Faculdade de Engenharia da Universidade do Porto
Decellularized matrices as platforms for studying
cancer cells
João Telmo Silva Vieira
Dissertation for master’s degree in
Bioengineering – Molecular Biotechnology
Supervisor: Dr. Joana Caldeira
Co-supervisors: Prof. Mário Barbosa
Dr. Ana Maria Magalhães
iii
Resumo
O cancro colorretal é a segunda causa de morte por cancro em todo o mundo. Apesar do sucesso reconhecido dos programas de rastreamento e do desenvolvimento de terapias adjuvantes, apenas uma minoria dos pacientes tem beneficiado de uma resposta favorável. Como tal, existe uma forte necessidade de novas ferramentas de diagnóstico e prognóstico, assim como de tratamentos personalizados, que permitam melhorar os resultados clínicos a longo-prazo de pacientes com cancro colorretal.
Os modelos tumorais in vitro têm tido uma grande influência na investigação na área do cancro. Tipicamente têm sido usados biomateriais como o colagénio, alginato e Matrigel para reproduzir características do microambiente tumoral, mas estas abordagens têm-se revelado curtas no que toca à recapitulação de várias propriedades destes tecidos. Mais recentemente, o uso de matrizes descelularizadas para estudos de cancro tem sido destacado devido à possibilidade de preservar a arquitetura de tecidos nativos e a composição bioquímica da matriz extracelular. No entanto, a obtenção destas matrizes biológicas envolve um processo de remoção de células que deve ser criteriosamente desenhado para garantir uma máxima remoção celular e assim prevenir efeitos imunogénicos do biomaterial. Para além disso, as propriedades nativas do tecido devem ser minimamente afetadas. A quantidade de glicosaminoglicanos retidos na matriz, em particular, é uma questão crítica no que toca à aplicação destes biomateriais para estudos de cancro, dada a sua reconhecida importância na remodelação da matriz extracelular de tecidos tumorais. O sulfato de condroitina, mais especificamente, tem sido descrito como estando sobre-expresso em diversos tumores humanos, daí o interesse na sua preservação na matriz extracelular após descelularização.
O presente trabalho teve como principais objetivos a descelularização de tecido intestinal de ratinho, tanto normal como tumoral, e a caracterização da eficácia do processo. Para isso, foi utilizado um protocolo químico-enzimático previamente estabelecido para tecido humano colorretal. A remoção de células e de fragmentos de ADN foi confirmada por histologia e métodos quantitativos. A matriz extracelular de ambas amostras nativas e descelularizadas foi também corada e o seu conteúdo em glicosaminoglicanos foi avaliado quantitativamente.
Os resultados quantitativos revelaram eficiências de descelularização superiores a 99% para ambos tecidos normal e tumoral, assim como uma manutenção parcial de alguns componentes principais da matriz. Em conformidade, a avaliação histológica demonstrou uma completa remoção celular e um decréscimo do conteúdo de moléculas da matriz, sobretudo de glicosaminoglicanos, em amostras descelularizadas.
Este trabalho constitui o primeiro passo para o desenvolvimento de uma plataforma 3D baseada em matrizes descelularizadas. Como tal, atividades futuras devem-se essencialmente focar na combinação de matrizes descelularizadas com diferentes quantidades de sulfato de condroitina a fim de produzir plataformas de diversas propriedades bioquímicas e biomecânicas. O objetivo final destes biomateriais seria a sua repopulação com células cancerígenas para avaliar o potencial desta abordagem em aplicações clínicas, nomeadamente no estudo de células cancerígenas derivadas de pacientes, na previsão da sua resposta terapêutica e no prognóstico da doença.
iv
Abstract
Colorectal cancer (CRC) is ranked as the second most deadly type of cancer worldwide. Despite the success of screening programs and the development of adjuvant therapies, only a minority of the patients have demonstrated a favorable response. As so, there is an urgent need for novel diagnostic and prognostic tools, as well as for personalized treatment options, that can improve long-term CRC patients’ outcomes.
In vitro tumor models have greatly impacted cancer research. Traditionally, biomimetic materials such as collagen, alginate and Matrigel have been used to resemble the tumor microenvironment, but they fail to recapitulate several of its key features. Recently, the use of biological scaffolds in cancer studies has been highlighted due to the preservation of tissue architecture and intricate extracellular matrix (ECM) composition, which is practically impossible to replicate using piecing together systems. Nevertheless, obtaining these acellular scaffolds involves a process of decellularization that must be carefully planned to guarantee maximal cell removal preventing graft rejection, while minimizing alterations of native ECM properties. The amount of glycosaminoglycans (GAGs) retained is a critical aspect regarding cancer applications given the importance of such molecules in tumor ECM remodeling. In particular, chondroitin sulfate (CS) is a natural building block of the ECM that has been described to be highly expressed in several human tumors. As so, retaining its original native composition is of particular interest.
Herein we proposed to successfully decellularize mice normal and tumoral intestinal tissue and characterize the effectiveness of the process. For that, a 0.1% SDS-based protocol previously established for human colorectal tissues was used. The confirmation of cell and DNA removal was assessed through histology and DNA quantification. All samples were stained with Alcian Blue/Sirius Red and quantified in terms of their GAG content to evaluate the preservation of ECM components.
With the SDS-based procedure, decellularization efficiency was higher than 99% for both normal and tumor tissues. Partial maintenance of major ECM molecules was also observed. In accordance, histological analysis showed no signs of cell remnants and a decrease of ECM components, particularly GAGs, in decellularized samples.
This work constitutes the first step towards the development of an initially intended dECM-based platform. As so, future activities should aim at supplementing mice decellularized matrices with different amounts of CS GAGs to generate 3D platforms of diverse biochemical and biomechanical properties. The final goal would be the repopulation with CRC cell lines to evaluate the potential of these constructs for clinically relevant applications, namely to study the behavior of patient-derived cancer cells, estimate therapeutic response and assess disease progression.
v
Acknowledgements
First, I would like to thank my supervisors, Dr. Joana Caldeira, Dr. Ana Maria Magalhães and Prof. Mário Barbosa for giving me the opportunity to do research and providing guidance throughout this work. To all of them, I am very thankful for the cooperation, knowledge and constant availability to assist me in this work.
