ISSN 0104-6632 Printed in Brazil
www.abeq.org.br/bjche
Vol. 32, No. 01, pp. 1 - 12, January - March, 2015 dx.doi.org/10.1590/0104-6632.20150321s00003020
Brazilian Journal
of Chemical
Engineering
PREPARING AGRICULTURAL RESIDUE BASED
ADSORBENTS FOR REMOVAL OF DYES FROM
EFFLUENTS - A REVIEW
D. S. Kharat
Central Pollution Control Board, East Arjun Nagar, Delhi-110032, India. Phone: + (91) (11) 43102443, Fax: + (91) (11) 22301955
E-mail: kharatds@hotmail.com
(Submitted: October 9, 2013 ; Accepted: February 11, 2014)
Abstract - Industries engaged in dyeing operation generate coloured effluent due to the presence of spent dyes. Adsorption is among the various treatment processes employed for removal of dyes from effluents. Activated carbon is mostly used as an adsorbent in the treatment process. Attempts have been made by researchers to use non-conventional, low-cost, naturally-occurring biomass as adsorbents, including fruit peals, seeds, leaves, bark, sawdust, straw, ash, sludge and others that are abundantly available. The literature indicates that the dye adsorption capacities of these non-conventional biomasses largely depend on the methods of processing and the types of dyes. This review highlights methods used to prepare the adsorbents, along with their adsorption capacity for removal of different dyes from effluents.
Keywords: Adsorption; Dye removal; Agricultural residue; Low-cost adsorbent.
INTRODUCTION
Annual production of dyes is to the tune of 7x105 tonnes worldwide (Allen and Koumanova, 2005). Dyes produced are classified as acid dyes, basic dyes, direct dyes, disperse dyes, reactive dyes and sulfur dyes according to their chemical constituents and application (Zollinger, 1991). Textile dyeing indus-tries, pulp and paper industries and tanneries are the main consumers of dyes and are also responsible for discharge of voluminous coloured effluents. About 10 to 15% of the dyes consumed in the dyeing proc-esses are disposed in the effluent (USEPA, 1997). In order to address the colour problems, industries are using various treatment processes including adsorp-tion. Activated carbon is the most used adsorbent in adsorption columns. There have been attempts by researchers to explore the adsorption potential of non-conventional, naturally-occurring agricultural residues in dye removal from effluents. In India
alone more than 400 million tonnes of agricultural residue is generated annually (Raghuvanshi et al., 2004), which includes rice husk, bagasse, stalk, coir pith etc. Exploring application of the agricultural residues for use as adsorbents can provide suitable alternatives for the removal of spent dyes from in-dustrial effluents.
REVIEW OF ADSORBENTS
Fruit Peels
Brazilian Journal of Chemical Engineering
to remove acid and dried at 150 °C for use as adsorb-ents for removal of Reactive red 2 dye from effluent. The study reported the adsorption capacities of NCDC, NCMC and NCAC as 0.608 mg/g, 0.580 mg/g and 0.566 mg/g respectively at an initial dye concentra-tion of 20 mg/g and adsorbent dose of 30 g/L. the adsorption potential of mosambi peel was studied by Ladhe et al. (2011) using Erichrome black T dye as adsorbate. The adsorbent was cleaned, ground to obtain powder (180-300 m) and dried. Then the powder was treated with concentrated sulfuric acid in a weight ratio of 1:1 for 24 hours, followed by washing with NaHCO3 solution and distilled water and drying. They observed Erichrome black T dye removal efficiency was 90% at a dye concentration of 50 mg/L and adsorbent dose of 0.004 g/cc. Parvathi and Maruthavanan (2010) performed adsorption studies using tapioca peel for removal of Megeta MB dye. They found that higher percentage removals were observed at solution pH 7 and the equilibrium was reached within 120 minutes of contact time. Jackfruit peel (0.84 mm in size) was used as adsorbent by Jayarajan et al. (2011) to remove Rhodamine dye. They reported a maximum colour removal of 25.3% at an adsorbent dose of 3.0 g/L and dye concentra-tion of 100 mg/L. In a study by Velmurugan et al. (2011), orange peel, neem leaves and banana peel were dried at 105 °C for 48 hours and ground to powder (600 m particle size). The study reported that the adsorption capacities of orange peel for dif-ferent dyes were in the order of Methyl orange > Methylene blue > Rhodamine B > Congo red > Me-thylene violet > Amido black 10B. An orange peel (Citrus sinensis L.) based adsorbent was also pre-pared and employed for the removal of Remazol brilliant blue dye from synthetic dye effluent by Mafra et al. (2013). They found that the equilibrium was reached in 15 hours of contact time with dye concentration 30-250 mg/L. The adsorption capacity of the orange peel adsorbent decreased with increase in temperature. The equilibrium data were reasona-bly described by the Langmuir and Freundlich iso-therms. The authors reported that the adsorption ca-pacities of orange peel adsorbent were 11.62 mg/g, 10.70 mg/g, 8.61 mg/g, 6.39 mg/g and 5.54 mg/g at 20 °C, 30 °C, 40 °C, 50 °C and 60 °C, respectively. In another study, grape juice waste was used by Ansari
et al. (2011) to evaluate adsorption of Methylene blue dye from aqueous solution. They reported that maxi-mum adsorption of Methylene blue dye was achieved at pH 10. Ong et al. (2010) studied adsorption of Methylene blue dye in a packed bed of durian peel powder (1 mm sieve size). The study established that durian peel is a potentially useful and attractive
adsorbent for removed of Methylene blue from aqueous solution. They reported that a flow rate of 15 mL/min showed an early breakthrough time.
Sugarcane Bagasse
Azhar et al. (2005) studied the removal of Methyl red dye using treated sugarcane bagasse and com-pared the results with those obtained using powered activated carbon. As per the study, one portion of ground bagasse with particle size between -80 to +230 mesh was treated with 1% formaldehyde in w/v ration of 1:5 at 50 °C for 4 hours followed by activation at 80 °C for 24 hours. The other portion of the bagasse was treated with sulfuric acid and heated in a muffle furnace for 24 hours at 150 oC, followed by soaking in 1% sodium bicarbonate solution over-night. The study reported adsorption efficiencies of the different adsorbents in the order of powdered activated carbon > bagasse treated with formalde-hyde > bagasse treated with sulfuric acid. Untreated, formaldehyde-treated and sulfuric acid-treated sugar-cane bagasse powders were used by Abdullah et al. (2005) for removal of Ethylene red dye from aque-ous solution. The method of preparing formalde-hyde-treated bagasse powder and sulfuric acid-treated sugarcane bagasse powder remained the same as that used by Azhar et al. (2005). The results reported indicated that sulfuric acid-treated sugar-cane bagasse gave better performance as compared to the formaldehyde-treated sugarcane bagasse. Amin (2008) studied the removal of Reactive orange dye from aqueous solutions by activated carbons pre-pared from sugarcane bagasse. The bagasse powder was sieved to an average size of 0.05 mm. One por-tion of bagasse was carbonized in the absence of oxygen at 600 °C for 1 hour. The second portion of bagasse powder was soaked in ZnCl2 solution (50% concentration) and the third portion of bagasse pow-der in H3PO4 solution (28% concentration) for 24 hours. After decantation, the samples were pyrolyzed in a muffle furnace in the absence of air at 600 °C for 1 hour. The study reported that the adsorption capacities of physically carbonized, ZnCl2-treated and H3PO4-treated bagasse powder samples were observed as 3.48 mg/g, 2.83 mg/g and 1.8 mg/g, respectively.
