Contents lists available atScienceDirect
Cell Calcium
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / c e c a
Glucose-induced calcium influx in budding yeast involves a novel calcium
transport system and can activate calcineurin
Silvia Groppi
a, Fiorella Belotti
a, Rogelio L. Brandão
b, Enzo Martegani
a, Renata Tisi
a,∗ aDep. Biotechnology and Biosciences, University of Milano-Bicocca, Milan, ItalybLaboratório de Biologia Celular e Molecular, Núcleo de Pesquisas em Ciências Biológicas, Escola de Farmácia, Universidade Federal de Ouro Preto, Ouro Preto, MG, Brazil
a r t i c l e
i n f o
Article history:
Received 21 July 2010
Received in revised form 15 February 2011 Accepted 17 March 2011
Available online 21 April 2011
Keywords:
Phospholipase C PLC1
Saccharomyces cerevisiae
a b s t r a c t
Glucose addition to glucose-starvedSaccharomyces cerevisiaecells triggers a quick and transient influx of calcium from the extracellular environment. In yeast at least two different carrier systems were identified: a high affinity system, requiring Cch1 channel, and a low affinity system. Here we report that another calcium transport system exists in yeast, not yet identified, that can substitute the two known systems when they are inactivated. This system was called GIC (for Glucose Induced Calcium) system and it is a high affinity calcium transport system, magnesium-sensitive but nickel-resistant. Moreover, GIC transport is sensitive to gadolinium and nifedipine, but it is not sensitive to inhibition by verapamil, which conversely behaves as an agonist on glucose response.
GIC transport is fully functional in conditions when calcineurin is active, a serine/threonine specificity phosphatase involved in the regulation of calcium homeostasis and in many other cellular phenomena such as tolerance to high concentrations of Na+and Li+, response to pheromones and gene transcription
regulation. Here it is reported for the first time that calcineurin can also be activated by nutrients: the activation of Crz1 transcription factor by calcineurin was observed in derepressed cells after addition of glucose in the presence of extracellular calcium.
© 2011 Elsevier Ltd. All rights reserved.
1. Introduction
As in all eukaryotic cells, in budding yeast cytosolic calcium con-centration is typically maintained at low levels (sub-micromolar) by calcium homeostasis mechanisms. Stimulus-dependent open-ing of Ca2+ channels in plasma membrane and/or in internal compartments triggers a rapid increase in calcium concentration in the cytosol, which represents a signal with specific spatial and tem-poral dynamics. In yeast, calcium signalling is essential for survival during conjugation, ion stress resistance, cell cycle progression, osmotic shock, vacuoles fusion[1–4].
At least two independent calcium influx systems were identified and characterized for calcium influx during mating process: a high affinity, low capacity, influx system (HACS), composed essentially of Mid1 and Cch1 proteins, and a low-affinity, high capacity, influx system (LACS), not yet fully characterized at the molecular level.
Abbreviations:GIC, glucose induced calcium; HACS, high affinity calcium influx system; LACS, low affinity calcium influx system; SM, synthetic medium; YPD, yeast extract peptone glucose medium.
∗Corresponding author at: Dip. Biotecnologie e Bioscienze, Università di Milano-Bicocca, P.za della Scienza 2, 20126 Milan, Italy. Tel.: +39 0264483522;
fax: +39 0264483551.
E-mail address:renata.tisi@unimib.it(R. Tisi).
In minimal medium, HACS is primarily responsible for pheromone induced calcium response, but in rich media HACS is strongly inhib-ited by calcineurin and LACS becomes essential for this response
[5,6].
Cch1 is homologous to the ␣1 catalytic subunits of L-type voltage-gated Ca2+ channels in mammals, and Mid1 appears to function as a regulatory subunit in yeast[7]. Cch1 and Mid1 are both required for HACS to be stimulated (up to 25-fold) in situ-ations causing depletion of secretory Ca2+pools, in a regulatory mechanism related to Capacitative Calcium Entry (CCE) in animal cells. Cch1 and Mid1 are likely both parts of the same calcium ion channel, with Cch1 forming the pore and Mid1 acting as a regu-latory subunit. Interestingly, recent evidence has suggested that Mid1 might be a stretch response activator of calcium influx, ini-tiating uptake in response to cell swelling[8]. HACS is stimulated also in Golgi Ca2+pumppmr1 mutants and in wild-type strains overexpressing either the vacuolar Ca2+-ATPase Pmc1 or the vac-uolar H+/Ca2+ exchanger Vcx1[9], in response to depolarization and to hypotonic shock[10], in case of ER stress or depletion of Ca2+stores in the ER/secretory pathway[11], and is required for cold stress and iron tolerance[12]. Calcineurin is known to inhibit channel activity, possibly through direct dephosphorylation. Only
Fig. 1protein has been identified up to now as a regulator or a com-ponent of LACS[13].Fig. 1is N-glycosylated and localizes to the plasma membrane via its four predicted transmembrane helices,
0143-4160/$ – see front matter© 2011 Elsevier Ltd. All rights reserved.
49 (2011) 376–386 377
Table 1
Yeast strains used in this study.
Strain Main genotype Source or reference
K601 MATa ade 2-1 can 1-11 his 3-11,15 leu 2-3,112 trp 1-1 ura 3-1 [9]
ELY117 K601cch1::TRP1 [9]
ELY138 K601mid1::LEU2 [9]
ELY151 K601mid1::LEU2 cch1::TRP1 [9]
RT960 K601yvc1::Sp his5+ This work
RT961 K601mid1::LEU2 yvc1::Sp his5+ This work
RT962 K601cch1::TRP1 yvc1::Sp his5+ This work
RT963 K601mid1::LEU2 cch1::TRP1 yvc1::Sp his5+ This work
RT970 K601fig1::Sp his5+ This work
RT971 K601mid1::LEU2 fig1::Sp his5+ This work
RT972 K601cch1::TRP1 fig1::Sp his5+ This work
RT973 K601mid1::LEU2 cch1::TRP1 fig1::Sp his5+ This work
RT974 K601mid1::LEU2 cch1::TRP1 fig1::Sp his5+yvc1::kanMX4 This work
RT1000 K601fig1::Sp his5+yvc1::kanMX4 This work
RT990 MATa ade 2-1 can 1-11 his 3-11,15 leu 2-3,112 trp 1-1 ura 3-1 cdc35::KanMX pde2::TRP1 yak1::LEU2 [44]
RT1170 K601cnb1::Sp his5+ This work
RT1174 K601mid1::LEU2 cch1::TRP1 fig1::Sp his5+cnb1::KanMX This work
characteristic of the PMP-22/EMP/MP20/Claudin protein super-family.Fig. 1, whose expression is strongly induced upon activation of pheromone signalling pathway, promotes Ca2+influx and eleva-tion of cytosolic free Ca2+concentration upon exposition to mating factor[13]and promotes mating factor–dependent programmed cell death independently of Ca2+[14], but up to now any calcium transport activity has been reported for this protein.
