w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Combined
administration
of
silymarin
and
vitamin
C
stalls
acetaminophen-mediated
hepatic
oxidative
insults
in
Wistar
rats
Saheed
Sabiu
a,∗,
Taofik
O.
Sunmonu
b,
Emmanuel
O.
Ajani
a,
Taofeek
O.
Ajiboye
baPhytomedicine,FoodFactorsandToxicologyResearchLaboratory,BiochemistryUnit,DepartmentofBiosciencesandBiotechnology,KwaraStateUniversity,Malete,Ilorin,Nigeria bPhytomedicineandPlantBiochemistryResearchLaboratory,BiochemistryUnit,DepartmentofBiologicalSciences,Al-HikmahUniversity,Ilorin,Nigeria
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14August2014 Accepted28November2014 Availableonline11February2015
Keywords:
Antioxidants Hepatoprotective Reactivemetabolite Therapeutic Lipidperoxidation
a
b
s
t
r
a
c
t
Oxidativeinsultbyfreeradicalshasbeenimplicatedindrug-inducedhepaticdamageandthishasresulted infrequentepisodesofliverdisorders.Therapeuticefficacyofantioxidantsmayprovideapossible solu-tiontothismenace.Thisstudywascarriedouttoinvestigatetheeffectofcombinedadministration ofsilymarinandvitaminCinrescuingacetaminophen-inducedhepatotoxicityinrats.Hepatotoxicrats wereorallyadministeredwithsilymarinandvitaminCat100and200mg/kgbodyweight,respectively. Attheendoftheexperiment,liverfunctionindices,antioxidantparametersandhistologicalanalysis wereevaluated.Weobservedthatthesignificantlyincreased(p<0.05)activitiesofalkalinephosphatase, alanineaminotransferase,aspartateaminotransferase,aswellaslevelsofthiobarbituricacidreactive substancesandserumtotalbilirubin,weremarkedlyreducedfollowingco-administrationofsilymarin andvitaminC.Thecompoundsalsoeffectivelyreversedthereducedactivitiesofsuperoxidedismutase, catalase,glutathioneperoxidase,glutathioneS-transferaseandtotalproteinconcentrationinthe hep-atotoxicrats.Thesefindingsareindicativeofhepatoprotectiveandantioxidantattributesofthetwo compoundswhicharealsosupportedbythehistologicalanalysis.Theavailableevidencesinthisstudy suggestthatthecomplementaryeffectsofsilymarinandvitaminCprovedtobecapableofameliorating acetaminophen-mediatedhepaticoxidativedamageandtheprobablemechanismisviaantioxidative action.
©2014SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Liver,thekeyorganinmaintenanceofhomeostasisaswellas metabolismandexcretion,hasanoverwhelmingtaskof detoxify-ingxenobioticsandchemotherapeuticagents(Ademuyiwaetal., 1994).Itsroleintransformingandclearingchemicalsrendersit susceptibletodamagefromtheseagents.Generally,thisusually goesunnoticedbecauseoftheconsiderablecapacity of hepato-cytestoregenerate.However,overstressedlivercompromisesits detoxificationrolewhichmayexposeittoavarietyofdiseasesand disorders(Amicetal.,2003).Liverinjurycausedbytoxicchemicals andcertaindrugshasbeenrecognizedasatoxicologicalproblem andlinkedtotheoccurrenceofoxidativestress(Wolfetal.,1997). Freeradicals’generation,arisingfromoxidativestress,isa com-monmechanismunderlyinghepatotoxicitycausedbydeleterious effectofdrugsandtoxicants.Oxidativestresshasalsobeen
impli-∗ Correspondingauthor.
E-mail:sabiu.saheed@kwasu.edu.ng(S.Sabiu).
catedinthepathogenesisofcellulardamagecausedbyanumber oftoxicagentsincludingarsenic,diclofenac,carbontetrachloride, rifampicinandacetaminophen(Deavalletal.,2012).
Acetaminophen(paracetamol)isawidelyusedantipyreticand analgesic which produces acute liver damage if overdoses are ingested.Thedrugismainlymetabolizedinthelivertoexcretable glucuronideandsulphate conjugates(Jollowet al.,1974;Wong etal.,1981).Hepatotoxicitycausedbyparacetamolingestionhas beenattributedtotheformationofahighlyreactivemetabolite,
N-acetyl-p-benzoquinoneimine(NAPQI),bytheactionofhepatic cytochromeP-450(Savidesand Oehne,1983).NAPQIisinitially detoxifiedbyconjugationwithreducedglutathione(GSH)toform mercapturicacid(Mooreetal.,1985).However,whentherateof NAPQIformationexceedstherateofdetoxificationbyGSH,it oxi-dizestissuemacromoleculessuchaslipidsorsulfylhydride(SH) groupofproteinandaltersthehomeostasisofcalciumafter deplet-ingGSH.