dye, Remazol brilliant blue R dye and Remazol brilliant red dye, respectively. Reghuvanshi et al. (2004) treated sugarcane bagasse with concentrated sulfuric acid at 150-160 °C for 36 hours. The carbon-ized material so obtained was washed, dried and ground to 0.33 mm size. The processed bagasse and raw bagasse were used as adsorbents for the removal of Methylene blue from solution. The average percentage of dye removal with processed bagasse was found to be 18% more than that obtained with raw bagasse. The work done by Wong et al. (2009) with natural and quartenary ammonium chloride treated sugarcane bagasse shown that the optimum pH for the removal of Basic blue 3 dye and Reactive orange 16 dye is between 6 to 8. For their study, quartenisation of bagasse was carried out by soaking in NaOH solution for 30 minutes. The adsorbent was then mixed with quartenary ammonium chloride (65% w/w) in water. The mixture was heated at 60-70 °C for 4 hours. It was then rinsed with distilled water and suspended in dilute HCl solution at pH 3 for 30 minutes. Then the adsorbent was washed with distilled water and dried at 50 °C in an oven over-night for use as modified adsorbent. The maximum adsorption capacities were reported as 37.59 mg/g and 34.48 mg/g for Basic blue 3 dye and Reactive orange 16 dye, respectively.
Ho et al. (2005) studied adsorption of three dyes, namely Basic violet 10, Basic violet 1 and Basic green 4, from aqueous solution onto sugarcane dust (par-ticle size 351-589 m). The Langmuir monolayer equilibrium capacities for these dyes were 50.4 mg/g, 20.6 mg/g and 13.9 mg/g for Basic violet 1, Basic green 4 and Basic violet 10, respectively. Bagasse ash has been used as a low cost adsorbent by Khandelwal and Gaikwad (2011) for investigating removal of Orange II dye. They reported that the percent adsorption of dye increased with decreasing flow rate from 2 L/hour to 1 L/hour, by increasing bed height from 15 cm to 45 cm, by decreasing the initial dye concentration from 150 mg/L to 100 mg/L and by increasing the column diameter from 2.54 cm to 3.50 cm. Kausik et al. (2008) used powdered sugar-cane bagasse, coconut coir pith, cow dung and euca-lyptus bark treated with 20% sodium hydroxide solu-tion. The adsorbents were aminated with a mixture of 10% amine and water at 70 °C and finally treated with acid for protonation. The adsorbents were used for treating Reactive blue 171, Reactive yellow 84 and Reactive red 141 dyes. The study reported that, among the above four adsorbents, bagasse had a slightly higher efficiency with 20-26% dye removal.
In another study, Ashoka and Inamdar (2010) used formaldehyde-treated bagasse and acid-treated
bagasse for adsorption of Methyl red dye. The ba-gasse powder (0.1456 mm particle size) was reacted with 1% formaldehyde (w/v ratio of 1:5) at 50 °C for 4 hours followed by washing and drying at 80 °C. Acid treatment involved treating bagasse with sulfu-ric acid (in the ratio of 1:1) and then heating in a muffle furnace for 24 hours at 150 °C and soaking in sodium bicarbonate solution overnight to remove acid, followed by washing and drying at 150 °C for 24 hours. As per the study, the dye removal effi-ciency of acid-treated bagasse was higher than that of formaldehyde-treated bagasse. Using a similar treatment method for bagasse, Mahesh et al. (2010) observed that the Crystal violet dye removal effi-ciency of formaldehyde-treated bagasse was more than that of acid-treated bagasse.
Husk
Ong et al. (2007) ground rice hull to pass through a 1 mm sieve and used it as natural rice hull (NRH). Ethylenediamine (EDA) modified rice hull was also prepared by treating natural rice hull with ethyl-enediamine in a ratio of 1.0 g rice hull to 0.02 mole of EDA in a well-stirred water bath at 80 °C for 2 hours to enable it to function as a sorbent for re-moval of Basic blue 3 and Reactive orange 16 dyes. They observed adsorption capacities, calculated from the Langmuir isotherm, of 14.68 mg/g and 6.24 mg/g for Basic blue 3 dye and Reactive orange 16 dye respectively. Sharma and Janveja (2008) conducted a study on the removal of Congo red dye from the effluent of a textile industry using rice husk carbon activated by steam. The study reported that a dose of 0.08 g/L of rice husk carbon removed 10 to 99% of dye from aqueous solution with an initial dye con-centration of 25 ppm within contact times from 20 to 200 minutes.
Sawdust
Experiments for removal of Ethylene blue dye us-ing saw dust (420-85 m) were performed by Gong
ca-Brazilian Journal of Chemical Engineering
pacity of 2.78 mg/g, 5.06 mg/g and 7.81 mg/g for Direct orange 26 dye, Acid orange 7 dye and Acid green 20 dye, respectively. El-latif et al. (2010) per-formed a study of removal of Methylene blue dye using oak sawdust. The sawdust was treated with 0.1N NaOH solution and immobilized on alginate biopolymer for use as an adsorbent. The study re-vealed an adsorption capacity of sawdust of 38.46 mg/g. The kinetics followed closely a Pseudo-second order model.