I also have to thank all the members of “Microenvironments for New Therapies” group for the friendly way they received me. My special thanks goes to Morena Fiordalisi for her great patience at all times helping me to develop this work.
Then, I have to thank my family for the unconditional support they gave me throughout my university years and for always encouraging me to take up new challenges and be successful. I am extremely grateful to them for their love, caring and sacrifices for educating and providing me everything I need.
Lastly, I have to thank my friends for the incredible moments we shared during these five years, both at parties, holidays and travel journeys, as on sleepless nights before exams. I hope we will be able to be all together very soon.
vi
List of Contents
List of figures ... viii
List of tables ... ix
Glossary ... x
Chapter 1: Introduction……….12
1. Epidemiology and pathophysiology of colorectal cancer ... 12
1.1
Colon structure ... 12
1.1.1
Mucosa ... 13
1.1.2
Submucosa ... 14
1.1.3
Muscularis propria ... 14
1.1.4
Serosa/Adventitia ... 14
1.2
Colon composition ... 15
1.3
Colon cancer ... 16
1.3.1
Pathophysiology ... 16
1.3.2
Pathological origin ... 16
1.3.3
Epidemiology ... 17
1.3.4
ECM alterations ... 20
2. Experimental models of colorectal cancer ... 21
2.1
In vivo models ... 21
2.2
In vitro models in cancer research ... 23
3. Biomimetic materials for cancer research ... 25
3.1
Hydrogels ... 25
3.1.1
Natural materials ... 26
3.1.2
Synthetic polymers ... 29
3.2
Decellularized extracellular matrix -based biomaterials... 29
3.2.1
Sources of decellularized ECM (dECM) ... 30
3.2.2
Decellularization methods ... 30
3.2.3
Characterization of the decellularization efficacy ... 31
3.2.4
Decellularized extracellular matrix (dECM)-based biomaterials ... 31
3.2.5
Decellularized matrices in colorectal cancer research ... 33
3.2.6
Chondroitin sulfate-based biomaterials ... 35
Chapter 2: Aims….……….36
Chapter 3: Materials and Methods……….37
vii
3.2 Methods ... 37
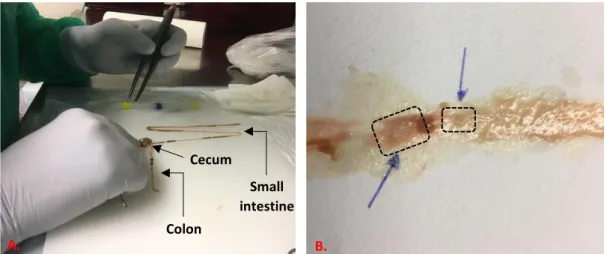
3.2.1 Mice intestine dissection ... 37
3.2.2 Tissue cleaning and freezing ... 38
3.2.3 Decellularization ... 39
3.2.4 Histological analysis ... 39
3.2.5 Viscoelastic properties ... 40
3.2.6 DNA evaluation ... 41
3.2.7 GAG quantification ... 41
Chapter 4: Results……….42
4.1 Efficiency of decellularization ... 42
4.2 Evaluation of extracellular matrix components upon decellularization ... 44
Chapter 5: Discussion………..46
5.1 SDS-based decellularization efficiently removed cells and DNA content
from normal and tumor tissues ... 46
5.2 SDS-based decellularization decreased matrix components levels ... 47
Chapter 6: Conclusions and future perspectives……….49
References ..………...50
viii
List of figures
Fig. 1 - Gross anatomy of the human large intestine ... 12
Fig. 2 - Schematic illustration of the different layers comprising the intestinal wall ... 13
Fig. 3 - Incidence and associated mortality in CRC worldwide in 2018 ... 17
Fig. 4 - Incidence and associated mortality of CRC by age and sex worldwide ... 18
Fig. 5 - Understanding CRC: a brief summary of the pathological process underlying CRC, first
signs and symptoms, risk factors, current screening methods and treatments. ... 20
Fig. 6 - Generation of a conditional knockout model for the tissue-specific inactivation of a
target gene ... 22
Fig. 7 - Schematic representation of in vitro 3D models used in cancer research ... 24
Fig. 8 - Stimuli responsive hydrogel for potential application in pharmaceutics ... 25
Fig. 9 - Chemical gels: radiation cross-linked polymers ... 26
Fig. 10 - A schematic representation of extracellular matrix-based biomaterials to be used in a
variety of applications. ... 32
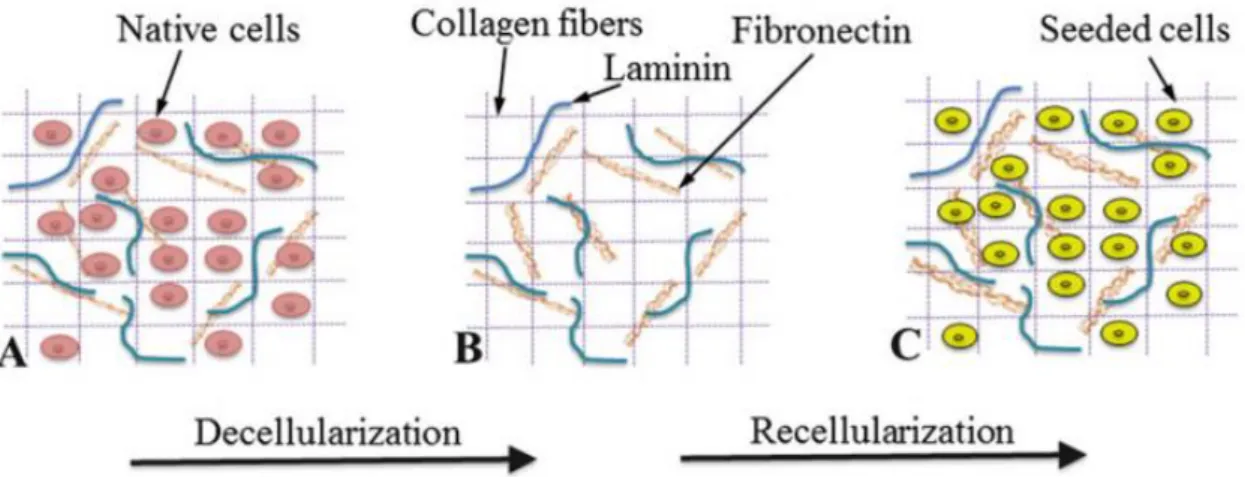
Fig. 11 - Decellularization and recellularization of decellularized matrices ... 33
Fig. 12 - Summary of the paper "Decellularized human colorectal cancer matrices polarize
macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via
CCL18” . ... 34
Fig. 13 - Mice intestine dissection ... 37
Fig. 14 - Representative images prior and following the intestine cleaning ... 38
Fig. 15 - Isolation of intestinal lesions ... 38
Fig. 16 - Weight distribution of both normal and tumor samples. ... 39
Fig. 17 - Schematic decellularization timeline ... 39
Fig. 18 - Step-by-step protocol for rheological characterization of healthy and tumor mice
colorectal tissue ... 40
Fig. 19 - Histological sections of normal and tumor tissues stained with DAPI. ... 42
Fig. 20 - Histological sections of normal and tumor tissues stained with H&E ... 43
Fig. 21 - Detailed examination of tumor tissue ... 43
Fig. 22 - Picogreen DNA quantification. ... 44
Fig. 23 - Sulfated GAGs (sGAG) quantification ... 45
Fig. 24 - Histological sections of normal and tumor tissues stained with Alcian Blue/ Sirius Red...
