w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
UPLC/Q-TOF-MS
profiling
of
phenolics
from
Canarium
pimela
leaves
and
its
vasorelaxant
and
antioxidant
activities
Juan
Wu
a,
Xiao’ai
Fang
a,
Yan
Yuan
a,
Yanfen
Dong
b,
Yanling
Liang
b,
Qingchun
Xie
a,∗,
Junfeng
Ban
a,
Yanzhong
Chen
a,
Zhufen
Lv
a,∗aGuangdongProvincialKeyLaboratoryofAdvancedDrugDeliverySystems,GuangongPharmaceuticalUniversity,Guangzhou510006,China
bCollegeofBasicMedicine,GuangongPharmaceuticalUniversity,Guangzhou510006,China
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received15September2017 Accepted26October2017 Availableonline15November2017
Keywords:
Phenolic UPLC/Q-TOF-MS Vasorelaxant Antioxidant Biologicalactivity HPLC-DAD
a
b
s
t
r
a
c
t
CanariumpimelaK.D.Koenig,Burseraceae,havealonghistoryofuseintheChinesetraditionalmedicine treatmentofvariousailmentsincludinghypertension,andourresearchteamhasreportedthe anti-hypertensiveactivityanddelineatedthemechanisminvolvedintheaction.Thefollowingresearchaimsto evaluatethevasorelaxantandantioxidantactivitiesofethanolextractfromC.pimelaleavesandtoanalyze itschemicalcompositionbyultra-performanceliquidchromatography-quadrupoletime-of-flightmass spectrometry(UPLC/Q-TOF-MS)thatmaycorrelatewiththeirpharmacologicalactivities.Theresults showedthatpre-incubationofaorticringswiththeextract(0.3,1,3,10,30and100mg/l)significantly inhibitedthecontractileresponseoftheringstonorepinephrine-inducedcontraction(p<0.01orp<0.05). Crudeethanolextractandrefinedethanolextractshowedahighestinhibitoryeffectagainst 2,2dipheyl-2-picrylhydrazylhydratescavengingactivity(IC50ofcrudeethanolextract=15.42±0.14g/mlandIC50 ofrefinedethanolextract=5.72±0.31g/ml)and2,2′-azinobis(3-ethyl-benzothiazoline-6-sulphonic acidammoniumsalt)(ABTS(IC50ofcrudeethanolextract=3.24±0.18g/mlandIC50ofrefinedethanol extract=1.88±0.07g/ml)scavengingactivity,whichwasconsiderablyhigherthanthatreportedfor butylatedhydroxytolueneandlowerofthatmeasuredforascorbicacid.Moreover,itschemical compo-sitionwasanalyzedbyUPLC/Q-TOF-MS.Sixteencompoundsincludingnineflavonoids,fourtannins,two phenolicacidsandonedianthronewereidentifiedforthefirsttimeasconstituentsofthisspecies.Andof this,sixmajorphenoliccomponentsweresimultaneousquantitativeanalysisbyHPLC-UV,chlorogenic acidisthemajorcompoundsinC.pimelaleaves.Theseresultsindicatethatthephenolic-richextractof
C.pimelaleavesisapromisingnaturalpharmaceuticalforcombatinghypertensionandoxidativestress. ©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
In2012and2013intheUnitedStates(Lindsley,2015),themost dispensedprescriptionswereanti-hypertensivedrugsinall dis-eases(698million).From2003to2013,thedeathrateattributable tohighbloodpressureincreased8.2%,andtheactualnumberof deathsrose34.7%(Mozaffarianetal.,2016), andthatthis prob-lemmayincreaseinthecomingdecade.However,thepathogenesis andpathophysiologyofhypertensionisacomplexheterogeneous disorder,ofwhichisapositiveanddirectcorrelationwithother cardiovascularandmetabolicabnormalities.Oxidativestressesor inefficientantioxidant defensesas one ofthe factorsappear to
∗ Correspondingauthors.
E-mails:xieqchun@163.com(Q.Xie),luzhufen@126.com(Z.Lv).
beinvolvedinthedevelopmentandprogressionofhypertension (Wilcox,2005;Elksetal.,2009).
In recent years, traditional and natural medicine have been extensively used to prevent and heal various cardiovascular problemsincludinghypertension(HoandJie,2007)andthe anti-hypertensiveactivity of plantextract has beencorrelated with its antioxidantproperties (Wilcox, 2005; Elks et al., 2009) and flavonoid contents (Hugel et al., 2016). As antioxidants, these compoundsmaypreventtheprogressiveimpairmentofvascular endothelialcells due tooxidativestress toimproveendothelial function.Inaddition,thereisgrowingevidencethatflavonoid-rich dietshavebeenlinkedtoimprovementsinendothelialfunctionand bloodpressure(Hugeletal.,2016).
CanariumpimelaK.D.Koenig(CPL)belongtothefamily Burs-eraceae, locallycalledas“Wulan”and “Chineseblack olive”or “MuWeizi”isthecharacteristiccashcropofGuangdongProvince (China)(Huang and Wang,2009)because ofits medicinalfood
https://doi.org/10.1016/j.bjp.2017.10.005
homology(JiangsuNew MedicalCollege,1986).It isindigenous tothesoutheastareaofChinaandhasbeenintroducedtoother Asian tropicaland semi-tropicalregions. Thisplant, whichwas madeintodecoction,wasusedinthefolkloremedicinetotreat anti-bacterium, anti-virus,anti-inflammation and detoxification in China(Ding,1999).In recent years,thestudy ofCPLmainly concentratedoncardiovascular.TheCPLleavesextracthas anti-hypertensiveactivity(Lietal.,1997).Itsextractcanhelptoimprove myocardialbloodperfusionandprotectthemyocardium.The liq-uidextractsfromCPLleaveshavea quickeffectofthenegative inotropicactiononisolatedfrogheartsandanti-positiveinotropic actionofCaCl2,andtheactioncanbeblockedbyatropine(Liang etal.,2006).TheextractsfromleavesofCPLhaveabi-directional effectonheartfunction.Aftertransientinhibition,myocardial con-tractilitywouldtransformtointensificationwiththedescending heartrate(Cenetal.,2012).Theuseofisolatedratheartperfusion LangendorffmodelshowedthatCPLleavesliquidextractionmaybe beneficialtoreducecardiacoxygenconsumption,maintainheart function,playaroleinprotectingthemyocardium(Cenetal.,2013, 2014).Thewaterextractsoffruit,leaves,andbarkofCPLall low-eredtheratsbloodpressurequickly,andtheeffectofextractsof fruitandleavesinloweringbloodpressurewasstrongerthanthatof thebarkextracts(Dongetal.,2006),anditwasfoundthattherewas norelationshipbetweenthedepressurizationeffectofCPLextract andthebasalbloodinstressedhypertensionrats(Dongetal.,2007). Intheisolatedratthoracicaorticannulusperfusiontest,foundthat theleaves, fruit,and barkallhavethevasodilatationeffect,and theleaveswerethemostobvious(Liangetal.,2011).Theleaves extractscanrelaxratthoracicringswithoutendothelium,which mechanismis associatedwithactivating-receptor,openingof Ca2+channelbythereceptordependentpathwayaswellas inhibi-tionofCa2+influxinthevascularsmoothmusclecell.Theextracts alsoincreaseaortaringstensioninpre-contractionwithKClbut arenotassociatedwith␣receptor(Dongetal.,2008).Alltheabove studiesarepointedoutthebenefitsofthisplantineffectof hyper-tensionactivities.However,onlythevolatileoilwasreported(Li etal.,2015),thechemicalcompositionofphenolicsofthisplantthat maycorrelatewithitspharmacologicalactivitiesisstillaproblem. In this context, and following our interest on the study of thechemicalcompositionandvalorizationofCPLleaves,theaim of thisstudy is toevaluatethepotentialof CPLas a cash crop forGuangdongProvinceinChina,determiningtheirvasorelaxant activepartsbythoracicaortaringsofrat,theirantioxidant prop-erties determined by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging and 2,2′-azinobis (3-ethyl-benzothiazoline-6-sulphonic acid ammonium salt) (ABTS) radical scavenging, analyzingtheirethanol:waterextractsbyUPLC/Q-TOF-MS, and alsoquantitativeanalyzedsixphenolic compounds(chlorogenic acid,vitexin,isovitexin,hyperin,rutin,quercetin)byHPLC-UV.