Sludge
Activated sludge was dried at 105 °C to a constant weight and sieved to < 205 m for use as an adsorb-ent in a study for removal of Rhodamine-B dye (Ju
et al., 2006). The results indicated that the adsorption capacity of activated sludge increased with de-creasing initial pH and temperature. The Langmuir monolayer adsorption capacities were 5.121 mg/g, 4.847 mg/g, 4.456 mg/g and 3.725 mg/g at tempera-ture of 5 °C, 15 °C, 25 °C, and 45 °C, respectively. Reddy et al. (2006) conducted a study of reactive dye removal from dyeing unit effluent using sewage sludge-derived activated carbons by pyrolysis. The pyrolysis was carried out in a muffle furnace in the absence of oxygen. Subsequently, 10 g of pyrolysed sludge was impregnated into 25 mL of activating agent (3M ZnCl2 solution) for 24 hours at room tem-perature, followed by washing with distilled water and drying at 105 °C for 24 hours. The Langmuir monolayer adsorption capacity of the sludge-derived carbon was 33.5 mg/g. The adsorption potential of granular sludge (from a pilot scale reactor treating waste water) with Acid orange 7 dye was investi-gated by Mendez-Paz et al. (2005). A dye removal efficiency of 92% was achieved in continuous treat-ment mode with dye loading rate of 590 mg/L.day. Won et al. (2006) studied the adsorption potential of protonated fermentation waste (Corynebacterium glutamicum) with Reactive black 5 dye. For that study, the protonated biomass was prepared by treating the biomass with 1N HNO3 solution, fol-lowed by washing with deionized distilled water and drying. They observed that, in the range of pH 1 to 3, removal of dye was 100%. The maximum dye up-takes using the Langmuir isotherm were 169.5 mg/g and 185.2 mg/L at 20 °C and 40 °C, respectively. The study further showed that the uptake of dye was not significantly affected by the concentration of salt in solution. Won et al. (2006a) studied different sludges from a water treatment plant, sewage treat-ment plant, anaerobic digestion and land fill for the removal of Reactive orange 16 dye. The sludges
were treated with 1 M HNO3 solution for 24 hours and dried at 60 °C to use these sludges as adsorb-ents. The maximum adsorption capacities using the Langmuir equation were 159.0 mg/g, 114.7 mg/g, 86.8 mg/g 47.0 mg/g for land fill sludge, sewage sludge, anaerobic sludge and water treatment plant sludge, respectively at pH 2.
Organisms
Kim et al. (2004) studied the adsorption of Reac-tive orange 16 dye using dead cell of brewery yeast. The yeast was washed with deionized water and dried at 80 °C. The dried biomass was ground to an average size of 112.5 m. An adsorption capacity of 0.604 mg/g, 0.090 mg/g and 0.50 mg/g were ob-served at solution pHs of 3, 7 and 10, respectively. Studies were conducted by Singh and Rastogi (2004) using baker’s yeast cells as an adsorbent for adsorp-tion of various dyes. For preparaadsorp-tion of the adsorb-ent, baker’s yeast (2 g) was suspended in saline (6 mL). The yeast was re-suspended in 6 ml of 0.1 M Acetate buffer with pH 4.6. The sediment was fur-ther suspended in acetate buffer to obtain a ca. 33% yeast suspension (v/v). Then 3 mL of yeast suspen-sion was added to 1 mL of ferrofluid, and incubated at room temperature for 1 hour. After this treatment, the majority of the yeast cells were modified. The modified yeast cells were heated in boiling water and then washed with saline and stored at 4 °C for use as adsorbent. The study reported that the adsorption capacities for Acridine orange dye, Aniline blue dye, Crystal violet dye, Malachite green dye and Sa-franine O dye were 82.8 mg/g, 430.2 mg/g 85.9 mg/g, 19.6 mg/g and 90.3 mg/g, respectively. The decol-ourization potential of fungus was performed by Akar et al. (2009) using Reactive red 198 dye. A maximum dye adsorption capacity of 1.03x10-4 mol/g was observed at pH 2 and adsorbent dose of 2.0 g/L. They observed an increase in the adsorption capacity with temperature, indicating that the adsorption proc-ess is endothermic. Fu and Viraghavan (2003) stud-ied the dye removal potential of immobilized fungal biomass (Aspergillus niger) with four dyes, namely Acid blue 29, Basic blue 9, Congo red and Disperse red 1. The adsorption capacities observed were 64.7 mg/g for Acid blue 29 dye, 8.9 mg/g for Basic blue 9 dye, 1.1 mg/g for Congo red dye and 0.1 mg/g for Disperse red 1 dye.
Grains
prepared from spent brewery grains. The spent brew-ery grains were suspended in 0.13 M sulphuric acid solution (20 g of grain per 100 mL of solution) for one hour. The grains were washed, dried and ground for use as adsorbent. They observed the highest ad-sorption capacity at pH 2 with an initial dye concen-tration of 150 mg/L, dose of adsorbent 0.5 g/L and contact time of 40 minutes.
Coconut
Theivarasu and Mylsamy (2010) conducted an ad-sorption study of Rhodamine-B dye on char prepared by treating the coconut shell with concentrated sulfu-ric acid at ratio of 1:1 (w/v). The activation was per-formed by heating in a muffle furnace at 550 °C for 7 hours, followed by washing and drying. The ad-sorption capacity of the treated coconut shell char was reported as 41.67 mg/g. For removal of Coomassie brilliant blue dye on coir pith as adsorb-ent, Prasad et al. (2008) conducted studies including the effects of time, initial dye concentration and dose of adsorbent. Treatment of coir pith includes dipping the coir pith in a one molar solution of HCl, washing with distilled water and drying in an oven at 55 °C. The study reported a maximum adsorption capacity of 31.84 mg/g and the adsorption capacity for the system was 6.43 mg/g.
Palm Shell
Rajavel et al. (2003) evaluated the removal effi-ciency of Dark green PLS dye from textile industry wastewater using carbons prepared from palm nut shell, cashew nut shell and broom stick. The carbons were prepared by treating 4 parts of each material with 2 parts of concentrated sulfuric acid and heating at 140-170 °C for 24 hours. The resultant materials were filtered, washed with water, dried at 105-110 °C and sieved to an average particle diameter of 0.5 mm for use as adsorbents. The adsorption capacities of palm nut shell carbon, cashew nut shell carbon and broom stick carbon were reported as 0.84 mg/g, 1 mg/g and 0.63 mg/g, respectively. The adsorption study by Rusly and Ibrahim (2010) involved palm shell activated carbon and Reactive red 3 BS dye. They observed that, upon increasing adsorbent dose and agitation, the efficiency of dye removal in-creased. The study reported that, at the optimal con-dition, the dye removal efficiency reached more than 90% and the adsorption capacity was more than 7 mg/g. Batch adsorption experiments were carried out by Sreelatha and Padmaja (2008) for removal of Methylene blue and Rhodamine 6G dyes from
solu-tion using palm shell powder. The sorpsolu-tion capacity was dependent on the operating parameters and the process was pH dependent above pH 4.0. The ad-sorption capacities were reported to be 121.5 mg/g and 105 mg/g for Methylene blue dye and Rhodomine 6G dye, respectively.