... 45
ix
List of tables
Table 1 – Iterative determinations of linear viscoelastic parameters of native mice intestine
tissue. ... 40
x
Glossary
Abbreviations
ADAMTs A Desintegrin and Metalloproteinase with Thrombospondin motifs
AOM Azoxymethane
APC Adenomatous polyposis coli
BM Basement membrane
CRC Colorectal cancer
Cre Cre recombinase
CS Chondroitin sulfate
dECM Decellularized extracellular matrix
DMH 1,2-dimethylhydrazine
ECM Extracellular matrix
EHS Engelbreth-Holm-Swarm
EMT Epithelial-mesenchymal transition
FAP Familial adenomatous polyposis
GAGs Glycosaminoglycans
HCC Hepatocellular carcinoma
HS Heparan sulfate
LOX Lysyl oxidase
MCTS Multicellular tumor spheroids
MMPs Matrix metalloproteinases
MMR Mismatch repair
PBS Phosphate buffer solution
PGs Proteoglycans
PGA Polyglycolic acid
PLA Polylactic acid
xi
SDC Sodium Deoxycholate
SDS Sodium dodecyl sulfate
sGAG Sulfated glycosaminoglycans
12 – Introduction
Chapter 1
Introduction
1. Epidemiology and pathophysiology of
colorectal cancer
1.1 Colon structure
The colon is a long, tube-like organ, of the digestive system that connects the ileum section of the small intestine to the rectum. It consists of four different portions – ascending, transverse, descending and sigmoid colon (Maqbool 2013).
The first part of the colon (ascending colon) is essentially involved in the absorption of remaining water and nutrients from the partly digested food that reaches the cecum. In combination with transverse colon, both segments perform critical functions regarding the completing of food processing and the generation of solid waste that will be stored in the descending colon. From there, the formed feces are moved into the sigmoid colon, where they are contracted to be finally propelled to the rectum (Azzouz and Sharma 2020; Levitan et al. 1962).
Although the colon represents the bigger portion of the large intestine, a detailed description of this organ should also include the cecum and the rectum (Fig.1). The cecum, located on the right side of lower abdominal cavity, marks the beginning of the large intestine. This pouch-like portion is an important element of the large intestine since it intermediates the junction between small intestine and ascending colon and also has attached to it a vermiform structure, called the appendix (Purysko et al. 2011). The rectum, by contrast, represents the terminal part. Its function relies essentially in storing feces before being eliminated through the anus (Shafik et al. 2006).
13 – Epidemiology and pathophysiology of colorectal cancer
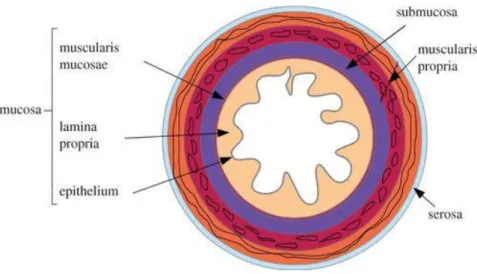
Besides the visible anatomy, the human colon also has distinctive features both on the outer and inner side of its wall that are well described in the literature. While the external surface is decorated in the entire length with omental appendices, haustra and teniae coli, the inner part is essentially marked by a characteristic microanatomy comprised of four different layers, namely mucosa, submucosa, muscularis propria and serosa/adventitia (Lamb and Kaiser 2015) (Fig. 2).
The same four layers compose the small bowel wall as well, although some functional differences do exist between these two main intestinal portions. Contrary to the colon, the primary job of the small intestine is the digestion and absorption of a variety of nutrients, including carbohydrates, proteins, fats, water, fat-soluble vitamins, minerals and micronutrients (Fish and Burns 2020). The first part of the small intestine, called duodenum, receives chyme from the stomach, pancreatic enzymes and bile from the liver, so here a more chemical digestion occurs. The jejunum and subsequent ileum portions, on the other hand, are more involved in the uptake of the products of the digestion. Together, both small and large intestine perform a massive and vital function in breaking down food and permitting the body to take in and use these food substances (Collins, Nguyen, and Badireddy 2020).
1.1.1
Mucosa
The colorectal mucosa is essentially constituted by three elements: epithelium, lamina propria and muscularis mucosa. The epithelium represents the innermost layer of the colorectal wall. It consists of a single layer of columnar cells serving three main functions: the establishment of a physical barrier between host and luminal environment, the absorption of water and ions from the waste material and the production of mucins which creates a protective mucus layer inside the colorectum. This simple epithelial tissue is also characterized by repetitive invaginations known as crypts of Lieberkühn that house, among other types of cells, actively proliferating stem cells that are responsible for renewing the lining of the colorectum. Similarly to the top surface epithelium, cells in these intestinal folds are organized into a single-cell layer (Leon and Gregorio 2001).