Materialsandmethods
Chemicals
MethanolwasofHPLCgradeandpurchasedfromLabscience, Inc. (Nevada, USA). Distilled water was supplied by Watson (Guangdong,China).Aceticacidandphosphoric acidof analyti-calgradewerepurchasedfromZhiyuanChemicals Ltd.(Tianjin, China).Rutin andquercetin(BatchNo. 100080-201409and No. 100081-201408)wereobtainedfromtheNationalInstitutesfor Food and Drug Control (Beijing, China). Other reference stan-dards (purity ≥98%) including chlorogenic acid (No. 150511), vitexin(No. 151102),isovitexin (No.151204) and hyperin(No. 150615)werepurchasedfromChengduPurechem-standardCo., Ltd.(Sichuan,China).Norepinephrine(No.20150414)were
pur-chasedfromGrandPharmaceutical(China)Co.,Ltd.Acetylcholine chloride(No.60-31-1)andDPPH(No.C10088334)werepurchased from Shanghai MacklinBiochemical Co., Ltd. (Shanghai,China). Isoprenalinehydrochloride(No.20150301)waspurchased from ShanghaiHarvestPharmaceuticalCo.,Ltd.(Shanghai,China).ABTS (No.F1719012)waspurchasedfromAladdinIndustrialCorporation (Shanghai,China).
Preparationofplantextract
ThefreshleavesofCanariumpimelaK.D.Koenig,Burseraceae, werecollectedduringthemid-August2015fromtheherbal gar-denofZhongshancity,Guangdongprovince,China.Theplantwas taxonomicallyidentifiedbyProfessorJizhuLiuandavoucher speci-men(No.20150825)wasdepositedatSchoolofTraditionalChinese Medicine,GuangdongPharmaceuticalUniversity.
Dried and powdered leaf(20g) of CPLwas transferredto a round-bottomflaskandwasextractedwithethanol:water(50:50, v/v) witha solid–liquidratio 1:50 (m/v).The flaskwasheated (70±1◦C) in an electric jacket under reflux for 80min. The suspensions were filteredand theextract wasvacuum-filtered, concentratedinarotaryvacuumevaporatorat50–60◦C(Jinetal., 2010)andfreeze-driedtoyieldthecrudeethanolextraction(CEE) (4.19g). CEEwasthenpurified byAB-8resinand elutedas fol-lows:water2-bedvolume(BV),30%2BV,50%3BVand95%ethanol 2BV.Whentheeluentwasclarified,beginningtocollectiontillthe end.Sugarswereelutedfirstandtheotherswerecombinedand driedasabovetoaffordabrownphenolicextract–refinedethanol extraction(REE)(2.68g).
EvaluationofCEEandREEonvasculartensioninrats
Adult male and female Sprague Dawley (SD)rats (weighing 280–320g) obtainedfromMedical LaboratoryAnimal Centerof GuangdongProvince(No.SCXK( )2013-0002)werehousedunder conventionallaboratoryconditionsthroughouttheperiodof exper-imentation. Animalexperimentswerecarried outfollowing the guide ofExperimental Animal Ethics Committeeof GuangDong PharmaceuticalUniversity(No.gdpu2016500)forthecareanduse ofLaboratoryanimals.Theywerefedwithstandard pelletsand wateradlibitumandmaintainedat22◦Ctemperature,85%relative humidity,anda12hdayandnightcycle.
Thepreparationofisolatedratthoracicaorticringswasbased ontheproceduresdescribedbyQinetal.(2014).Contractionof aortaringswasinducedwithnorepinephrine (NE,0.1mM),and inducedtherelaxationwithacetylcholine (Ach,1M). The ten-sionwasmeasuredusingforce–displacementtransducer(Beijing, China)coupledwithaMedLabsbiologicalsignalacquisitionsystem (Nanjing,China).
Relaxation,ameasureofinhibitionofcontractioninaorticring pre-contractedwithphenylephrinewasmeasuredinpercentage andcalculatedasEq.(1):
% Relaxation=Tc−Tt
Tc ∗100 (1)
whereTcstandsforchangeintensionaftercontractionwith norepi-nephrine,whileTtstandsforchangeintensionafteraddingextract. Valueswereexpressedasmean±standarddeviation(SD).
EvaluationofantioxidanteffectsofCEEandREE
DPPHradicalscavengingassay
TheantioxidantactivityoftheextractofCPLleavesevaluated by2,2dipheyl-2-picrylhydrazylhydrate(DPPH)radicalscavenging. Theextractwasdissolvedinethanoltopreparestocksolutionsover theconcentrationrange0.005–0.1mg/ml,whichwasmixedwith 2mlofDPPHsolution.Then,themixturewasincubatedatroom temperaturefor30mininthedark.Theabsorbanceofthe solu-tionwasmeasuredat517nm.Theantioxidantactivitywasalso expressedinmgofascorbicacidequivalents/gofextract(mgAAE/g ofextract).ThescavengingratewascalculatedusingEq.(2):
Radicalscavenging rate (%)=
1−Asample Acontrol
×100 (2)
ABTSradicalcationdecolourizationassay
TheantioxidantactivityoftheextractofCPLleaveswasalso evaluated by ABTS radical cation decolourization assay, which were carried out as reported by ˇSliumpait ˙e et al. (2013) and witha slightly modified.Stock solution(7mM)of 2,2′-azinobis (3-ethyl-benzothiazoline-6-sulphonicacidammoniumsalt)(ABTS) waspreparedby dissolvingit in 2.45mMpotassium persulfate solutionandstoring themixturein thedarkat room tempera-turefor15–16hbeforeuse.TheABTS•+stocksolutionwasdiluted withphosphatebuffer(PBS,10mM,pH7.4)beforemeasurements toobtaintheabsorptionof0.800±0.030at734nm.Theextractwas dissolvedinPBSanddilutedtoaseriesofconcentrations(0.05,0.10, 0.20,0.23,0.26,0.30mg/ml).A4mlofABTS•+solutionwasmixed with40loftheextractsolutioninthedark,theabsorptionwas readafter10min.Allmeasurementswereperformedintriplicate. AnditsabilitytoscavengeABTS•+iscomparedwithascorbicacid.
