure 2.1 we have a representation of the endothelial cells that form the BBB, as well as of its TJs and of microglia, astrocytes, pericytes and neurons, which comprise some of the capillaries’ neighboring glial cells that are known to release vasoactive agents and cytokines that can modify TJ assembly and barrier permeability.3
Figure 2.1 – Schematic representation of the BBB. The BBB is formed by endothelial cells that form tight junctions at their
borders, responsible for reducing the paracellular pleonastic diffusional pathway. These endothelial cells are surrounded by pericytes and smooth muscle, thus forming a capillary within the basal lamina. The bond between the glial endfeet and the brain parenchyma form an extracellular matrix, BL2, different in composition from BL1. Surrounding the capillaries are the astrocytic endfeet, which offers connections to the neurons and microglia, thus forming close and complex cell associations that are important in inducing and maintaining the properties of the barrier. Adapted from [4].
4
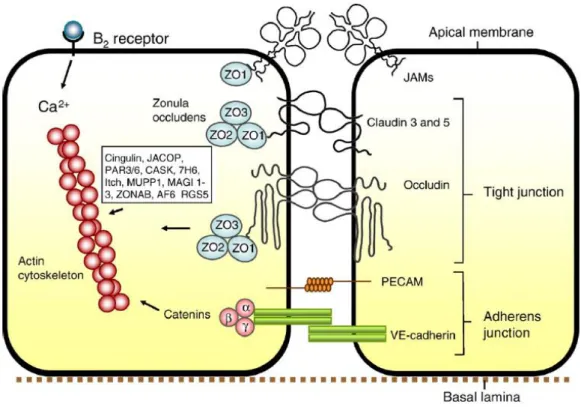
Besides TJs, the junctional complexes between endothelial cells also include adherens junctions (AJs). AJs are situated in the basal region of the lateral plasma membrane and contain cadherin proteins that are linked into the cell cytoplasm by α, β and γ catenin proteins. These junctions hold the cells together giving the tissue structural support and they are essential for the formation of TJs, as their disruption is associated with barrier disruption.10 In figure 2.2 we can see in more detail the structure of the BBB tight and adherens junctions.
Amongst the most important functions of the BBB, is its crucial role in maintaining brain homeostasis, protecting the brain from the extracellular environment and providing the nutrients needed for its normal function. Brain homeostasis is achieved through the regulation of ion balance and of compound influx/efflux.7 By a combination of specific ion channels and transporters, the BBB helps keeping the ionic composition ideal for synaptic signaling and neural function.4 As for the case of compounds influx/efflux, it both prevents many macromolecules from entering the brain and expels some of them from it, shielding the CNS from neurotoxic substances circulating in the blood, such as endogenous metabolites or proteins.4 A good example of this function is the specialized drug efflux P-glycoprotein. Regarding nutrition, in order to provide the necessary nutrients and metabolites required by the nervous tissue, specific transport systems are expressed in the BBB besides paracellular pathways. These transport mechanisms are illustrated in figure 2.3.
Figure 2.2 – Structure of BBB tight and adherens junctions. Occludins, claudins 3 and 5 and possibly other claudins comprise
the tight junctional complex, whereas the cadherins of the adherens junctions provide the structural integrity and the attachment between the endothelial cells that is needed for the formation of tight junctions. Such tight junctions are formed by the bonding of the claudins and occludins to the zonula occludens scaffolding proteins and are the main responsible for the extremely low permeability of the BBB. Adapted from [4].