Hepatotoxicityisoneofverycommonailmentsresultinginto seriousdebilitiesrangingfromseveremetabolicdisordersto mor-tality(Pateletal.,2008).Agoodnumberofantioxidantshavebeen
http://dx.doi.org/10.1016/j.bjp.2014.11.012
exploitedinthemanagementofliverdisorders.Theseagentsplay animportantroleinscavengingfreeradicalstherebyproviding pro-tectionagainstinfectionsanddegenerativediseases(Subramaniam etal.,2000).SilymarinandvitaminChavereceivedconsiderable attentionovertheyearsduetotheirdiverseantioxidantand hep-atoprotectiveactivities(McDowell,1989;Siesetal.,1992;Burtis andAshwood,1994;Shakeretal.,2010;Tongetal.,2011).
Silymarin(1)isaflavonoidextractedfromtheseedofSilybum marianum(L.)Gaertn.(milkthistleplant)whichisa memberof theasterfamily (Asteraceae).Silybin (silibinin),silychristinand silydininhavebeenidentifiedasitsotheractiveprinciples(Rui, 1991). S. marianum is one of the oldest and most thoroughly researchedplantsinthetreatmentofliverdiseases(Morazzoniand Bombardelli,1995).Seedsoftheplanthavebeenusedformorethan 2000yearstotreatarangeofliverandgallbladderdisorders includ-inghepatitis,cirrhosis andicterus (Morazzoniand Bombardelli, 1995).Itisalsocommonlyusedasinherbaltherapyespeciallyfor treatingliverdiseasespartlyduetoitsantioxidantactivity(Tong etal.,2011).Indrug-andchemically-inducedoxidativestress, sily-marinhasbeenreportedastheprimarytherapeuticmodalityof choice(Ferencietal.,1989;BlázovicsandFehér,2001;Féherand Lengvel,2012).
HO
OH
OH OH
O
1 O
O CH2OH
OCH3 O
VitaminCisasix-carboncompoundstructurallyrelatedto glu-cose.Itconsistsoftwo inter-convertiblecompounds:l-ascorbic acid,whichisastrongreducingagent,anditsoxidizedderivative,l -dehydroascorbicacid(EliasandOputiri,2013).VitaminCisfound incitrus,softfruitsandleafygreenvegetableswhilekidneyand liveraregoodanimal-derivedsourcesofthecompound(Stangeland etal.,2008).VitaminC ishydrophilicandexertsitsantioxidant actionbyinhibitinglipidperoxidationandoxidativecelldamage (Xavieretal.,2007).Pathogenicdysfunctionoftissuesowingtocell deathviaapoptosisisoneoftheimportantoutcomesofoxidative stressthatcouldbeamelioratedbyvitaminC(Santosetal.,2009). Erguletal.(2010)havealsoreportedtheeffectofvitaminCon oxidativeliverinjuryinducedbyisoniazidinrats.Theyfoundthat isoniazid-mediatedhepaticonslaughtwasassociatedwith oxida-tivestressandtreatmentwithvitaminCamelioratedliverdamage appreciably.
Accordingly,sincetheattentionoftoday’sclinicalpracticeis focusedonantioxidantconsumptionagainstdrug-induced oxida-tive stress, the present study examined the combined effects ofsilymarinandvitaminConacetaminophen-mediatedhepatic oxidativeinsultinrats.
Materialsandmethods
Chemicalsandreagents
Silymarinwasprocured fromSigma-AldrichChemicals Com-pany(St.Louis,Mo,USA).ParacetamolandvitaminCwereproducts of EmzorPharmaceuticals, Lagos,Nigeria. Assay kitswere pur-chased fromRandox Laboratories limited,United Kingdom and Sigma-AldrichChemicalsCompany(St.Louis,Mo,USA).Distilled waterwasobtainedfromBiochemistryLaboratory,KwaraState University,Malete,Ilorin,Nigeria.Otherchemicalsandreagents wereofanalyticalgrade.
Experimentalanimals
Wistarstrainalbinoratswithameanweightof180.00±2.33g wereobtainedfromtheAnimalHouseofAl-HikmahUniversity, Ilorin,Nigeria. The animalswerekeptin clean metaboliccages placedinawell-ventilatedroomwithoptimumcondition (tem-perature:23±1◦C,photoperiod:12hnaturallightand12hdark, relative humidity: 45–50%). They were acclimatized to animal house conditionsfor ten daysand were allowedfree accessto foodandwateradlibitum.Theresearchwascarriedoutfollowing approvalfromtheEthicalcommitteeontheuseofLaboratory Ani-malsofAl-HikmahUniversity,Ilorin,Nigeria.Aclearancenumber HUI/ECULA/014/04/002wasassignedandissuedfortheresearch.
Inductionofliverdamage
Hepatotoxicity(liver damage)wasinduced inratsaccording totheproceduredescribed byKanchanaand Mohamed(2011). Briefly,theanimalswereorallyadministeredwith400mg/kgbody weight(b.w.)ofacetaminophenoncedailyforsevendays.Feedand waterweremadeavailabletotheanimalsadlibitumthroughoutthe inductionperiod.