Determinationoftotalphenolicsandflavonoidscontents
Thetotalphenoliccontentsoftheextractsweredeterminedby Folin–CiocalteumethodbasedontheproceduresdescribedbySiger etal.(2008).Gallicacidwasusedtocomputethestandard calibra-tioncurve(2,4,6,8,10g/ml).Alloftheresultswereexpressedas mgofgallicacidequivalentspergramextract(mgGAE/gofextract). Theanalyseswerecarriedoutintriplicateandtheaveragevalue wascalculatedineachcase.
TotalflavonoidswerecarriedoutasreportedbyIbrahimetal. (2015).Rutinwasusedasstandardandtheequivalents(w/w)were determinedfromastandardconcentrationcurve(0.05,0.10,0.20, 0.30,0.40,0.50mg/ml).Determinationswerecarriedoutin trip-licates;resultswererepresentedasthemean values±standard deviationsandexpressedasmgrutinequivalentspergramextracts (mgRE/gofextract).
UPLC-Q-TOF/MSanalysis
Theleafpowderwasdissolvedinaqueousmethanol(50%(v/v)) inanultrasonic bath(Shumei KQ-300DAultrasonic instrument, Kunshan,China)for30minandthemixturewascentrifugedthrice with9055.8×g for 10min.The clearsupernatant wasused for UPLC-Q-TOF/MSanalysis.
AcquityUPLCsystem(WatersCo.,Massachusetts,USA)coupled withMicro-massQ-TOFmicro(WatersCo.,Massachusetts,USA). UPLCwasusedasaseparationmeansequippedwithabinary sol-ventdeliverysystem,anauto-sampler,andanAcquityUPLCTMBEH C18column(4.6×150mm,5m,WatersCo.,USA).Agradient elu-tionwasachievedusingtwomobilephases:0.1%(v/v)aqueous aceticacid(solventA)andmethanol(B)ataflowrateof0.21ml/min withthefollowinggradientconditions:0min,22%B;3.6min,29% B;4.0min,33%B;12.6min,35%B;15.6min,70%B;17.6min,70%
CEE REE
80
70
60
50
40
30
20
10
0
Chlorogenic acid Vitexin
Isovitexin hyperin Rutin Quercetin
Content(mg/g of e
xtr
act)
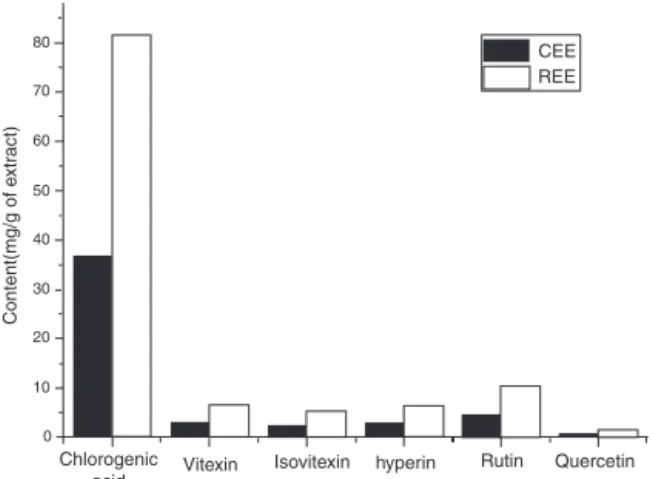
Fig.1.ThedifferencesinthecontentsofthesixcomponentsinCEEandREEwere determinedbyHPLC-UV.
B;22min,22%B.Thesampleinjectionvolumewas5landthe columntemperaturewasmaintainedat30◦C.
MassspectrometricanalyseswereperformedonMicro-mass Q-TOFMicroSystemequippedwithanelectrosprayionization(ESI) sourceinnegativeion mode.Theoptimized parametersforthe mass spectrometric analysiswere as follows: capillary voltage, 3000V;sampleconevoltage,30V;extractionconevoltage,2.0V; desolvationgasflowrate,600l/h;desolvationgastemp,300◦C; collisionenergies,30–35eV.Allmassdataacquisitionwere per-formedonMassLynxsoftware(Version4.1,Waters,Cicero,USA) usinganindependentreferencesprayviaaLockSprayTMinterface toensureaccuracy,lockingthe[M−H]− ofleucineenkephalinat m/z554.2615andtheQ-TOFacquisitionratewassetas0.1sand theinter-scandelayas0.02(Wuetal.,2015).
Quantification
QuantitativeanalyseswereperformedonanAgilent1260 Infin-ity series equipped with a UV detector. The chromatographic separationwascarriedoutonanAgilentZORBAXSB-C18column (250mm×4.6mm,5m,AgilentCo.,USA)withthemobilephase consistingof0.5%(v/v)aqueousphosphoricacidandmethanol(B). Thegradientelutionprogramwasasfollows:0min,23%B;8min, 32%B;30min,35%B;50min,70%B;55min,23%B.Theflowrate wasat1ml/min,theUVdetectionwavelengthwassetat360nm, theinjectionvolumewas20landthecolumntemperaturewas maintainedat30◦C.
CalibrationcurveswereobtainedbyHPLCinjectionof chloro-genicacid, vitexin,isovitexin,hyperin,rutin,quercetinstandard solutionsin50%(v/v)aqueousmethanolwiththeconcentration of571.87,25.70,34.96,25.06,55.14and9.91g/ml,respectively. Thedatarelevantforobtainingthecalibrationcurvesareshown inTable1.Quantificationofindividualcompounds(Table2,Fig.1) wasobtainedusingthecalibrationdataofthecommercialstandard. Compoundsconcentrationswerecalculatedintriplicateandthe meanvaluecalculatedineachcase.
Statisticalanalysis
Table1
Regressionequation,correlationcoefficients,linearityranges,LODandLOQforsixanalytes.
Compounds Linearityranges(mg/l) Calibrationcurvea (r2) LODb(mg/l) LOQc(mg/l)
Chlorogenicacid 57–570 y=0.2402x+0.3208 0.9998 0.13 0.48 Vitexin 2.5–25 y=0.3108x− 0.1698 0.9996 0.52 1.89 Isovitexin 3.5–35 y=0.6288x− 0.3924 0.9994 0.44 1.12 Hyperin 2.5–25 y=0.642x − 0.3703 0.9995 0.41 1.02 Rutin 5.5–55 y=0.5521x−0.3689 0.9995 0.97 2.53 Quercetin 1–10 y=0.9747x−0.4955 0.9978 0.21 0.65
ay=peakarea,x=concentrationinmg/lofextract. bLOD,limitofdetection.
c LOQ,limitofquantification.