5
Due to its highly selective permeability, the BBB only allows molecules smaller than, approximately, 500 Da to easily cross it, which means that it not only protects the human brain from a great amount of toxic substances but also inhibits several drug candidates from entering brain tissues from capillaries, therefore posing a great hurdle to drug delivery to the brain. Moreover, loss of barrier function is highly associated with several neurological disorders, such as brain tumors, multiple sclerosis, Alzheimer’s and Parkinson’s diseases, brain edema, and many more. For example, the loss of BBB integrity and barrier functionality caused by the impairment of the tight junctions of claudin-3, but not claudin-5 or occludin, has been reported to be associated with conditions such as experimental allergic encephalomyelitis (EAE) and glioblastoma multiform (GBM).4,12 Therefore, the need for more intensive pre-clinical studies is clear, not only to develop new drug treatments and better understand their passage across the BBB, but also to study the role of barrier function on CNS disease progression and test innovative methods of delivery,15 as directing therapies to protect or repair the endothelium may provide an effective way to reduce severity of neurological symptoms or delay onset of neurodegeneration for many pathologies.2
2.2 Ways to study the BBB
Since the importance of the BBB has been understood, its physiology and pathology have been widely studied through in vivo, ex vivo, in silico and in vitro studies, although in vivo and in vitro are the mainly adopted approaches.
In vivo studies present the most relevant and direct method to study BBB physiology. With these type of experimentations, in the case of the BBB, valuable information regarding the entire Figure 2.3 – Main routes of molecular transport across the BBB. A) Water-soluble agents, such as polar drugs, have their
diffusivity reduced by the tight junctions between adjacent endothelial cells. B) Alternative to the paracellular pathways, the transcellular pathway across the lipid membranes of the endothelium offers an effective diffusive route for lipid-soluble agents. C) Glucose, amino acids, purine bases, nucleosides, choline and other substances have their own specific transport proteins, or carriers, across the BBB, whereas D) some transporters, such as P-glycoprotein, are energy-dependent efflux transporters. E) Proteins like insulin and transferrin are taken up by specific receptor-mediated endo and transcytosis and F) albumin and other cationic plasma proteins can have their uptake increased by adsorption-mediated endo and transcytosis. G) Monocytes, macrophages or other immune cells can be used to carry any drugs or drugs incorporated in liposomes or nanoparticles. Adapted from [11].
6
microenvironment of the brain and biological processes in live animals can be obtained, which can be extremely advantageous when testing and validating other models.7 However, due to the differences between humans and animals, most of these models fail to replicate the human response accurately16, 17 and therefore much attention has to be given when translating the obtained results from animal models to humans. Furthermore, we also have to take into account not only the fact that these tests may be highly costly and labour intensive,17 especially due to the costs for buying the animals and keeping them alive, but also all the ethical and moral questions addressed to them.
Ex vivo studies are a type of experiments where a living tissue, which can be a whole organ, a portion of it, or even a larger organ system, is analyzed outside of the organism in an artificial setup with as little change as possible to in vivo conditions, therefore allowing more controlled conditions when compared to in vivo experiments. Moreover, these experiments may also be performed on tissues collected at autopsies and they avoid all the ethical and moral questions of performing experiments in living subjects, which makes them a viable alternative to in vivo studies. In the case of the brain, one of the most common ex vivo methods for its study are slices, where interactions between all the components of the neurovascular unit (NVU) are preserved in a fashion close to the in vivo situation.7
In silico studies are carried out via computer simulations. In these methods, computational models of the BBB are constructed based on in vivo experimental data, allowing for robust predictions, with little need to recur to animals.18,19 Despite the fact that these models can be very useful in predicting, for example, brain permeability to drugs or the properties of the different transport mechanisms across the BBB, they still present some limitations as they do not fully take into account the complex nature of the BBB.