Animalgroupingandtreatments
Thirtyalbinoratswererandomizedintofivegroupsofsixrats each.Group1animalsservedasnormalcontrolandweregiven distilledwater.Group2comprisedanimalsinducedwithliver dam-agebutnottreated.Animalsingroups3–5werehepatotoxicrats administeredwiththerapeuticdosesofsilymarin(200mg/kgb.w.), vitaminC(200mg/kgb.w.)andbothcompoundsco-administered (100mg/kgb.w.each)respectively(Mongietal.,2011;Jánosand Gabriella,2012;SabzevarizadehandNajafzadeh,2012;Santhrani etal.,2012).Alladministrationsweredoneoncedailyforsevendays usingoralintubatorwithadlibitumprovisionoffoodandwater throughouttheexperimentalperiod.
Preparationofserumandexcisionofliver
Twenty-four hours after the last treatment, the rats were humanelysacrificedbydiethyletheranaesthetization.Bloodwas collectedbycardiacpunctureintocentrifugetubesandallowedto stayfor20minbeforecentrifugingat3000×gfor15minusinga benchcentrifuge(BeckmanandHirsch,Burlington,IO,USA).Serum wascarefullyaspiratedandusedforliverfunctiontests.Theliver wasexcised,cleanedoffatandslicedintotwoportions.Aportionof theliverwashomogenizedinTris–HClbuffer(0.05mol/lTris–HCl and1.15%KCl,pH7.4)forantioxidantanalyses,whiletheother portionwasfixedinsalineformaldehydesolutionforhistological examination.
Liverfunctionindices,antioxidantanalysesandhistopathological examination
lipidperoxidationmeasuredintermsofthiobarbituricacid reac-tivesubstances(TBARS)wasdeterminedintheliverhomogenate. Histopathologicalexaminationoftheliverwascarriedoutusing themethodofBancroftandStevens(1990).
Statisticalanalysis
All data were subjected to one-way analysis of variance (ANOVA)usingSPSSsoftwarepackageforwindows(Version16) andexpressedasmean(X)±standarderrorofmean(SEM)(n=6). Significantdifferencebetweenthetreatmentmeanswas deter-minedat5%confidencelevelusingDuncan’sMultipleRangeTest.
Results
Liverfunctiontests
Table1shows theeffectsof silymarinand vitaminC onthe activitiesofserumALP,ALT,ASTaswellasconcentrationsoftotal bilirubinandtotalproteinintheexperimentalrats.Oral adminis-trationof400mg/kgb.w.ofacetaminophenforsevendayscaused asignificant(p<0.05)increaseintheactivitiesoftheseenzymes andleveloftotalbilirubinaswellassignificantreduction(p<0.05) in the concentrationof total protein when compared withthe normal control. Theelevatedactivities of theassayedenzymes andtotalbilirubinconcentrationinducedbyacetaminophenwere significantlyattenuated(p<0.05)followingtreatmentwitheither silymarin/vitaminCor both.Thereduced concentrationof total proteinwasalsosignificantlyincreased(p<0.05)aftertreatment witheithersilymarin/vitaminC orbothcompounds.Theeffects werehowevermorepronouncedintheratswhollytreatedwith vitaminCandthoseco-administeredwithbothsilymarinand vita-minC.
Antioxidantanalyses
TheeffectsofsilymarinandvitaminContheantioxidant sta-tusofratliverarepresentedinTable2.TBARSwassignificantly increased(p<0.05)inthehepatotoxicrats.Separateandcombined treatments with silymarin and vitamin C significantly reduced (p<0.05)thelevelofTBARScomparabletonormal.Asignificant reduction(p<0.05)wasalsoobservedintheactivitiesofSOD,CAT, GPxandGSTinliverofhepatotoxicratswhencomparedwith nor-malcontrol.Co-treatmentwithsilymarinandvitaminCresulted insignificantincrease(p<0.05)intheactivitiesoftheseenzymes whichwascomparabletonormal.
Histopathologicalanalysis
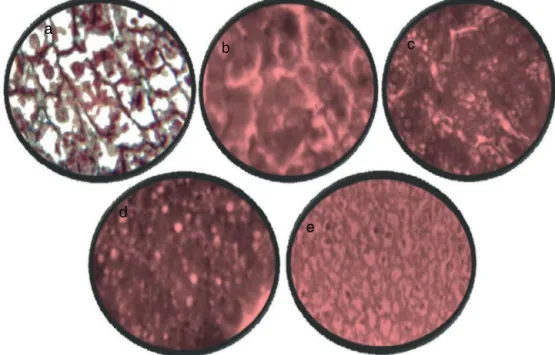
ThelivermicrographsoftheratsarepresentedinFig.1.The liverofthecontrol ratsshowednormal architecturewith well-preservedcordsofhepatocytes,well-demarcatedsinusoidsandno areaofinfiltrationbyinflammatorycells(Fig.1A).Thisisin con-trasttothefeaturesobservedintheliverofhepatotoxicrats.There weredrasticalterationsinliverarchitecturerangingfromextensive fattychange,distendedhepatocytes,vacuolatedcytoplasm, com-pressedsinusoids,fattydegenerationareaofnecrosis,toinfiltration byinflammatorycells(Fig.1B).However,thelivermicrographsof ratstreatedwithsilymarin/vitaminCorbothshoweddistinctand essentiallynormalcordsofhepatocytewithnon-prominentfatty change(Fig.1C–E).