Saccharomyces cerevisiaeresponds with a transient elevation in cytosolic calcium not only to exposure to pheromones, but also to addition of hexoses after carbon source limitation. The glucose induced calcium increase requires glucose phosphoryla-tion[15,16], phospholipase C activity and Gpa2-associated glucose receptor Gpr1[17,18]and leads to plasma membrane H+-ATPase activation[19]. GIC response is mediated mainly by an influx of cal-cium from the extracellular space, which was reported to require Mid1 channel in synthetic minimal medium[18]but not in rich media[19]. Here we show that an additional channel exists in yeast that is responsible for glucose induced calcium influx in rich media. In yeast, excess calcium is removed from the cytosol by the vacuolar H+/Ca2+ antiporter Vcx1 and Ca2+-ATPase Pmc1, as well as the Golgi-located Ca2+-ATPase Pmr1. The calcium-calmodulin/calcineurin signal transduction pathway stimulates the activities of both Pmc1 and Pmr1, whereas it represses the activity of Vcx1 [20–22]as well as it represses HACS activity, as men-tioned above. Calcineurin is a Ca2+/calmodulin-dependent protein phosphatase required for yeast to adapt to a variety of environ-mental stresses[23], primarily via calcium homeostasis regulation and transcriptional control exerted by the calcineurin activated transcription factor Crz1[24]. Calcineurin involvement in glucose response is here investigated.
2. Materials and methods
2.1. Strains and cultural conditions
Cells were grown in YPD, containing 2% glucose, 2% tryptone, 1% yeast extract and 2% agar by Biolife, USA, supplemented with 50 mg/l adenine, or in minimal medium, containing 2% glucose, 0.67% YNB (Difco, USA) and supplemented with 50 mg/l adenine, histidine, tryptophan and leucine, with shaking, at 30◦C. Selective
solid media were prepared with complete supplemented syntheti-cal medium, containing 2% glucose, 0.67% YNB, the appropriate CSM drop–out (BIO101, USA) according to supplier instructions, 2.5% agar (Biolife, USA), supplemented with 50 mg/l tryptophan. Cells density was determined by measuring optical density (OD600) or by Coulter Counter (Coulter Electronics Z2).
2.2. Plasmids and strains construction
YVC1, FIG1 and CNB1 genes were deleted in wild-type and mutant strains (Table 1) using a disruption cassette generated by PCR according to the method first described by Wach et al.[25], using pFA6a-His3MX6 plasmid[26], containing an expression cas-sette for the heterologous markerhis5+fromSchizosaccharomyces, as a template (primers
ATTCAGTTATAAAATATAATAT-TACTAGAACAGGAGCATTCGGATCCCCGGGTTAATTAA and
TTCTGAGAAATTAATTAAGCAGTATTTGAACACATGTCGTTGGATCT-GATATCATCGATG for YVC1 deletion, GTAAACAAACAAACAAA-CAAACAAAAAAAAAAAAAAAAAACGGATCCCCGGGTTAATTAA and TTTTATCCTCAAATAAACATATAAGTTTTGAGCGGATATTTGGATCTG-ATATCATCGATG forFIG1deletion;
TTAAAAATCACTAGTTTCTTTTT-TAGCGGAATGCAATAAACGGATCCCCGGGTTAATTAA and
CGTATTATTCTTCTTTTCTTAAAAATATTGGCATACCATATGGATCT-GATATCATCGATG for CNB1 deletion). The deleted strains were selected on synthetic complete medium lacking histidine and the integration of the cassette disruption in the correctlocuswas verified by PCR.
YVC1andCNB1deletion in themid1::LEU2 cch1::TRP1 fig1:: Sp his5+mutant strain were performed by using a disruption cassette generated by PCR using pFA6a-KanMX4[26]as template and the same primers described above. The deleted strains were selected on YPD medium with 500g/ml G418 and 400g/ml G418 respec-tively and the integration of the cassette and disruption at the correctlocuswere verified by PCR.
For luminescence assays yeast cells were transformed by lithium acetate method with the multicopy pVTU-AEQ plasmid
[19]. For-galactosidase assays yeast cells were transformed with multicopy pAMS366 plasmid kindly provided by M. Cyert (Stanford University)[27], containing4xCDRE::lacZreporter.
2.3. Bioluminescence assay
Exponentially growing cells (5–6×106cells/ml) in YPD or in minimal medium at 30◦C, were harvested by filtration on
nitro-cellulose filters (Millipore, pore sizes 0.22m), washed twice with cold water (1 volume/wash) and then resuspended in 0.1 M 2-(N-morpholino)-ethanesulphonic acid (MES)/Tris, pH 6.5 (MES/Tris buffer) at a density of about 108cells/ml.
reconstitute functional aequorin, 50M coelenterazine (Molecular Probes; stock solution 1g/l dissolved in 99.5% methanol, con-served in the dark at−20◦C) was added to the cell suspension and
the cells were incubated for 20 min at room temperature in the dark. Excess coelenterazine was removed by washing cells three times with 0.1 M MES/Tris buffer pH 6.5 (200l/wash) and by cen-trifugation at 7000 rpm for 1 min. For each treatment, 200l of the cell suspension (in 0.1 M MES/Tris buffer pH 6.5) were transferred to a luminometer tube, in the presence of solution of CaCl2,of the calcium chelator ethylene glycol tetraacetic acid (EGTA) and of the indicated metals (stock solutions prepared in 0.1 M MES/Tris buffer pH 6.5) or inhibitors (gadolinium chloride hexahydrate, Nifedip-ine dissolved in DMSO, Verapamil hydrochloride, Sigma–Aldrich), at the final concentrations indicated in the figures. Light emission was monitored with a Berthold Lumat LB 9507 at intervals of 10 s for 1 min before and for at least 6 min after the addition of glu-cose to a final concentration of 100 mM, and reported in relative luminescence units/s (RLU/s).
At the end of each experiment aequorin expression and activity was tested by lysing cells with 0.5% Triton X-100 in the presence of 10 mM CaCl2(stock solution 0.1 M in MES/Tris buffer pH 6.5) moni-toring light emission for 18 min. This maximum intensity was used to normalize light emission according to the amount of aequorin expressed.
Free calcium concentration in the presence of chelators was determined using an on-line available MaxChelator program[28]. Dose–response curves were constructed by calculating the ini-tial shape of the peak in light emission curve, and plotting it in a semi-log plot as a function of external calcium concentra-tion. ApparentKMwas calculated by fitting the curve with a Hill function in the form y=y0+ (ymax−y0)[Ca2+]/(KM+ [Ca2+]). Com-petition curves were constructed by plotting the percentage of inhibition of the maximal response as a function of the con-centration of the added competitive metal. Apparent IC50 was calculated by fitting the curve with a Hill equation in the form I(Me) = [1 + (IC50/[Me])n]−1, where IC50is the concentration at half maximal block, andnis the Hill coefficient.Kiwas calculated by plotting apparentKMvalues, calculated in the presence of differ-ent concdiffer-entrations of metal on Lineweaver–Burk plots, on inhibitor concentration, and fitting them to the linear equation: apparent
KM=KM(1 + [I]/Ki).