Fig. 2 - Schematic illustration of the different layers comprising the intestinal wall, from Balbi and Ciarletta 2013.
14 – Introduction
Just below the colorectal epithelium it is found the lamina propria which is a thin layer of loose connective tissue containing a wide variety of cells, particularly with immune regulation functions. Therefore, it provides an important support for the local defense against microbes and other harmful agents (Bates 2004; Okumura and Takeda 2016).
Ultimately, the muscularis mucosa is a thin layer of smooth muscle that lies at the base of the lamina propria. Although not found immediately beneath the epithelial layer, the muscularis mucosa coordinates the physiological activity of the epithelium by altering the shape of the mucosa through muscle contractions (Leon and Gregorio 2001).
1.1.2
Submucosa
The submucosa is the layer of connective tissue underlying the mucosa. It is constituted by a variety of elements that resemble the composition of lamina propria, including lymphocytes, fibroblasts, macrophages, mast cells and fibrous tissues. This area is also endowed with many blood and lymphatic vessels, with particular emphasis on an extensive network of neurons forming the submucosal plexus that controls the secretions of the colorectal epithelium (Arbizu and Nurko 2016).
1.1.3
Muscularis propria
Muscularis propria is the term given to the muscular layer that extends from the submucosa to the serosa. It consists of smooth muscle cells arranged into an inner circular layer and an outer longitudinal layer (Feher 2017). The main function of this muscular layer is to trigger peristaltic movements that allow waste material to move forward and out of the gastrointestinal tract, although muscle contractions may also help the food break down. The frequency and strength of these contractions are controlled by a plexus of neurons, called the myenteric plexus, found between the two sublayers of smooth muscle (Gropper, Smith, and Carr 2016).
1.1.4
Serosa/Adventitia
The outer layer of the large intestine is a serosa over the intraperitoneal regions, while the retroperitoneal regions are covered by adventitia (Amerongen 2010). The same logic applies to the small intestine, with a slight difference on the lining of the retroperitoneal regions (i.e. duodenum and very terminal portion of the ileum) that consists on both types of tissue (Shroyer and Kocoshis 2010). The serosa is a serous membrane composed of areolar connective tissue and simple squamous epithelium (Tortora and Derrickson 2008). It displays many fat-filled pouches along the colon, but these are absent in the rectum (Gartner and Hiatt 2011). The adventitia is a loose connective tissue containing fibroblasts, blood vessels, nerves, as well as the arterial supply and venous drainage passing through it (Lowe, Anderson, and Anderson 2019). This particular outer coat surrounds the ascending colon, descending colon and rectum (Backman, Wax, and Zhang 2018).
15 - Epidemiology and pathophysiology of colorectal cancer
1.2 Colon composition
The extracellular matrix (ECM) of the colorectal tissue comes in two major forms: basement membrane (BM), that provides a foundation to the overlying epithelium and separates it from the underlying stroma; and connective tissue matrix that provides the bulk tissue structure (Bonnet 2018). Under normal conditions, the BM of the human colon is mainly constituted by collagen IV, the proteoglycan perlecan and glycoproteins such as laminin, fibronectin and nidogen (Crotti et al. 2017).
Type IV collagen is the most abundant constituent of the BM and commonly denominated the BM backbone (Sand et al. 2019). The main form of collagen found in the BM of the colon, as indeed is the case in all digestive tract, is composed by a trimerization of alpha chains (two α-1
and one α-2) (Khoshnoodi, Pedchenko, and Hudson 2008). These units are arranged in a triple helical molecule that presents a major structural importance in the BM, forming network-like structures that provide a scaffold for epithelium anchorage. Besides, type IV collagen has many binding partners in the BM, displaying interactions with several molecules and regulating many cellular processes (Sand et al. 2019).
Perlecan is another important component of the BM that belongs to the family of
proteoglycans, and more specifically to the class of heparan sulfate proteoglycans (Costell et al. 1999). Proteoglycans (PGs) are a family of molecules composed of a core protein decorated with one or more covalently attached linear chains of glycosaminoglycans (GAGs), such as chondroitin sulfate (CS) or heparan sulfate (HS). These molecules can be found in the cytoplasm of cells, cell membrane as well as in the ECM, playing a key role on the biomechanical properties of these structures (Frevert and Wight 2006; Wight and Mecham 1987). Since PGs are generally composed of sulfated GAGs (negatively charged molecules), they attract opposite charged ions (Na+) that pull water into the matrix, causing the swelling of the matrix. The amount of water
gathered consequently influences the viscoelastic properties of the tissues, being highly hydrated ECMs generally associated to tissues that are object of higher stresses (Atkins, Kerr, and Goodlad 2010; Stecco et al. 2015).
Glycoproteins are also a very important class of constituents of the BM because they greatly contribute for a cohesive network of molecules. Besides linking structural components of the BM, these molecules intermediate the junction between ECM and cells (J. Xu and Mosher 2011). A well-known example is the laminin which is the primary multiadhesive matrix molecule found in the BM. This molecule interacts with cells through cell-surface receptors, such as integrins, thus mediating cell attachment and several cellular processes such as proliferation, differentiation and motility (Ishihara et al. 2018).
Besides the BM, there still exists another form of ECM in the colorectal tissue that fundamentally provides the tissue structure, called the stromal ECM. Both forms are composed by similar components, with just a particular difference on the main type of collagen that forms the stromal ECM. In the stromal ECM, type IV collagen is substituted by collagen I (COL1 A1, COL1 A2) which provides a 3D and less rigid structure, thanks to the fibrillar properties and the absence of disulfide bridges in this type of collagen, respectively (Crotti et al. 2017).
Overall, the ECM composition of the human intestine is very similar along its extension. Similarly to the colorectum, the epithelial basement membrane of the small intestine is mainly composed of type IV collagen, heparan sulfate proteoglycans and several glycoproteins including heterotrimeric laminins (laminin-1, laminin-2 and laminin-5), fibronectin, tenascins and nidogen.