Table2
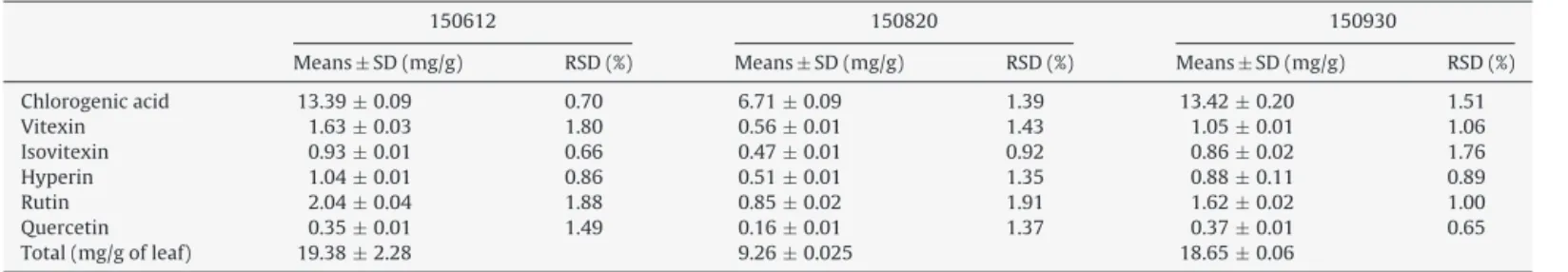
HPLCquantificationofsixcomponentsidentifiedinCPLleavesextracts.
150612 150820 150930
Means±SD(mg/g) RSD(%) Means±SD(mg/g) RSD(%) Means±SD(mg/g) RSD(%)
Chlorogenicacid 13.39±0.09 0.70 6.71±0.09 1.39 13.42±0.20 1.51
Vitexin 1.63±0.03 1.80 0.56±0.01 1.43 1.05±0.01 1.06
Isovitexin 0.93±0.01 0.66 0.47±0.01 0.92 0.86±0.02 1.76
Hyperin 1.04±0.01 0.86 0.51±0.01 1.35 0.88±0.11 0.89
Rutin 2.04±0.04 1.88 0.85±0.02 1.91 1.62±0.02 1.00
Quercetin 0.35±0.01 1.49 0.16±0.01 1.37 0.37±0.01 0.65
Total(mg/gofleaf) 19.38±2.28 9.26±0.025 18.65±0.06
Allvaluesareexpressedasmean±standarddeviation(n=3).
Resultsanddiscussion
Totalphenolsandflavonoidscontents
TotalphenolsandflavonoidscontentsofCEEandREEofC.pimela
leavesweredeterminedinthisstudy(Table3).Thevalueofthe totalphenoliccontentofCEEandREEwere259.1±55(mgGAE/g ofextract)and 415.6±2.37(mgGAE/g ofextract),respectively. AndthatofthetotalflavonoidsofCEEandREEwere373.9±6.56 (mgRE/gofextract)and824.1±5.86(mgRE/gofextract), respec-tively.Theresultrevealedthatthetotalphenolic(approximately 1.6-fold)andflavonoids(approximately2.2-fold)contentofREE wereincreasedcomparedwithCEE,andthedifferencesbetween CEEandREEweresignificant(p<0.01orp<0.001).Thatwaslikely duetotheproteins,carbohydratesorothersmallpolarhydrophilic moleculesremoval.
EffectofCEEandREEonvasculartensioninrats
Having a relaxingeffect onblood vessels is themost direct effecttoantihypertension. Studieshave reportedthat theeffect ofrelaxingtheaorticringshowedanon-endothelium-dependent vasodilation by Dong et al. (2008). So the relaxation effect of extracts on endothelium-intact aortic rings pre-contracted with 0.1mM NE was evaluated at the addition of cumulative concentrations (0.3, 1.0, 3.0, 10.0, 30.0 and 100mg/ml) in the presentstudy.
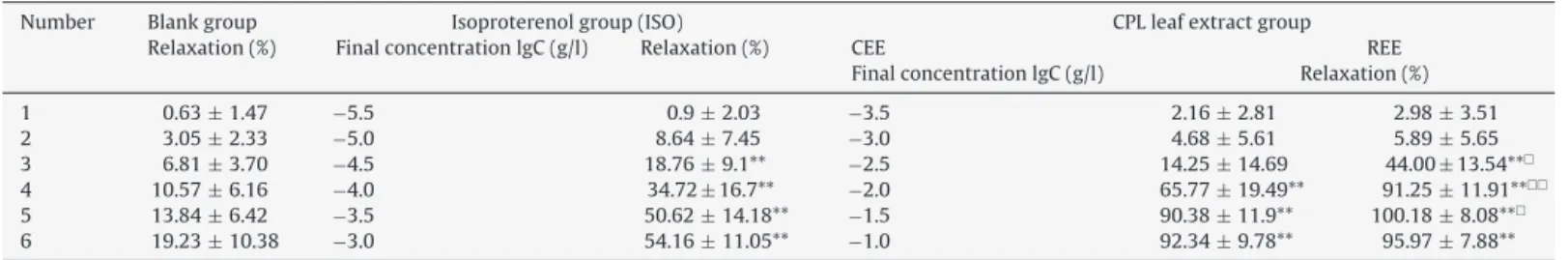
The result revealed that CEE (Emax=92.34±9.78%) and REE (Emax=100.18±8.08%)ofCPLleavesextractsboth havea relax-ingeffectonthoracicaortaringspre-contractedwithNEcompared to the normal control group (saline group), and showing a concentration-dependentrelaxeffects(Fig.2).Isoproterenol(ISO), areceptoragonist,whichcanexpansionofperipheryblood ves-selasapositivecontrolgroup.ThevasorelaxanteffectofCEEgroup (10,30and100mg/ml),REEgroup(3,10,30and100mg/ml)and ISOgroup(3,10,30and100mg/ml)showsalargesignificance com-paredtothenormalcontrolgroup(p<0.05orp<0.01)(Table4).In addition,bothCEEgroupandREEgrouphaveasimilareffecttoISO group(EC50=0.73±0.00064mg/l)(Fig.2).Atthesame concentra-tion,thevasorelaxanteffectofREEgroup(EC50=5.2±0.002mg/l)
150
100
50
0
-50
-3 -2
IgCa(g/L)
CEE REE Control
Relaxtion(%)
-1 0
Fig.2. EffectsofCEEandREEofCPLleafonvasorelaxantactivityinrataorticrings. Allvaluesareexpressedasmean±standarddeviation(n=10).aIgC,cumulative
concentration.
wasstrongerthanCEEgroup(EC50=10±0.007mg/l).Basedonthe resultsofthetotalphenolicandflavonoidscontentoftheextracts, itisspeculatedthattheactivecompoundsofvasodilationmaybe relatedtoitsphenolicandflavonoidscomponents.
Antioxidantactivity
Table3
Extractionyields,totalphenoliccontentandflavonoidscontentofCEEandREEofCPLleafextracts.