2.4. Calcineurin reporter assay
Exponentially growing cells (5–6×106cells/ml) in YPD or mini-mal medium, with or without addition of either 3 mM cAMP, 1 mM CaCl2or 1 mM EGTA when indicated, were harvested by filtration on nitrocellulose filters (Millipore, pore sizes 0.22m), washed twice with cold water (1 volume/wash) and then resuspended in 0.1 M 2-(N-morpholino)-ethanesulphonic acid (MES)/Tris, pH 6.5 (MES/Tris buffer) at a cell density of 1.25×107cells/ml. To mea-sure the-galactosidase activity in exponentially growing cells, the method described by Kiechle et al.[29]was used. Briefly, 200l of the cell suspension (‘exponentially growing’ treatment) was taken immediately after the filtration, cells were collected by centrifu-gation at 13000 rpm for 2 min, resuspended in 550l of pre-cold Z-buffer (75 mM Na2HPO4, 50 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4) and quickly frozen in dry ice and conserved at−80◦C.
The remaining cell suspension was incubated for 1.5 h at room temperature and then 200l-aliquots were separated and collected either immediately after starvation (‘90-min starved’ treatment), or after the induction with 100 mM glucose for 1 h at room temperature in the presence of 1 mM CaCl2(‘CaCl2+ glc’ treat-ment) or 1 mM ethylene glycol tetraacetic acid (EGTA) (‘EGTA + glc’ treatment) and processed as described above.
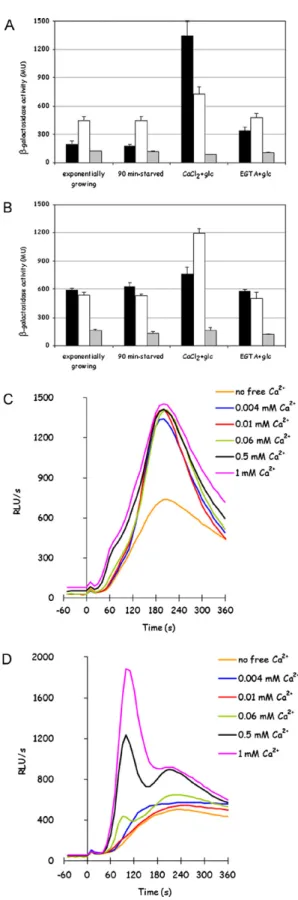
Fig. 1.Glucose-induced calcium response is different in rich medium. Apoaequorin-expressing K601 cells exponentially growing in rich medium were collected, washed by filtration and resuspended in Mes/Tris buffer. After aequorin reconstitution with coelenterazine (see Section2), light emission was registered before and after 100 mM glucose addition, in presence of the indicated extracellular free calcium con-centrations. The apparentKMfor calcium of the GIC response in YPD media grown wild-type K601 cells, evaluated as described in Section2, is 56.2±4.2M (panel B).
For detection, all samples were simultaneously thawed, a 100l-aliquot was removed and used to determine the OD600. The remaining cells were incubated for 1 h at 37◦C with 100L
zymolyase solution (zymolyase T20, ICN, USA; 0.5 mg/ml in Z-buffer). To measure -galactosidase activity, 100l of 4 mg/ml CPRG (chlorophenol-red--D-galactopyranoside, Sigma–Aldrich) in Z-buffer were added to each sample and after incubation for suitable time at 37◦C the reaction was stopped with 200l of 1 M
Na2CO3. Then samples were centrifuged at 13000 rpm for 15 min and the supernatant was read at 574 nm.-galactosidase activ-ity was calculated as follows and expressed in Miller Units (MU): MU = [(OD574−OD574ctrl)×1000]/(OD600×tinc). Each value is the average of two independent extracts of the same strain. The exper-iments were repeated at least three times giving similar results.
3. Results
3.1. Glucose-induced calcium (GIC) signalling is modulated by nutrients
49 (2011) 376–386 379
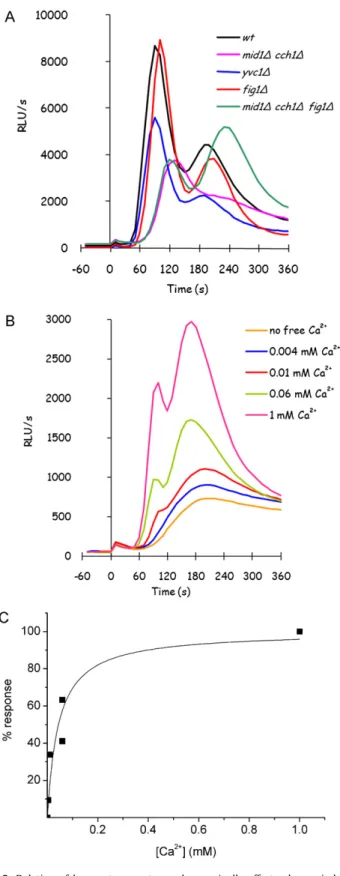
strain, where two peaks are usually observed, the first and gen-erally higher at 60 s and a second one at 180 s, but this is only typical for this genetic background. The absence of extracellular calcium reduces calcium peak to a very faint signal, indicating that calcium response depends mainly on calcium influx from the exter-nal medium. This response is also depending on exterexter-nal calcium concentration (Fig. 1A).
While Mid1/Cch1 transporter was characterized, in cells over-expressing both the proteins, as a unique channel with aKMof 12M[30], LACS system was reported with aKM of∼3 mM in response to pheromones[5]. Glucose-induced calcium response is unlikely involving any such low affinity system: though the quite large standard deviation, intrinsically related to bioluminescence methods, the estimation ofKMin the YPD grown wild-type strain was 56.2±4.2M (Fig. 1B), suggesting that the systems involved in this response are high affinity calcium transport systems.
3.2. Different transporters are involved in glucose-induced calcium influx
In order to identify the transporters involved in GIC response,
FIG1gene, encoding the only component of the LACS system iden-tified up to now, was deleted either alone or together withMID1
andCCH1genes.
Glucose-induced calcium response was then analyzed inmid1,
cch1,mid1cch1,fig1andmid1cch1fig1mutants. As can be observed inFig. 2A, none of the mutants showed a com-pletely impaired response to glucose when they were grown in rich medium, suggesting that the contributions of the HACS and LACS systems to this calcium influx are not essential in rich media: deletion of HACS subunits affected the signal more seriously than
FIG1deletion, which has a negligible effect, suggesting a role for HACS but not for LACS in this calcium influx. Actually, HACS sys-tem was reported to be repressed by calcineurin in rich media[5], whileFig. 1is poorly expressed during exponential growth[13].
Anyway, the deletion of HACS subunit/s or of LACS compo-nent didn’t affect the biphasic signature of the response, that was affected only by co-deletion of HACS and LACS components in the
mid1cch1fig1mutant: in this mutant, in fact, the later peak at 180 s becomes the major component of the response.
A different transport system is thus expected to drive the main part of glucose-induced calcium response in such conditions, which we will refer to as GIC (Glucose-Induced Calcium) transporter here-after.
As it was found for the wild-type strain, inmid1cch1fig1
mutant GIC mediated response is still completely dependent on phospholipase C activity, since it is abolished by addition of the phospholipase C inhibitor, 3-nitrocoumarin[31](data not shown); moreover, in this mutant glucose-induced calcium influx is still appreciable even in the presence of external sub-millimolar cal-cium concentrations (Fig. 2B), with an intensity comparable to the wild-type strain, indicating that the unknown transporter is not a low affinity transporter. In fact, apparentKMfor calcium in themid1cch1fig1mutant GIC response was calculated as 43.8±10.3M (Fig. 2C), not far from theKM calculated for the wild-type strain in the same conditions.
