w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Phytochemical
screening
of
the
dichloromethane–ethanolic
extract
of
Eriosema
campestre
var.
macrophylum
roots
and
its
antiproliferative
effect
on
human
peripheral
blood
lymphocytes
Michaelle
G.
Santos
a,
Valéria
G.
Almeida
a,
Bethânia
A.
Avelar-Freitas
b,
Cristiane
F.F.
Grael
c,
Luiz
E.
Gregório
d,
Wagner
F.
Pereira
a,
Gustavo
E.A.
Brito-Melo
a,∗aLaboratóriodeImunologia,DepartamentodeFarmácia,UniversidadeFederaldosValesJequitinhonhaeMucuri,Diamantina,MG,Brazil bInstitutodeCiênciaeTecnologia,UniversidadeFederaldosValesdoJequitinhonhaeMucuri,Diamantina,MG,Brazil
cLaboratóriodeFarmacognosia,UniversidadeFederaldosValesdoJequitinhonhaeMucuri,Diamantina,MG,Brazil dLaboratóriodeCiênciasAmbientais,QuímicaseFarmacêuticas,UniversidadeFederaldeSãoPaulo,Diadema,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received19February2015 Accepted13August2015 Availableonline9October2015
Keywords: Eriosemacampestre
Flavonoids Cellularproliferation IL-2
a
b
s
t
r
a
c
t
Eriosemacampestrevar.macrophylum(Grear)Fortunato,Fabaceae,isanativeplantoftheBrazilian
Cer-radoandthedecoctionofitsrootshasbeenusedbyfolkmedicineforthetherapyofinflammatory diseases.Inthisstudyweaimedtoinvestigatetheeffectofthedichloromethane–ethanolicextract
ofE.campestrerootsontheproliferativeresponseoflymphocytesandtoexaminetheprofileof
IL-2production.Theeffectofdichloromethane–ethanolicextractofE.campestreontheproliferationof phytohemagglutinin-stimulatedlymphocyteswasevaluatedbyusingflowcytometryandthecell super-natantswereassayedforIL-2concentrationsbyusinganenzyme-linkedimmunosorbentassay.The phytochemicalscreeningofE.campestrerootswasperformedtodeterminethemainsecondary metabo-litesthroughchromogenicandprecipitationreactionsandbyusingHPLC-PAD.Inadditiontothepresence ofsubclassesofflavonoids(flavonesandflavonols)indichloromethane–ethanolicextractofE.campestre, weobservedthattheextractinducedaconcentration-dependentdecreaseinIL-2levelsonthe super-natantofthecellculturesaswellasanantiproliferativeeffectonTlymphocytes,includingCD4+andCD8+ cells.Theanti-inflammatoryeffectsattributedtoE.campestrebyfolkmedicinemaypartlybeexplained byitsantiproliferativeactiononTlymphocytes.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Inflammationisacompleximmuneresponsethatinvolves mul-tiplefactors.Theinitialeventsresultintheactivationof innate immuneresponsesandinvolvementofenzymesystems(Nathan,
2002;Medzhitov,2008).Inlateevents,cooperationbetweenTand
Blymphocytescontributes toantigen-specificantibody produc-tion;Tlymphocytesinitiateacell-mediatedimmune-inflammation processinsituandmaintaintheactivationofphagocyticcellsby transformingthemintotissue-destructiveeffector cells(Barton, 2008).In chronicinflammatory diseases,thepermanent activa-tionandproliferationofTlymphocytesamplifytissuedamageand contributetotheclinicaloutcomeobservedinthesediseases,in additiontothecellularinteractionsoccurringintheaffectedsites (Caietal.,2012;Chimentietal.,2013).
∗ Correspondingauthor.
E-mail:gustavomelo@ufvjm.edu.br(G.E.A.Brito-Melo).
Sincechronicinflammatorydisordersarecharacterizedbythe highactivationandproliferationofTlymphocytes,most immuno-suppressivedrugsaimtoblockthecellcycleprogressionofthese cells(Macián,2005).Inadditiontothedrugsregisteredforfirst-line therapy,whichmayhavemanyandsometimesseveresideeffects
(De Mattos etal., 2000), there are numerousalternative herbal
treatmentswithpromising,butnot yetproven,efficacy(Reuter etal.,2010).
