www.revportcardiol.org
Revista
Portuguesa
de
Cardiologia
Portuguese
Journal
of
Cardiology
ORIGINAL
ARTICLE
The
hybrid
approach
for
palliation
of
hypoplastic
left
heart
syndrome:
Intermediate
results
of
a
single-center
experience
Sérgio
Laranjo
a,∗,
Glória
Costa
a,
Isabel
Freitas
a,
José
Diogo
Ferreira
Martins
a,
Luís
Bakero
b,
Conceic
¸ão
Trigo
a,
Isabel
Fragata
c,
José
Fragata
b,
Fátima
F.
Pinto
aaServic¸odeCardiologiaPediátrica,HospitaldeSantaMarta---CHLC,EPE,Lisboa,Portugal bServic¸odeCirurgiaCardio-Torácica,HospitaldeSantaMarta---CHLC,EPE,Lisboa,Portugal cServic¸odeAnestesiologia,HospitaldeSantaMarta---CHLC,EPE,Lisboa,Portugal
Received30September2014;accepted15November2014 Availableonline5May2015
KEYWORDS
Hypoplasticleftheart syndrome;
Hybridapproach; Ductusarteriosus stent
Abstract
Introduction:Hypoplasticleftheartsyndrome(HLHS)isamajorcauseofcardiacdeathduring the firstweek oflife.The hybridapproach isareliable, reproducible treatmentoption for patientswithHLHS.Hereinwereportourresultsusingthisapproach,focusingonitsefficacy, safetyandlateoutcome.
Methods:WereviewedprospectivelycollecteddataonpatientstreatedforHLHSusingahybrid approachbetweenJuly2007andSeptember2014.
Results:Ninepatientshadastage1hybridprocedure,withsevenundergoingacomprehensive stage 2 procedure.One patient completed the Fontan procedure.Five patients underwent balloonatrialseptostomyafter thehybridprocedure;inthreepatients,astentwas placed acrosstheatrialseptum.Therewerethreedeaths:twoearlyafterthehybridprocedureand oneearlyafterstagetwopalliation.Overallsurvivalwas66%.
Conclusions: In our single-center series, the hybrid approach for HLHS yields intermediate resultscomparabletothoseoftheNorwoodstrategy.Theexistenceofdedicatedteamsforthe diagnosis andmanagementofthesepatients,preferablyinhigh-volumecenters,isofmajor importanceinthiscondition.
© 2014SociedadePortuguesade Cardiologia.Publishedby ElsevierEspaña,S.L.U.Allrights reserved.
∗Correspondingauthor.
E-mailaddress:sergiolaranjo@gmail.com(S.Laranjo).
http://dx.doi.org/10.1016/j.repc.2014.11.015
PALAVRAS-CHAVE
Síndromedocorac¸ão esquerdohipoplásico; Abordagemhíbrida;
Stentcanalarterial
Abordagemhíbridadepaliac¸ãodesíndromedecorac¸ãoesquerdohipoplásico ---resultadosintermédios:experiênciadeumcentro
Resumo
Introduc¸ão:Asíndromedocorac¸ãoesquerdohipoplásico(SCEH)éumadasprincipaiscausasde morteduranteaprimeirasemanadevida.Aabordagemhíbridaéumaopc¸ãodepaliac¸ãopara doentescomSCEH.Reportamososnossosresultadoscomestaabordagem,comfocoparticular nasuaeficácia,seguranc¸aeresultadofinal.
Métodos: Trabalhoprospetivo.RevisãodosdadosclínicosdedoentescomSCEH,submetidosa abordagemhíbridadepaliac¸ãoentrejulhode2007esetembrode2014.
Resultados: Novedoentesforamsubmetidosaprimeiroestadio híbridodepaliac¸ão.Destes, sete completaram segundo estadioe um doente foisubmetido a cirurgia deFontan. Cinco doentesforamsubmetidosaatriosseptostomiacombalão.Emtrêsprocedeu-seaimplantac¸ão destentnoseptointerauricular.Verificaram-setrêsóbitos:doislogoapósoprimeiroestadio híbridoeumapósosegundoestadio.Asobrevidaglobalfoide66%.
Conclusões:Na nossa experiência, aabordagem híbrida para SCEHproduz resultados com-paráveis aos da estratégia de Norwood. A necessidade de uma equipa dedicada para o diagnósticoemanejodestesdoentes,depreferênciaemcentrosdealtovolume,édegrande importâncianestacondic¸ãoparticular.
©2014SociedadePortuguesadeCardiologia.PublicadoporElsevierEspaña,S.L.U.Todosos direitosreservados.