The involvement of an intracellular, vacuolar calcium channel, Yvc1, was also investigated. In yeast, vacuole serves as a major store for Ca2+, for the purposes of both detoxification and signalling. The Yeast Vacuolar Channel Yvc1, a homolog of the constitutively active inwardly rectifying calcium channels in mammals known as TRP (Transient Receptor Potential) channels[32], likely repre-senting a calcium-activated calcium channel, has been shown to release Ca2+from the vacuole into the cytosol in response to hyper-osmotic shock both by mechanical activation and Ca2+-induced calcium release[33]; thus, Yvc1 channel is a calcium-responsive
channel, and it is involved in the amplification of several calcium signals[34].
The yvc1mutant showed only a partial reduction in glu-cose response: our results suggest that Yvc1 could be involved in glucose-triggered calcium signal amplification also (Fig. 2A).
3.3. Glucose-induced calcium signalling is sensitive to magnesium
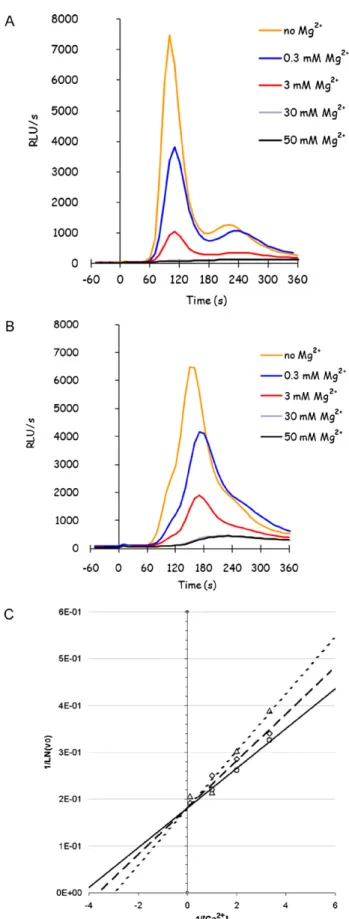
In order to better characterize the still unknown GIC transporter, the sensitivity to magnesium of the glucose-induced calcium response was tested. In fact, Mg2+is the most abundant divalent cation in cells, where it acts as a counter-ion for solutes, as a cofactor in catalytic processes and as a stabilizer for membranes, physiological structures and proteins conformation. Cellular Mg2+ concentration is in the millimolar range, 3 orders of magnitude higher than Ca2+. The two ions appear to affect each other in a competitive way: the impaired growth of yeast cells in high cal-cium environments is ameliorated by Mg2+salts in the medium. Recently, it was reported that Ca2+influx pathways in yeast become (re)activated upon withdrawal of extracellular Mg2+[35].
Exposure to different concentrations of Mg2+ revealed that glucose-induced calcium response is very sensitive to low con-centrations of magnesium, and is already significantly reduced by [Mg2+] lower than 1 mM (Fig. 3A), showing an apparent IC
50 of 0.52±0.03 mM. The sensitivity to magnesium is only partially relieved by deletion of HACS components, and is still very high in
mid1cch1fig1mutant, where the total response shows an apparent IC50of 1.04±0.05 mM (Fig. 3B). The difference in sensi-tivity is reliable, and confirms that the target of inhibition is calcium transport and not some other component of the signalling pathway. Fitting of the curves of inhibition to Hill function gavenvalues not far from 1, suggesting a simple competitive inhibition to the same ligand site. In order to verify this issue, the initial slope of GIC responses was determined in themid1cch1fig1mutant while co-varying the calcium and magnesium concentrations: the kinetics are typical of a competitive inhibition (Fig. 3C), giving aKI of 0.33±0.05 mM.
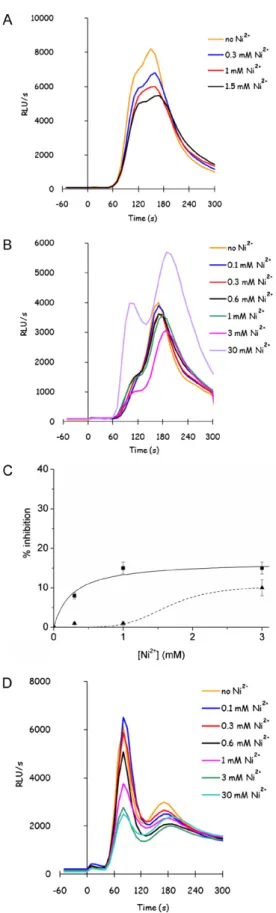
3.4. The unknown channel is nickel insensitive
GIC response sensitivity to several bivalent cations was tested in the wild-type strain and in themid1cch1fig1mutant, in order to characterise the unknown channel sensitivity to metal ions. No differences were found in the sensitivity of GIC response to Zn2+ or Mn2+in the wild-type and in the mutant strain, showing only half inhibition of the response in presence of millimolar levels of metals (data not shown), while the sensitivity to Ni2+was different. GIC response is not very sensitive to Ni2+in YPD grown wild-type strain (Fig. 4A), but it is completely resistant to submillimolar con-centrations of Ni2+in themid1cch1fig1mutant cells (Fig. 4B and C). Furthermore, 30 mM NiCl2 seems even to stimulate GIC response (Fig. 4B): in order to verify if this effect could be related on an agonist effect of Ni2+on Yvc1-dependent signal amplification, sensitivity of calcium response to Ni2+was also tested on amid1
cch1fig1yvc1mutant (Fig. 4D), revealing that Yvc1 is actually responsible for signal stimulation at higher concentrations of Ni2+. Anyway, even in the absence of Yvc1, Ni2+is not able to completely abolish GIC transport, giving a maximal inhibition of less than 30%.
3.5. GIC transport is sensitive to inhibition by gadolinium and nifedipine, but is resistant to verapamil
Several different subtypes of voltage-gated calcium channels have been identified, depending on their electrochemical and bio-physical properties. The calcium channel blockers in current use all act inhibiting calcium entry through L-type channels. Among them
Fig. 3.Glucose-induced calcium response is strongly inhibited by Mg2+.
Glucose-induced calcium response was analyzed as previously described, in presence of 1 mM CaCl2and the indicated concentration of MgCl2, in K601 (panel A) andmid1 cch1fig1(panel B) strains grown in YPD medium. The Lineweaver–Burk plot of calcium response initial slope (v0) was reported in panel C in the presence of the
49 (2011) 376–386 381
Fig. 4.Glucose-responsive unknown transporter is not sensitive to Ni2+ ions.
Glucose-induced calcium (GIC) response was analyzed as previously described, in presence of 1 mM CaCl2and the indicated concentration of NiCl2: K601 (panel A), mid1cch1fig1(panel B) andmid1cch1fig1yvc1(panel D) strains were grown in YPD medium, and the calcium response to glucose was detected as described after addition of different concentrations of NiCl2. The percentage of
inhibition of total calcium response was reported for a comparison between wild-type (squares and solid line) andmid1cch1fig1(triangles and dashed line) in panel C.
we chose the compounds that were reported to affect HACS chan-nel in yeast[30]: gadolinium, which is specific for stretch-activated ion channels; nifedipine, a dihydropyridine inhibitor, specific for blood vessels smooth muscle L-type calcium channel; verapamil, a phenylalkylamine inhibitor, effective on both vascular and cardiac L-type calcium channels[36].