16 - Introduction
Moreover, type I collagen is also the one predominantly found in the connective tissue (Beaulieu 2001; Graham et al. 1988).
1.3 Colon cancer
1.3.1 Pathophysiology
The earliest changes seen in the colon that may lead to cancer are thought to occur in the colon mucosa with the appearance of aberrant crypts (Orlando et al. 2008; Takayama et al. 1998). The mechanisms underlying the promotion and initiation of these events involve interactions among environmental influences, germ-line factors and accumulated somatic mutations in the colorectal epithelium which make this disease to not be confined to a single cause (Markowitz and Bertagnolli 2009). Even so, a common feature to about 80% of all colon tumors is the existence of mutations that inactivate the Adenomatous Polyposis Coli (APC) tumor suppressor gene located on the long arm of the human chromosome 5 (Kwong and Dove 2009). This gene is critical in colorectal cancer (CRC) development since it produces the APC protein that regulates the levels of β-catenin in the cells. When damaged, it is observed an accumulation of β-catenin and its consequent translocation into the nucleus, thus activating the Wnt signaling pathway in inadequate proportions. So, ultimately, there is an increase in the expression of oncogenes that may lead to cancer (Markowitz and Bertagnolli 2009; Shang, Hua, and Hu 2017).
1.3.2 Pathological origin
Colon cancer and polyps that arise from mutations in the APC gene may have a hereditary or sporadic origin. The hereditary form, called familial adenomatous polyposis (FAP), is an autosomal dominant condition that confers a lifetime risk of CRC of 100%. The clinical expression of the disease is marked by the development of hundreds to thousands of adenomatous polyps, which increased attention is usually paid due to the close association of this type of polyps with colorectal malignancies (Jasperson et al. 2010). Generally, most of polyps are benign, but those histologically classified as neoplastic (adenomas) have potential to acquire traits of malignancy and spread to the nearby layers of the colon wall. In that state, they are called adenocarcinomas (Aarons, Shanmugan, and Bleier 2014; Unal and Ozturk 2015). The other common form of hereditary CRC is called, the Lynch syndrome, and involves the inactivation of DNA mismatch repair (MMR) genes (e.g. Msh2). In contrast to the FAP disease, the presence of polyps is rare in this case, but the risk to develop CRC is still very high (Jasperson et al. 2010; Stoffel et al. 2009). Finally, somatic defects are the most representative cause of CRC with approximately 75% of CRCs occurring in people showing no genetic signs indicative of being at risk from the disease (PDQ Cancer Genetics Editorial Board 2020). In this subgroup, the most common mechanism associated with CRC is the chromosomal instability that drives the loss of heterozygosity in tumor suppressor genes (ex. APC) (Lengauer, Kinzler, and Vogelstein 1997). Other events underlying the disease, although less expressed, are somatic defects in MMR genes that leads to a DNA
17 - Epidemiology and pathophysiology of colorectal cancer
microsatellite instability (MSI) and the epigenetic silencing of DNA mismatch repair genes mediated by aberrant DNA methylation (Issa 2004; Markowitz and Bertagnolli 2009).
1.3.3 Epidemiology
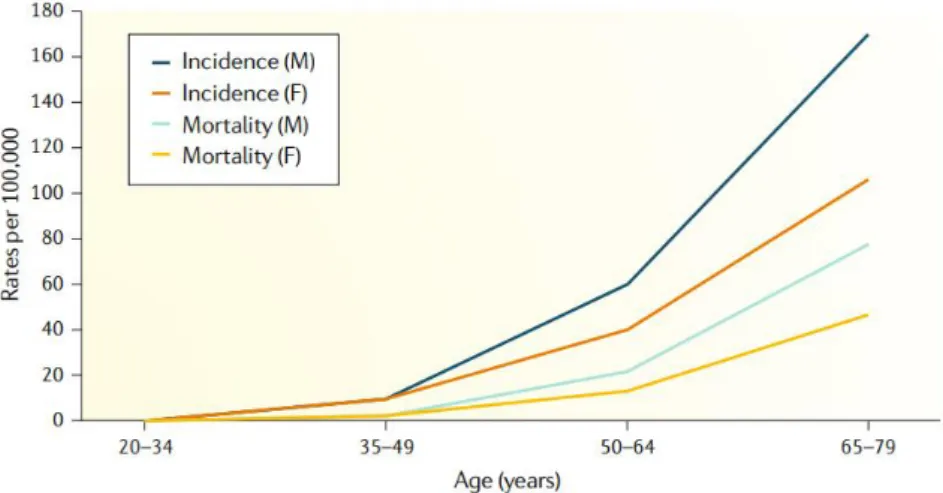
According to World Health Organization (WHO) stats, CRC is the third most incident type of cancer and the second leading cause of cancer-related deaths worldwide (Fig.3). More than half of the cases and deaths are observed in Asia which makes this region to be the most affected by the disease, followed by Europe and North America, respectively (International Agency for Research on Cancer 2018). Importantly, advanced age and male sex are risk factors associated with a higher incidence of this type of cancer (Low et al. 2020). In men, both the incidence and associated mortality are approximately 25% higher than in women. Older people are also more vulnerable to the disease, as confirmed by the fact that 90% of the cases reported are diagnosed in people aged over 50 (International Agency for Research on Cancer 2018) (Fig.4).
Fig. 3 - Incidence (upper) and associated mortality (lower) in CRC worldwide in 2018 (WHO, International Agency for Research on Cancer 2018)
18 - Introduction
Following the same reasoning, a study conducted in the USA demonstrated the most common anatomical location of CRC to follow the trend observed in older people, which shows a highest incidence of CRC in the proximal colon. Conversely, in people aged below 50, it was observed a higher predominance of tumors in the rectum and distal colon (Siegel et al. 2020). For the next decade, the global burden of the disease is expected to continue to grow, reaching more than 2.2 million people and causing 1.1 million deaths (Arnold et al. 2017). Surprisingly, an increase in the number of cases in adults younger than 50 has been observed in the last years. Lifestyle and dietary habits might contribute to these worrying predictions, but the role of genetics, family and personal health histories, obesity and smoking cannot be neglected (Dekker et al. 2019; World Cancer Research Fund International/ American Institute for Cancer Research 2018).