Introduction
Hypoplasticleftheartsyndrome(HLHS)represents1.4---3.8% of congenitalheart diseases but is responsible for 23% of cardiacdeathsduringthefirstweekoflife.1---3HLHSis
char-acterizedbyvariabledegreesofunderdevelopmentofleft
heartstructures.4---6TraditionalmanagementofHLHS,either
withstagedsurgicalpalliation(Norwood,Glenn,Fontan)7,8
orcardiactransplantation,9remainsachallengeinmost
cen-ters,particularlyinhigh-riskpatientssuchasthosewithlow
birthweight(<3kg)anddiminutiveaortas(<2mm).10---12For
thissubgroup of patients, a hybrid approach--- combining
transcatheterandsurgicaltechniques---wasdevisedinthe
lastdecade,aimingtocombinethebestcharacteristicsof
surgicalandinterventionalcardiologytechniques.13---15
Originally reported in 1993,16 hybrid stage 1
pallia-tionconsistsof bilateralbranchpulmonary arterybanding
and stenting of the ductus arteriosus, without
cardiopul-monarybypass, during the neonatalperiod. Assurance of
a nonrestrictive atrial septal defect is the next step,
througheitherballoonatrialseptostomyorstenting.Later
ininfancy, amorecomplexcomprehensive stage2 is
per-formedatapproximatelysixmonthsofage,intheformofa
Norwood-typereconstructioncombinedwithabidirectional
cavopulmonary(Glenn)anastomosis.Althoughinitially
sug-gestedforhigh-riskcandidatesfortheclassicNorwoodstage
1and2palliation,14,17ithassincebeenadoptedasthe
pre-ferredfirstoptionbyseveralcenters.14,18,19
In 2007,our center starteda hybrid programfor HLHS
palliationinhigh-riskcases.Ouraimistoreportourinitial
resultswiththistechnique,focusingonitsefficacy,safety
and lateoutcome, with particular emphasis on morbidity
andmortality,need forunplannedreintervention,andthe
currentstatusofthepatients.
Methods
Patientpopulation
Ourcohortcomprisedpatientswhounderwentahybridstage
1procedurebetweenJuly2007andSeptember2014.Allhad
typicalHLHS(aorticatresiaor criticalstenosis withmitral
atresiaorstenosis).Initially,ourcenterchoseonlyhigh-risk
patientsforthehybridpalliation,definedbythepresence
of low birth weight (<2.5 kg), prematurity, severe aortic
archhypoplasia,poorrightventricularfunction,morethan
mild tricuspid regurgitation, highly restrictive atrial
sep-taldefect,andthepresenceofnon-cardiacmalformations.
Lateron, wewidened ourcriteriaand optedfor a hybrid
approachfor all HLHSpatients. A reviewof prospectively
collecteddataincludinginformationfromallplannedstaged
procedures,anyunplannedreinterventions,andinterstage
morbidityandoutcomesarediscussedherein.Follow-upwas
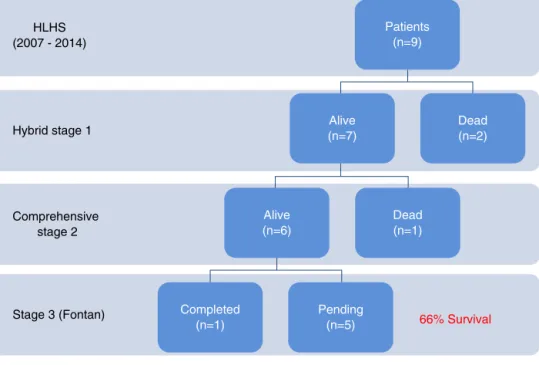
completeinallpatients(Figure1).
Technique
Hybridstage1
The procedurewasperformed inthecatheterization
labo-ratory,adaptedasahybridsuite,undergeneralanesthesia.
ThetechniquewasbasedonreportsbyGalantowiczetal.18
Briefly, through a median sternotomy, and without
car-diopulmonarybypass,bilateralbranchpulmonaryartery(PA)
bandswereplacedusinga3.5-mmGore-Textubegraft(WL
Gore& Associates,Flagstaff,AZ,USA);the bandwasfirst
placedontheleftPA.The circumferenceofthebandwas
calculated according to the caliber of the branch PA and
HLHS (2007 - 2014)
Hybrid stage 1
Comprehensive stage 2
Stage 3 (Fontan) Completed 66% Survival
(n=1) Pending (n=5) Dead (n=1) Alive (n=6) Alive (n=7) Dead (n=2) Patients (n=9)
Figure1 Clinicaloutcomesofpatientswhounderwentthehybridprocedure.HLHS:hypoplasticleftheartsyndrome.
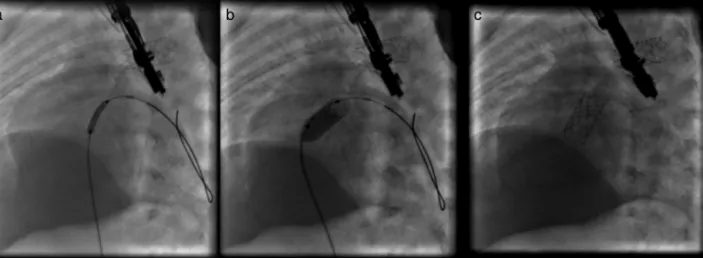
Figure2 Angiographicimages:(a)stentpositioningintheductusarteriosus;(b)fullyexpandedstent.
a
b
c
PA PA Left PA band Right PA band Stent Stent Stent Asc Ao Desc AoFigure3 Angiographicimagesoftheanatomyattheendofhybridstage1palliation.AscAo:ascendingaorta;DescAo:descending aorta;PA:pulmonaryartery.