Glucose-induced calcium response was investigated inmid1
cch1fig1yvc1mutant in order to get rid of any interference by intracellular calcium release from the vacuole. GIC transport was found to be extremely sensitive to gadolinium (apparent IC50 16.7±1.7M) (Fig. 5A and D) and to nifedipine (apparent IC50 0.36±0.13M) (Fig. 5B and D), while it is only slightly affected by 20M verapamil. Surprisingly, an agonist effect was observed for higher concentrations of verapamil (Fig. 5C), inducing the response up to the double than in the absence of the drug.
The inhibitory effect of nifedipine is likely to be specific for cal-cium transport, since any other target would show no difference in sensitivity in the mutant strain when compared to the wild-type: in contrast, glucose-induced calcium transport in the wild-type show an IC50for nifedipine of 1.8±0.005M, six-times higher than in the mutant strain (data not shown). Inhibition mechanism was inves-tigated by co-varying nifedipine and calcium (data not shown), which suggested a mixed competition affecting bothKMandVmax.
3.6. Glucose-induced calcium signalling is modulated by the cultural medium
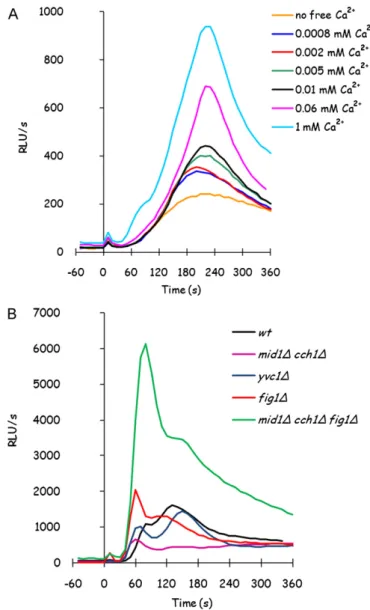
Mid1 HACS subunit was reported in literature as essential for glucose-induced calcium peak[18], but those experiments were performed in minimal medium, where the signal is much lower than when yeast cells are grown in rich media (Fig. 6A). This discrepancy could be explained by the calcineurin dependent reg-ulation of calcium transporters, that was already found for HACS system and could be important for other calcium transport systems involved in glucose response. In detail, HACS system was reported to be repressed by calcineurin in rich media[5].
In minimal medium, the effect of the deletion either ofCCH1
alone andMID1andCCH1together is more serious (Fig. 6B), con-sistently with previously observed role of HACS system in minimal medium. Surprisingly, an increase in the response was observed in minimal media-grownmid1cch1fig1mutant: this mutant makes no more difference in calcium transport either if it is grown in minimal or in rich medium (Figs. 6B and 2A). The unknown trans-porter involved in GIC response, sustaining calcium influx inmid1
cch1fig1mutant, seems to be inhibited byFig. 1protein when cells are grown in minimal medium, since it becomes fully active in this condition only inFig. 1absence.
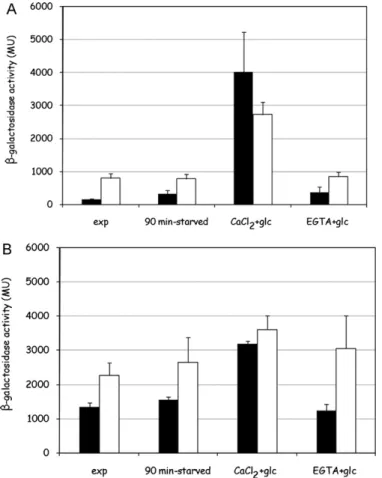
3.7. Glucose addition to minimal medium grown glucose–deprived cells activates calcineurin dependent transcription
The main effector of calcium availability in the extracellular medium is the Ca/calmodulin/calcineurin pathway, consequently the activity of calcineurin after glucose addition to nutrient deprived cells was investigated. The activation of calcineurin was detected taking advantage of a Crz1-responsive element (CDRE) in the promoter of a LacZ reporter gene [27]: once activated, calcineurin dephosphorylates the Zn-finger transcription factor Crz1/Tcn1, causing it to accumulate in the nucleus where it acti-vates gene expression, thus promoting adaptation to stress.
Fig. 5.GIC transporter is sensitive to gadolinium and to nifedipine, but not to ver-apamil. GIC response was analyzed as previously described, in presence of 1 mM CaCl2and the indicated concentration of inhibitor:mid1cch1fig1yvc1strain
was grown in YPD medium, and the calcium response to glucose was detected as described after addition of different concentrations of gadolinium (panel A), nifedip-ine (panel B) or verapamil (panel C). The percentage of inhibition of total calcium response was reported for a comparison between gadolinium (squares and solid line) and nifedipine (triangles and dashed line) in panel D.
Fig. 6.Deletion of known transporters seriously affects glucose-induced calcium response only in minimal medium. Glucose-induced calcium response was ana-lyzed as previously described, in presence of the indicated concentration of free extracellular calcium, in K601 (panel A), and in the presence of 1 mM extracellular calcium chloride in K601,mid1cch1,yvc1,fig1,mid1cch1fig1strains (panel B), grown in minimal medium.
in rich medium (Fig. 7A and B andTable 2), where the basal level of expression of the calcineurin-responsive reporter was already much higher than in minimal medium. Both the effects, higher basal level in rich medium-grown cells and glucose-responsive Crz1-dependent transcription, are calcineurin dependent since none of them is observed in a calcineurin-deficientcnb1strain (Fig. 7A and B).
The reporter gene is activated by glucose when calcium is avail-able in the external medium, but it is not activated by 1 mM extracellular calcium alone (data not shown) or by glucose when calcium is not available in the external medium, indicating that glucose-induced calcium influx is required (Fig. 7A and B). In con-trast, the reporter was equally activated in minimal medium in all the mutants in known calcium channels mentioned above (data not shown), showing that residual calcium signal is in any case sufficient to sustain the response.
49 (2011) 376–386 383
Fig. 7.Calcineurin-dependent transcription is activated by glucose re-addition after starvation to cells grown in minimal medium, but not to cells grown in rich medium. Crz1-responsive promoter activity was assayed as described in Section2in wild-type strain grown in minimal (panel A) or in rich medium (panel B), with (open bars) or without (closed bars) the addition of either 1 mM CaCl2to minimal medium or
1 mM EGTA to YPD medium during cell growth. Grey bars represent the same results obtained incnb1strain in minimal (panel A) or in rich medium (panel B). GIC response was analyzed as previously described, in presence of the indicated con-centrations of free extracellular calcium, in K601 strain grown in minimal medium with 1 mM CaCl2added (panel C) or in YPD medium with 1 mM EGTA (panel D).
Table 2
Calcineurin activation stimulation-fold in wild-type K601 strain. Crz1-responsive promoter activity was assayed as described in Section2in wild-type strain grown in the indicated media supplemented either with 1 mM EGTA or 1 mM CaCl2when
indi-cated. Stimulation-fold upon addition of 100 mM glucose in the presence of 1 mM CaCl2was calculated on at least three independent experiments.
EGTA CaCl2 Stimulation-fold
Minimal medium – – 7.1±2.4
– + 1.7±0.1
YPD medium – – 1.2±0.2
+ – 2.3±0.2
[5]. Nevertheless, among the differences between rich and minimal medium, there is also calcium availability: calcium concentration is far higher in rich media than in minimal medium, and this could be sufficient to impinge on basal Crz1-dependent response in dif-ferently cultivated cells.