Although less common, it is also relevant to mention that small intestinal cancers are on the rise in developing countries (Barsouk et al. 2019). Besides developing similarly to CRC, they share some of the factors affecting colon cancer incidence. The main differences, however, contributing for the much lower incidence are believed to reside in quicker transit time which results in shorter time of exposure to carcinogens, lower bacterial load, increased IgA, less reactive oxygen species and a different response to DNA damage (Pan and Morrison 2011). They also share common genetic mutations in KRAS, p53 and APC genes, among others. However, small bowel mucosal cells have higher proliferation rates and may inhibit malignant cell growth through competition (Delaunoit et al. 2005).
1.3.3.1
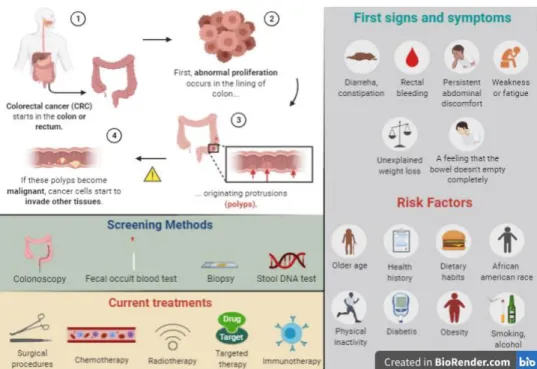
Screening Methods
The adoption of preventive measures like monitoring intestinal health right from risk ages can produce better outcomes, especially when the case is a largely silent disease. For that, there are several screening methods that can be employed for detecting abnormalities in the colon and rectum (Fig.5). Colonoscopy is the screening method of choice since it enables to visualize the inside of the entire colon and directly evaluate the presence of typical or precursor lesions of CRC (Medical Advisory Secretariat 2009). Nevertheless, a positive fecal occult blood test is often
Fig. 4 - Incidence and associated mortality of CRC by age and sex worldwide, from Low et al. 2020
19 - Epidemiology and pathophysiology of colorectal cancer
indicative of complications in the digestive tract and so, it is also considered as a useful screening test for the disease. The same reasoning applies to a stool DNA test which searches for specific mutations associated with CRC in cells shed in stool. Although these tests do not directly validate the presence of a tumor, they are seen as good first line screening tests for reasons of affordability and patient’s acceptability given their non-invasive nature (Wu and Sung 2012). Besides these medical tests, biopsies are also usually performed to examine a suspicious area under microscope. In fact, the identification and subsequent management of a malignant condition are currently based on a histological evaluation of the tissue and so, a biopsy is always required even though enough evidence of the pathology already exists (Dekker et al. 2019; National Institute for Health and Care Excellence 2011). Finally, imaging methods such as computed tomographic (CT) colonography can complement the information obtained from the colonoscopy (Pickhardt 2013).
1.3.3.2
Current treatments
As usual in cancer care, the choice of the medical treatment to be used must be based on several factors, including the type and stage of the cancer, possible side effects, patient’s preferences and health condition. Looking to the available treatment options, the surgical procedures are the most common techniques employed to treat CRC (M. T. G. Lee et al. 2017). In particular, the polypectomy and endoscopic treatment, when an early-stage cancer is referred, and the laparoscopy have become standard methods worldwide (Dekker et al. 2019). Additionally, other treatments such as chemotherapy, radiotherapy, targeted drug therapy, immunotherapy and palliative care are often used along with surgical procedures for a more effective treatment of CRC (Fig.5) (Andre and Schmiegel 2005).
Even though there is a variety of approaches directed to the treatment of cancer, many challenges remain, as it is the case of a poor response to therapies and/or a reduced quality of life. The development of new, safe, effective and improved treatments is, therefore, essential to help patients who fight cancer and hope for an increased life expectancy. In particular, addressing the lack of response or patient’ resistance to anti-cancer therapies, with customized in vitro 3D platforms, could provide a successful management of the disease right from the very beginning. In these cases, patients would be submitted to alternative treatments early on, what could result in better outcomes.
20 - Introduction
1.3.4 ECM alterations
In cancer, the ECM composition and microstructure differ according to the tissue type and malignancy features (Emon et al. 2018; Ioachim et al. 2002; Kharaishvili et al. 2011; Naba et al. 2012). Typically, the ECM conversion into a cancerous condition is characterized by alterations in ECM biochemistry. These changes include an excessive deposition of structural components (i.e. different types of collagen I,II,III,V,IX (Bonnans, Chou, and Werb 2014; Handorf et al. 2015; Huijbers et al. 2010; Wei et al. 2017), cross-linker glycoproteins (Ioachim et al. 2002) and proteoglycans as is the case of HS and CS (Brauchle et al. 2018), as well as a secretion/regulation of several growth factors (Witsch, Sela, and Yarden 2010), cytokines (Iijima, Konno, and Itano 2011), ECM-transforming enzymes (ex. MMPs) (Bonnans, Chou, and Werb 2014), transglutaminase (J. Lee et al. 2015) and lysyl oxidase (LOX), whose high expression is reported to be correlated with CRC progression and invasion (Wang, Hsia, and Shieh 2017; Wei et al. 2017). Besides alterations in the composition, the ECM also suffers a structural reconfiguration, with the alignment of ECM fibers which ultimately contributes to an anisotropic arrangement of the tumor microenvironment (Crotti et al. 2017; Emon et al. 2018).
As a consequence of all these modifications, the ECM biomechanical properties are also affected, thus suggesting them to be critical in the course of carcinogenic events. For instance, tumor ECM is reported to be considerably stiffer than surrounding normal tissue ECM (Levental et al. 2009; Mierke et al. 2018). Also, an association between higher cancer staging and stiffer tissues (Acerbi et al. 2015; Wei et al. 2017) seems to exist. In the particular case of CRC, it is observed an upregulation of collagen I, LOX and consequently increased stiffness, at later stages of CRC when compared to early phases (Wei et al. 2017). Due to this, the role of the gradual stroma stiffening has been emphasized as a potential target for new therapies against cancer, as well as being recognized as a potential tool for diagnostic and prognostic evaluation, to estimate both the malignancy and progression of cancer (Emon et al. 2018).