Discussion
Drug-inducedliverdisorders occurredfrequentlyand canbe life threatening. Oxidative stress occasioned by highly reactive intermediates(freeradicals)hasbeenlinkedto acetaminophen-mediated hepatotoxicity in rats (Balamurugan, 2007). The catastrophic freeradical eventssuchas lipidperoxidation, pro-tein oxidation and DNA oxidation are rarely the cause of cell deathinrealisticinvivocondition.Thisisbecausetheantioxidant defensearsenal inlivercellsiscapableofdetoxifyingfree radi-calsandrepairdamageresultingfromhighlyreactivemetabolites (Jaeschke etal., 2003).However, whenthe antioxidantdefense systemisoverwhelmed,freeradicalsmayinflictdirectoxidative damagetocellularmacromolecules,leadingtocelldeath(Sabiu etal.,2014).Timelyinterventionwithexogenousantioxidants aug-mentsthecellulardefensesystemtopreventtheseilleffectson cellularmacromolecules.Studieshavereportedtheantioxidants andcytoprotectiveactivitiesofsilymarinandvitaminC(Crocenzi et al., 2003; Santos et al., 2009; Mor and Ozmen, 2010). This study,thus, demonstratestheantioxidant andhepatoprotective potentialsofsilymarinandvitaminCinacetaminophen-mediated hepaticoxidativedamageinrats.
IncreasedactivitiesofserumAST,ALTandALPareindicativeof cellularleakageandlossoffunctionalintegrityoflivercell mem-brane.Theextentofdrug-inducedhepatotoxicityisassessedbythe release oftheseintracellularenzymesviathehepatocyte mem-braneintocirculation (Sabiuetal.,2014).Specifically,increased activityofASTisindicativeofliverdamageduetoviralhepatitis, cardiacinfarctionandmuscleinjury.ALTismorespecifictotheliver andthusabetterparameterforassessingliverinjury(Willianson etal.,1996)whileserumALPactivitygivesacluetothe function-ality ofthehepatocytes.ElevatedactivityofserumALP maybe attributedtoincreasedsynthesisinthefaceofincreasingbiliary pressure(GiniandMuraleedhara,2010).Inthepresentstudy, ele-vatedactivitiesofAST,ALTandALPinacetaminophen-treatedrats
Table1
EffectsofsilymarinandvitaminConliverfunctionindicesofacetaminophen-inducedhepatotoxicrats(n=6,X±SEM).
Treatments AST(U/L) ALT(U/L) ALP(U/L) Totalbilirubin(mg/dl) Totalprotein(g/dl)
Control 85.36±4.51a 47.15±2.35a 75.23±5.31a 0.92±0.06a 9.32±0.32a
Acetaminophen induced
147.81±9.20b 160.28±4.52b 126.21±3.36b 1.98±0.09b 5.46±0.06b
Acetaminophen induced+silymarin
110.17±5.32c 63.65
±2.25c 90.32
±2.63c 0.70
±0.04c 7.75
±0.32c
Acetaminophen
induced+vitamin
C
100.36±4.63a 60.87
±3.19a 70.25
±9.61a 0.80
±0.05a 8.99
±0.15a
Acetaminophen
induced+silymarin+vitamin C
90.04±4.68a 55.39±2.86a 73.61.±2.92a 0.88±0.02a 9.25±0.35a
Table2
EffectsofsilymarinandvitaminConantioxidantstatusofacetaminophen-inducedhepatotoxicrats(n=6,X±SEM).
Treatments TBARS(mM/100gtissue) SOD(U/mgprotein) CAT(U/mgprotein) GPX(U/mgprotein) GST(U/mgprotein)
Control 14.64±1.75a 7.25
±0.90a 3.98
±0.35a 18.56
±0.31a 1.02
±0.05a
Acetaminopheninduced 18.80±1.55b 5.65±0.93b 2.18±0.25b 16.42±0.79b 0.59±0.03b
Acetaminophen induced+silymarin
15.30±1.75a 6.69±0.52a 2.99±0.23a 17.48±0.30a 0.82±0.02a
Acetaminophen
induced+vitaminC
14.01±1.63a 6.99
±0.32a 3.55
±0.97a 18.27
±0.08a 0.92
±0.02a
Acetaminophen induced+silymarin
+vitaminC
14.52±1.09a 7.20
±0.59a 3.81
±0.05a 18.48
±0.21a 0.99
±0.02a
Valueswithdifferentsuperscriptsalongthesamecolumnforeachparameteraresignificantlydifferent(p<0.05).