Eriosema campestre var. macrophylum (Grear) Fortunato,
Fabaceae,isaplantpopularlyknownas“pustemeira”andisnative totheBrazilianCerrado(Grear,1970;Fortunato,1999).Thisspecies consistsofsmallanderectherbsrangingfrom14to17.5cm/high, withyellowflowersandfusiformroots(RogalskiandMiotto,2011). ThedecoctionofE.campestrerootshasbeendescribedandusedby folkmedicineforthetherapyofinflammatorydiseasesincluding inflammatoryskindisorderssuchaspsoriasis.However,nostudy ofthechemicalcompositionofthisplantoranybiologicalanalysis hasyetprovidedevidenceregardingtheeffectivenessofthisplant asananti-inflammatory.
http://dx.doi.org/10.1016/j.bjp.2015.08.009
Wedecidedtoperformaphytochemicalscreeningto charac-terizethemainclasses ofsecondary metabolitespresent inthe plantextractaswellastoinvestigatetheprofileofitschemical composition.Moreover,inadditiontotheroleofTlymphocytesin thepathologicalprocessofchronicinflammatorydiseases,wealso investigatedtheeffectofthedichloromethane–ethanolicextract
ofE.campestreroots(DEEC)ontheproliferativeresponseof
lym-phocytesaswellastheprofileof IL-2production, thecytokine essential for the expansion of these cells during the adaptive immuneresponse.
Materialsandmethods
Plantmaterial
FreshEriosemacampestrevar.macrophylum(Grear)Fortunato, Fabaceae,plantswerecollectedinthecityofDatas,MinasGerais, Brazil (S 18◦27.318′, W 43◦39.764′, 1223m altitude). Botanical
identification was performed by Dr. Ana Paula Fortuna Perez, Curator ofHerbario BOTU, Department ofBotany, Universidade Estadual Paulista, Botucatu, SP, Brazil, and a specimen was depositedundervouchernumber881atJeanineFelfiliDendrologic HerbariumoftheFederalUniversityofJequitinhonhaandMucuri Valleys.
Preparationofplantextracts
TherootsofE.campestreweredriedtoaconstantweightatroom temperature.Thedriedmaterial(450g)wasgroundandmacerated inamixtureofdichloromethaneandethanol(1:1v/v)for72hat roomtemperature.Themaceratewasseparatedbyfiltrationand concentrated undervacuum ona rotatoryevaporator(Fisatom, model801,Brazil)at40◦Ctofurnishabrownresidue(12.1g).A
5.0mg/mlsolutionoftheresidue(DEEC)inDMSO(Sigma,USA) wasprepared.Smallstockaliquotswerekeptat−20◦Cuntilthe
timeofuse.NewdilutionsoftheextractinDMSOwereperformed toobtaintherequiredconcentrationinthecellculturemedium.
Chromatographicprofileusinghighperformanceliquid
chromatography(HPLC)
Reagents
HPLC-grademethanolwasobtainedfromJ.T.Baker(Ecatepec, Mexico),HPLC-grade acetonitrilewasobtainedfromTediaHigh PuritySolvents(Fairfield,USA),trifluoroaceticacidwaspurchased fromSynth(SãoPaulo,Brazil)andHPLC-qualityultrapurewater waspreparedbyusingaMilliporeMilli-QDirect-8System (Biller-ica,USA).
Chromatographicanalysis
SamplesofDEECdilutedto10mg/mlweredissolvedin HPLC-grademethanol,filteredthrougha0.2mmembraneandanalyzed ona ThermoAccelaHPLCsystem(ThermoFisherScientificInc., Waltham, USA) with a solvent delivery unit, on-line degasser, columnovenandautosampler,andequippedwithaphotodiode array detector (PAD). For data analysis, we used ChromQuest software (Version 5.0, Thermo Fisher Scientific Inc., Waltham, USA).ALunaC18(2)analyticalcolumn(150×4.6mm;particlesize 3m;Phenomenex,USA)wasused.Thesamplewaselutedbya gradientelutionemployingultrapurewater(SolventA)and ace-tonitrile(SolventB),bothcontaining0.1%v/vtrifluoroaceticacid: 0–5min,90%ofA,50min,100%B;after10minthecolumnwas re-equilibratedwith90%A.Thecolumntemperaturewasmaintained at40◦C.Analysiswasperformedataflowrateof1.0ml/minand
wasmonitoredat288nm.
Biologicalsamplesandpreparationofperipheralblood
mononuclearcells(PBMC)
Peripheralbloodwasobtainedfrom22healthyadultdonors. Volunteerswithanyinfectious,autoimmunediseasesormaking useofantibiotics,anti-inflammatorymedication,corticosteroids orotherimmunosuppressivedrugswerenotconsideredforblood donation. Informedwritten consentwasobtainedfromall par-ticipants. The study was approved by the Ethical Committee at the UFVJM, Diamantina, Minas Gerais, Brazil (register code 569.313/2014).
PBMCwasisolatedfromheparinizedhumanperipheralblood samples(15ml)byusingtheFicoll-Paque(specificgravity1.077) gradientdensitymethod,asdescribedpreviouslybyBicalhoetal.