Exstracts Extractionyield(%) Totalphenoliccontent(mgGAEa/gofextract) Flavonoidscontent(mgREd/gofextract)
CEEb 20.95 259.1±5.5 373.9±6.56
REEc 13.4 415.6± 2.37** 824.1± 5.86
ComparewiththetotalphenoliccontentofCEE,**p<0.01. ComparewiththeflavonoidscontentofCEE,p<0.001.
Valuesoftotalphenoliccontentareexpressedasmean±standarddeviation(n=3).
aGAE,gallicacidequivalents. b CEE,crudeethanolextract. c REE,refinedethanolextract. d RE,rutinequivalents.
Table4
VasorelaxationeffectsofCEEandREEonrataorticrings.
Number Blankgroup Isoproterenolgroup(ISO) CPLleafextractgroup
Relaxation(%) FinalconcentrationlgC(g/l) Relaxation(%) CEE REE
FinalconcentrationlgC(g/l) Relaxation(%)
1 0.63±1.47 −5.5 0.9±2.03 −3.5 2.16±2.81 2.98±3.51
2 3.05±2.33 −5.0 8.64±7.45 −3.0 4.68±5.61 5.89±5.65
3 6.81±3.70 −4.5 18.76±9.1** −2.5 14.25±14.69 44.00±13.54**
4 10.57±6.16 −4.0 34.72±16.7** −2.0 65.77±19.49** 91.25±11.91**
5 13.84±6.42 −3.5 50.62±14.18** −1.5 90.38±11.9** 100.18±8.08**
6 19.23±10.38 −3.0 54.16±11.05** −1.0 92.34±9.78** 95.97±7.88**
Comparewithblankgroups,**p<0.01;comparedwithCFE,p<0.05,p<0.01. Allvaluesareexpressedasmean±standarddeviation(n=10).
Table5
Antioxidantactivity.
IC50ofDPPH •
(g/ml)
IC50ofABTS •+
(g/ml)
mgAAEa/gof
extracts Ascorbicacid 4.23± 0.23 1.78± 0.01 – BHT 20.87± 0.40 – – CEE 15.42± 0.14 3.24± 0.18 274.39± 2.54 REE 5.72±0.31*** 1.88±0.07 740.52±40.65
ComparewithCEE,***p<0.001,p<0.001.
Allvaluesareexpressedasmean± standarddeviation(n=3).
aAAE,ascorbicacidequivalents.
observed that the ascorbic acid (IC50=4.23±0.23g/ml) was onlyslightly more effective DPPH• scavenger compared toREE (IC50=5.72±0.31g/mlofextracts),andREEwasmoreeffective DPPH• scavenger compared to CEE(IC50=15.42±0.14g/ml of extracts)andBHT(IC50=20.87±0.40g/ml),inaddition,the dif-ferencesweresignificant(p<0.001).
ABTS•+ decolorization assay gave quite similar results com-paredtothoseobtainedbytheDPPH• assay(Table5).Statistical analysisprovedthat thereweresignificantdifferencesbetween CEE(IC50=3.24±0.18g/ml)andREE(IC50=1.88±0.07g/ml)in ABTSradicalcationdecolourizationassay(p<0.001),andthe ascor-bicacid(IC50=1.78±0.01g/ml)wasonlyslightlymoreeffective scavengercomparedtoREE.
Theantioxidantactivityresultsrevealed thatleavesextracts haveanantioxidantactivityconsiderablyhigherthanthatreported for BHTand lower of thatmeasured for ascorbicacid, and the antioxidantactivityofREEisbetterthanCEE.Sotheextractsmaybe couldprotectthebloodvesselsfromthedetrimentaleffects pro-ducedbyoxidativestressandplayaroleinrelaxingbloodvessels andloweringbloodpressure(Hugeletal.,2016).
AnalysisphenoliccompoundsintheleafextractofCPL
Theidentificationofthecomponentswascarriedoutby HPLC-UV,UPLC-MS/MS.Atotal16compoundshavebeenidentifiedand tentativeidentifiedbycomparingtheirspectraldatawithreference compounds,whenavailable,orcorroboratedwiththeliterature.
100
%
0
2.00 4.00 6.00 8.00 10.00 12.00 14.00 16.00
Relativ
e ab
undance
1
3 4 5 6
7 8
9 10
11 13 14 15
16 17
12 2
Fig.3.BasepeakchromatogramsdatadetectedinCanariumpimelaL.leaves.
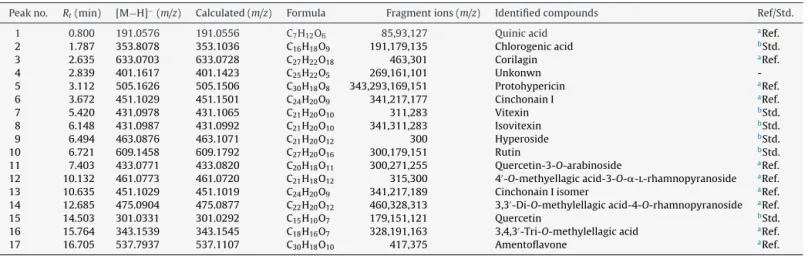
Thebase-peakchromatographyof themethanolextractfromC. pimelaleaveswasdisplayedinFig.3.UPLC-MSdataforthe iden-tifiedcompounds,namelytheirretentiontime,themolecularion [M−H]−andthemainproductionsobtainedbyUPLC-MS/MSwere providedinTable6.Identifiedphenoliccompoundsbelongedto variousclassesincludingnineflavonoids,fourtannins,two pheno-licacidsandonedianthrone.
Twophenolicacidscompounds(1and2)wereidentified accord-ingtothefragmentation.Peak1wasidentifiedasthequinicacid inthenegative mode,revealing thediagnosticprecursorion of m/z191at 0.800min andproduct ionspectra withm/z 85 and 93,consistingofdatafromtheliterature.Peak2wasassignedto chlorogenicacid(3-O-caffeoylquinicacid)duetothepresenceof characteristicproductionsatm/z191[quinicacid-H]−,179[caffeic acid-H]−and161[caffeicacid-H
2O-H]−.Itwasfurtherconfirmed byreferenceaswell.
Table6
PhytochemicalconstituentsidentifiedinCanariumpimelaleavesextracts.
Peakno. Rt(min) [M−H]−
(m/z) Calculated(m/z) Formula Fragmentions(m/z) Identifiedcompounds Ref/Std.
1 0.800 191.0576 191.0556 C7H12O6 85,93,127 Quinicacid aRef.
2 1.787 353.8078 353.1036 C16H18O9 191,179,135 Chlorogenicacid bStd.
3 2.635 633.0703 633.0728 C27H22O18 463,301 Corilagin aRef.
4 2.839 401.1617 401.1423 C25H22O5 269,161,101 Unkonwn
-5 3.112 505.1626 505.1506 C30H18O8 343,293,169,151 Protohypericin aRef.
6 3.672 451.1029 451.1501 C24H20O9 341,217,177 CinchonainI aRef.
7 5.420 431.0978 431.1065 C21H20O10 311,283 Vitexin bStd.
8 6.148 431.0987 431.0992 C21H20O10 341,311,283 Isovitexin bStd.