In vitro techniques refer to models that allow the study of cells, or other biological features, outside their normal biological context. As these models are fast, high-throughput, simple, can be thoroughly analyzed and can be set up with healthy, modified or diseased human tissue20 and they can overcome significant disadvantages posed by the models mentioned above, the pursuit for a reliable in vitro model of the BBB has greatly increased. The ideal in vitro model should not only be simple and reproducible, but it should also mimic as closely as possible the in vivo barrier both functionally and anatomically in order to allow the study of BBB-related issues and drug delivery to the CNS.7 Essentially, in vitro BBB models consist of primary cell cultures and cell lines of brain endothelial cells used to assess a wide range of cell features and disease mechanisms. Not only can these models be designed for a specific research question, granting the researcher control over a variety of parameters, as they often provide cells with a controlled environment, making them relatively robust, reproducible, easy to analyze and more fit for high-throughput screening than animal studies.20 Good examples of such in vitro models are the Petri dish and the Transwell culture systems. Petri dish cultures form the most simple culture systems, where cells are seeded on a dish with culture medium, and although they are too simple to carry out some studies they are helpful when evaluating, e.g., the cytotoxicity of a certain drug. On the other hand, the Transwell systems are the standard models for culturing barrier forming tissues and consist of an improved culture setup with a suspended filter membrane normally made of polycarbonate or polyester. This membrane divides the culture chamber in an apical and basolateral region and allows more complex and functional studies, such as permeability and transendothelial electrical resistance (TEER) measurements, and also the possibility to co-culture different types of cells on both sides of the membrane, thus increasing the relevance of the model. The large surface area of these systems, however, presents a considerable disadvantage as the difficulty in growing a uniform and confluent cell layer increases and a single rupture can lead to erroneous results in permeability and TEER assays. Furthermore, Transwell inserts only allow static culture conditions, therefore lacking the ability to mechanically modulate the cell layer and assess the influence of fluidic shear stress exposure on cell differentiation and migration. Such drawbacks may also influence permeability studies, as the analytes cannot be introduced in the system at a constant rate and that may influence the transport of the
7
molecules across the membrane.23 To address these shortcomings, attempts have been made to microengineer and integrate more realistic key aspects of the cell’s natural environment in these models. One of these attempts are the organs-on-chips platforms.
Organs-on-chips are a class of three-dimensional microdevices, based on the principles of microchip technology, designed to incorporate human living cell cultures that are grown in dynamic engineered microenvironments derived from the organ. These engineered cell structures mimic the minimal functional units of human healthy and diseased organs and tissues and allow biological, chemical or physical manipulation, and analysis, through microfluidics, mechanical and electrical stimulation, microelectronics, imaging and other high-tech methods, thus granting a high level of spatiotemporal control over the culture conditions.20
Microfluidics is the science and technology that uses systems with channels with dimensions in the order of tens to hundreds of micrometers to process small volumes of fluids.24 The main fields of application of this technology are engineering, physics, chemistry, biology and nanotechnology and among some of its advantages are its reduced costs and the fact that it offers the possibility to perform analysis with high resolution and sensitivity in short times and with high throughputs.25 With the development of new fabrication methods and components, such as valves to direct the flow, pumps to supply the fluids and samples and mixers to mix those fluids and samples, this technology allows a great control over a wide variety of aspects, thus both buying time and reducing risks in the experiments,25 which makes it an attractive technology for in vitro researchers. Depending on the design, the organ-on-chip devices may have one or more microfluidic chambers that can contain multiple cell types in a 3D culture, thus allowing cells to grow in controlled and sterile conditions and interact as much as they might do in in vivo tissue. Moreover, due to their small dimensions, these chambers reduce the need for high volumes of cells, nutrients or drugs and allow the continuous supply of such agents, as well as bacteria or viruses depending on the question to be addressed.
To more closely mimic the three-dimensional shape and tissue environment of the blood-brain barrier, there have also been organ-on-chip models described in literature where polymers with a high water content, commonly known as hydrogels, are used as scaffolds on which cells are grown. The most commonly used hydrogel for mimicking the cell’s microenvironment is a collagen gel. Collagen is the most abundant protein in mammals and, depending on the degree of mineralization, can be present in, e.g., scar tissue, tendons, ligaments, the organic part of the bone26 and in neurons of the human central nervous system.27 Therefore, the use of collagen as a support in/on which cells are cultured is easy to understand, as it provides a greater level of biomimicry than the one offered when cells are grown, for example, in permeable inserts. This can be achieved by mixing a suspension of cells with the hydrogel solution and, after pipetting the solution into the platform that is being used, gelating the hydrogel by UV-photopolymerization or changes in temperature and pH, for instance.