In order to assess if what observed in glucose-induced cal-cineurin signals was related to the different level of calcium availability and consequent calcineurin activity during cell growth, calcineurin activity was assayed in wild-type cells either grown in YPD medium or in minimal medium, either in exponential growth, after nutrient-starvation or after glucose stimulation in presence or in absence of extracellular calcium.
Calcineurin activity dependence on calcium availability in the medium was investigated adding 1 mM CaCl2to minimal medium, in order to raise the free calcium concentration in this medium to levels comparable to YPD medium, or 1 mM EGTA to YPD medium, in order to low down the concentration of free calcium in this medium: these modifications were aimed to abolish the difference in calcium content in the two cultural media. Cells exponentially growing in these modified media were collected, starved for nutrients for 2 h and then tested for calcineurin depen-dent transcriptional activity. As can be observed inFig. 7A and
Table 2, calcium availability during growth in minimal medium caused a rise in basal Crz1-dependent transcriptional activity, as expected, but most of all strongly inhibited stimulation upon glu-cose addition: this indicates that high basal calcineurin activity counteracts its glucose-responsiveness. Conversely, EGTA addition to YPD medium only marginally rescued calcineurin susceptibil-ity to glucose, and had almost no effect on Crz1 basal activsusceptibil-ity level (Fig. 7B andTable 2). This could be due to inefficient calcium chelat-ing by EGTA, but this is unlikely since the same treatment showed an evident effect on calcium signalling (see later), and actually a partial relief in glucose-triggered calcineurin-dependent transcrip-tional activation is observed in YPD/EGTA grown cells (Fig. 7B).
The effect of calcium content in the medium on glucose-induced calcium response was also analyzed, to reveal if modifications in calcium flux could justify what observed in glucose-induced Crz1-dependent transcription. As above, wild-type cells were grown either in minimal medium with the addition of 1 mM CaCl2, or in YPD medium in the presence of 1 mM EGTA, then starved for nutrients and exposed to glucose in the presence of extracellular calcium. As Crz1-dependent transcription, GIC response was also affected by external calcium availability during cell growth: EGTA added to YPD medium exposed cells to low calcium availability during growth, and GIC response became lower, even if the shape of peaks and their sensitivity to extracellular calcium did not change (Fig. 7D). In contrast, GIC response was only slightly enhanced in wild-type cells grown in minimal medium with calcium added (Fig. 7C).
antagonis-Fig. 8.PKA activity is involved in the inhibition of glucose-induced Crz1-dependent transcription in cells grown in YPD medium. Crz1-responsive promoter activity was assayed as described in Section2incyr1pde2yak1strain grown in minimal medium (panel A) or in rich medium (panel B), with (open bars) or without (closed bars) the addition of 3 mM cAMP in the cultural medium.
tically in yeast: actually, PKA opposes calcineurin signalling by directly phosphorylating the Crz1 nuclear localization signal (NLS), thereby preventing its import into the nucleus[37]. In order to ana-lyze the contribute of PKA signalling on glucose activation of CDRE promoter, acyr1pde2yak1strain was tested in minimal and in rich medium with or without the addition of 3 mM cAMP in the growth medium: since this strain is cAMP permeable, the presence of cAMP activates PKA even in the absence of a functional adenylate cyclase. The Crz1-dependent promoter was activated by glucose in minimal medium cultured mutant cells, like in the wild-type strain (Fig. 8A andTable 3). However, incyr1pde2yak1strain, grown in rich medium, CDRE promoter activation is responding to glucose more than in the wild-type strain, although weakly (Fig. 8B). The inhibitory effect of PKA on Crz1 transcription factor can be observed when cAMP is added to these mutant cells, but the response is not completely abolished (Table 3).
Table 3
Calcineurin activation stimulation-fold in cyr1 pde2 yak1 strain. Crz1-responsive promoter activity was assayed as described in Section2incyr1pde2 yak1strain grown in the indicated media supplemented or not with 3 mM cAMP when indicated. Stimulation-fold upon addition of 100 mM glucose in the presence of 1 mM CaCl2was calculated on at least three independent experiments.
3 mM cAMP Stimulation-fold
Minimal medium – 13.3±1.5
+ 3.6±1.0
YPD medium – 2.1±0.2
+ 1.4±0.3
Fig. 9.Calcineurin deficiency affects GIC transport functionality. GIC response was analyzed as described in Section2in thecnb1(A) and in themid1cch1fig1 cnb1(B) mutant strains grown in YPD medium.
Taken together, these results suggest that both factors, cal-cium levels in external medium and PKA activity, seem to act on calcineurin-dependent transcriptional activity regulating its responsiveness to nutrient signals.
3.8. Calcineurin is required for nutrient availability dependent regulation of functionality of calcium transporters
49 (2011) 376–386 385
major contribution of the HACS transport, characterized by higher affinity than GIC system itself.
4. Discussion
Several yeast proteins show homology with human calcium channels that have been implicated in channelopathies, allowing for study of the genotype/phenotype correlation in several diseases
[38].
Recently, conservation between yeast calcium system and mammalian cardiac myocytes was described. This suggests that knowledge on calcium transport in yeast can be used to help understanding of cardiac disease [39] or to create a model for channelopathies research. Thus, insights in calcium homeosta-sis/signalling in yeast can be very useful to understand homologous systems in other eukaryotic cells.
Electrophysiological recordings of ion channel activity in the plasma membrane of live yeast cells require non-physiological modification of the cells in order to remove the cell wall, but genetic and cellular biology tools available in yeast have allowed to dissect the different calcium transporters as they are mainly involved in responses induced by different stimuli: like pheromone-induced calcium influx, glucose-induced calcium influx involves several transporters on the plasma membrane. Two proteins, Mid1 and Cch1, composing a high affinity calcium transport system (HACS), are required for GIC response in cells growing in minimal medium (Fig. 2). This is not true for cells growing in rich medium, which again sustains the hypothesis of a nutrient-dependent regulation of calcium transporters, relying on different calcineurin activity related to nutrient and calcium availability in the medium[5]. Consistently, glucose-induced calcium influx response is very sen-sitive to cultural conditions, being much higher in YPD growing cells than in minimal medium cultured cells (Figs. 1 and 2). This is probably related to a differential expression or functionality of calcium transporters: HACS system was already reported to be negatively regulated by calcineurin in rich media[5]. Another cal-cium specific, high affinity (KM ∼50M) calcium transport has been identified in this work, physiologically fully working in rich medium but not in minimal medium. This putative calcium flux was defined as Glucose Induced Calcium flux (GIC). Differently from known calcium transporters in yeast it is rather resistant to nickel (Fig. 5); GIC transport is also resistant to verapamil, which acts as an agonist at high concentration. Conversely, GIC channel is sensitive to magnesium, with an apparent IC50of 1.04±0.05 mM (Fig. 4), to gadolinium (apparent IC5016.7±1.7M) and to nifedip-ine (apparent IC500.36±0.13M) (Fig. 5). This is peculiar since both verapamil and nifedipine are inhibitors of L-type voltage-gated calcium channels, even if with a slightly different specificity. Anyway, these two classes of inhibitors act on different target sites in L-type voltage-gated channels[36], consequently there could be no relation between their effect on a non-conventional calcium channel. This peculiar pharmacological trait in fact could suggest that GIC transport would rely on a novel class of calcium chan-nels; consistently,S. cerevisiaegenome does not encode for any protein homolog to known calcium channels, besides the already characterized channels described above.