(1981). Peripheral blood was diluted with phosphate-buffered
saline(PBS;pH7.2)andcentrifugedinaFicoll-Histopaque(Sigma, USA) discontinuous gradient at 400×g at room temperature for 30minfor obtaining thecharacteristic layer containing the mononuclear cells. PBMCwas collected, washed with PBS and centrifuged(240×gatroomtemperaturefor7min).Cellswere sus-pendedataconcentrationof1×107cells/mlinPBSorRPMI-1640
medium (Sigma, USA) supplemented with10%heat-inactivated fetalcalfserum(FCS;Gibco,InvitrogenCorporation,USA),2mM
l-glutamine(Sigma, USA)and anantibiotic/antimycoticcocktail
(100UI/ml penicillinG, 100g/ml streptomycinand 250ng/ml amphotericinB–Sigma,USA).ThePBMCfromeachresearch sub-ject wasobtained separately and used for cell culture analysis individually.
Cellviabilityanalysis
PBMC (5×105) were cultured with 0.5% dimethyl sulfoxide
(DMSO),assolventcontrol,or DEEC(100,50,25or12.5g/ml) at37◦Cinahumidifiedincubatorwitha5%CO2-airatmosphere
for24hor5days.UntreatedPBMCwasusedastheunstimulated cellculturecontrol(Ctrl).Intheendoftheincubation,cellswere washedwithPBS,centrifuged(240×g,roomtemperature,7min) andresuspendedwith0.5mlPBS.Then,10lofcellsuspension wasmixedwithanequalvolumeof0.4%trypanblue(Sigma,USA). Total,viableandnonviablecellnumberswerecountedunderthe microscopebyusingahemocytometerNeubauerchamber.
Lymphocyteproliferativeresponse
PBMC (1×107 cells) were resuspended in PBS and labeled
with 1M of BD HorizonTM Violet Proliferation Dye 450 (VPD 450,BDBiosciences,USA)for15minat37◦C(Lyons,1999).The
VPD450-stainedPBMC(5×105 cells/wellin24-wellplate)were
culturedinRPMI-1640containing10%FCS(Gibco,Invitrogen Cor-poration,USA),2mMl-glutamineandtheantibiotic–antimycotic
cocktail(Sigma,USA),withorwithoutphytohemagglutinin(PHA 5g/ml). Cells were also stimulated with PHA in combination with 0.5% DMSO (solvent control) or different concentrations of DEEC (25,12.5 or6.25g/ml). Cells stimulatedwithPHA in combinationwithdexamethasoneat8g/ml(Aché,Brazil)were used as the inhibition control (DEXA). The plate was kept in a humidified incubator with a 5% CO2 air atmosphere for five
days at 37◦C. After incubation, cells were harvested, washed
102
0 500
M1 1000
1500
0
500 B
C
D A
400
300
200
100
0 500
350 300 250 200 150 100 50 0 400
300
200
100
103 104 105
102 103 104 105
102 103 104 105
102 103
VPD450 (FI)
M5 M4
M3
M2 M1
M5
M6
M4
M3
M2 M1
M5 M4
M6
Count
M3
M2 M1
104 105
Fig.1.AnalysisofcellproliferationusingVioletProliferationDye(VPD)450inflow cytometry.A,VPD450-stainedcellsderivedfromunstimulatedcultures;B,VPD 450-stainedcellsinculturesstimulatedwithphytohemagglutinin(PHA),C,VPD 450-stainedcellsinculturesstimulatedwithPHAandtreatedwithDMSO(solvent control),D,VPD450-stainedcellsinculturesstimulatedwithPHAandtreatedwith DEEC25g/ml.
fluorescencehistograms(Fig.1)byusingthefollowing formula, asdescribedbyAnguloandFulcher(1998):PI=(100−Y)/Y,where
Y (%)=X0+X1/2+X2/4+X3/8+X4/16+X5/32; X0 represents the
percentageofT-cellsthatdidnotdivide(locatedinM1),andX1–X5 representsthepeaksofgradualdivision(locatedinM2–M6).
IL-2production
PBMC (5×105 cells/well on 24-well plates) were cultured
in RPMI-1640 containing 10% FCS (Gibco, Invitrogen Corpora-tion,USA),2mMl-glutamine,theantibiotic–antimycoticcocktail
(Sigma,USA) andPHA (5g/ml)with(i)PBS(control),(ii)0.5% DMSO(solventcontrol)or(iii)differentconcentrationsof DEEC (25,12.5or6.25g/ml).CellsstimulatedwithPHAin combina-tionwithdexamethasoneat8g/mlwereusedastheinhibition control(DEXA).Theplatewasmaintainedat37◦C ina
humidi-fiedincubatorwitha5%CO2airatmospherefor36h,followedby
centrifugationfor10min,at240×gandatroomtemperature.The cellsupernatantswerecollectedandassayedforIL-2 concentra-tionsbyusinganenzyme-linkedimmunosorbentassay(ELISA;R&D Systems,USA)accordingtothemanufacturer’sinstructions.