Fig. 5 – Understanding CRC: a brief summary of the pathological process underlying CRC, first signs and symptoms, risk factors, current screening methods and treatments.
21 – Experimental models of colorectal cancer
2. Experimental models of colorectal
cancer
2.1 In vivo models
CRC research has been performed resorting to animal models and cell cultures, with both methods aiming to replicate carcinogenic situations found in humans, both sporadic origin or hereditary. CRC animal models are currently represented by induced colorectal tumors and genetically modified mice (De-Souza and Costa-Casagrande 2018). Looking to the first case, several studies addressing the induction of CRC in mice found hyper caloric dietary habits as well as exposure to chemical inducers such as azoxymethane (AOM) and dimethylhydrazine (DMH) to have a carcinogenic potential. More specifically, a diet enriched in fat and low fiber content was demonstrated to trigger relevant mutations for CRC development and to promote spontaneous colorectal neoplasms (Newmark et al. 2009; K. Yang et al. 2008). Also, AOM and DMH showed to be inducers of colorectal carcinogenesis as mice exposed to these substances presented signs of first-staged colorectal tumorigenesis as well as higher expressions of pro-inflammatory markers (Aachary et al. 2015; Lahouar et al. 2014; Umesalma and Sudhandiran 2010; Youssef et al. 2015; Yu et al. 2015).
On the other hand, genetically modified mice have been generated through mutation of genes involved in CRC development. A well-known case is the APC gene mutation. APCmin animals are great representative models of hereditary CRC since they don’t require multiple genetic mutations or the concurrent use of chemical methods to induce colorectal tumorigenesis. Animals that are heterozygous for the mutation develop intestinal tumors as well as colorectal adenomas, making them good models for studying inherited disorders, namely the FAP disease (De-Souza and Costa-Casagrande 2018; Yamada and Mori 2007).
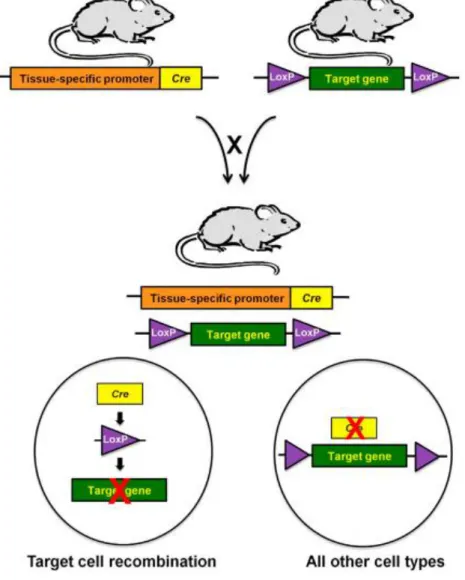
Meanwhile, MMR gene mutations have been also kept on the route to study hereditary CRC, in particular Lynch syndromes, and to cause sporadic CRCs. So far, at least six genes involved in the MMR system have been identified to be altered in these disorders, namely MSH2, MSH3, MSH6, MLH1, PMS1 and PMS2 (Kheirelseid et al. 2013), with MSH2 being one of the most commonly mutated MMR genes (Fishel et al. 1993; Lothe et al. 1993; Marsischky et al. 1996). This particular gene encodes the MSH2 protein that forms DNA repair complexes with either MSH3 or MSH6, and these dimers recognize different types of DNA damage, including base-base and insertion/deletion mismatches. Loss of MSH2 function, therefore, conducts to a higher predisposition to develop tumors and cancer, as result of a deficient DNA damage response repair (Hsieh 2013; Kheirelseid et al. 2013). Considering that information, researchers have generated MSH2 knockout mouse models for studying intestinal cancer, but the increased predominance of lymphomas in these animals has limited its use. In the attempt of more closely recapitulate the CRC phenotype, a conditional knockout model has been developed with the specific inactivation of the MSH2 gene in the intestinal mucosa (Fig.6) (Kucherlapati et al. 2010). This mouse model basically results from a cross-breeding process involving animals expressing Cre recombinase (Cre) under the control of the villin promoter, an intestinal-specific promoter, and animals whose MSH2 gene is flanked by loxP sites. By controlling the expression of Cre recombinase, the villin
22 – Introduction
promoter confines the activity of the Cre-loxP system to the intestinal mucosa, which then recognizes two directly repeated loxP sites and inactivates the target gene (e.g. MSH2) (Harno, Cottrell, and White 2013; H. Kim et al. 2018; Kucherlapati et al. 2010). Finally, the offspring produced from this mating will display an inoperative MMR system in the intestinal epithelium, leading to an exclusive appearance of neoplasms there (Kucherlapati et al. 2010).
To sum up, the use of animal models for research in CRC is very important to obtain models that are representative of the human CRC disease features and to address different biological issues, such as the molecular mechanism involved in initiation and development of the disease or the evaluation of possible treatments. A major drawback of using genetically engineered mice for this purpose is, however, related to the multifactorial characteristics of cancer pathogenesis, which makes a broad array of genes to be associated with a predisposition to develop CRC. Consequently, most models require the association of different forms of induction to become effective representative models of human CRC (De-Souza and Costa-Casagrande 2018). Particularly regarding mutations in DNA repair genes (e.g. MSH2), the potential of these models to be used for preclinical modelling of Lynch syndromes has been reported, but the higher
Fig. 6 - Generation of a conditional knockout model for the tissue-specific inactivation of a target gene from Harno, Cottrell, and White 2013
23 – Experimental models of colorectal cancer
predominance of tumors observed in the small intestine is a significant disadvantage when it comes to obtain more accurate phenotypes of the disease (Kucherlapati et al. 2010). Alternatively, transplantation models are seen as another attractive option for designing representative mouse cancer models due to the possibility of engrafting patient-derived tumors in orthotopic sites, making it possible to maintain the histopathological features of human cancerous tissue into organs of origin (Day, Merlino, and Van Dyke 2015). Several studies employing this strategy have proven effective in paralleling human outcomes (Malaney, Nicosia, and Davé 2014), but the higher costs and challenges associated to preclinical therapeutic studies has limited its use (Day, Merlino, and Van Dyke 2015). Moreover, these models still require animal testing and the physiological differences between humans and other species must not be relativized.