Figure4 Angiographicimages:(a)stentpositioningattheleveloftheinteratrialseptum;(b)stentexpansion;(c)fullyexpanded stent.
responseofsystemicbloodpressureandoxygensaturation
(O2Sat),aimingforO2Satpercentagesinthehigh70stolow
80s.Afterbilateralpulmonarybandingasheathwasinserted
throughacontrolledarteriotomydirectly inthemain
pul-monary artery and a balloon-expandable stent (Genesis,
Cordis,Johnson& Johnson,Miami, FL,USA)wasdeployed
tocompletelycovertheductusarteriosus(Figures2and3).
Whenever there was evidence of a restrictive interatrial
septaldefect(ASD)(meanDopplergradientby
echocardiog-raphy≥8mmHg),itwasrelievedinaseparateprocedure,20
eitherinterventional(Figures4and5)orsurgical.
Interstagemonitoring
After discharge, patients were closely monitored every
one to two weeks with serial O2 Sat and weight
mea-surements and with complete cardiology assessments:
echocardiogramswere performed to monitor for
obstruc-tion of pulmonary venous return at the atrial septum
or obstruction to aortic arch blood flow (antegrade,
ret-rograde or through the patent ductus arteriosus stent).
Decreasedrightventricularfunctionorincreased
atrioven-tricularvalveregurgitationwereconsidered earlymarkers
ofincreasedafterload.Patientsweremaintainedonchronic
afterloadreductionwithanangiotensin-convertinginhibitor
plusdiureticsanddigoxin.Prophylacticantiplatelettherapy
withaspirin3---5mg/kg/daywasuniformlyadministered.
Comprehensivestage2
Patientsunderwentcomprehensivestage2surgeryatsixto
ninemonthsofage,dependingonclinicalassessment.
Car-diac catheterizationwaselectivelyperformed inall cases
priortothecomprehensivestage2proceduretoobtain
diag-nosticdata(suchasassessmentofphysiologyorassociated
anomalies)and/ortorelieveanyobstruction(systemicorat
theatrialseptum).
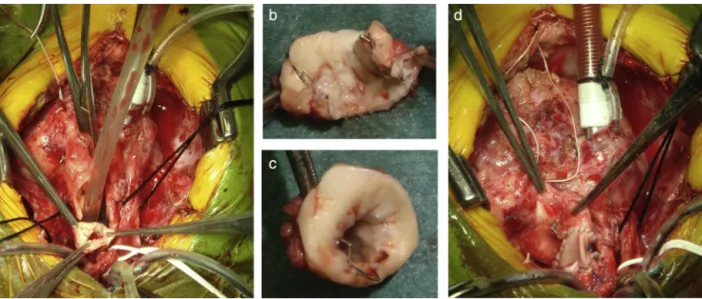
Thecomprehensivestage2surgeryconsistedof
deband-ing of the branch pulmonary arteries, resection of the
stentedductusarteriosus,reconstructionoftheaorticarch
andpulmonaryarteries(ifneeded),divisionofthe
diminu-tiveascending aortawithreimplantationintothemain PA
toreconstructsystemicoutflow(aNorwood-likeprocedure
---Figure6),atrial septectomy,andconstruction ofa
bidi-rectional cavopulmonary anastomosis (Glenn procedure).
The right pulmonary band site was incorporated in the
Figure5 Transthoracicechocardiographyofapatientwith hypoplasticleftheart syndromeafterstentimplantationinto the interatrialseptum.(aandb)Two-dimensionalimage;(c)colorDopplerimagingoftheflowthroughtheimplantedstent.
Figure6 Comprehensivestage2surgery.(a)Insituductusarteriosusstent;(bandc)resectionofthestentedductusarteriosus, withendothelializedlumen;(d)reconstructionofthesystemicoutflow(Norwood-likeprocedure).
Glennshuntandenlargedifneeded;aleftpulmonarypatch
enlargementwasperformedwhenjudgednecessary.
Stage3
The totalcavopulmonarycirculation (stage3surgery)was
completed with an extracardiac fenestrated conduit
con-nectingtheinferiorvenacavatothepulmonaryarteriesviaa
Gore-Texconduit(WLGore&Associates,Elkton,Maryland).