All the devices described in this work are examples of organ-on-chip models and aim at providing a microfluidic platform that can be used to closely mimic and study the physiological functions of the BBB.
2.3 In vitro BBB model evaluation
Although there are several aspects that need to be taken into account when evaluating the physiological relevance and functionality of a microfluidic BBB model, the most important ones that constitute the biggest standardization challenges are the used cell line and whether the model allows for shear stress, permeability assays, and TEER measurements. These are discussed in more detail here.
8
2.3.1 Cells
When developing a BBB model it is extremely important to choose a cell line that closely mimics the functions of the human BBB. For this reason, the used cell line constitutes one of the most important aspects of the model and therefore needs to be well-thought through and evaluated before deciding. Cells from porcine, bovine and rodent origin have been used in several BBB models reported in literature6, 13, 28, 29, 30
as an alternative to human cells. Bovine and porcine cells are relatively easy to obtain and isolate, with only one brain yielding large quantities of cells. As for rodent derived brain cells, such as the ones from rats and mice, these are generally used to investigate the feasibility of certain compounds in treating some brain pathologies.6 However, translating the results obtained in such animal in vitro models to humans is quite challenging and therefore, to better resemble what happens in vivo, models with human brain endothelial cells have been developed. Such cells are usually derived either from autopsies or biopsies,6 which makes them preferable when it comes to human research purposes as they might have the appropriate expression profile or possibly a distorted expression profile that is worth studying. This, among other qualities, has made these models valuable when, e.g., studying drug and nanoparticle transport across the barrier.6 However, not only the use of such cells is limited by both ethical and moral questions as they are also scarce and difficult to obtain. Moreover, these cells are relatively fragile when in culture, thus being difficult to maintain the same phenotype after several passages, and can pose great batch-to-batch variability,6 which decreases the reproducibility of these models and may hamper the comparison between different platforms. As an alternative, there have been attempts to immortalize primary brain endothelial cells to overcome the limited tissue availability. Such cell lines are also attractive as they can be established with small amounts of tissue and have shown better maintenance of in vivo functions when comparing to the models previously mentioned. One example of such cell lines is the immortalized human cerebral microvascular endothelial cell line (hCMEC/D3) that was chosen for the BBB model in this work, which has already been used in more than 100 scientific publications6, 31 and will be thoroughly described in section 5.1.4. Unfortunately, one common drawback among commercially available immortalized cell lines is the fact that most of them show poor development of complex tight junctions between the endothelial cells when these are grown as a cell monolayer on a porous membrane.6 In addition, advances have also been made in the field of personalized medicine, with brain endothelial cells derived from human induced pluripotent stem cells (hiPSC) holding great promise. As these cells can be derived from both healthy and diseased subjects, conditions that happen in vivo can be mimicked and studied in vitro,22 bringing more hope when treating pathologic states of the brain that are highly dependent on patient-specific factors, such as genetic defects.
Besides brain endothelial cells, also astrocytes, pericytes, neurons and other cell types from the neurovascular unit have been shown to enhance barrier integrity when co-cultured with brain endothelial cells.3, 13, 28, 32 Such models are physiologically more relevant than those that only have a monoculture of endothelial cells and, when not excessively complex, can provide more accurate and valuable information regarding the human brain.