Leaves
Brazilian Journal of Chemical Engineering
furnace. The resulting carbon was washed with dis-tilled water and then dried at 100 °C for 4 hours for use as adsorbent. The adsorption capacities were 21.491 mg/g, 20.267 mg/g, 20.069 mg/g and 18.928 mg/g at 30 °C, 40 °C, 50 °C and 60 °C, re-spectively in the case of Congo red dye. With Mala-chite green dye, the observed adsorption capacities were 9.737 mg/g, 9.624 mg/g 9.633 mg/g and 9.569 mg/g at 30 °C, 40 °C, 50 °C and 60 °C, respectively.
Waste tendu (Diospyros melanoxylon) leaf cut-tings were processed and used as an adsorbent for removal of Crystal violet dye by Nanda and Ghole (2008). Tendu leaf cuttings were powdered and sieved with 80 mesh (called TLR). Carbons were prepared by treating 5 parts of TLR with 3 parts of concentrated sulfuric acid at 120-130 °C for 24 hours. The carbonized mass was freed from acid by soaking in 1% solution of sodium bicarbonate, fol-lowed by drying and sieving through 80 mesh sieve for use as adsorbent (called TLR-CM). One portion of LTR was treated with 5 parts of 2N sulfuric acid for 24 hours. Then the material was washed, dried, powdered and used as adsorbent (called TLR-2N). The study reported adsorption capacities of 67.57 mg/g, 42.92 mg/g and 22.47 mg/g for TLR-2N, TLR and TLR-CM, respectively. El-Zawahry and Kamel (2004) used water hyacinths (Eichhornia crassipes) powder as adsorbent for removal of acid and reactive dyes from aqueous solution. The samples were cut to smaller size, air dried and ground and sieved to 0.147-1.5 mm size. The powder was subjected to a chemical scouring treatment (boiling with 20 g/L sodium hydroxide for 2 hours at 120-130 °C in a 20:1 ratio). The material was filtered, washed and air dried for use as adsorbent. The study reported that the higher nitrogen percent of hyacinths showed higher adsorption capacities. Vijayaraghavan and Yun (2008) studied adsorption of Reactive black 5 dye using seaweed (Luminaries sp.). The adsorbent was ground to an average size of 0.4-0.6 mm. Protonation of adsorbent involved treatment with 10 g/L of 0.1 M HCl solution followed by washing and drying. The maximum dye uptake of 101.5 mg/g was observed at pH 1.0 and 40 °C.
Ncibi et al. (2007) studied adsorption of Reactive red 228 on sea grass leaf sheaths with variables in-cluding temperature, pH and chemical pre-treatment, and observed maximum colour removal at pH 5.0. Pre-treatment of the absorbent with phosphoric acid and nitric acid solution increased the adsorption effi-ciency up to 80%. Aquatic plant (Hydrilla verticillata) biomass was experimented for removal of Malachite green dye from solution by Rajeshkannan et al. (2010) and the study showed an adsorption capacity
of 91.97 mg/g at pH 8.0. Purai and Rattan (2009) used ash prepared from cow dung and commercial activated carbon. The ash was prepared in muffle furnaces at 500 °C. The study revealed that 4.98 mg/g and 4.67 mg/g of dye were adsorbed dye adsorbed by cow dung ash and activated carbon, respectively, at pH 3.33. The authors noted that at pH 8.76, the adsorption was the least for both the adsorbents.
Tree Bark
Patil et al. (2011) carried out adsorption studies of Methylene blue dye using teak tree bark with various process parameters. The maximum adsorption of Methylene blue dye was 333.33 mg/g. The study revealed an increase in dye adsorption efficiency with increasing pH, increasing temperature and de-creased particle size of adsorbent.
Straw
Abdualhamid and Asil (2011) conducted adsorp-tion studies for removal of Methylene blue dye using barley, wheat and oat straws as adsorbents. The straws were cut into pieces of 1 cm size, washed and dried at 65 °C overnight. One portion of each straw was subjected to soaking by immersing in water at room temperature for 20 days and then dried at 60 °C over-night for use as adsorbent. In the study, the maxi-mum dye adsorption capacity for the straws before soaking followed the order: barley > oat > wheat, with values of 27.72 mg/g, 17.54 mg/g and 8.34 mg/g, respectively. The maximum dye removal capacity of straws after soaking in water was found in the order of oat > barley > wheat, with values of 50.00 mg/g, 22.22 mg/g and 11.11 mg/g.
Seeds
solution to remove remaining acid, followed by dry-ing and sievdry-ing to 125-250 m size. Dye adsorption capacities of 8.52 mg/g, 40.48 mg/g and 25.91 mg/l were observed for Methylene blue dye, Methylene red dye and Malachite green dye, respectively. De-colourization studies of Acid orange dye 7 by char-coal prepared from coffee grounds were performed by Nakamura et al. (2003). For preparing the adsorb-ent, extracted residues of coffee beans was dried to reduce the moisture content by 50% and the same was carbonized in a furnace at 800 °C, 1000 °C and 1200 °C and sieved through 10-20 mesh. The carbons were washed with distilled water and dried at 110 °C for use as adsorbents. The studies indicated that the equilibrium adsorption of Acid orange 7 dye was higher for charcoal carbonized at higher temperatures.