Inbrief,plates werecoatedwithmousecaptureantibodyto humanIL-2andincubatedovernightatroomtemperature.Wells werewashedfivetimeswithPBScontaining0.05%Tween20(PBST) and incubated with 1% bovine serum albumin in PBS contain-ing0.05%sodiumazide.Differentconcentrationsofrecombinant humanIL-2dilutedinPBS(standardcurve)orcellsupernatants wereaddedandincubatedatroomtemperaturefor2h.After wash-ingwithPBST,goatbiotinylatedantibodiestohumananti-IL-2were added,andtheplatewasincubatedatroomtemperaturefor2h priortoaddingstreptavidinhorseradishperoxidase.Afterdetection withtetramethylbenzidine(Sigma,USA),sulfuricacidwasadded toblocktheenzymaticreaction.Theabsorbanceat450nmwas determinedinamicrotiterplatereader(SpectraMax190, Molecu-larDevices,USA).ConcentrationsofIL-2insampleswereestimated bycomparisonwithastandardcurve.
Statisticalanalysis
GraphPadPrism,version5.0forWindows(GraphPadSoftware, USA)wasusedforthestatisticalanalysis,andp-values0.05were consideredtobestatisticallysignificant.Dataarereportedasthe mean±standarddeviation(SD).TheShapiro–Wilktestwasused toevaluatethenormalityofthedata.Analysisofvariance (one-wayANOVA)wasemployed,followedbyTukeyposthocpairwise comparisons.
Resultsanddiscussion
E.campestreisaplantnativetoBrazilanditstraditionaluseas
atherapyagainstchronicinflammatorydisordersiswidespreadin theBrazilianCerrado.However,therearenoreportsofany chem-icalorbiological-toxicologicalstudiesforthisplantthusfar.Thus, thecurrentstudyprovidedthefirstinvestigativecontributionto thechemicalcharacterizationandbiologicalanalysisofthisplant species.
The phytochemical analysis (Farnsworth, 1966; Bustamante etal.,2010)ofDEECshowedthepresenceofflavonoidsand ter-penes.
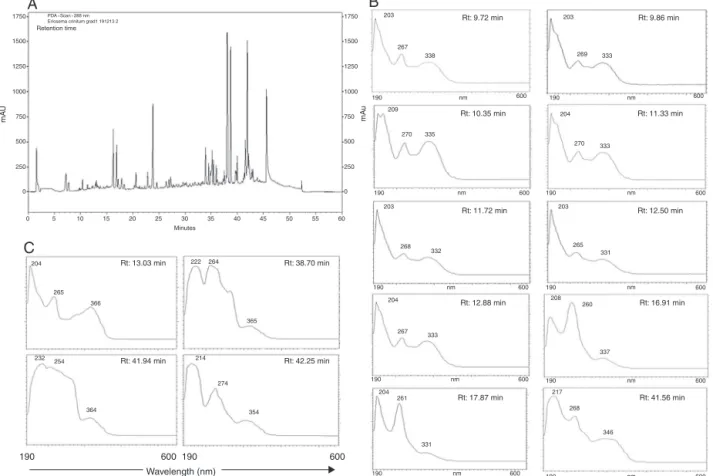
A complex chemical profile for DEEC was observed in the HPLC-PADanalysis,indicatingthepresenceofsubstancesofhigh, mediumandlowpolarity(Fig.2A).Highpolaritycompoundsare representedbypeakswithshorterretentiontimes,elutedbetween 1and20min,becausethesesubstanceshaveahigheraffinityforthe mobilephase.Somepeakswereelutedbetween20and35min,a factthatdemonstratesthepresenceofmediumpolaritymolecules. Thelateelutingpeaksobservedbetween35and47min,which rep-resentahigheraffinityforthenon-polarstationaryphase(C18), aredistinguishedbyahigherintensityandtheyseemtoconstitute majoritycompoundscomparedwiththeotherpeaks.This obser-vationindicatesthatthesubstancesoflowpolaritypredominatein theextract.