Results
Hybridstage1
Thehybridstage1wasperformedonninepatientswithHLHS
atamedianageof9.5daysandweight3kg.Patient
char-acteristicsarelistedinTable1.Onlytwopatientsreceived
blood products.Most(84%) wereweanedfromventilation
onthesecond postoperativeday(mediantwodays;range
2---15days).Medianlengthofstayintheintensivecareunit
was9.5 days(range3---42 days).The median timetofirst
enteralfeedwaspostoperativeday2(range2---20days).No
patient experienced infectious complications, necrotizing
enterocolitis or acuterenal failure.Therewas 77%
hospi-talsurvival(7outof9).Duringthisperiodtwopatientsdied
afterstage1palliationwithlowcardiacoutputsyndrome:
one due toacute ductus arteriosus occlusion, becauseof
incompleteductalstenting, unresponsivetoprostaglandin
infusion and unableto be takenback to the
catheteriza-tionlaboratoryforplacementofanadditionalcoaxialductal
stent;and the other due tostent-related retrograde
aor-ticarchobstruction.Postoperative outcomesareshownin
Table2.
Balloonatrialseptostomyoratrialseptectomywas
per-formed as a separate procedure after the hybrid stage
1. Five patients underwent balloon atrial septostomy at
a median of 10 days after the hybrid procedure; in
two of these, due to increasingly restrictive atrial septal
flow, a surgical atrial septectomy waslater performed at
26---47daysafterseptostomy. Inthreepatients,duetoan
unusually thickatrial septum witha superiorly positioned
atrial septal defect, making balloon septostomy difficult,
an 8 mm×15 mm stent (Palmaz Genesis, Cordis, Johnson
& Johnson, Miami, FL,USA), wasplaced across the atrial
septum(Figures4and5).Oneofthesepatientsunderwent
emergentatrialseptectomyandstentremovalduetostent
migrationtotherightatrium.
Interstage1---2
Therewerenointerstagedeaths.Priortothecomprehensive
stage2,theechocardiographicassessment ofright
ventri-cularfunctionwasgradedasnormalinallbuttwopatients,
inwhomitwasgradedasmildlydepressed.Tricuspid
regur-gitationwastrivialinallbuttwopatients.
There were reinterventions in the catheterization
lab-oratory (balloon angioplasty) in two patients due to
recoarctation,bothsuccessful.Allpatientsproceededtoa
comprehensivestage2.
Comprehensivestage2
Acomprehensivestage2procedurewasperformedinseven
patientsatamedianageofninemonthsand6.3kgweight.
Medianbypassandcross-clamptimeswere284and90min,
respectively.Lowdoseinotropicsupport(dopamineor
mil-rinone) was used in the early post-operative period. No
patient required a delayed sternal closure. The
postop-erative echocardiographic assessment of right ventricular
functionwasgradedasnormalin80%,withonlyonepatient
havinggreaterthan milddysfunctionor greaterthan mild
tricuspidregurgitation.Fourofsixpatientswereextubated
within 24 hours (median 25 hours; maximum 32 hours).
Medianlactatelevelwas20mg/dl(range10---35mg/dl)on
arrivalattheICU,and10mg/dl(range7---22mg/dl)on
post-operativeday1.The medianlengthofstay intheICU was
sevendays(range4---16days).Nopatienthadrenalfailure.
Allpatientswereinnormalsinusrhythmandtherewereno
arrhythmias.
One patient out of seven died after comprehensive
Table1 Diagnosisandclinicalprofile. Diagnosis Age1 (days) Weight1 (kg) Ascending aorta(mm) Stage2 Age2 (months) Weight2 (kg) Stage3 Current status Stageof death Causeof death
1 AA/MA 9 2.4 2.3 Yes 8 6.3 Yes Alive
2 AA/MA 18 3.3 2.7 Yes 9 6.6 Pending Alive
3 AA/MS 14 3 3 Yes 9 6.3 No Dead 2 LCOS
4 AA/MA 10 3.23 2 No No Dead 1 Retrograde
aorticarch obstruction
5 AA/MA 90 3.43 2 Yes 9 6 Pending Alive
6 AA/MA 5 3 2 Yes 9 6.9 Pending Alive
7 AA/MS 7 2.6 2.3 No No Dead 2 Incomplete
ductal stenting
8 AA/MA 9 3.1 2.6 Yes 8 6.1 Pending Alive
9 AS/MS 12 2.4 3 Yes 7 6 Pending Alive
Mean 20.25 3.01 2.36 8.4 6.37
Median 9.5 3.05 2.3 9 6.3
AA:aorticatresia;AS:aorticstenosis;LCOS:lowcardiacoutputsyndrome;MA:mitralatresia;MS:mitralstenosis.
Table2 Hybridstage1results.
Intervention Timetoextubation(days) Timetoenteralfeeding(days) ICUstay(days) LOS(days)
1 PAB+PDAstent 2 2 6 22
2 PAB+PDAstent 2 13 13 150
3 PAB+PDAstent 2 2 14 60
4 PAB+PDAstent 0 0 2 2
5 PAB 2 2 3 19
6 PAB+PDAstent 2 2 13 26
7 PAB+PDAplasty 0 0 3 3
8 PAB+PDAplasty 15 20 42 85
9 PAB+PDAstent 2 2 4 20
Mean 3.125 5.125 12 45.875
Median 2 2 9.5 24
ICU:intensivecareunit;LOS:lengthofstay;PAB:pulmonaryarterybanding;PDA:patentductusarteriosus.
postoperativeday1fromlowcardiacoutputsyndromeand Glennobstruction.