2.3.2 Shear stress
Endothelial cells in the brain capillaries are exposed to fluidic shear stress caused by the circulating blood. This shear stress has been reported to be between 0.3 and 2 Pa and exposure to such shear rates can influence cell growth, morphology and proliferation as well as enhance the tightness of the cell barriers.33, 34 As it was explained before, one of the major advantages of the organs-on-chips when comparing to the standard Transwell culture systems is that they allow the dynamic culture of cells, which can be easily achieved by flowing solutions, such as normal culture medium, through the channels of the devices. Therefore, more physiologically relevant microenvironments are created, thus more
9
closely mimicking what happens in vivo. In the case of a microfluidic device with channels with rectangular cross-sections, such as the 2D model that will be described in detail in this thesis, the fluidic shear stress is related to the volumetric flow rate according to:35
𝜏 =6µ𝑄 𝑤ℎ2· (1 + ℎ 𝑤) · 𝑓 · ( ℎ 𝑤) [Pa]
in which τ is the shear stress (Pa), µ the viscosity of the fluid that is flushed through the microfluidic channels (Pa·s), which might for instance be normal culture medium (viscosity of water at 37ºC, which is 0.7 mPa·s, can be used to approximate the viscosity of culture medium), Q is the flow rate (mm3/s), w is the width of the channel (mm) and h its height (mm). The values of function f(x) for the most common heights and widths of microfluidic channels were listed by Son et al.35 In the case that the width of the channel is much bigger than the height, the previous equation is reduced to:
𝜏 =6 · µ · 𝑄 𝑤 · ℎ2 [Pa]
When exposing cells to shear stress it is important that the shear rates are uniform on all cells across the channel width so cells in the middle region and cells close to the walls of the channel are not exposed to higher and lower shear rates, respectively. For this reason, when designing a microfluidic device it is important to make the width of its microfluidic channels much bigger than the height, in order to achieve a flat flow profile across the channel width. The flow profile, in a channel with a rectangular cross section, can be approximated by:
𝑢𝑥,𝑦 𝑢𝑚ax = [1 − ( 2𝑥 ℎ) 2 ] [1 − |2𝑦 𝑤| 𝑚 ] ; 𝑚 =𝑤 ℎ√2 + 0.89 ℎ 𝑤
where 𝑢𝑥,𝑦 is the fluid velocity at a given position (x,y) inside the channel cross-section, 𝑢𝑚ax the maximum velocity, h is the channel height and w is the channel width (w>h).36 With this in mind, it is clear that although cells inside microfluidic devices can be modulated by shear stress, care needs to be taken when designing such devices if one wants to make sure no differences in cell behavior arise.
2.3.3 Permeability
Low permeability to almost every molecule constitutes one of the main features of the blood-brain barrier, as it helps maintaining brain homeostasis. Therefore, BBB models should be able to accurately simulate this feature. As it was mentioned before, the BBB, or more specifically its TJs, only allows molecules smaller than 500 Da to easily cross it, whereas molecules such as ions, proteins, peptides or hormones have serious difficulties to enter the brain due to their different size, charge, or polarity, needing specific transport systems. In this way, a common way of investigating the tightness and functionality of an endothelial cell layer inside a microfluidic device is to introduce molecules, which can be either hydrophilic or lipophilic, in the blood compartment and assess what concentration ends up in the brain compartment. Generally, large molecules, such as long-chain dextran or sucrose, are blocked by the tight junctions between the endothelial cells and thus have a hard time crossing the barrier. On the opposite, small lipophilic molecules are known to easily cross it. Therefore, if molecules that are known to cross the barrier without major obstructions fail to end up in the brain compartment of a BBB model, or if molecules that are known to not be able to cross the BBB easily cross the BBB in a microfluidic model, the model cannot be considered physiologically relevant nor functional for transport studies and further improvements need to be done. However, and if proven fit, such model can still be used to study other features of the BBB, such as TJ expression or barrier exposure to certain agents.
(2.1)
(2.2)
10
As it was mentioned before, some of the hurdles of conducting permeability assays in Transwell culture systems are their big surface areas and the fact that a single hole in the cell layers can result in an increased diffusion of the introduced compound. Microfluidic platforms, on the other hand, not only have channels with considerably smaller dimensions that require less cells to create a tight cell barrier and thus reduce the probability of having a gap in it, but also have the ability of providing the analyte to the luminal channels and collecting it from the basal channels at a constant flow rate. This helps keeping the concentration difference of the analyte across the membrane constant during the entire assay, thus preventing underestimations regarding its actual permeability coefficient.