Wild-type yeast growing in rich medium was previously reported to accumulate calcium primarily by a low-affinity Ca2+ uptake system. Raising extracellular Mg2+from 1 to 10 mM strongly inhibited a low affinity calcium transport, exposing a Mg2+ -resistant high affinity Ca2+ uptake system [9]. More recently, a mathematical model was constructed to reproduce calcium transients in yeast [40] which suggested the existence of two Mg2+-sensitive influx pathways (indicated as transporter X and transporter M), both targets of rapid Ca2+-dependent feedback
inhibition. Computational analysis revealed the existence of a transporter, called X, which should be inhibited by magnesium at very low concentrations and characterized by high affinity for calcium, consistently with the characteristic revealed for the trans-porter involved in glucose response.
GIC transport seems to be fully functional in rich medium, and almost completely inactivated in minimal medium. Glucose-induced calcium signal seems to be enhanced by calcineurin activity, since calcium addition to minimal media activates calcineurin-dependent transcription and can rise the faint calcium influx which is typical in this medium: it is possible that calcineurin is responsible for the responsiveness of GIC channel, which is in fact fully functional only in rich medium (Fig. 2), where calcineurin activity is higher (Fig. 7). In fact, calcineurin activity deficiency implies the severe inhibition of GIC transport in amid1cch1
fig1cnb1strain (Fig. 9B).
Molecular identification of GIC channel is not a trivial challenge, since no genes encoding mammalian calcium channels homologs were identified inS. cerevisiaegenome, besidesCCH1and YVC1, either in literature or by bioinformatic researches we have per-formed (data not shown). Anyway, Mid1 itself, which was reported to generate a stretch-activated calcium channel when expressed in mammalian cells, doesn’t show any evident similarity to mam-malian calcium channels. Further work on genetic screenings will take advantage of GIC channel pharmacological properties here identified.
Activation of calcineurin has been recognized as being essential for survival under diverse stress conditions, such as pheromones or ion-induced stress [23], which is in contrast with the effect of nutrient availability on calcineurin-responsive Crz1 transcrip-tion factor: here it is reported for the first time that calcineurin can be responsive to nutrients. A relation between calcineurin and carbon sources was already suggested[41], since many genes encoding carbohydrate-metabolizing enzymes were reported to be regulated by calcium/calcineurin pathway. Consistently, PKA and calcineurin signalling converge on regulation of Crz1 transcription factor. The effect on glucose-induced Crz1-dependent transcrip-tional response of these two main regulators of yeast metabolism was considered, in order to clarify their role in regulating calcium influx effect in these two nutritional conditions, revealing that the effect of glucose-induced calcium influx on calcineurin-dependent transcription is counteracted not only by PKA, as expected, but also by calcium availability in the medium during exponential growth. The phosphoinositide specific phospholipase C Plc1 acts together with the membrane receptor Gpr1 and the associated G␣protein Gpa2 in a pathway separated from Ras1/Ras2 and converging on activation of adenylate cyclase and PKA[42]:plc1strain displays phenotypes characteristic of cells with decreased PKA activity, such as increased expression of stress-responsive genes mediated by decreased PKA-mediated inhibitory phosphorylation of Msn2 stress-responsive transcription factor[42], while calcium stress seems to act both on Msn2/Msn4 transcription factors and Snf1 pro-tein kinase regulatory phosphorylation[43]. However, the complex interaction of glucose-induced calcineurin activation and classi-cal glucose activated pathways (Snf3/Rgt2-1, Snf1/Mig1, and PKA pathways) is far from being elucidated and deserves further study.
Conflict of interest statement
There are no conflicts of interest concerning this work.
Acknowledgements
References
[1] N. Rispail, D. Soanes, C. Ant, R. Czajkowski, A. Grünler, R. Huguet, E. Perez-Nadales, A. Poli, E. Sartorel, V. Valiante, M. Yang, R. Beffa, A. Brakhage, N. Gow, R. Kahmann, M. Lebrun, H. Lenasi, J. Perez-Martin, N. Talbot, J. Wendland, A. Di Pietro, Comparative genomics of MAP kinase and calcium-calcineurin sig-nalling components in plant and human pathogenic fungi, Fungal Genet. Biol. 46 (2009) 287–298.
[2] H. Iida, Y. Yagawa, Y. Anraku, Essential role for induced Ca2+influx followed by
[Ca2+]
irise in maintaining viability of yeast cells late in the mating pheromone
response pathway. A study of [Ca2+]
iin singleSaccharomyces cerevisiaecells
with imaging of fura-2, J. Biol. Chem. 265 (1990) 13391–13399.
[3] P. Kraus, J. Heitman, Coping with stress: calmodulin and calcineurin in model and pathogenic fungi, Biochem. Biophys. Res. Commun. 311 (2003) 1151–1157.
[4] R. Burgoyne, M. Clague, Calcium and calmodulin in membrane fusion, Biochim. Biophys. Acta 1641 (2003) 137–143.
[5] E. Muller, E. Locke, K. Cunningham, Differential regulation of two Ca(2+) influx systems by pheromone signaling inSaccharomyces cerevisiae, Genetics 159 (2001) 1527–1538.
[6] M. Bonilla, K. Cunningham, Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast, Mol. Biol. Cell 14 (2003) 4296–4305.
[7] M. Bonilla, K. Nastase, K. Cunningham, Essential role of calcineurin in response to endoplasmic reticulum stress, EMBO J. 21 (2002) 2343–2353.
[8] H. Yoshimura, T. Tada, H. Iida, Subcellular localization and oligomeric structure of the yeast putative stretch-activated Ca2+channel component Mid1, Exp. Cell
Res. 293 (2004) 185–195.
[9] E. Locke, M. Bonilla, L. Liang, Y. Takita, K. Cunningham, A homolog of voltage-gated Ca(2+) channels stimulated by depletion of secretory Ca(2+) in yeast, Mol. Cell Biol. 20 (2000) 6686–6694.
[10] A. Batiza, T. Schulz, P. Masson, Yeast respond to hypotonic shock with a calcium pulse, J. Biol. Chem. 271 (1996) 23357–23362.
[11] M. Bonilla, K. Cunningham, Calcium release and influx in yeast: TRPC and VGCC rule another kingdom, Sci. STKE 2002 (2002) PE17.
[12] E. Peiter, M. Fischer, K. Sidaway, S. Roberts, D. Sanders, TheSaccharomyces cere-visiaeCa2+channel Cch1p Mid1p is essential for tolerance to cold stress and
iron toxicity, FEBS Lett. 579 (2005) 5697–5703.
[13] E. Muller, N. Mackin, S. Erdman, K. Cunningham, Fig1p facilitates Ca2+influx
and cell fusion during mating ofSaccharomyces cerevisiae, J. Biol. Chem. 278 (2003) 38461–38469.
[14] N. Zhang, D. Dudgeon, S. Paliwal, A. Levchenko, E. Grote, K. Cunningham, Multi-ple signaling pathways regulate yeast cell death during the response to mating pheromones, Mol. Biol. Cell 17 (2006) 3409–3422.