Flavonoidshavetwoabsorptionbandsintheultraviolet–visible region:bandIIwithamaximumabsorptionbetween240nmand 285nm,correspondingtothebenzoylgroup,andbandIwitha max-imumabsorptionregionfrom300to550nm,whichcorrespondsto thecinnamoylgroup.TheanalysisofbandsIandII(absorption max-imumandintensity)canassistinidentifyingthetypeofflavonoid
(MarstonandHostettmann,2006).TheHPLC-PADspectrashowed
1750
A
C
B
1500 Retention time
1250
1000
750
500
250
0
1750
1500
1250
1000
750
500
250
0
0 5 10 15 20 25 30
Minutes
204
232
364
Rt: 41.94 min
190 600190 600
Wavelength (nm)
Rt: 13.03 min Rt: 38.70 min
Rt: 42.25 min
254 214
274
354 365 222 264
Rt: 9.72 min Rt: 9.86 min
Rt: 12.50 min
Rt: 16.91 min
Rt: 41.56 min Rt: 10.35 min
Rt: 11.72 min
Rt: 12.88 min
Rt: 17.87 min
Rt: 11.33 min 209
270
270 335
190 600 600
204
203
265 331
260 208
337
268
346 217 190
nm nm
mA
u
mA
U
nm nm
190
203
268
204
267
204 261
331 333
600
190 nm
600
190 nm
600
190 nm
332
190 600
nm
190 600
nm
190 600
nm
190 600
600
203 203
267
269 333
333 338
265 366
35 40 45 50 55 60
Fig.2.ChemicalanalysisofDEEC.HPLC-PADchromatogramofDEEC,monitoredat288nm(A)andUV/VISspectra(190–600nm),indicatingthepresenceofflavones(B)and flavonols(C).
SeveralstudieswithotherspeciesoftheEriosemagenushave reportedthatflavonoidsareresponsibleforthebiologicalactivity attributedtotheseplants.Insomestudies,authorshavereported thepresenceofseveralnovelflavonoidssuchasKraussianones1–7, observedintherootsofE.kraussianum(Drewesetal.,2002,2004), ErioschalconesAandB,isolatedfromE.glomerata(Awouafacketal., 2008),RobusflavonesAandB,extractedfromtheaerialpartsofE.
robustum(Awouafacketal.,2013)andKhonklonginolsA–H,
iso-latedfromtherootsofE.chinense(Sutthivaiyakitetal.,2009).It ispossiblethatE.campestrecontainsnovelmolecules.Thisidea supportsthenecessityforfurtherchemicalstudiesofitsbioactive compounds.
Thesubstancespresentinplantextractsmightincludethose withcytotoxicactivities(Veiga-Júnioretal.,2005),andthe tox-icityof thesecompounds maydefine theuseof theplantas a phytomedicine. To investigate this issue, the first trials in our study aimedto evaluate the toxicity of DEEC in human PBMC by using the trypan blue exclusion test. After 24h of culture in the presence of different concentrations of the extract, we observed that DEEC at 12.5, 25 and 50g/ml didnot decrease cellviability(12.5g/ml=97.56±0.32%;25g/ml=95.09±1.71%; 50g/ml=93.88±1.46%) compared with the control cultures (96.75±1.49%)andsolventcontrol(DMSO=95.66±1.56%).Data showedcytotoxicityonlywhenPBMCwastreatedwithDEECat 100g/ml(56.60±7.24%)(Fig.3A).
After five days of cell culture, DEEC in concentrations lower than 25g/ml showed no cytotoxic effect on PBMC (12.5g/ml=93.56±3.03%; 25g/ml=83.75±6.67%) compared withtheuntreated(Ctrl=91.50±2.86%)andsolventcontrol cul-tures(DMSO=85.47±7.75%).However,asignificantreductionin cellviabilitywas observedin cultures treatedwithDEEC at 50
(65.47±10.37%)and100g/ml(54.70±2.90%)comparedwiththe DMSO-treatedanduntreatedcellcultures(Fig.3B).
TheseresultsallowedustochooseconcentrationsofDEEClower than25g/mlastheoptimumconcentrationsforuseincell pro-liferationassayssincethecellsarekeptincontactwithanextract forfivedays.
Ctrl 0 20 40 60 80 100
0 20 40 60 80 100
DMSO 12.5 25 50 100 Ctrl DMSO 12.5 25 50 100
a,b
a,b a
DEEC (μg/ml)
A
B
DEEC (μg/ml)
Cell viability
, %
Cell viability
, %
Fig.3.EffectofDEEConcellviabilityafter24h(A)andfivedays(B)ofculture.PercentagesofviablePBMCinPBS-treated(Ctrl),DMSO-treatedandDEEC-treatedcellswere assessedbyusingthetrypanblueexclusiontest(n=8).Theresultsareexpressedasthemean±SDofthepercentageofcellviabilityindifferentgroups.Significantdifferences (p0.05,ANOVAwithposthocTukeytest)areidentifiedbytheletters“a”and“b”forcomparisonsrelativetothecontrolandDMSOcellcultures,respectively.