Quantification of this permeability is extremely important as it enables the comparison of microfluidic BBB models and other in vitro and in vivo data described in literature. For passive transport where an analyte is added to the luminal side of the barrier under constant flow and it crosses the cell barrier towards the basal side of the membrane, the permeability coefficient can be obtained by:22
𝑃𝑚𝑒as = 𝑚̇a 𝐴 · (𝐶𝑙− 𝐶𝑏)=
𝐶𝑏· 𝑄
𝐴 · (𝐶𝑙 − 𝐶𝑏) [𝑐𝑚/𝑠]
In the previous formulas, Pmeas is the apparent permeability coefficient (cm/s); ṁ the molar a transport rate across the membrane (mol/s), obtained by multiplying the concentration of analyte in the basal channel, Cb (mol/ml), by the flow rate, Q (ml/s), inside the channels; A is the area of the membrane through which transport takes place (cm2) and C
l is the analyte concentration in the luminal chamber (mol/ml). The permeability coefficient of the cell barrier, Pendo, can then be calculated by subtracting the apparent permeability measured in a blank device (without any cells), P0, from the calculated permeability of a device with an endothelial cell layer according to:22
1 𝑃𝑒𝑛𝑑𝑜= 1 𝑃𝑚𝑒𝑎𝑠− 1 𝑃0 [𝑠/𝑐𝑚]
By comparing the coefficient of the endothelial permeability to a certain analyte with permeability coefficients for the same analyte obtained in other platforms found in literature, the physiological relevance of the BBB model in question can be evaluated.
2.3.4 Transendothelial electrical resistance
Apart from permeability, transendothelial electrical resistance (TEER) is another widely used technique to assess the integrity of TJs, as it provides a great way of measuring the electrical resistance across a cellular monolayer and is a very sensitive and reliable method to confirm the integrity and permeability of the monolayer.37 As this technique is mostly based on quantifying the amount of ions that are able to cross the cellular monolayer formed by the endothelial cells, it is easy to understand that the tighter the endothelial cells are, the less ions will cross the barrier, thus resulting in a higher electrical resistance and a smaller electrical conductivity, and vice-versa. Among the most important advantages of this technique are the fact that it is quick, non-invasive, and can be performed in real time to monitor the different stages of cell growth and differentiation without damaging the cells.
The two main approaches to perform TEER measurements are the Ohm’s law method and impedance spectroscopy (IS). In the Ohm’s law approach two electrodes (one placed in an upper compartment and the other in a lower compartment) are used and a defined direct current (DC) voltage is applied to both electrodes, resulting in a current that is measured and then used to obtain the ohmic resistance with Ohm’s law (R = U/I, where R is the resistance, U the applied DC voltage and I the measured current). Between the electrodes there normally is a porous membrane with cells cultured on it and culture medium, which means that when measuring TEER, the measured resistance is the sum of the TEER, the medium and the membrane resistances. Therefore, to accurately determine the TEER (2.4)
11
resistance of a cell layer, the TEER value from a setup filled only with culture medium and without any seeded cells needs to be measured and subtracted from the measurements obtained from the setup with the cell layer, after which it is normalized by multiplying it by the area through which the resistance has been measured, according to:
𝑇𝐸𝐸𝑅 = 𝑅𝑒𝑛𝑑𝑜· 𝐴 = (𝑅𝐶− 𝑅𝐵) · 𝐴 [Ω · cm2]
Where RC and RB are the measured resistances (Ω) of the setup with cells and of the blank setup, respectively, and A is the area (cm2).
As DC currents can easily damage both the cells and the electrodes by build-up of charge,37 systems that use an alternating current (AC) voltage signal with a square waveform, such as the Epithelial Voltohmmeter (EVOM, World Precision Instruments), are widely used. Although these systems may not damage the setup in the same way as the ones that use DC, the resulting TEER measurements are highly dependent on the electrode positions. When measuring the TEER of a cell layer cultured on a Transwell system, for instance, great care is required while placing the electrodes in each of the compartments of the well in order to avoid erroneous measurements.37 On the other hand, IS is a highly reliable technique to measure the transendothelial electrical resistance38 that, when combined with a fitting algorithm, provides a more accurate representation of TEER values than traditional DC/single-frequency AC measurement systems.39 By applying an AC voltage with a frequency sweep to the two electrodes the amplitude and phase response of the resulting current can be measured, giving the total impedance (Z) information not only about the TEER but also about the capacitance of the cell layer, which can be extracted and provided as a readout parameter38 and reveals additional features of the properties of the barrier such as cell shape and the degree of cell-substrate adhesion.6 Moreover, IS also allows the continuous analysis over hours to days.