[15] Y. Eilam, M. Othman, D. Halachmi, Transient increase in Ca2+influx in Saccha-romyces cerevisiaein response to glucose: effects of intracellular acidification and cAMP levels, J. Gen. Microbiol. 136 (1990) 2537–2543.
[16] J. Nakajima-Shimada, H. Iida, F. Tsuji, Y. Anraku, Monitoring of intracellular cal-cium inSaccharomyces cerevisiaewith an apoaequorin cDNA expression system, Proc. Natl. Acad. Sci. U.S.A. 88 (1991) 6878–6882.
[17] R. Tisi, S. Baldassa, F. Belotti, E. Martegani, Phospholipase C is required for glucose-induced calcium influx in budding yeast, FEBS Lett. 520 (2002) 133–138.
[18] M. Tökés-Füzesi, D. Bedwell, I. Repa, K. Sipos, B. Sümegi, A. Rab, A. Mis-eta, Hexose phosphorylation and the putative calcium channel component Mid1p are required for the hexose-induced transient elevation of cytoso-lic calcium response inSaccharomyces cerevisiae, Mol. Microbiol. 44 (2002) 1299–1308.
[19] M. Trópia, A. Cardoso, R. Tisi, L. Fietto, J. Fietto, E. Martegani, I. Castro, R. Brandão, Calcium signaling and sugar-induced activation of plasma membrane H(+)-ATPase inSaccharomyces cerevisiaecells, Biochem. Biophys. Res. Commun. 343 (2006) 1234–1243.
[20] K. Cunningham, G. Fink, Calcineurin inhibits VCX1-dependent H+/Ca2+
exchange and induces Ca2+ATPases inSaccharomyces cerevisiae, Mol. Cell. Biol.
16 (1996) 2226–2237.
[21] T. Pozos, I. Sekler, M. Cyert, The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+exchange and is related to mammalian Na+/Ca2+
exchang-ers, Mol. Cell. Biol. 16 (1996) 3730–3741.
[22] A. Miseta, L. Fu, R. Kellermayer, J. Buckley, D. Bedwell, The Golgi apparatus plays a significant role in the maintenance of Ca2+homeostasis in thevps33Delta
vac-uolar biogenesis mutant ofSaccharomyces cerevisiae, J. Biol. Chem. 274 (1999) 5939–5947.
[23] M. Cyert, Calcineurin signaling inSaccharomyces cerevisiae: how yeast go crazy in response to stress, Biochem. Biophys. Res. Commun. 311 (2003) 1143–1150.
[24] D. Matheos, T. Kingsbury, U. Ahsan, K. Cunningham, Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in
Saccharomyces cerevisiae, Genes Dev. 11 (1997) 3445–3458.
[25] A. Wach, A. Brachat, R. Pöhlmann, P. Philippsen, New heterologous modules for classical or PCR-based gene disruptions inSaccharomyces cerevisiae, Yeast 10 (1994) 1793–1808.
[26] A. Wach, A. Brachat, C. Alberti-Segui, C. Rebischung, P. Philippsen, Heterologous HIS3 marker and GFP reporter modules for PCR-targeting inSaccharomyces cerevisiae, Yeast 13 (1997) 1065–1075.
[27] A. Stathopoulos, M. Cyert, Calcineurin acts through theCRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast, Genes Dev. 11 (1997) 3432–3444.
[28] C. Patton, S. Thompson, D. Epel, Some precautions in using chelators to buffer metals in biological solutions, Cell Calcium 35 (2004) 427–431.
[29] M. Kiechle, P. Manivasakam, F. Eckardt-Schupp, R. Schiestl, A. Friedl, Promoter-trapping inSaccharomyces cerevisiaeby radiation-assisted fragment insertion, Nucleic Acids Res. 30 (2002) e136.
[30] J. Teng, R. Goto, K. Iida, I. Kojima, H. Iida, Ion-channel blocker sensitivity of voltage-gated calcium-channel homologue Cch1 inSaccharomyces cerevisiae, Microbiology 154 (2008) 3775–3781.
[31] R. Tisi, P. Coccetti, S. Banfi, E. Martegani, 3-Nitrocoumarin is an efficient inhibitor of budding yeast phospholipase-C, Cell Biochem. Funct. 19 (2001) 229–235.
[32] X. Dong, X. Wang, H. Xu, TRP channels of intracellular membranes, J. Neu-rochem. 113 (2010) 313–328.
[33] X. Zhou, A. Batiza, S. Loukin, C. Palmer, C. Kung, Y. Saimi, The transient receptor potential channel on the yeast vacuole is mechanosensitive, Proc. Natl. Acad. Sci. U.S.A. 100 (2003) 7105–7110.
[34] Y. Chang, G. Schlenstedt, V. Flockerzi, A. Beck, Properties of the intracellular transient receptor potential (TRP) channel in yeast, Yvc1, FEBS Lett. 584 (2010) 2028–2032.
[35] G. Wiesenberger, K. Steinleitner, R. Malli, W. Graier, J. Vormann, R. Schweyen, J. Stadler, Mg2+deprivation elicits rapid Ca2+uptake and activates
Ca2+/calcineurin signaling inSaccharomyces cerevisiae, Eukaryot. Cell 6 (2007)
592–599.
[36] G. Hockerman, B. Peterson, B. Johnson, W. Catterall, Molecular determinants of drug binding and action on L-type calcium channels, Annu. Rev. Pharmacol. Toxicol. 37 (1997) 361–396.
[37] K. Kafadar, M. Cyert, Integration of stress responses: modulation of calcineurin signaling inSaccharomyces cerevisiaeby protein kinase A, Eukaryot. Cell 3 (2004) 1147–1153.
[38] D. Wolfe, D. Pearce, Channeling studies in yeast: yeast as a model for chan-nelopathies? Neuromolecular Med. 8 (2006) 279–306.
[39] J. Cui, J. Kaandorp, P. Sloot, C. Lloyd, M. Filatov, Calcium homeostasis and sig-naling in yeast cells and cardiac myocytes, FEMS Yeast Res. (2009). [40] J. Cui, J. Kaandorp, O. Ositelu, V. Beaudry, A. Knight, Y. Nanfack, K.
Cunning-ham, Simulating calcium influx and free calcium concentrations in yeast, Cell Calcium 45 (2009) 123–132.
[41] A. Ruiz, R. Serrano, J. Ari ˜no, Direct regulation of genes involved in glucose utilization by the calcium/calcineurin pathway, J. Biol. Chem. 283 (2008) 13923–13933.
[42] A. Demczuk, N. Guha, P. Nguyen, P. Desai, J. Chang, K. Guzinska, J. Rollins, C. Ghosh, L. Goodwin, A. Vancura,Saccharomyces cerevisiaephospholipase C reg-ulates transcription of Msn2p-dependent stress-responsive genes, Eukaryot. Cell 7 (2008) 967–979.
[43] T. Ohdate, S. Izawa, K. Kita, Y. Inoue, Regulatory mechanism for expression ofGPX1in response to glucose starvation and Ca inSaccharomyces cerevisiae: involvement of Snf1 and Ras/cAMP pathway in Ca signaling, Genes Cells 15 (2010) 59–75.