Other Biomasses
Habib et al. (2006) performed adsorption studies using tuberose sticks as adsorbent for removal of Methylene blue dye. The dried tuberose sticks were cut into small pieces, powdered and then sieved with a 425 µm sieve for use as adsorbent. The maximum dye removal of 80% was achieved at pH 11, adsorb-ent dose of 1 g/L and dye concadsorb-entration in solution of 40 mg/L. In another study by Ramakrishna and Nagarajan (2009), flame tree (Delonix regia) pods were used for preparing adsorbents. The flame tree pods were crushed into smaller pieces and soaked with concentrated sulfuric acid in a 1:1 ratio (weight of material to volume of acid) for 48 hours and acti-vated at 160 °C for 6 hours. The carbon so prepared was washed with distilled water and dried at 105 °C for 2 hours to prepare the adsorbent. The data were reported to fit well to the Langmuir and Freundlich isotherms. The maximum adsorption capacity ob-served with Crystal violet dye was 16.70 mg/g. A granule prepared from leaf, fruits and twigs of
Muntingia calabura was utilized by Santhi et al. (2009) for adsorption of Methylene blue, Methylene red, and Malachite green dyes. The maximum
adsorp-tion capacities were 20 mg/g, 58 mg/g and 32 mg/g for Methylene blue dye, Methylene red dye and Malachite green dye, respectively. Sivakumar and Palanisamy (2010) prepared an adsorbent by treating precursor wood with H3PO4 solution followed by activation at 800 °C. The adsorbent so processed had a surface area of 918 cm2/g. The adsorbent was used to remove Acid blue 92, Basic blue 29, Reactive red 4, and Direct blue 53 dyes from aqueous solution. Mittal
et al. (2007) investigated the removal of Tartrazine dye by using hen feathers. To remove the adhering organic matter, feathers were treated with hydrogen peroxide followed by washing and drying. The study reported dye removal efficiencies of 47%, 52% and 55% at 30 °C, 40 °C and 50 °C, respectively. Piccin
et al. (2011) tested adsorption potential of chitosan for removal of commercial dye (FD&C Red n° 40) at different temperatures (298 to 338 K). The maxi-mum adsorption capacity of chitosan was observed as 1065.8 μmol/g, 1061.4 μmol/g, 800.8 μmol/g, 508.5 μmol/g at 308 K, 318 K, 328 K and 338 K, respectively. The authors observed that the adsorp-tion process was exothermic in nature. Adsorpadsorp-tion studies of Reactive red 120 and Reactive black 5 dyes onto cotton fibre were performed by Gamal et al. (2010). They observed the monolayer adsorption capacities of 11.63 mg/g and 6.22 mg/g for Reactive red 120 dye and Reactive black 5 dye, respectively. Schimmel et al. (2010) studied the adsorption poten-tial of commercial activated carbon for Turquoise blue QG reactive bye. The adsorption studies were conducted to obtain isotherm and kinetic data under different experimental conditions. They observed maximum dye removal at a pH of 2 and temperature of 30 °C. The equilibrium data were reasonably de-scribed by the Langmuir and Freundlich isotherms. The authors reported that the adsorption capacity of activated carbon was 140.14 mg/g. The kinetics fol-lowed closely a Pseudo-second order model. Table 1 show different agricultural residues used to prepare the adsorbents, along with adsorption capacities for removal of different dyes from effluents.
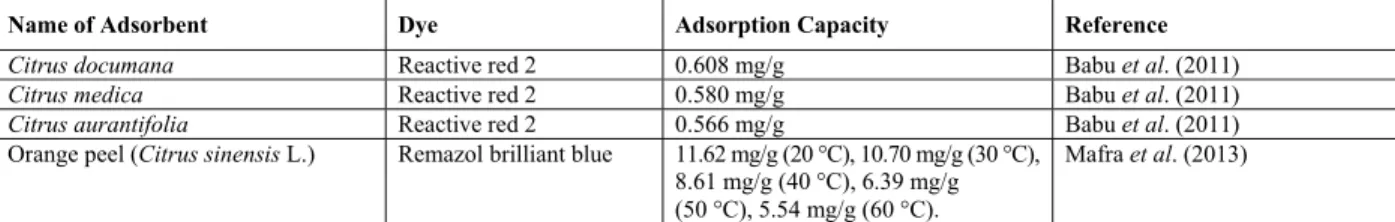
Table 1: Adsorption capacities of some agricultural residue based adsorbents for removal of different dyes from effluents.
Name of Adsorbent Dye Adsorption Capacity Reference
Citrus documana Reactive red 2 0.608 mg/g Babu et al. (2011)
Citrus medica Reactive red 2 0.580 mg/g Babu et al. (2011)
Citrus aurantifolia Reactive red 2 0.566 mg/g Babu et al. (2011)
Orange peel (Citrus sinensis L.) Remazol brilliant blue 11.62 mg/g (20 °C), 10.70 mg/g (30 °C), 8.61 mg/g (40 °C), 6.39 mg/g (50 °C), 5.54 mg/g (60 °C).
Mafra et al. (2013)
Brazilian Journal of Chemical Engineering
Continuation Table 1
Name of Adsorbent Dye Adsorption Capacity Reference
Mosambi peel Erichrome black T 90% (Initial dye concentration 50 mg/L & adsorbent dose 4 g/L)
Ladhe et al. (2011)
Palm nut shell carbon Dark green PLS 0.84 mg/g Rajavel et al. (2003) Cashew nut shell carbon Dark green PLS 1 mg/g Rajavel et al. (2003) Broom stick carbon Dark green PLS 0.63 mg/g Rajavel et al. (2003)
Coconut shell char Rhodamine-B 41.67 mg/g Theivarasu and Mylsamy (2010) Coir pith char Coomassie brilliant 31.84 mg/g Prasad et al. (2008)
Palm shell activated carbon Reactive red 3 BS 7 mg/g Rusly and Ibrahim (2010) Palm shell powder Methylene blue
Rhodamine 6G
121.5 mg/g Sreelatha and Padmaja (2008) 105 mg/g
Sugarcane bagasse Reactive orange 3. 48 mg/g Amin (2008) Sugarcane bagasse (ZnCl2 treated) Reactive orange 2.83 mg/g Amin (2008) Sugarcane bagasse (H3PO4 treated) Reactive orange 1.8 mg/g Amin (2008) Sugarcane bagasse fly ash Remazol Black B
Remazol brilliant blue R Remazol Brilliant red
16.42 mg/g 32.468 mg/g 18.282 mg/g
Rachakornkij et al. (2004)
Sugarcane bagasse Basic blue 3 Reactive orange 16
37.59 mg/g 34.48 mg/g
Wong et al. (2009)
Sugarcane dust Basic violet 1 Basic violet 10 Basic green 4
50.4 mg/g 13.9 mg/g 20.6 mg/g
Ho et al. (2005)
Rice hull Basic blue 3
Reactive orange 16
14.68 mg/g 6.24 mg/g
Ong et al. (2007)
Rice husk carbon Congo red 10 to 99% (Initial dye concentration 25 ppm & adsorbent dose 0.08 g/L)
Sharma and Janveja (2008)
Saw dust Ethylene blue 87.7 mg/g (natural saw dust). 188.7 mg/g (treated saw dust)
Gong et al. (2008)
Beech wood saw dust Direct orange 26 Acid green 20 Aid orange 7
2.78 mg/g 7.81 mg/g 5.06 mg/g
Izadyar and Rahimi (2007)