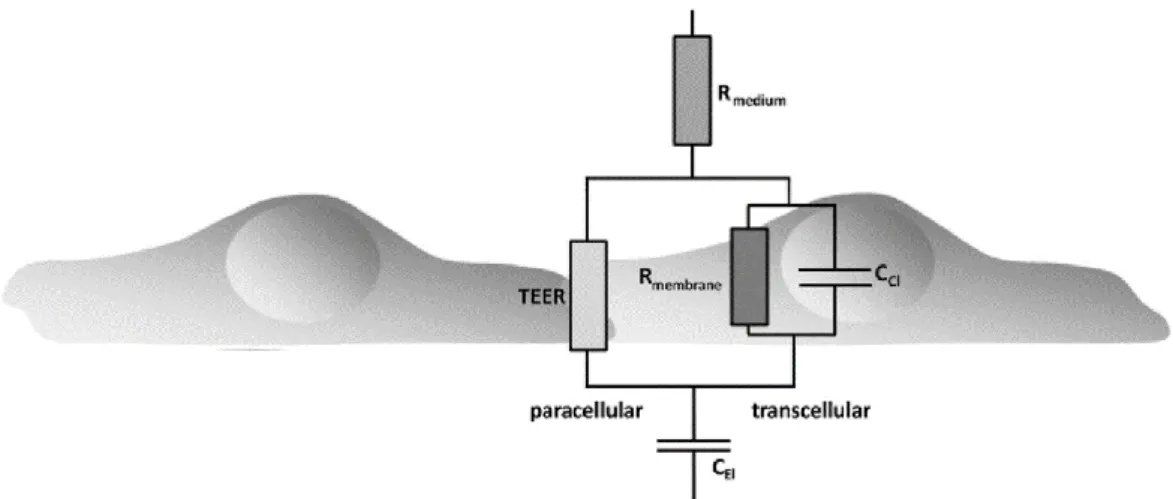
In figure 2.4 a schematic illustration of the equivalent circuit model of a cell monolayer is depicted. In the circuit displayed in the figure, the double layer of ions that is formed at the interface between the electrodes and culture medium when an AC signal is applied is modeled as the capacitance of the electrodes, CEL, and the resistance of the surrounding culture medium as Rmedium. As was explained
before, when cells are cultured on a porous membrane both the membrane and the cell layer add elements to the circuit that contribute to the measured TEER. In case the membrane has pores, the medium inside the confinement of the pores results in a resistance, which is not depicted in the circuit in figure 2.4 but is usually modeled as Rpor. The cell layer, on the other hand, is modeled by the electrical resistance of
the membranes (Rmembrane), the resistance of the paracellular transport pathway (TEER), and the
additional capacitances formed by the interfaces between the apical and basolateral lipid bilayer membranes and the medium, summarized in the figure by CCL. The capacitances at the cell membranes, CCL, and the resistance of the membranes, Rmembrane, form the transcellular pathway, while TEER is the
resistance of the paracellular pathway, or the transendothelial resistance.