The influence of DEEC on the proliferation of human lymphocytesbyusingcell-basedassayswascomparedwith phar-macologicaldosesofdexamethasone,whichisthebenchmarkof immunosuppressive pharmaceuticalsto defineits immunother-apeutic activity. DEEC exhibited a concentration-dependent
inhibitionofthecelldivisionoftheactivatedTlymphocytes, includ-ingCD4andCD8T-cells.Incomparisonwithdexamethasone,the inhibitionpromotedbyDEECat25g/mlonTlymphocytesand CD4T-cellspresentedefficacylevelsof71.65%and53.14%, respec-tively.In theCD8T-cell analysis,DEEC exhibited anefficacyof
4
1000 300
500 450
400 350 300 250 200 150 100 50 0
350 300 250 200 150 100 50 0 350
300 250 200 150 100 50 0 400 300 200 100 0
250 200 150 100 50
102 103 104 105 102 103 104 105
102 103 104 105
102 103 104 105
102 103 104 105
102 103 104 105 0
750 500 250 0
CD4+ cells
Proliferation index (PI)
CD8+ cells Lymphocytes
A
B
Untreated
PI=0.01
PI=3.55
PI=0.30 PI=0.49
PI=2.61
PI=1.11
PHA+DEEC 6.25μg/ml
PHA+DEEC 12.5μg/ml
Count
PHA+DEEC 25μg/ml PHA+DEXA (8μg/ml)
VPD450 (FI)
PHA
**
***
***
***
***
***
*
***
3
2
1
0 4
3
2
1
0 5
4
3
2
1
0
PHA (5 μg/ml) –
–
–
– +
–
–
– +
–
+
–
+
12.5
–
– +
25
–
– +
–
–
+ +
6.25
–
– DEEC (μg/ml)
DMSO (0.5%)
DEXA (8 μg/ml)
Fig.4.InfluenceofDEEContheproliferationofactivatedlymphocytes.VPD450-labeledhumanlymphocytes(5×105cells)wereculturedinthepresenceofmedium, dexamethasone(DEXA,8g/ml)ordifferentconcentrationsofDEEC(6.25,12.5and25g/ml)andactivatedwithPHA(5g/ml)forfivedays.Thelymphocyteproliferation
PHA (5 μg/ml)
**
*** ***
–
–
–
– +
–
–
– +
–
+
–
+
12.5
–
– +
25
–
– +
–
–
+ +
6.25
–
–
IL-2 (pg/ml)
80
60
40
20
0
DEEC (μg/ml)
DMSO (0.5%)
DEXA (8 μg/ml)
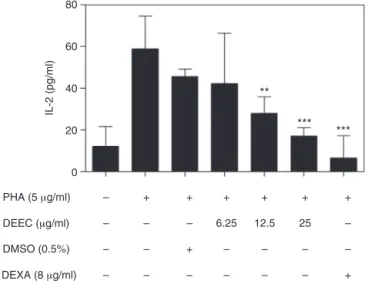
Fig.5.AnalysisofIL-2productioninthesupernatanthumanPBMCcultures acti-vatedbyPHAandtreatedwithDEEC.HumanPBMC(5×105cells)wereculturedin thepresenceofmedium,DMSO(0.5%),dexamethasone(DEXA,8g/ml)ordifferent
concentrationsofDEEC(6.25,12.5and25g/ml)andactivatedwithPHA(5g/ml)
for36h.TheamountofIL-2wasmeasuredinthesupernatantofthecellculturesby usinganELISA-basedcytokinedetectionmethod.Theresultsarepresentedasthe mean±SDofsixindependentexperiments.ThestatisticalmethodusedwasANOVA followedbytheTukeytest.
167.94%,anantiproliferativeeffecthigherthanthosepresentedby dexamethasone.T-CD8lymphocytesplayanimportantroleinviral infections,cancerandtransplantrejectionaswellasinskinlesion and tissue damagemaintenance in autoimmuneand degenera-tiveinflammatorydiseasescharacterizedbyaheightenedcellular adaptiveimmuneresponse(Gaoetal.,2009).Theinhibitoryeffect ofDEECcouldthusbeapromisingtherapeuticstrategy inthese inflammatorysituations,openingnewperspectivesforresearch.
Tlymphocyteproliferation is initiatedby bondingtheT-cell receptor to external stimuli that initiate the secretion of the autocrinegrowthfactorIL-2,which,inturn,promotesthe inter-actionwiththeCD25receptorthatisupregulatedonthesurface ofactivatedT-cells(Gangulyetal.,2001;Malek,2008).Theeffect ofDEEConIL-2productioninthesupernatantofPBMCcultures wasanalyzedbyusingELISA(Fig.5).DEECinhibitedtheproduction ofIL-2inthePHA-stimulatedculturestreatedwiththeextractat 12.5g/ml(28.01±7.95pg/ml)and25g/ml(17.02±4.04pg/ml) comparedwiththecellculturesstimulatedonlywithPHAcultures (58.77±15.70pg/ml).Onceagain,DMSOwasnotabletoinhibitthe productionofIL-2(DMSO=39.79±15.7pg/ml).