TBARS,thiobarbituricacidreactivesubstances;SOD,superoxidedismutase;CAT,catalase;GPX,glutathioneperoxidase;GST,glutathioneS-transferase.
AST,aspartateaminotransferase;ALT,alanineaminotransferase;ALP,alkalinephosphatase.
maybeanindicationofliverdamageandcellnecrosisresulting fromformationofNAPQIinexcessofGSHdetoxificationcapacity. ThisagreeswithearlierreportsbyBalamurugan(2007),Giniand Muraleedhara(2010),andKanchanaandMohamed(2011).These authorsopinedthatoverdoseofacetaminophencouldbetoxicto thehepatocytes.Conversely,thesignificantreductioninenzyme activitiesofratstreatedwithsilymarinandvitaminCsuggeststhat bothcompoundswereabletoamelioratethedeleteriouseffectsof acetaminophen.Theeffectsweremorepronouncedinthevitamin C-treatedratsaswellasthoseco-treatedwithbothsilymarinand vitaminC.
The serum levels of total protein and bilirubin may indi-catethestateoftheliverand thetype ofdamage (Sabiu etal., 2014).Hypoproteinemiaisafeatureofliverdamagewhichmay be attributed to a decrease in protein synthesis (Oloyede and Sunmonu, 2009). In this study, hypoproteinemia was observed in the acetaminophen-treated rats and may be a consequence ofimpairedhepato-cellular functions.In addition,theobserved hyperbilirubinemia may be due to excessive heme destruction andblockageofbiliarytractinacetaminophen-treatedrats.This obstructionmighthaveresultedtomassinhibitionofconjugation reactionandreleaseofunconjugatedbilirubinfromdamagedand deadhepatocytes(Wolfetal.,1997).Thisagreeswiththeearlier reportsbyAjiboyeetal.(2010),KanchanaandMohamed(2011),
andChinnasamyetal.(2011)whereacetaminophenwasreported tohavecausedalterationinserumconcentrationsoftotal biliru-binandtotalprotein.Co-treatmentswithsilymarinandvitamin C,however,reducedthelevelofbilirubin andincreasedprotein concentrationsuggestingthattheyofferedconsiderablelevelof hepatoprotectionatthetestedregimen.
Oxidativeinsulttoacellinducesperoxidationof membrane-bound lipids whose toxic products cause damage of macro-molecules.In thepresent study,the increasedconcentrationof TBARSintheliverofhepatotoxicratsissuggestiveoffacilitatedlipid peroxidationleadingtotissuedamageandfailureofbody’s antiox-idantdefensemechanismstopreventformationofexcessivefree radicals.Ithasbeenreportedthatacetaminophencaused signifi-cantincreaseinhepaticlipidperoxidationduetofreeradicalinjury innecroticliversofrats(GiniandMuraleedhara,2010).Reactive oxygenspeciesinconjunctionwithNAPQIarerequiredtoinitiate lipidperoxidationwhichhasbeenopinedasanimportant initia-tioneventinthetoxicitymechanismofacetaminophen(Thabrew etal.,1987).ThesignificantlyreducedconcentrationofTBARSinthe liverofsilymarinandvitaminC-treatedratsindicatestheirpossible antiperoxidativeattributeandthusantioxidativepotential.
Thebodyisendowedwithaneffectivemechanismtocounter theravagingeffectoffreeradical-induceddamage.Thisisattained
viaendogenousantioxidantenzymes,suchasSOD,CAT,GPXand
a
b
c
d
e
GST.Animbalancebetweenfreeradicalsproductionand antiox-idant defense system resultsin oxidative stress which further deregulatescellularfunctionsleadingtovariouspathological con-ditions.Inthepresentstudy,thereducedactivityofantioxidant enzymesinacetaminophen-treatedratsisan obviousreflection ofexcessiveformationoffreeradicalsresultingintissuedamage. However,thesignificantincreaseintheiractivitiesfollowing treat-mentwithsilymarinandvitaminCisanindicationofantioxidant effect.
Inthepresentstudy,theprotectionofferedbysilymarinagainst acetaminophen-inducedhepatotoxicity maybegenerallylinked toitsbeneficialattributesasrevealedbyValenzuelaandGarride (1994).Theseincludeitsabilitytoscavengefreeradicals,increase cellularGSHcontentand regulate membranepermeability. The authorsalsoreportedthatsilymarinhasthecapacitytoregulate nuclearexpressionbymeansofasteroid-likeeffect.