In figure 2.5 are displayed a simulation of a simplified circuit of a cell monolayer on a chip and the corresponding simulated impedance measurements. As the resistances of the wires, the membrane and the cell membranes are small compared to the induced resistance of other components, these were excluded from the model. In this circuit, the double-layer capacitances of the electrodes are modeled as Cdl,1-2, the resistance of the medium as Rmed,1-2, the capacitances at the cell membranes as Cmem, the
resistance of the paracellular pathway as RTEER and the parasitic capacitance of the system as Cpar. If we
look at the impedance spectrum on the right, it is possible to distinguish four different regimes. From the lower to the higher frequencies, first we have the regime of the double-layer capacitance, Cdl,
characterized by a decrease in impedance and a shift in phase. The next regime corresponds to the medium and TEER resistances, RTEER+Rmed, and is characterized by a plateau in impedance and a local
maximum in phase. Next, we have the regime of the cell membrane capacitances, Cmem, characterized
by a decrease both in impedance and in phase, and finally we have the medium resistance, Rmed, regime,
12
which is characterized by a plateau in impedance and a shift in phase. At higher frequencies the regime of the parasitic capacitance is also visible, despite it is not highlighted in the figure, and is characterized by another shift in phase and a decrease in impedance.
When performing an impedance spectroscopy measurement, an AC signal is applied and the generated current can either cross the cell layer through transcellular pathways, passing the cell membranes and cytoplasms, or through paracellular pathways, where it encounters the transendothelial, or leak, resistances, which are related to cell confluence. Despite the fact that both the transcellular and the paracellular pathways contribute to the measured TEER, it is assumed that the resistance of the transcellular pathway is much higher than the resistance of the paracellular pathway, thus the latter being more dominant in overall TEER as current tends to follow the path of least resistance, especially at the beginning of the barrier culture when adherent or tight junctions between the cells have not yet formed.48 In this way, it becomes clear that the higher the cell confluence the higher the leak, or transendothelial, resistance will be, with the peak happening when complex tight junctions are formed between the endothelial cells, whereas non-confluent surfaces will result in smaller resistances as the generated current can easily cross the cell layer through paracellular paths. Moreover, TEER is sensitive to temperature and the ionic composition of the culture medium,33 factors that need to be kept constant in order to arrive at reliable values.
For these reasons, it is obvious that great care and planning needs to be taken when performing TEER assays in order to achieve valid TEER values that can be used to quantify the tightness of a cell barrier and compared with different platforms.
Figure 2.4 - Equivalent circuit diagram describing the contribution of the trans- and paracellular pathway to the total impedance, Z, of the cellular system. TEER, transendothelial electric resistance; CEl, capacitance of the electrodes; CCl,
capacitance of the cell layer; Rmedium, ohmic resistance of the medium; Rmembrane, ohmic resistance of the membranes. Adapted
13
Figure 2.5 – Impedance simulation of a cell monolayer on a chip. On the left we have a simplified circuit of a cell monolayer
on a microfluidic chip with realistically chosen values for each component, while on the right we have the corresponding impedance spectroscopy plot. The blue and red lines represent the simulations done for the case where a device has no cells (blank) and RTEER = 1 Ω and the case where a cell layer is seeded inside a device, with RTEER = 2800 Ω, respectively. The four
dash-dotted circles represent the four different regimes, which are, from the lower to the higher frequencies, the double-layer capacitance regime, Cdl, the sum of the TEER and medium resistances regime, RTEER + Rmed, the cell membrane capacitance
regime, Cmem, and the medium resistance regime, Rmed. If we look at the straight lines, the impedance spectrum when a cell
layer is simulated (straight red line) displays a clear plateau in the form of a shoulder, which is not visible in the control spectrum (straight blue line), where no cells were simulated. This is due to the fact that when cells are not present there is no influence of RTEER and Cmem. The local maximum of the phase curve indicates the frequency at which RTEER must be determined
(fTEER), represented by the dot-dashed line around 10 kHz. In the figure on the right, the phase is shifted by half a period,
meaning that 180º in this figure corresponds to a phase shift of 0º, and 90º corresponds to -90º (reprinted with permission from Bjorn de Wagenaar [40]).



![Figure 2.6 - Models of the BBB reported in literature. I) Booth and Kim [13]; II) Yeon et al](https://thumb-eu.123doks.com/thumbv2/123dok_br/15157497.1013431/38.892.110.781.239.910/figure-models-bbb-reported-literature-booth-kim-yeon.webp)