Theseresultsindicatedthattheantiproliferativeaction exhib-ited bytheextract onTlymphocytes wasdue toitsinhibitory action onthe production of IL-2. Theinhibitory effect of DEEC (25g/ml) on the secretion of IL-2 presented an efficacy of 38.33%incomparisontothoseobservedinculturestreatedwith dexamethasone. The expression of IL-2 involves the activation of transcription factors, mainly NF-kB. The recognition of an antigen bytheT lymphocytereceptor/CD3 complex, aswellas co-stimulatorystimuliinitiatestheactivationofthesignaling path-wayrelatedtoNF-kB.ThistranscriptionfactorisessentialforT-cell proliferation,cytokineproductionandthedevelopmentofa well-establishedcell-mediatedimmuneresponse,cytokineproduction andconsequentlyanincreaseofinflammatorymediatorssuchas prostaglandinsandleukotriene(Kaneetal.,2002;LiandVerma,
2002).
The inhibition of IL-2 production is the target for several immunosuppressive drugs (Schreiber and Crabtree, 1992). BecauseoftheinhibitoryactionofDEEConcellproliferationand itsinhibitory effect onthesecretion of IL-2, inaddition tothe
predominantpresence offlavonoids intheE.campestreextract, moleculesbelongingtothischemical groupmightbetheactive compounds responsible for the effects presented here. Several studies haveidentified flavonoids asthe agents responsiblefor the inhibition of lymphocyte proliferation, and this effect has been shown to be associated with lower levels of IL-2 in cell cultures(Gaoetal.,2009;Changetal.,2008;Gharagozlooetal., 2010).FurtherstudiesusingfractionatedDEECwillbeperformed toelucidate thestructure of thesubstances responsiblefor the effectdemonstratedhereaswellastheperformanceoftheactive fractionsininflammatorymodelsinvivo.
The results suggest that the anti-inflammatory activity attributedtoE.campestrebyfolkmedicinemaybe,atleastinpart, theresultoftheactionofitsflavonoidsontheactivationand pro-liferationofTlymphocytes.ThisfindingmeansthatE.campestre isapromisingnaturalsourceforthedevelopmentofnew effec-tivephytomedicinesforthetreatmentofthedamageobservedin autoimmuneandinflammatorydegenerativediseases,especially thosemediatedbyTlymphocytes.
Authors’contributions
MGS collected plant sample, performed the experiments, interpreted the results and drafted the manuscript. VGA and BAAF contributed to biological studies and to critical read-ing of the manuscript. CFFG contributed to prepare the dichloromethane–ethanolicextractofE.campestreroots.LEG con-tributedto chromatographicanalysis. WFP contributedto draft andcriticalreadingofthemanuscript.GEABMdesignedthestudy, supervisedthelaboratoryworkandcontributedtocriticalreading ofthemanuscript.Alltheauthorshavereadthefinalmanuscript andapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
WearegratefultoDr.DavidLeeNelsonforthetechnicalreview ofthemanuscriptandtotheBrazilianfundingagenciesFAPEMIG (CBB-APQ-00581-11),CNPqandCAPESforfinancialsupport,aswell astothePost-GraduatePrograminPharmaceuticalScienceofthe UFVJM(PPGCF/UFVJM).
References
Angulo,R.,Fulcher,D.A.,1998.MeasurementofCandida-specificblastogenesis: com-parisonofcarboxyfluoresceinsuccinimidylesterlabellingofTcells,thymidine incorporation,andCD69expression.Cytometry34,143–151.
Awouafack,M.D.,Kouam,S.F.,Hussain,H.,Ngamga,D.,Tane,P.,Schulz,B.,Green, I.R.,Krohn,K.,2008.AntimicrobialprenylateddihydrochalconesfromEriosema glomerata.PlantaMed.74,50–54.
Awouafack,M.D.,Tane,P.,Eloff,J.N.,2013.Twonewantioxidantflavonesfromthe twigsofEriosemarobustum(Fabaceae).Phytochem.Lett.6,62–66.
Barton,G.M.,2008.Acalculatedresponse:controlofinflammationbytheinnate immunesystem.J.Clin.Invest.118,413–420.
Bicalho,H.M.S.,Gontijo,M.C.,Nogueira-Machado,J.A.,1981.Asimpletechniquefor simultaneoushumanleukocytesseparation.J.Immunol.Methods40,115–116. Bustamante,K.G.L.,Lima,A.D.F.,Soares,M.L.,Fiuza,T.S.,Tresvenzol,M.F.,Bara,M.T.F., Pimenta,F.C.,Paula,J.R.,2010.Avaliac¸ãodaatividadeantimicrobianadoextrato etanólicobrutodacascadasucupirabranca(PterodonemarginatusVogel)– Fabaceae.Rev.Bras.PlantasMed.13,341–345.
Cai,Y.,Fleming,C.,Yan,J.,2012.NewinsightsofTcellsinthepathogenesisof psoriasis.CellMol.Immunol.9,302–309.