Similarly,theabilityofvitaminCtotrapfreeradicals,protect biomembranes fromperoxide damage and effectively scavenge reactive oxygen species as reported by Sminorff and Wheeler (2000)maybesuggestiveofitseffectexhibitedinthetreatment groups.Bendich(1990)hasalsoreportedvitaminCasanexcellent electrondonortofreeradicalswhichsubsequentlyquenchtheir deleteriousactivityoncellularmacromolecules,thusplayingarole inantioxidantmechanism.Inaddition,the200mg/kgb.w.of vita-minCexploitedinthisstudymaybesufficientenoughtocontribute toitstherapeuticpotentialexhibitedinthetreatedrats.Thisagrees withthereportofMongietal.(2011)wherevitaminCwasreported tohaveofferedprotectionagainstbiochemicaltoxicityinducedby deltamethrininmaleWistarrats.This,perhaps,couldalsobea ten-ablefactfortheratswhollyadministeredwithvitaminCandthose co-administeredwithsilymarinforhavingpronounced hepatopro-tectiveandantioxidantpotentialscomparedtoratstreatedwith onlysilymarin.Thissubmissionisinconformitywiththereportof SabzevarizadehandNajafzadeh(2012)thatvitaminCmodulates myoglobinurichepaticfailurebetterthansilymarinbybindingto variousharmfulsubstancesinrats.
Anotherimportantconsideration in assessing theefficacyof potentialtherapeuticagentsagainsthepaticinjuryistheireffect onhistology.Theeffectsaremanifestationsofinflammatoryinsult ontheliverandoftencomplementenzymeanalysis(Adesokanand Akanji,2007).Theapparentlyannulleddegenerativethreatsposed byacetaminophenonthearchitecturalfeaturesofhepatocytesin theratswhollytreatedwithvitaminCandincombinationwith silymarinsuggestthatthetwocompoundsconferredareasonable levelofintegrityontheliver.Infact,architecturalorganizationof someofthehepatocyteswasalmostcompletelyrestoredto nor-mal.Theeffects noticedwereinconsonancewiththeresultsof biochemical analysisobtainedand in agreementwiththe find-ingofBalamurugan(2007),whererecoverytowardsnormalization ofserum enzymesand liverhistologicalarchitecturecaused by acetaminophenwereattributedtoantioxidantagents.
Conclusively,therestoration ofdegenerative insultsinflicted by 400mg/kg b.w. acetaminophen by co-administration with silymarinandvitaminCisanindicationoftheirinherent hepato-protectiveandantioxidantattributesinrats.Though,theeffects wereprominentlyexhibited byvitaminC, theircomplementary efficacy is formidable and thus recommended against hepatic oxidativedamage.
Authors’contributions
SS and SOT designed the study and were involved in the manuscript preparation. AOT evaluated the compounds in acetaminophen-treated rats. SS, AOE and SOT performed
biochemicalestimationsandhistopathology.Allauthorsreadand approvedthefinalmanuscript.
Conflictofinterest
Allauthorshavenothingtodeclare.
Acknowledgements
TheauthorsareimmenselythankfultotheBiochemistryUnits ofKwaraStateUniversity,Malete,Ilorin,NigeriaandAl-Hikmah University,Ilorin,Nigeriaforprovidingamplesupportandaccess toresearchfacilities.
References
Ademuyiwa,O.,Adesanya,O.,Ajuwon,O.R.,1994.VitaminCinCC14hepatotoxicity –apreliminaryreport.Hum.Exp.Toxicol.13,107–109.
Adesokan,A.A.,Akanji,M.A.,2007.Effectofrepeatedadministrationofaqueousstem extractofEnantiachloranthaontheintegrityofratlivercellularsystem.Nig.J. Biochem.Mol.Biol.22,19–22.
Ajiboye,T.O.,Salau,A.K.,Yakubu,M.T.,Oladiji,A.T.,Akanji,M.A.,Okogun,J.I.,2010.
AcetaminophenperturbedredoxhomeostasisinWistarratliver:protectiverole ofaqueousPterocarpusosunleafextract.DrugChem.Toxicol.33,77–87.
Amic,D.,Davidovic-Amic,D.,Beslo,D.,Trinajstic,N.,2003.Structure-radical scav-engingactivityrelationshipofflavonoids.Croat.Chem.Acta76,55–61.
Balamurugan,M.,2007.Restorationofhistoachitectureintheparacetamol-induced liverdamagedratbyearthwormextract,Lampitomauritii(Kinberg).Eur.Rev. Med.Pharmacol.Sci.11,407–411.
Bancroft,D.J.,Stevens,A.,1990.TheoryandPracticeofHistopathological Tech-nigques,3rded.ChurchillLivingston,NewYork,pp.126–129.
Bendich,A.,1990.In:Bendich,A.,Chandra,R.K.(Eds.),Micro-nutrientsandImmune Functions.AcademySciences,NewYork,p.175.
Blázovics,A.,Fehér,J.,2001.Oxidativestressandliver.In:Fehér,J.,Lengyel,G.(Eds.), Hepatologia.Medicina,Budapest,pp.50–88.
Burtis,C.A.,Ashwood,E.R.,1994.TietzTextbookofClinicalChemistry,2nded.WB SaundersCo.,Philadelphia,pp.1275–1512.