9 6.494 463.0876 463.1071 C21H20O12 300 Hyperoside bStd.
10 6.721 609.1458 609.1792 C27H20O16 300,179,151 Rutin bStd.
11 7.403 433.0771 433.0820 C20H18O11 300,271,255 Quercetin-3-O-arabinoside aRef.
12 10.132 461.0773 461.0720 C21H18O12 315,300 4′-O-methyellagicacid-3-O-␣-l-rhamnopyranoside aRef.
13 10.635 451.1029 451.1019 C24H20O9 341,217,189 CinchonainIisomer aRef.
14 12.685 475.0904 475.0877 C22H20O12 460,328,313 3,3′-Di-O-methylellagicacid-4-O-rhamnopyranoside aRef.
15 14.503 301.0331 301.0292 C15H10O7 179,151,121 Quercetin bStd.
16 15.764 343.1539 343.1545 C18H16O7 328,191,163 3,4,3′-Tri-O-methylellagicacid aRef.
17 16.705 537.7937 537.1107 C30H18O10 417,375 Amentoflavone aRef. aRef.:Identifiedbycomparisonwithreferences.
bStd.:Identifiedbycomparisonwithastandard.
hexoside, ellagicacidand gallic acidrespectively (Zhang et al., 2016).Peak3exhibitedtheprecursorion[M−H]−atm/z633and ionfragmentswere463[M-galloyl-H2O-H]−and301[ellagic acid-H]−,coincidentwithagallicgroupandhexosylresidue,indicating thatthiswasprobablyanHHDP-hexosyl-gallate,thuspeak3was identifiedascorilagin(Daetal.,2010).Themolecularionofpeak 12was[M−H]−,m/z461.Ityieldedthemajorfragmentatm/z315 withalossofarhamnoseresidue(146u),andafurtherlossofa methylradical(15u)presentanothercharacterizedfragmentat m/z300,whichwasproposedas4′-O-methylellagicacid-3-O-␣-l -rhamnopyranosewiththereference(Lietal.,2013).Peak14shared thesimilarfragmentationregularitieswithpeak12,whichisthe lossofrhamnoseresidueandmethyl,withtheexceptionthatpeak 14losttwomethylradicals(15u)duringtheprocess.Thus,peak 14wastentativelyidentifiedas3,3′-di-O-methylellagic acid-4-O-rhamnopyranose(Siratetal.,2010).Peak16wasassignedtobe methylellagicacidduetoitssamefragmentpatternwithreference identifiedas3,4,3′-tri-O-methylellagicacidwiththemolecularion atm/z343anddiagnosticfragmentsatm/z328,191and163(Gao etal.,2014).
Furthermore,nineflavonoids(6,7,8,9,10,11,13,15and17) wereidentifiedintheCPLleavesextract.Peak6and13were ten-tativelyproposedascinchonainIandcinchonainIisomer(Zhang etal.,2016)basedontheirsamemolecularion[M−H]−atm/z451 (C24H20O9)andsimilarmajorfragmentionsat341[M-C6H6O2-H]− and217[M-2C6H6O2-CH2-H]−,whichrevealedthepresenceoftwo dehydrobenzeneringsandtwomethylene.Peak7and8exhibited thesameprecursorion[M−H]−atm/z431inthenegativemode ofESI/MSspectra.Twomainfragmentionspresentedatm/z341 ([M−H−90]−),and311([M
−H−120]−),whichconfirmedthatthese compoundsaremono-C-glycosideflavonoids.Ithasbeen demon-stratedthataslightdifferencetodistinguishthepositionofthe sugarresidueistoobservethepeakofthe[M−H−18]−fragment ion.Whenthisispresent,itmeansthesugarsubstituentlieson the6positionasincompound8(isovitexin).Onthecontrary,its absencerevealsthatthesugarislocatedinposition8ascompound (7vitexin)(Gattusoetal.,2006).Thesefragmentationsare consis-tentwiththedatafromliteratureandonlinedatabases(Daetal., 2010).Bothtwocompoundswerefurtherconfirmedbythe authen-ticstandardsaswell.Peak9,peak10,peak11,peak15andpeak 17presentedthesamefragmentionatm/z301withtheformula C15H9O7,whichiscorrespondingtotheaglyconflavonolquercetin. Peak9, peak 10 andpeak 15 werecharacterized ashyper side (quercetin-3--d-galactoside),rutin(quercetin-3--d-rutinoside)
andquercetin,andconfirmedbytheauthenticstandardsand
lit-1
2
2
0 10 20 30 40 50 60 70
3 4 6
6
t / min
A
B C
5
5 4 3 1
Fig.4.HPLCchromatogramofmixedreferencessolution(A),samplesolution(B), andblanksolution(C)at360nm.(1)Chlorogenicacid,(2)vixetin,(3)isovitexin,(4) hyperin,(5)rutin,(6)quercetin.
erature(Hsuetal.,2017;Zhouetal.,2014)accordingtotheMS andMS/MSdata.Peak11wasconsideredtobequercetin-3-O-␣-l
-arabinofuranoside,yieldtheprecursorion[M−H]−atm/z433and majorfragmentionatm/z300([M−H−133]−),atypicallossof arab-inoseresidue,whichisconsistentwithfragmentionsreportedin theliterature(Lietal.,2009)andMassbankdatabase.Peak17with themolecularion[M−H]−atm/z537andmajorMS2fragmentsat m/z417and375,wasassignedtoamentoflavoneduetoitssame fragmentpatternwithreference(Zhouetal.,2014).
Peak5presentamolecularion[M−H]−atm/z505(C
30H18O8), andMS2fragmentsatm/z343,293,169and151weretentatively identifiedasprotohypericinaccordingtoreference(Holscheretal., 2009).
acid(69.1%,72.5%,and72.0%)possessedthehighestshareamong thesixcomponentsfromtheextractinthreebatches.Andthetotal amountsofsixcompoundsofCEEandREE(Fig.1)identifiedby HPLCfollowsatrendsimilartothatoftotalphenoliccontentand flavonoidscontent,whichwasfurthervalidatedthatpolyphenols maybeactiveingredients.
Inthecurrentstudy,16compoundsoftheextractwere char-acterized by using UPLC/Q-TOF-MS technique, and all the 16 compoundsarereferencedforthefirsttimeasconstituentsofCPL leaves.UPLC/Q-TOF-MSanalysisrevealedthataqueousmethanol extractcontainsacomplexmixtureofphenoliccompounds includ-ingphenolic acids and flavonoids, of which chlorogenicacid is themajorcompoundsin C.pimelaleaves. Additionally, mostof thesewerereportedtohavevasorelaxantandantioxidanteffects.It wasreportedthatchlorogenicacidcanreduceoxidativestressand improvesNObioavailability,leadingtotheattenuationof endothe-lialdysfunctionandhypertension(Suzukietal.,2006).Quercetin was reported to possess anti- hypertensive action (Marunaka etal.,2017).Isovitexinandvitexinwerereportedtohavea relax-ationeffectonagonist-inducedvascularcontractionregardlessof endothelialfunction(Je etal.,2014).Rutincan improvekidney andheartstructureandfunction(bloodpressure,leftventricular stiffness,vascular responses,echocardiog., histol.)(Diwan etal., 2017).However,furtherseparationandpurificationofbioactive compoundsintheleafextracts,aswellastheelaborationoftheir molecularmechanismsarewarrantedinthenextstudy.