Chimenti,M.S.,Ballanti,E.,Perricone,C.,Cipriani,P.,Giacomelli,R.,Perricone,R., 2013.Immunomodulationinpsoriaticarthritis:focusoncellularandmolecular pathways.Autoimmun.Rev.12,599–606.
DeMattos,A.M.,Olyaei,A.J.,Bennett,W.M.,2000.Nephrotoxicityof immunosup-pressivedrugs:long-termconsequencesandchallengesforthefuture.Am.J. KidneyDis.35,333–346.
Drewes,S.E.,Horn,M.M.,Munro,O.Q.,Dhlamini,J.T.B.,Meyer,J.J.M.,Rakuambo, N.C.,2002.Pyrano-isoflavoneswitherectile-dysfunctionactivityfromEriosema kraussianum.Phytochemistry59,739–747.
Drewes,S.E.,Horn,M.M.,Khan,F.,Munro,O.Q.,Dhlamini,J.T.B.,Rakuambo,C.,Meyer, J.J.M.,2004.Minorpyrano-isoflavonesfromEriosemakraussianum:activity-, structure-,andchemicalreactionstudies.Phytochemistry65,1955–1961. Farnsworth,N.R.,1966.Biologicalandphytochemicalscreeningofplants.J.Pharm.
Sci.55,225–276.
Fortunato,R.H.,1999.CâmbiosNomenclaturalesenEriosema(Fabaceae: Papil-ionoideae,Cajaninae)II.Kurtziana27,371–382.
Ganguly,T.,Bandheka,L.P.,Sainis,K.B.,2001.ImmunomodulatoryeffectofTylophora indicaonConAinducedlymphoproliferation.Phytomedicine8,431–437. Gao,X.,Deeb,D.,Liu,Y.,Dulchavsky,S.A.,Gautam,S.C.,2009.
Immunomodula-toryactivityofxanthohumol:inhibitionofTcellproliferation,cell-mediated cytotoxicity and Th1 cytokine production through suppression of NF-B. Immunopharmacol.Immunotoxicol.31,477–484.
Gharagozloo,M.,Velardi,E., Bruscoli,S.,Agostini,M.,Sante,M.D., Donato,V., Amirghofran,Z.,Riccardi,C.,2010.SilymarinsuppressCD4+Tcellactivationand proliferation:effectsonNF-kappaBactivityandIL-2production.Pharmacol.Res. 61,405–409.
Grear,J.W.,1970.ArevisionoftheAmericanspeciesofEriosema(Leguminosae– Lotoideae).Mem.NewYorkBot.Gard.20,1–98.
Kane,L.P.,Lin,J.,Weiss,A.,2002.It’sallRelative:NF-kappaBandCD28costimulation ofT-cellactivation.TrendsImmunol.23,413–420.
Li,Q.,Verma,I.M.,2002.NF-kappaBregulationintheimmunesystem.Nat.Rev. Immunol.2,725–734.
Lyons,A.B.,1999.Dividedwestand:Trackingcellproliferationwith carboxyfluo-resceindiacetatesuccinimidylester.Immunol.CellBiol.77,509–515. Macián,F.,2005.NFATproteins:keyregulatorsofT-celldevelopmentandfunction.
Nat.Rev.Immunol.5,472–484.
Malek,T.R.,2008.Thebiologyofinterleukin-2.Annu.Rev.Immunol.26,453–479. Marston,A.,Hostettmann,K.,2006.Separationandquantificationofflavonoids.In:
Andersen,O.M.,Markham,K.R.(Eds.),Flavonoids:Chemistry,Biochemistry,and Applications.Taylor&Francis,BocaRaton,pp.1–36.
Medzhitov,R.,2008.Originandphysiologicalrolesofinflammation.Nature454, 428–435.
Nathan,C.,2002.Pointsofcontrolininflammation.Nature420,846–852. Reuter,J.,Merfort,I.,Schempp,C.M.,2010.Botanicalsindermatology:an
evidence-basedreview.Am.J.Clin.Dermatol.11,247–267.
Rogalski,L.D.,Miotto,S.T.S.,2011.OgêneroEriosema(DC.)Desv. (Leguminosae-Papilionoideae)nosestadosdoParanáedeSantaCatarina.Brasil.Rev.Bras. Bioci.9,350–370.
Schreiber,S.L.,Crabtree,G.R.,1992.ThemechanismofactionofcyclosporinAand FK506.Immunol.Today13,136–142.
Sutthivaiyakit,S.,Thongnak,O.,Lhinhatrakool,T.,Yodchun,O.,Srimark,R., Dow-taisong,P.,Chuankamnerdkarn, M., 2009.Cytotoxic andantimycobacterial prenylatedflavonoidsfromtherootsofEriosemachinense.J.Nat.Prod.72, 1092–1096.