Activated sludge Rhodamine-B 5.121 mg/g (5 °C), 4.847 mg/g (15 °C), 4.456 mg/g (25 °C), 3.725 mg/g (45 °C)
Ju et at. (2006)
Sewage sludge activated carbons Reactive dye 33.5 mg/g Reddy et at. (2006) Granular activated sludge Acid orange 7 92% (at dye loading rate of
590 mg/L.day
Mendez-Paz et al. (2005)
Fermentation waste
(Corynebacterium glutamicum)
Reactive black 5 169.5 mg/g (20 °C), 185.2 mg/g (40 °C)
Won et al. (2006)
Sewage treatment plant sludge Rhodamine B 5.121 mg/g (5 °C), 4.847 mg/g (15 °C), 4.456 mg/g (25 °C), 3.72 5 mg/g (45 °C)
Ju et al. (2006)
Water treatment plant sludge Reactive orange 16 47.0 mg/g Won et al. (2006a) Sewage treatment plant sludge Reactive orange 16 114.7 mg/g Won et al. (2006a) Anaerobic digestion sludge Reactive orange 16 86.8 mg/g Won et al. (2006a) Land fill sludge Reactive orange 16 159.0 mg/g Won et al. (2006a) Brewery yeast dead cell Reactive orange 16 0.604 mg/g (pH 3), 0.090 mg/g
(pH 7), 0.50 mg/g (pH 10)
Kim et al. (2004)
Baker’s yeast cells Acridine orange Aniline blue Malachite green Safranine O Crystal violet
82.8 mg/g, 430.2 mg/g 19.6 mg/g 90.3 mg/g 85.9 mg/g
Singh and Rastogi (2004)
Fungus Reactive red 198 1.03x10-4 mol/g Akar et al. (2009) Fungal biomass (Aspergillus niger) Acid blue 29
Disperse red 1 Congo red Basic blue 9
64.7 mg/g 0.1 mg/g 1.1 mg/g 8.9 mg/g
Fu and Viraghavan (2003)
Gulmohor leaves Methylene blue 120 mg/g (293 K), 178 mg/g (303 K) and 253 mg/g (313K)
Ponnusami et al. (2009)
Continuation Table 1
Continuation Table 1
Name of Adsorbent Dye Adsorption Capacity Reference
Used tea leaves carbon Malachite green Methylene blue
444.44 mg/g 454.5 mg/g
Singh and Rastogi (2004)
Agave (Americana (L.) leaves fibres Alpacide yellow 16.97 mg/g(20 °C), 15.79 mg/g (30 °C) and 21.41 mg/g (50 °C)
Hamissa et al. (2008)
Pandanus leaves Congo red 21.491 mg/g (30 °C), 20.267 mg/g (40 °C), 20.069 mg/g (50 °C), 18.928 mg/g (60 °C)
Hema and Arivoli (2007)
Pandanus leaves Malachite green 9.737 mg/g (30 °C), 9.624 mg/g (40 °C), 9.633 mg/g (50 °C), 9.569 mg/g (60 °C)
Hema and Arivoli (2007)
Tendu (Diospyros melanoxylon) leaf Crystal violet 67.57- 22.47 mg/g depending on processing of adsorbent.
Nagda and Ghole (2008)
Tuberose sticks Methylene blue 80% (at pH 11, adsorbent dose 1 g/L, 40 mg/L dye concentration)
Habib et al. (2006)
Flame tree (Delonix regia) pods Crystal violet 16.70 mg/g Ramakrishna and Nagarajan (2009)
Muntingia calabura Leaves Methylene blue Methylene red Malachite green
20 mg/g 58 mg/g 32 mg/g
Santhi et al. (2009)
Water hyacinths (Eichhornia crassipes)
Acid and reactive dyes Higher N2 percent of hyacinths showed higher adsorption capacities
El-Zawahry and Kamel (2004)
Seaweed (Luminaries sp.) Reactive black 5 101.5 mg/g Vijayaraghavan and Yun (2008) Sea grass leaf sheaths Reactive red 228 80% dye removal efficiency at pH 5 Ncibi et al. (2007)
Aquatic plant (Hydrilla verticillata) Malachite green 91.97 mg/g Rajeshkannan et al. (2010) Teak tree bark Methyleme blue 333.33 mg/g Patil et al. (2011) Oak saw dust Methylene blue 38.46 mg/g El-latif et at. (2010) Barley straw Methylene blue 27.72 mg/g Abdualhamid and Asil (2011) Wheat straw Methylene blue 17.54 mg/g Abdualhamid and Asil (2011)
Oat straw Methylene blue 8.34 mg/g Abdualhamid and Asil (2011)
Papaya seeds Methylene blue 250.0 mg/g (esterified adsorbent) 200 mg/g (natural adsorbent)
Nashuha et al. (2011)
Annona squamosa seeds Methylene blue Methylene red Malachite green
8.52 mg/g 40.48 mg/g 25.91 mg/g
Santhi et al. (2010)
Hen feathers Tartrazine (azo dye) 47% (30 °C), 52% (40 °C), 55% (50 °C)
Mittal et al. (2007)
Chitosan FD&C Red n° 40 dye 1065.8 μmol/g (308 K),
1061.4 μmol/g (318 K), 800.8 μmol/g (328 K), 508.5 μmol/g (338 K)
Piccin et al. (2011)
Cotton fibres Reactive red 120 Reactive black 5
11.63 mg/g 6.22 mg/g
Gamal et al. (2010)
Commercial activated carbon Turquoise blue QG reactive bye
140.14 mg/g Schimmel et al. (2010)
CONCLUSIONS
Agricultural residues are abundantly available. For using as adsorbents, the agricultural residues are required to be properly treated. The treatments em-ployed by researchers involve physical and chemical processes such as washing, drying, size reduction, burning to produce ash, burning in the absence of oxygen to obtain char, carbonizing and specific treat-ment to effect chemical modifications. This literature review shows that it is possible to develop agricul-tural residues for use as adsorbents in colour removal from effluents. The adsorption capacity data reported in the literature indicate that dye removal through the
use of agricultural residue is feasible. Although in-tensive studies have been undertaken on the lab scale with different processes parameters, there is a need to explore the adsorption potential of the agriculture residues through pilot plant studies to establish the treatment process at commercial level.
REFERENCES
Brazilian Journal of Chemical Engineering
Technol., 2(1), 8-13 (2005).
Abdualhamid, S. A. and Asil, A. A., The effect of soaking process of agricultural wastes on the ad-sorption of Methylene blue dye. International Food Research J., 18(3), 977-981 (2011).
Akar, S. T., Akar, T. and Cabuk, A., Decolorization of a textile dye Reactive red 198 (RR 198), by As-pergillus parasiticus fungal biosorbent. Brazilian Journal of Chemical Engineering, 26(02), 399-405 (2009).
Allen, S. J. and Koumanova, B., Decolourisation of water/wastewater using adsorption (Review). J. University of Chemical Technology and Metal-lurgy, 40(3), 175-192 (2005).
Amin, K. N., Removal of reactive dye from aqueous solution by adsorption onto activated carbons pre-pared from sugarcane bagasse pith. Desalination, 223, 152-161 (2008).