Fontancompletion
OnepatientunderwentasuccessfulFontancompletionwith nomortality,whilefiveothersareawaitingFontan comple-tion;survival tocompletionof stage2palliationis 66%of thepatients(sixoutofnine).
Discussion
Wereport ourinitial resultswith thehybrid approachfor palliationof patientswithHLHS.These resultsreflectour technicalchallengesandlearningcurve.
In recent years, several strategies have had a notice-ableimpactonthediseaseburden of HLHS,mostnotably therightventricletopulmonaryarterySanomodification21
and the development of dedicated teams for close
monitoring of HLHS patients between stages 1 and 2 of
palliation.22 It has been shown, however, that there are
subgroups of patientswithin thespectrum of HLHS which
havepooreroutcomeswhentreatedbytraditionalsurgical
palliation.10,23Also,theclassicalstagedpalliationapproach
appears to have reached a stagnation point,24 no further
improvements beingpossible withthe currently available
technology, with recent cumulative early and interstage
mortality of 5%---30% for standard-risk patients25---27 but as
high as 30%---50% in high-risk patients.11,12 A recent
Pedi-atricHeartNetwork-sponsoredmulticenterrandomizedtrial
comparedtheNorwoodprocedurewithamodifiedBTshunt
versustheSanomodification,26withtheprimaryendpointof
deathortransplantationatoneyearandthesecondary
end-pointsofhospitalcourse, RVfunctionby echo,pulmonary
arterysizebyangiography,unintendedcardiovascular
inter-ventions,andseriousadverseeventsandcomplications.This
study failedtoshowclear superioritybetween anyof the
groupwasfoundtohaveastatisticallysignificantlowerrisk
ofmortalityat the12-monthendpoint, thiswasnolonger
significant withalonger follow-up.The complication rate
wasalsohigherintheSanogroup,althoughthepercentage
ofinfants withatleast onecomplicationwasthesamein
bothgroups.26
Hybrid palliation has evolvedasan alternative to
Nor-woodpalliationbasedontheassumptionthatalessinvasive
stage1palliationinthehybridstrategywouldimprove
over-allsurvivalandneurologicaloutcomes.28Thegoalsofhybrid
stage 1 palliation include the following29: (1) to ensure
unimpededsystemiccardiacoutputthroughapatent
duc-tusarteriosus;(2)toobviatetheneedforandcomplications
associatedwithlong-termprostaglandinE1(PGE1)infusion;
(3)toimprovebalanceofthepulmonary andsystemic
cir-culations by restricting pulmonary blood flow; and (4) to
relieveanyobstructiontopulmonaryvenousreturnat the
atrialseptumlevel.Oneofthemostimportantadvantages
of hybrid palliationis to defer themost complexsurgical
interventiontolaterininfancy,reducingtheriskof
neuro-logicalcomplications.Inourseriesthemajorityofpatients
wereextubated in thefirst 48hoursand weredischarged
homeafteramedianhospitalstay of24days,significantly
shorter than for the traditional Norwood procedure, and
haveanormalneurologicalassessmenttodate.
The hybrid approach is also more flexible than the
conventional staged approach.30 Some authors have even
modifiedthehybridapproachandproposeapartialhybrid
stageI palliationin theformof bilateral PAbanding with
continuous PGE1 administration.31 It may be used as a
bailout or salvage approach,32 providing excellent
pallia-tion for patients for whom transplantation is considered
themostappropriatelong-termapproach. Insuch
circum-stances the time pressure to find a suitable donor heart
is relieved and thechild is likely tobe exposedto fewer
bloodproducts,reducingproblemswithlaterorgan
match-ing.Likewise,somepatientswithaborderlineleftventricle
maybepalliatedusingthehybridapproach,allowingfurther
growth or recovery of cardiac function so that a
two-ventricle repair may ultimately be attempted.33,34 While
it is possible to take down a Norwood stage 1, this is a
much larger undertaking than converting a hybrid
proce-dure.
Despite the constant evolution of interventional
tech-niques,thehybridapproachstillfacescertainlimitations.