Conclusion
Inthiswork,wehaveevaluatedthevasorelaxantand antiox-idant activity of the ethanolic of CPL leaves, determined the phenolic as active ingredients, and analyzed the compounds by UPLC-MS. Sixteen compounds were identified or tenta-tive identified including two phenolic acids, quinic acid and chlorogenic acid, four tannins, corilagin, 4′-O-methyl ellagic acid-3-O-␣-l-rhamnopyranose,3,3′-di-O-methylellagic
acid-4-O-rhamnopyranose and 3,4,3′-tri-O-methyl ellagic acid, and nine flavonoids, cinchonain I, cinchonain I isomer, vitexin, isovi-texin, hyperin, rutin, quercetin-3-O-arabinoside, quercetin and amentoflavone,andananthrone,protohypericin.Allthe compo-nentsweredeterminedforthefirsttime asconstituentsof this species.Furthermore,sixofthesecompounds,including chloro-genicacid,vitexin,isovitexin,hyperin,rutin andquercetinwere simultaneouslyanalyzedbyHPLC-UVforthefirsttime.The cur-rentresultsindicatethatthephenolic-richextractofCPLleaves isapromisingnaturalpharmaceuticalforcombatinghypertension andoxidativedamages.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethattheyhave fol-lowed theprotocolsof theirworkcenter onthepublication of patientdata.
Right to privacy and informed consent. The authors have
obtainedthewritteninformedconsentofthepatientsorsubjects mentionedinthearticle.Thecorrespondingauthorisinpossession ofthisdocument.
Authors’contributions
YFDandYLLcollectedandidentifiedthesamples.JWandYY performedtheexperimentsinterpretedtheresults,andJWdrafted themanuscript.XAFconductedtheUPLC/Q-TOF-MSanalysisand designedthestudy.QCXandYZCsupervisedthelaboratorywork. ZFLandJFBinterpretedtheresultsandcontributedtocritical read-ingofthemanuscript.Alltheauthorshavereadthefinalmanuscript andapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Theauthorsgratefullyacknowledgethetechnicalhelpprovided by Professor Rui in Central Laboratory, Guangdong Pharma-ceutical University. This research was financiallysupported by theGuangdongScienceand TechnologyDepartment (GrantNo. 2013B020311020) and Guangdong R&D Team for Formulation Innovation(GrantNo.2015KCXTD026).
References
Cen,B.H.,Jiang,W.N.,Liu,P.F.,Lin,J.C.,Liang,Y.L.,Dong,Y.F.,2012.Experimental studyontheeffectoftheliquidextractsofleavesofCanariumpimelaKoening onthecardiachemodynamic.J.Med.TheoryPract.25,747–749.
Cen,B.H.,Jiang,W.N.,Liu,P.F.,Lin,J.C.,Dong,Y.F.,Liang,Y.L.,2013.Experimental studyontheeffectoftheliquidextractsofCanariumpimelaLeenhontherats’
exvivocardiacperfusion.ChinaPract.Med.8,4–5.
Cen,B.H.,Jiang,W.N.,Liu,P.F.,Lin,J.C.,Liang,Y.L.,Dong,Y.F.,Lv,Z.F.,2014.Theimpact ofthediverseextractsofCanariumpimelaLeenhleavesonisolatedheartofthe rat.Mod.Med.J.China01,1–4.
Ding,B.P.,1999.PharmacologyofQingguopillsonrelievingcough.ChinaTrad. PatentMed.21,27–28.
Dong,Y.F.,Liang,Y.L.,Luo,J.P.,Luo,Y.,2006.Experimentontheeffectofdifferent partsofCanariumpimelaKoeningonnormalrats’bloodpressureandheartrates. Int.Med.HealthGuidanceNews17,8–9.
Dong,Y.F.,Liang,Y.L.,Wang,J.H.,Luo,Y.,2007.Depressurizationeffectofextractsof leaveofCanariumpimelaKoeninginstress-inducedhypertensiverats.Tianjin Med.J.35,587–589.
Dong,Y.F.,Liang,Y.L.,Luo,Y.,Li,Y.L.,2008.TheeffectofCanariumpimela Koen-ingleavesonratthoracicaortasmoothmusclecells.GuangdongMed.J.29, 1780–1783.
Da,S.C.,Trevisan,M.T.,Rios,J.B.,Erben,G.,Haubner,R.,Pfundstein,B.,Owen,R.W., 2010.Secondaryplantsubstancesinvariousextractsoftheleaves,fruits,stem, andbarkofCaraipadensifoliaMart.FoodChem.Toxicol.48,1597–1606.
Diwan,V.,Brown,L.,Gobe,G.C.,2017.Theflavonoidrutinimproveskidneyand heartstructureandfunctioninanadenine-inducedratmodelofchronickidney disease.J.Funct.Foods33,85–93.
Elks,C.M.,Mariappan,N.,Haque,M.,Guggilam,A.,Majid,D.S.,Francis,J.,2009.
ChronicNF-{kappa}Bblockadereducescytosolicandmitochondrialoxidative stressandattenuatesrenalinjuryandhypertensioninSHR.Am.J.Physiol.Renal Physiol.296,F298–F305.
Elks,C.M.,Reed,S.D.,Mariappan,N.,Shukitt-Hale,B.,Joseph,J.A.,Ingram,D.K., Fran-cis,J.,2011.Ablueberry-enricheddietattenuatesnephropathyinaratmodelof hypertensionviareductioninoxidativestress.PLoSONE6,e24028.
Gao,X.,Wu,J.,Zou,W.,Dai,Y.,2014.Twoellagicacidsisolatedfromrootsof San-guisorbaofficinalisL.promotehematopoieticprogenitorcellproliferationand megakaryocytedifferentiation.Molecules19,5448–5458.
Gattuso,G.,Caristi,C.,Gargiulli,C.,Bellocco,E., Toscano,G.,Leuzzi, U.,2006.
Flavonoidglycosidesinbergamotjuice(CitrusbergamiaRisso).J.Agric.Food Chem.54,3929–3935.
Ho,J.W.,Jie,M.,2007.Pharmacologicalactivityofcardiovascularagentsfromherbal medicine.Cardiov.Hematolog.AgentsMed.Chem.5,273–277.
Holscher,D.,Shroff,R.,Knop,K.,Gottschaldt,M.,Crecelius,A.,Schneider,B.,Heckel, D.G.,Schubert,U.S.,Svatos,A.,2009.Matrix-freeUV-laserdesorption/ionization (LDI)massspectrometricimagingatthesingle-celllevel:distributionof sec-ondarymetabolitesofArabidopsisthalianaandHypericumspecies.PlantJ.60, 907–918.