Ansari, R., Khan, A. M. and Mosayebzadeh, Z., Ap-plication of unripe grape juice waste as an effi-cient low cost biosorbent for dye removal. Annals of Biological Research, 2(5), 323-328 (2011). Ashoka, H. S. and Inamdar, S. S., Adsorption
re-moval of Methyl red from aqueous solution with treated sugarcane bagasse and activated carbon- a comparative study. Global J. Environ. Res., 4(3), 175-182 (2010).
Azhar, S. S., Liew, A. G., Suhardy, D., Hafz, K. F. and Hatm, H. D. F., Dye removal from aqueous solution by using adsorbent on treated sugarcane bagasse. American J. Appl. Sci., 2(11), 1499-1503 (2005).
Babu, C. S., Chakrapani, C. C. and Rao, K. S., Equi-librium and kinetic studies of reactive red 2 dye adsorption onto prepared activated carbons. J. Chem. Pharm. Res., 3(1), 428-439 (2011).
El-Zawahry, M. M. and Kamel, M. M., Removal of azo and anthraquinone dyes from aqueous solu-tions by Eichhornia crassipes. Water Research, 38, 2967-2972 (2004).
El-Latif, M. M. A., Ibrahim, A. M. and El-Kady, M. F., Adsorption equilibrium, kinetics and ther-modynamics of Methylene blue from aqueous so-lutions using biopolymer oak sawdust composite. J. American Science, 6(6), 267-283 (2011). Fu, Y. and Viraraghavan, T., Column studies for
biosorption of dyes from aqueous solutions on immobilised Aspergillus niger fungal biomass. Water SA, 29, 465-472 (2003).
Gamal, A. M., Farha, S. A. A., Sallam, H. B., Mah-moud, G. E. A. and Ismil, L. F. M., Kinetic study and equilibrium isotherm analysis of reactive dyes adsorption onto cotton fiber. Nature and Science, 8(11), 95-110 (2010).
Gong, R., Liu, X., Feng, M., Liang, J., Cal, W. and Li, N., Comparative study of Methylene blue sorbed on crude and monosodium glutamate func-tionalized sawdust. J. Health and Sci., 54(6), 623-628 (2008).
Habib, A., Hassa, Z., Rahman, A. S. M. S. and Alam, A. S. M., Tuberose sticks as an adsorbent in the removal of Methylene blue from aqueous solution. Pak J. Environ. Chem., 7(2), 112-115 (2006). Hamissa, A. M., Ncibi, M. C., Mahjoub and Seffen,
M., Biosorption of metal dye from aqueous solu-tion onto Agave americana (L.) leaves. Int. J. En-viron. Sci. Tech., 5(4), 501-508 (2008).
Hema, M. and Arivoli, S., Comparative study on the adsorption kinetics and thermodynamics of dye onto acid activated low cost adsorbent. Interna-tional J. Physical Science, 2(1), 10-17 (2007). Ho, Y. S., Chiu, W. T. and Wang, C. C., Regression
analysis for the sorption of basic dyes on sugar-cane dust. Bioresource Technology, 96, 285-1291 (2005).
Izadyar, S. and Rahimi, M., Use of beach wood saw dust for adsorption of textile dyes. Pakistan J. Bio-logical Science, 10(2), 287-293 (2007).
Jaikumar, V. and Ramamurthi, V., Adsorption of acid yellow by spent brewery grain in a batch system: Equilibrium and kinetic modeling. International J. of Biology, 1(1), 21-29 (2009).
Jayarajan, M., Arunachalam R. and Annaduria, G., Agricultural wastes of jackfruit peel nano-porous adsorbent for removal of Rhodamine dye. Asian J. Applied Science, 43, 263-270 (2011).
Ju, D. J., Byun, I. G., Lee, C. H., An, G.H., Park, T. J., Biosorption characteristics of reactive dye onto dried activated sludge. Water Practice & Technol-ogy, 1(3), (2006).
Khandelwal, S. K. and Gaikwad, R. W., Removal of dyes from dye effluent using sugarcane bagasse ash as an adsorbent. International J. Chemical Engineering and Applications, 2(3), 309-317 (2011).
Kaushik, N., Kaushik, C. P., Tateja, R. and Sharma, J. K., Studies on adsorption of Triazine dyes by natural and chemical modified agro waste materi-als. Rasayan J. Chem., 1(4), 819-827 (2008). Kim, T. Y., Baek, S. J., Rho, S. G., Kim, S. J. and
Cho, S. Y., Adsorption characteristics of reactive dye onto biosorbent. Theories and Application of Chem. Eng., 10(2), 1402-1404 (2004).
Mafra, M. R., Igarashi-Mafra, L., Zuim, D. R., Vasques, E. C. and Ferreira, M. A., Adsorption of Remazol brilliant blue on an orange peel adsorb-ent. Brazilian J. Chemical Engineering, 30(3), 657-665 (2013).
Mahesh, S., Kumar, V. G. and Agrawal, P., Studies on the utility of plant cellulose waste for the bioadsorption of Crystal violet dye. J. Environ-ment Biology, 31, 277-280 (2010).
Mendez-Paz, D., Omil, F. and Lema, J. M., Anaero-bic treatment of azo dye Acid orange 7 under fed-batch and continuous conditions. Water Research, 39, 771-778 (2005).
Mittal, A., Kurup, L. and Mittal, J., Freundlich and Langmuir adsorption isotherms and kinetics for the removal of Tartrazine from aqueous solutions using hen feathers. J. Hazardous Materials, 146, 243-248 (2007).
Nakamura, T., Tokimoto, T., Tamura, T., Kawasaki, N. and Tanada, S., Decolourisation of acid dye by charcoal from coffee grounds. J. Health Science, 49(6), 520-523 (2003).
Nanda, G. K. and Ghole, V. S., Utilisation of ligno-cellulosic waste from bidi industry for removal of dye from aqueous solution. Int. J. of Environ-mental Research, 2(4), 385-390 (2008).
Nasuha, N., Zurainan, H. Z., Maarof, H. I., Zubir, N. A. and Amri, N., Effect of cationic and anionic dye adsorption from aqueous solution by using chemically modified papaya seed. International Conference on Environment Science and Engi-neering, IPCBEE, Singapore, 8, 50-54 (2011). Ncibi, M. C., Mahjoub, B. and Seffen, M.,
Adsorp-tive removal of textile reacAdsorp-tive dye using Posido-nia oceanica (L.) fibrous biomass. Int. J. Environ. Sci. Tech, 4(4), 433-440 (2007).