Aparticularly seriousone is theoccurrence ofretrograde
aortic arch obstruction, leading to suboptimal cerebral
and coronary artery perfusion.18,15 In our series the two
deaths after stage 1 palliation were directly attributable
toobstructed systemiccardiac outputthroughthe ductus
arteriosus. Retrograde aortic arch obstruction can occur
in utero, postnatally beforesurgical treatment begins, or
afterwardin acute or more insidiousfashion after ductus
stenting.35Itsincidencerangesfrom8%to30%.Some
cen-ters have managed this problem by stenting the stenotic
isthmus,36 thusrenderingreconstructivearchsurgeryeven
more challenging, or by creating a prophylactic reverse
Blalock-Taussig shunt,37 while others consider evidence
of restricted flow into the retrograde transverse aorta
from the ductus arteriosus an exclusion criterion for the
hybrid procedure.15 Nevertheless, it is highly desirable
toavoidahybridprocedureinpatientswithobstructionto
retrogradearchbloodflow,whichleadstopoorerresultsand
significantlyincreasedmortality.
Furthermore, several authors argue that the hybrid
approachfailstoattainsomeoftheessentialprerequisites
foranoptimalFontan-typecirculation,classicallydescribed
byChoussat andFontan.38 Fortheseauthorsspecific
phys-iologicalrisk factors for a failing Fontan prevail, related
toventricular performance, atrioventricularand systemic
valve function, and pulmonary and systemic circulation.
Someofthepotentialproblemsreportedinclude:(1)riskof
residualaorticarch obstruction, duetoeither
inappropri-atestentdeploymentleavingdistalductaltissueuncovered
or to intrastent occlusion15,39; (2) increased single
ven-tricle afterload due to the non-compliant/non-expansible
nature of the stent placed in the ductus arteriosus and
to excessively tight PA bands that may also (3) fail to
provide adequate pulmonary blood flow or to promote
adequatePAgrowthoreven40(4)induceiatrogenicPA
steno-sis,necessitating PA reconstructionin the comprehensive
stage2operation41;(5)presenceofincreasinglyrestrictive
atrialcommunication,leadingtohigherpulmonary venous
resistance;(6)decreasedsingle-ventriclefunction(systolic,
diastolicorboth), duetoalltheabove-mentionedfactors
and leadingto (7) increased atrioventricular valve
regur-gitation. In our series, prior to the comprehensive stage
2, the echocardiographic assessment of right ventricular
function was graded as normal in all but two patients,
in whomit wasgraded asmildly depressed and whohad
mildtomoderatetricuspidregurgitation.Thesepatientshad
their aortic arch obstruction corrected percutaneously in
theinterstagecardiaccatheterizationprocedure.In three
patients PA reconstruction was deemed necessary. These
patientsarenowwaitingforcompletionoftheir
bicavopul-monarycirculation,butourseriesisstillsmallandlacksa
longerfollow-up.
Inourlimitedexperience,thetimingoftheatrial
inter-ventionalprocedureisthemostdifficultdecision.Ingeneral
wetendtoperformitaround10daysafterthehybrid
pro-cedurebut,eitherduetoourlimitedexperienceorbecause
of morphologicallydifficult atrial septums, several of our
patientsneededsurgicalseptectomy.
Toobviatethesepotentiallimitations,thehybrid
tech-nique has evolved during the last decade.15 Several
strategies have been proposed, including: (1) deferring
theatrialseptostomytoasecond procedure,immediately
beforedischarge, allowingfor amoredurable septostomy
byusingalargerballooninalargerleftatrium;(2)useof
cuttingandstaticballoonsfor thethickatrialseptum;(3)
useofself-expandingductalstents; and(4)performingan
earliercomprehensivestage2 proceduretoavoid(a)
pro-longedbranchPAbanding,promotingenhancedPAgrowth
andobviatingtheneedforbranchPAreconstruction;(b)
pro-longedsingle-ventriclestrainagainstanon-compliantstent,
preservingventricularfunctionandavoidingatrioventricular
valveregurgitation;(c) pulmonary venous return
obstruc-tion,sothatthepatientisinabetterconditionforthefinal
Fontanoperation.
Inhigh-volumecenterswithmodernsurgical,anesthetic
andintensivecarepractices,surgicalresultsforaverage-risk
patientsbornwithHLHStocompletionofstage2palliation
arestillsuperiortothoseofferedbythehybridapproach.
stage 2 surgery, a long and complexoperation. For
high-risk patients, the results appear to be equivalent, with
50%anticipatedsurvival.Ourresultsareinagreementwith
reportedinternationalseries,withsurvivaltocompletionof
stage2palliationof66%.However,thereisalearningcurve
effectthatwould suggest thatresults of hybridpalliation
willimprovewith moreexperience,even for average-risk
patients.
Inconclusion,therehasbeenconsiderableprogresswith
thehybridapproach. Thoughit isrelatively early,current
international results suggest there may be subgroups of
patientsthatarebetter palliatedwiththisapproachthan
bysurgery.Inmostinstitutionssurgicalresultsarecurrently
superiorforothersubgroupsofpatients.However,as
expe-rience in interventional palliation increases,this position
willneedtobereassessedwithmulticenterprospective
tri-als comparing both classic and hybrid approaches. Above
all,theexistenceofdedicatedteamsforthediagnosisand
managementof thesepatients, preferably inhigh-volume
referralcenters,isofmajorimportanceinthiscondition.