Huang,H.Y.,Wang,Z.M.,2009.Theproductivesituationandcountermeasuresof
CanariumpimelaLeethinPuningcity.ChinaFruitNews26,30.
Hsu,B.Y.,Lin,S.W.,Inbaraj,B.S.,Chen,B.H.,2017.Simultaneousdeterminationof phenolicacidsandflavonoidsinChenopodiumformosanumKoidz.(djulis)by HPLC-DAD-ESI-MS/MS.J.Pharm.Biomed.Anal.132,109–116.
Ibrahim,R.M.,El-Halawany,A.M.,Saleh,D.O.,ElNaggar,E.M.B.,El-Shabrawy,A.E.O., El-Hawary,S.S.,2015.HPLC-DAD-MS/MSprofilingofphenolicsfromSecurigera securidacaflowersanditsanti-hyperglycemicandanti-hyperlipidemic activi-ties.Rev.Bras.Farmacogn.25,134–141.
JiangsuNewMedicalCollege,1986.TheDictionaryofChineseMedicinalPlant, Shanghai.ShanghaiSci.Technol.Press,pp.467–472.
Jin,S.N.,Wen,J.F.,Kim,H.Y.,Kang,D.G.,Lee,H.S.,Cho,K.W.,2010.Vascular relax-ationbyethanolextractofXanthocerassorbifoliaviaAkt-andSOCE-eNOS-cGMP pathways.J.Ethnopharmacol.132,240–245.
Je,H.G.,Hong,S.M.,Je,H.D.,Sohn,U.D.,Choi,Y.S.,Seo,S.Y.,Min,Y.S.,Chung,S.J.,Shin, Y.K.,Lee,T.J.,Park,E.S.,Jeong,J.H.,2014.Theinhibitoryeffectofvitexinonthe agonist-inducedregulationofvascularcontractility.Pharmazie69,224–228.
Li,J.,Dong,Y.F.,Liang,Y.L.,Tang,H.Q.,1997.Studyontheantihypertensiveeffectof aqueousextractofCanariumpimelaKoeningleaf.Inform.Trad.Chin.Med.6,39.
Liang,Y.L.,Dong,Y.F.,Luo,Y.,Luo,J.P.,2006.Experimentontheeffectoftheaqueous solublepartofCanariumpimelaKoeningoftheleavesextractsonbloodpressure andheartratesofrats.ActaAcad.Med.Jiangxi46,42–45.
Li,C.,Du,H.,Wang,L.,Shu,Q.,Zheng,Y.,Xu,Y.,Zhang,J.,Zhang,J.,Yang,R.,Ge, Y.,2009.Flavonoidcompositionandantioxidantactivityoftreepeony(Paeonia
sectionmoutan)yellowflowers.J.Agric.FoodChem.57,8496–8503.
Liang,Y.L.,Luo,Y.,Li,Y.L.,Dong,Y.F.,2011.EffectoffruitofCanariumpimelaKoening onvasculartensioninrats.Chin.J.Gerontol.31,3099–3100.
Li,L.,Zhao,Y.,Liu,W.,Feng,F.,Xie,N.,2013.HPLCwithquadrupoleTOF-MSand chemometricsanalysisforthecharacterizationofFoliumTurpiniaefrom differ-entregions.J.Sep.Sci.36,2552–2561.
Lindsley,C.W.,2015.2013Trendsandstatisticsforprescriptionmedicationsin theUnitedStates:CNShighestrankedandrecordnumberofprescriptions dis-pensed.ACSChem.Neurosci.6,356–357.
Li,Z.F.,Li,K.,Li,F.Y.,Chen,X.,Zhao,H.H.,Du,Y.Y.,Li,Q.P.,2015.GC–MSanalysis andantioxidantactivityofvolatileoilfromleavesofCanariumpimelaLeenh.in Rongxiancounty.J.SouthernAgric.46,317–321.
Mozaffarian,D.,Benjamin,E.J.,Go,A.S.,etal.,2016.Executivesummary:heart diseaseandstrokestatistics-2016update:areportfromtheAmericanHeart Association.Circulation133,447–454.
Marunaka,Y.,Marunaka,R.,Sun,H.,Yamamoto,T.,Kanamura,N.,Inui,T.,Taruno, A.,2017.Actionsofquercetin,apolyphenol,onbloodpressure.Molecules22,
http://dx.doi.org/10.3390/molecules22020209,209/1–209/12.
Newman,D.J.,Cragg,G.M.,2007.Naturalproductsassourcesofnewdrugsoverthe last25years.J.Nat.Prod.70,461–477.
Qin,X.,Hou,X.,Zhang,M.,Liang,T.,Zhi,J.,Han,L.,Li,Q.,2014.Relaxationofrataorta byfarrerolcorrelateswithpotencytoreduceintracellularcalciumofVSMCs.Int. J.Mol.Sci.15,6641–6656.
Suzuki,A.,Yamamoto,N.,Jokura,H.,Yamamoto,M.,Fujii,A.,Tokimitsu,I.,Saito, I.,2006.Chlorogenicacidattenuateshypertensionandimprovesendothelial functioninspontaneouslyhypertensiverats.J.Hypertens.24,1065–1073.
Siger,A.,Nogala-Kalucka,M.,Lampart-Szczapa,E.,2008.Thecontentand antioxi-dantactivityofphenoliccompoundsincold-pressedplantoils.J.FoodLipids15, 137–149.
Sirat,H.M.,Rezali,M.F.,Ujang,Z.,2010.Isolationandidentificationofradical scav-engingandtyrosinaseinhibitionofpolyphenolsfromTibouchinasemidecandra
L.J.Agric.FoodChem.58,10404–10409.
ˇSliumpait ˙e,I.,Venskutonis,P.R.,Murkovic,M.,Pukalskas,A.,2013.Antioxidant propertiesandpolyphenolicscompositionofcommonhedgehyssop(Gratiola officinalisL.).J.Funct.Foods5,1927–1937.
Wilcox,C.S.,2005.Oxidativestressandnitricoxidedeficiencyinthekidney:a criticallinktohypertension?Am.J.Physiol.Regul.Integr.Comp.Physiol.289, R913–R935.
Wu,X.,Li,Y.,Wang,Q.,Li,W.,Feng,Y.,2015.Effectsofberberineandpomegranate seedoilonplasmaphospholipidmetabolitesassociatedwithrisksoftype2 diabetesmellitusbyU-HPLC/Q-TOF-MS.J.Chromatogr.B1007,110–120.
Zhang,L.,Tu,Z.,Xie,X.,Lu,Y.,Wang,Z.,Wang,H.,Sha,X.,2016.Antihyperglycemic, antioxidantactivitiesoftwoAcerpalmatumcultivars,andidentificationof phenolicsprofilebyUPLC-QTOF-MS/MS:newnaturalsourcesoffunctional con-stituents.Ind.Crop.Prod.89,522–532.
Zhou,D.,Jin,Y.,Yao,F.,Duan,Z.,Wang,Q.,Liu,J.,2014.ValidatedLC–MS/MSmethod forthesimultaneousdeterminationofhyperosideand2′′-O-galloylhyperinin