Chinnasamy,A.,Balasubramanium,R.,Jajapalu,K.,2011.ProtectiveeffectofPisonia aculeateonparacetamolinducedhepatotoxicityinrats.J.Exp.Integr.Med.1, 167–172.
Crocenzi,F.A.,Sanchez,P.E.J.,Pellegrino,J.M.,Rodriguez,E.A.G.,Mottino,A.D.,Roma, M.G.,2003.Preventiveeffectofsilymarinagainsttaurolithocholate-induced cholestasisintherat.Biochem.Pharmacol.66,355–364.
Deavall,G.D.,Elizabeth,A.M.,Judith,M.H.,Ruth,R.,2012.Drug-inducedoxidative stressandtoxicity.J.Toxicol.,http://dx.doi.org/10.1155/2012/645460. Devasagayam,T.P.,Tarachand,U.,1987.Decreasedlipidperoxidationintherat
kidneyduringgestation.Biochem.Biophys.Res.Commun.145,134–138.
Elias,A.,Oputiri,D.,2013.HepatoprotectiveeffectofvitaminC.Pharmacol.Pharm. 4,84–92.
Ergul,Y.,Erkan,T.,Uzun,H.,2010.EffectofvitaminConoxidativeliverinjurydue toisoniazidinrats.Ped.Inter.52,69–74.
Féher,J.,Lengvel,G.,2012.Silymarininthepreventionandtreatmentofliver dis-easesandprimarylivercancer.Curr.Pharm.Biotechnol.13,210–217.
Ferenci,P.,Dragosics,B.,Dittrich,H.,Frank,H.,Benda,L.,Lochs,H.,Meryn,S.,Base, W.,Schneider,B.,1989.Randomizedcontrolledtrialofsilymarintreatmentin patientswithcirrhosisoftheliver.J.Hepatol.9,105–113.
Gini,C.K.,Muraleedhara,G.K.,2010.HepatoprotectiveeffectofSpirulinalonaron paracetamolinducedliverdamageinrats.AsianJ.Exp.Biol.Sci.1,614–623.
Jaeschke,H.,Knight,T.R.,Bajt,M.L.,2003.Theroleofoxidantstressandreactive nitrogenspeciesinacetaminophenhepatotoxicity.Toxicol.Lett.144,279–288.
János,F.,Gabriella,L.,2012.Silymarininthepreventionandtreatmentofliver diseasesandprimarylivercancer.Curr.Pharm.Biotechnol.13,210–217.
Jendrassik,L.,Grof,P.,1938.Acolorimetricmethodforthedeterminationofdirect andtotalbilirubin.Biol.Chem.297,81.
Jollow,D.J.,Thorgeirsson,S.S.,Potter,W.Z.,Hashimoto,M.,Mitchell,J.R.,1974.
Acetaminopheninducedhepaticnecrosis.VI.Metabolicdispositionoftoxicand non-toxicdosesofacetaminophen.Pharmacology12,251–271.
Kanchana,N.,Mohamed,A.S.,2011.HepatoprotectiveeffectofPlumbagozeylanicaon paracetamolinducedlivertoxicityinrats.Int.J.Pharm.Pharm.Sci.3,151–154.
Lowry,O.H.,Rosebrough,N.J.,Farr,A.L.,Randall,R.J.,1951.Proteinmeasurement withtheFolinphenolreagent.J.Biol.Chem.193,265–275.
Marklund,S.,Marklund,G.,1974.Involvementofsuperoxideanionradicalinthe autooxidationofpyrogallolandaconvenientassayforsuperoxidedismutase. Eur.J.Biochem.47,469–474.
McDowell,L.R.,1989.VitaminsinAnimalNutritionComparativeAspectstoHuman NutritionVitaminC.AcademicPress,London,pp.93–131.
Mongi,S.,Mahfoud,S.,Amel,M.,Kamel,B.,Abdel-fattahel,J.,2011.Protective effectsofvitaminCagainsthaematologicalandbiochemicaltoxicityinduced bydeltamethrininmaleWistarrats.Ecotoxicol.Environ.Saf.74,1765–1769.
associatedwiththiodepletionandincreasedcytosolicCa2+.J.Biol.Chem.260, 13035–13040.
Mor,F.,Ozmen,O.,2010.EffectofvitaminCinreducingthetoxicityofendosulfan inliverinrabbits.Exp.Toxicol.Pathol.62,75–80.
Morazzoni,P.,Bombardelli,E.,1995.Silybummarianum(Carduusmarianus). Fitoter-apia66,3–42.
Oloyede,O.B.,Sunmonu,T.O.,2009.Potassiumbromatecontentofselectedbread samplesinIlorin,CentralNigeriaanditseffectonsomeenzymesofratliverand kidney.FoodChem.Toxicol.47,2067–2070.