Ethical
disclosures
Protection of human and animal subjects.The authors
declarethat the proceduresfollowed were in accordance
withtheregulationsoftherelevantclinicalresearchethics
committeeandwiththoseoftheCodeofEthicsoftheWorld
MedicalAssociation(DeclarationofHelsinki).
Confidentialityofdata.Theauthorsdeclarethattheyhave
followedtheprotocolsoftheirworkcenteronthe
publica-tionofpatientdata.
Righttoprivacyandinformedconsent.Theauthorshave
obtainedthe written informed consentof the patients or
subjectsmentionedinthearticle.Thecorrespondingauthor
isinpossessionofthisdocument.
Conflicts
of
interest
Theauthorshavenoconflictsofinteresttodeclare.
References
1.MorrisCD,OutcaltJ,MenasheVD.Hypoplasticleftheart syn-drome:naturalhistoryinageographicallydefinedpopulation. Pediatrics.1990;85:977---83.
2.Samanek M,Slavik Z, Zborilova B, et al. Prevalence, treat-ment, andoutcome ofheartdisease inlive-bornchildren: a prospectiveanalysisof91,823live-bornchildren.Pediatr Car-diol.1989;10:205---11.
3.Talner CN. Report of the New England Regional Infant Cardiac Program, by Donald C. Fyler, MD, Pediatrics, 1980;65(suppl):375---461.Pediatrics.1998;102:258---9.
4.LevM.Pathologicanatomyandinterrelationshipofhypoplasia oftheaortictractcomplexes.LabInvest.1952;1:61---70.
5.NoonanJA,NadasAS.Thehypoplasticleftheartsyndrome;an analysisof101cases.PediatrClinNorthAm.1958;5:1029---56.
6.BharatiS,LevM.Thesurgicalanatomyofhypoplasiaofaortic tractcomplex.JThoracCardiovascSurg.1984;88:97---101.
7.AzakieA,McCrindleBW,BensonLN,etal.Totalcavopulmonary connectionsinchildrenwithapreviousNorwoodprocedure.Ann ThoracSurg.2001;71:1541---6.
8.Norwood WI, Lang P, Hansen DD. Physiologic repair of aor-tic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308:23---6.
9.RazzoukAJ, ChinnockRE, Gundry SR, et al. Transplantation as a primary treatment for hypoplastic left heart syn-drome:intermediate-termresults.AnnThoracSurg.1996;62: 1---7.
10.Tabbutt S, Ghanayem N, Ravishankar C, et al. Risk fac-torsfor hospital morbidityand mortality afterthe Norwood procedure: a report from the Pediatric Heart Network Sin-gleVentricle Reconstructiontrial. JThoracCardiovasc Surg. 2012;144:882---95.
11.GaynorJW,MahleWT,CohenMI,etal.Risk factorsfor mor-talityaftertheNorwoodprocedure.EurJCardiothoracSurg. 2002;22:82---9.
12.StasikCN,GelehrterS,GoldbergCS,etal.Currentoutcomes andriskfactorsfortheNorwoodprocedure.JThoracCardiovasc Surg.2006;131:412---7.
13.AkintuerkH,Michel-BehnkeI,ValeskeK,etal.Stentingofthe arterialductandbandingofthepulmonaryarteries:basisfor combinedNorwoodstageIandIIrepairinhypoplasticleftheart. Circulation.2002;105:1099---103.
14.BachaEA,DavesS,HardinJ,etal.Single-ventriclepalliation forhigh-riskneonates:theemergenceofanalternativehybrid stageI strategy.JThoracCardiovasc Surg.2006;131:163---71, e162.
15.Galantowicz M, Cheatham JP. Lessons learned from the development of a new hybrid strategy for the manage-ment of hypoplastic left heart syndrome. Pediatr Cardiol. 2005;26:190---9.
16.GibbsJL,Wren C,WattersonKG,et al.Stentingofthe arte-rialductcombinedwithbandingofthepulmonaryarteriesand atrialseptectomyorseptostomy:anewapproachtopalliation forthehypoplasticleftheartsyndrome.BrHeartJ.1993;69: 551---5.
17.PizarroC,DerbyCD,BaffaJM,etal.Improvingtheoutcomeof high-riskneonateswithhypoplasticleftheartsyndrome:hybrid procedureorconventionalsurgicalpalliation?EurJ Cardiotho-racSurg.2008;33:613---8.
18.GalantowiczM,CheathamJP,PhillipsA,etal.Hybridapproach forhypoplasticleftheartsyndrome:intermediateresultsafter thelearningcurve.AnnThoracSurg.2008;85:2063---70 [discus-sion2070---1].
19.CaldaroneCA,BensonL,HoltbyH,etal.Initialexperiencewith hybridpalliationforneonateswithsingle-ventriclephysiology. AnnThoracSurg.2007;84:1294---300.