Ong, S. T., Keng, P. S., Voon, M. S., Lee, S. L. and Hung, Y. T., Sorption of basic dye from aqueous solution by durian peel (Durio zibethinus murray). World Applied Science J., 9(3), 245-249 (2010). Ong, S. T., Lee, C. K. and Zainal, Z., Removal of
basic reactive dyes using Ethylenediamine modi-fied rice hull. Bioresource Technology, 98, 2792-2799 (2007).
Parvathi, C. and Maruthavana, T., Adsorptive re-moval of Megeta MB cold brand reactive dye by modified activated carbons derived from agricul-tural waste. Indian J. Science and Technology, 3(4), 408-410 (2010).
Patil, S., Renukdas, S. and Patel, N., Removal of Methylene blue, a basic dye from aqueous solu-tions by adsorption using teak tree (Tectona gran-dis) bark powder. International J. Environmental Sciences, 1(5), 711-726 (2011).
Piccin, J. S., Dotto, G. L. and Pinto, L. A. A., Ad-sorption isotherms and thermochemical data of FD&C Red n° 40 binding by Chitosan. Brazilian J. Chemical Engineering, 28(2), 295-304 (2011). Ponnusami, V., Aravindhan, R., Karthiraj, N.,
Ramadoss, G. and Srivastawa, S. N., Adsorption of Ethylene blue onto gulmohar plant leaf powder: Equilibrium, kinetic and thermodynamic analysis. J. Environmental Protection Science, 3, 1-10 (2009). Prasad, R. N., Viswanathan, S., Devi J. R., Rajkumar,
J. and Parthasarathy, N., Kinetics and equilibrium studies on biosorption of CBB by coir pith. American-Eurasian J. Scientific Research, 3(2), 123-127 (2008).
Purai, A. and Rattan, V. K., Removal of Basic green 4 from wastewater by adsorption on biomass ash and activated carbon. Indian Chemical Engineer, 51(4), 287-299 (2009).
Rachakornkij, M., Ruangchuay, S. and Teachakulwiroj, S., Removal of reactive dyes from aqueous solu-tion using bagasse fly ash. Songklanakarin J. Sci-ence and Technology, 26 (2004).
Raghuvanshi, S. P., Singh, R. and Kaushik, C. P., Kinetics study of methyene blue bye bioadsorp-tion on baggase. Applied Ecology and Environ-mental Research., 2(2), 35-43, (2004).
Rajavel, G., Anathanarayanan, C., Prabhakar, L. D. and Palanivel, C., Removal of Dark green PLS dye from textile industrial waste through low cost carbons. Indian J. Environ. Health, 45(3), 195-202 (2003). Rajeshkannan, R., Rajasimman, M. and Rajamohan,
N., Removal of Malachite green from aqueous solution using Hydrilla verticillata - optimization, equilibrium and kinetic studies. International J. Civil and Environmental Engineering, 2(4), 222-229 (2010).
Ramakrishnan, M. and Nagarajan, S., Utilization of waste biomass for the removal of basic dyes from water. World Applied Science J., 5 (Special Issue for Environment), 114-121 (2009).
Reddy, S. S. and Kotaiah, B., Reddy, N. S. P. and Velo, M., Removal of composite reactive dye from dyeing unit effluents using sewage sludge derived activated carbon. Turkish J. Engineering and Environmental Science, 30, 367-377 (2006). Rusly, S. M. and Ibrahim, S., Adsorption of textile
reactive dye by palm shell activated carbon, Re-sponse Surface Methodology. World Academy of Science, Engineering and Technology, 67, 892-895 (2010).
Brazilian Journal of Chemical Engineering
Schimmel, D., Fagnani, K. C., Santos, J. B., Barros, M. A. S. D. and Silva, E., Adsorption of turquoise blue QG reactive bye commercial activated car-bon in batch reactor: Kinetic and equilibrium studies. Brazilian J. Chemical Engineering, 27(2), 289-298 (2010).
Sharma, J. and Janveja, B., A study on removal of Congo red dye from the effluent of textile indus-try using rice husk activated by steam. Rasayan J. Chemistry, 1(4), 653-958 (2008).
Singh, D. K. and Rastogi, K., Adsorptive removal of basic dyes from aqueous phase onto activated car-bon of used tea leaves: A kinetic and thermody-namic study. J. Environmental Science and Engi-neering, 46(4), 293-302 (2004).
Sivakumar, P. and Palanisamy, N., Mechanistic study of dye adsorption on to a novel non-conventional low-cost adsorbent. Library Advances in Applied Science Research, 1(1), 58-65 (2010).
Sreelatha, G. and Padmaja, P., Study of removal of cationic dyes using palm shell powder as adsorb-ent. J. Environmental Protection Science, 2, 63-71 (2008).
Tahir, H., Hammed, U., Jahanzeb, G. and Sultan, M., Removal of fast green dye from and aqueous solution using Azadirachta leaf powder as low cost adsorbent. Asian Technology of Biotech-nology, 7(21), 2906-2911 (2008).
Theivarasu, C. and Mylsamy, S., Equilibrium and kinetic adsorption studies of Rhodmine-B from aqueous solutions using cocoa (Theobroma
cacao) shell as a new adsorbent. International J. Engineering, Science and Technology, 2(11), 6284-6292 (2010).
USEPA, Profile of Textile Industry (Publication No. EPA/310-R-97-009). U.S. Environmental Protec-tion Agency, Washington, 40-41 (1997).
Velmurugan, P., Kumar V. R. and Dhinakaran, G., Dye removal from aqueous solution using low cost adsorbent. Int. J. Environmental Science, 1(7), 1492-1503 (2011).
Vijayaraghavan, K. and Yun, Y. S., Biosorption of C.I. Reactive Black 5 from aqueous solution using acid-treated biomass of brown seaweed
laminaria sp. Dyes and Pigments, 76, 726-732 (2008).
Won, S. W., Kim, H. J., Choi, S. H., Chung, B. W., Kim, K. J. and Yun, Y. S., Performance, kinetics and equilibrium in biosorption of anionic dye Re-active black 5 by the waste biomass of Coryne-bacterium glutamicum as a low-cost biosorbent. Chemical Engineering Journal, 121, 37-43 (2006). Won, S. W., Choi, S. B. and Yun, Y. S., Performance
and mechanism in binding of Reactive orange 16 to various types of sludge. Biochemical Engineer-ing Journal, 28, 208-214 (2006a).
Wong, S. Y., Tan, Y. P., Abdullah, A. H. and Ong, S. T., The removal of basic and reactive dyes using quartenised sugar cane bagasse. J. Physical Sci-ence, 20(1), 59-74 (2009).