Patel,R.K.,Patel,M.M.,Patel,M.P.,Kanzaria,N.R.,Vaghela,K.R.,Patel,N.J.,2008. Hep-atoprotectiveactivityofMoringaoleiferaLam.Fruitonisolatedrathepatocytes. Phcog.Mag.4,118–123.
RecGSCC,1972.Optimisedstandard colorimetricmethods.J.Clin.Chem.Clin. Biochem.10,182.
Reitman,S.,Frankel,S.,1957.Acolorimetricmethodforthedeterminationofserum glutamic-oxaloacetateandglutamic-pyruvatetransaminase.Am.J.Clin.Pathol. 28,56–61.
Rotruck,J.T.,Pope,A.L.,Ganther,H.E.,Swanson,A.B.,Hafeman,D.G.,Hoekstra,W.G., 1973.Selenium:biochemicalroleasacomponentofglutathioneperoxidase. Science179,588–590.
Rui,Y.C., 1991. Advances inpharmacologicalstudiesof silymarin.Mem.Inst. OswaldoCruz86,79–86.
Sabiu,S.,Wudil,A.M.,Sunmonu,T.O.,2014.CombinedadministrationofTelfaira occidentalisandVernoniaamygdalinaleafpowdersamelioratesgarlic-induced hepatotoxicityinWistarrats.Pharmacologia5,191–198.
Sabzevarizadeh,M.,Najafzadeh,H.,2012.Comparisoneffectofsilymarinand vita-minConliverfunctioninmyoglobinuricstatusinrats.WorldAppl.Sci.17, 228–232.
Santos,I.M.S.,DaRocha-Tome,A.,Saldanha,G.B.,Ferreira,P.M.P.,Militao,G.C.G.,De Freitas,R.M.,2009.Oxidativestressinthehippocampusduringexperimental seizurescanbeamelioratedwiththeantioxidantVitaminC.Oxid.Med.Cell. Longev.2,214–221.
Santhrani,T.,Maheswari,E.,Saraswathy,G.R.,2012.Ameliorationofcarbamazepine inducedoxidativestress andhematotoxicity by vitamin C.Spatula DD 2, 173–180.
Savides,M.C.,Oehne,F.W.,1983.Acetaminophenanditstoxicity.J.App.Toxicol.3, 95–111.
Shaker,E.,Mahmoud,H.,Mnaa,S.,2010.Silymarin,theantioxidantcomponent andSilybummarianumextractspreventliverdamage.FoodChem.Toxicol.48, 803–806.
Sies,H.,Stahl,W.,Sundquist,A.R.,1992.Antioxidantfunctionsofvitaminsvitamin EandC,caroteneandothercarotenoids.Ann.N.Y.Acad.Sci.669,7–20.
Sinha,A.K.,1972.Colorimetricassayofcatalase.Anal.Biochem.47,389–394.
Sminorff,N.,Wheeler,G.L.,2000.Ascorbicacidinplantsbiosynthesisandfunction. Crit.Rev.Biochem.Mol.Biol.19,267–290.
Stangeland,T.,Remberg,S.F.,Lye,K.A.,2008.Totalantioxidantactivityin35Ugandan fruitsandvegetables.FoodChem.113,85–91.
Subramaniam,A.,Evans,D.A.,Rajasekharan,S.,Pushpangadam,P.,2000.Effect ofTrichopuszeylanicusGaertn(activefraction)onphagocytosisbyperitoneal macrophagesandhumoralimmuneinresponseinmice.Ind.J.Pharmacol.32, 221–225.
Thabrew,M.I.,Joice,P.D.,Rajatissa,W.,1987.Acomparativestudyoftheefficacyof PavettaindicaandOsbeckiaoctandainthetreatmentofliverdysfunction.Planta Med.53,239–241.
Tong,S.,Chu,C.,Wei,Y.,Wang,L.,Gao,X.,Xu,X.,Yu,J.,2011.Preparationandeffects of2,3-dehydrosilymarin,apromisingandpotentantioxidantandfreeradical scavenger.J.Pharm.Pharmacol.63,238–244.
Valenzuela,A.,Garride,A.,1994.Biochemicalbasesofthepharmacologicalaction oftheflavonoidssilymarinandofitsstructuralisomersilibinin.Biol.Res.27, 105–112.
Willianson,E.M.,Okpako,D.T.,Evans,F.J.,1996.Selection,Preparationand Pharma-cologicalEvaluationofPlantMaterial.JohnWiley,England.
Wolf,A.,Diez-Fernadez,C.,Trendelenburg,C.F.,Prieto,P.,Hary,S.,Trammer,W.E., 1997.Paracetamolinducedoxidativestressinrathepatocytes.J.Pharmacol.Exp. Ther.280,1328–1334.
Wong, L.T., Whitehouse, L.W., Solemonraj, G., Paul, C.J., 1981. Pathways of acetaminophenconjugateinthemouse.Toxicol.Lett.9,145–151.
Xavier,S.M.,Barbosa,C.O.,Barros,D.O.,Silva,R.F.,Oliveria,A.A.,Freitas,R.M.,2007.