20.HolzerRJ,Wood A,ChisolmJL,etal.Atrialseptal interven-tionsinpatientswithhypoplasticleftheartsyndrome.Catheter CardiovascInterv.2008;72:696---704.
21.SanoS,IshinoK,KawadaM,etal.Rightventricle-pulmonary arteryshuntinfirst-stagepalliationofhypoplasticleftheart syndrome.JThoracCardiovascSurg.2003;126:504---9 [discus-sion509---10].
22.Ghanayem NS, Hoffman GM, Mussatto KA, et al. Home surveillanceprogram prevents interstage mortalityafterthe Norwood procedure. J Thorac Cardiovasc Surg. 2003;126: 1367---77.
23.HehirDA,Dominguez TE, BallwegJA,et al. Risk factorsfor interstage deathafter stage 1 reconstruction of hypoplastic leftheart syndrome and variants.JThorac CardiovascSurg. 2008;136:94---9,99e91---93.
24.FeinsteinJA,BensonDW,DubinAM,etal.Hypoplasticleftheart syndrome:currentconsiderationsandexpectations.JAmColl Cardiol.2012;59:S1---42.
25.JacobsML,BlackstoneEH,BaileyLL.Intermediatesurvivalin neonateswithaortic atresia:a multi-institutionalstudy.The CongenitalHeartSurgeonsSociety.JThoracCardiovascSurg. 1998;116:417---31.
26.OhyeRG,SleeperLA,MahonyL,etal.Comparisonofshunttypes intheNorwoodprocedureforsingle-ventriclelesions.NEnglJ Med.2010;362:1980---92.
27.TweddellJS,HoffmanGM,MussattoKA,et al.Improved sur-vivalofpatientsundergoingpalliationofhypoplasticleftheart syndrome:lessonslearnedfrom115consecutivepatients. Cir-culation.2002;106:I82---9.
28.GalliKK,ZimmermanRA,JarvikGP,etal.Periventricular leuko-malaciais commonafter neonatalcardiac surgery. JThorac CardiovascSurg.2004;127:692---704.
29.HonjoO, Caldarone CA. Hybrid palliationfor neonates with hypoplasticleft heartsyndrome:currentstrategiesand out-comes.KoreanCircJ.2010;40:103---11.
30.DiBardinoDJ,McElhinneyDB,MarshallAC,etal.Areviewof ductalstenting inhypoplasticleftheartsyndrome:bridgeto transplantationand hybridstage Ipalliation.PediatrCardiol. 2008;29:251---7.
31.SakuraiT,Kado H,Nakano T,et al.Earlyresultsofbilateral pulmonaryarterybandingforhypoplasticleftheartsyndrome. EurJCardiothoracSurg.2009;36:973---9.
32.MitchellMB,CampbellDN,BoucekMM,etal.Mechanical limi-tationofpulmonarybloodflowfacilitateshearttransplantation inolder infantswithhypoplastic left heartsyndrome. EurJ CardiothoracSurg.2003;23:735---42.
33.BallardG,TibbyS,MillerO,etal.Growthofleftheartstructures followingthehybridprocedureforborderlinehypoplasticleft heart.EurJEchocardiogr.2010;11:870---4.
34.Akinturk H, Michel-Behnke I, Valeske K, et al. Hybrid transcatheter-surgical palliation: basis for univentricular or biventricularrepair:theGiessenexperience.Pediatr Cardiol. 2007;28:79---87.
35.EganMJ,HillSL,BoettnerBL,etal.Predictorsofretrograde aorticarchobstructionafterhybridpalliationofhypoplasticleft heartsyndrome.PediatrCardiol.2011;32:67---75.
36.StoicaSC,PhilipsAB,EganM,etal.Theretrogradeaorticarch inthehybridapproachtohypoplasticleftheartsyndrome.Ann ThoracSurg.2009;88:1939---46[discussion1946---7].
37.Caldarone CA, Benson LN, Holtby H, et al. Main pulmonary arterytoinnominate arteryshuntduringhybridpalliationof hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2005;130:e1---2.
38.Choussat A, Fontan F, Besse P, et al. Selection criteria for Fontan’sprocedure.In:AndersonRN,ShinebourneEA,editors. Paediatriccardiology.1sted.Edinburgh:ChurchillLivingstone; 1977.p.559---66.
39.Nadorlik HA, Egan MJ, Hill SL, et al. Predictors of duc-tus arteriosus in-stent stenosis in the hybrid approach to hypoplastic left heart syndrome. Pediatr Cardiol. 2013;34: 656---60.
40.Chen Q, Parry AJ. The current role of hybrid proce-dures in the stage 1 palliation of patients withhypoplastic left heart syndrome. Eur J Cardiothorac Surg. 2009;36: 77---83.
41.HonjoO,BensonLN,MewhortHE,etal.Clinicaloutcomes, pro-gramevolution,andpulmonaryarterygrowthinsingleventricle palliationusinghybridandNorwoodpalliativestrategies.Ann ThoracSurg.2009;87:1885---92[discussion1892---83].