2.2 In vitro models in cancer research
Alternatively to the use of animals, other (in vitro) systems that can reproduce the tumorigenic microenvironment found in patients have been explored. These models are key for fundamental research on cancerous conditions, identification of new targets for cancer treatment, drug testing and preclinical validation, discovery of diagnostic and prognostic biomarkers, among others. 2D cell cultures represent a cornerstone of the research performed until now, not only in the cancer field but also in other areas of biomedical sciences, mainly due to the convenience, cost-effectiveness and ease of use associated to this type of models (Ryan et al. 2016). However, 2D models have the cellular interactions with the microenvironment limited to a bidimensional space, what does not reflect what happens in living organisms. As a consequence, the experimental results obtained using 2D approaches can lead to distorted interpretations (Hoarau-Véchot et al. 2018).
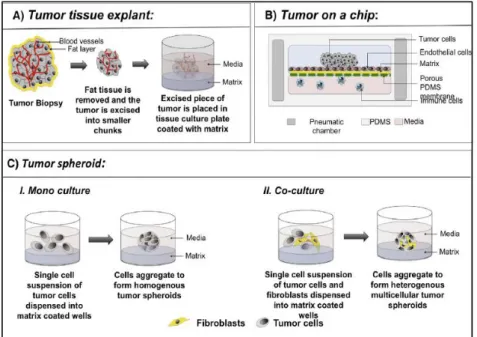
In this context, in vitro 3D models are seen as better alternatives towards more realistic assays since they provide a higher complexity of both cell-cell and cell-matrix interactions and are able to reproduce the spatial and temporal cell heterogeneity found in many tissues or pathological conditions, as it is the case of solid tumors (Hoarau-Véchot et al. 2018; H. Ma et al. 2012). Moreover, 3D systems were already demonstrated to present more comparable drug responses and gene expressions to in vivo systems than 2D models, thus stressing the importance of spatial interactions (Hoffman 1993). The various 3D models employed in cancer research are represented by tumor tissue explants, “tumor-on-a-chip” devices and 3D spheroids (Fig.7) (Nath and Devi 2016).
Tumor tissue explants or ex vivo models have been essentially used as drug-testing platforms to evaluate the efficacy of therapeutic agents (Hidalgo et al. 2014). They involve the culture of patient-derived tumors in tissue culture plates (Ritter et al. 2007), which is attracting for in vitro studies because the tissue architecture and histological features of tumors are retained.
In turn, “tumor-on-a-chip” devices are recent approaches that use microfluidic techniques to recreate the complex tumor microenvironment (Wan, Neumann, and Leduc 2020). By co-culturing tumor cells with other types of cells and molecules that play an active role in tumor transformations, such as endothelial cells, stromal cells and matrix components, in a compartmentalized disposition that resembles the native organization, it is developed a model that can be usefully applied in drug and genomic screening (Jeong et al. 2016; Kwak et al. 2014; Wan, Neumann, and Leduc 2020). Moreover, since this technology uses micro-channels to allow
24 – Introduction
the movement of molecules and cells between compartments, there is the possibility to real-time monitor this flow through incorporation of electronic biosensors into the chip (Y. Chen et al. 2018).
Ultimately, 3D spheroids represent the most well-characterized in vitro 3D model systems used in cancer research (Chambers et al. 2015; Chandrasekaran et al. 2014; Cichon et al. 2012; Harma et al. 2010; G. Y. Lee et al. 2007; Luca et al. 2013; Rofstad and Sutherland 1989; Tong et al. 2015). The generation of tumor cell-based 3D spheroids is very suitable in this context because it allows to reproduce important tumor cell characteristics that are key for a successful recapitulation of human solid tumors. As is the case with in vivo solid tumors, large multicellular tumor spheroids (MCTS) are characterized by a core enriched with necrotic cells, a middle layer of quiescent cells and a proliferating rim that is due in large measure to the physiochemical gradients found through the spheroid (Sant and Johnston 2017). Indeed, the 3D nature of this approach causes tumor cells to face different levels of oxygen, nutrients and accumulation of metabolic waste that consequently impact cell signaling and behavior. As a result, large MCTS present a structural organization and cell heterogeneity that resemble in vivo tumors (Friedrich et al. 2009). Besides, the formation of tumor spheroids can be addressed with different degrees of complexity. It is possible to generate spheroids composed of tumor cells alone, in combination with other types of cells such as fibroblasts, or to include scaffold materials in its composition (Baal et al. 2009; Nath and Devi 2016). The latter culture system, usually termed scaffold-based MCTS, has the main advantage of incorporating external components that not only support cellular organization but also provide external cues that guide cellular behavior. For this reason, several methods have been developed to generate this type of tumor spheroids including matrix-on-top, matrix-embedded, matrix encapsulation, spinner flasks and micropatterned plates approaches (Nath and Devi 2016).
Fig. 7 - Schematic representation of in vitro 3D models used in cancer research, from Nath and Devi 2016.
25 – Biomimetic materials for cancer research
3. Biomimetic materials for cancer
research
3.1 Hydrogels
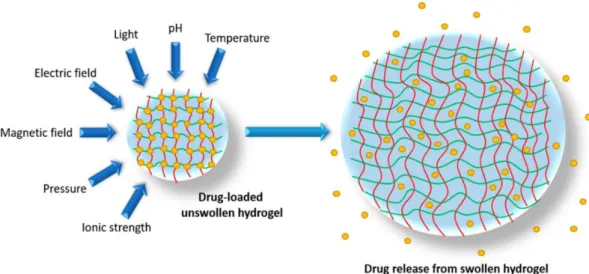
Hydrogels are tridimensional structures composed of polymeric networks that absorb large amounts of water (usually > 80% wt) (Hua, Ng, and Fei 2018). Due to their biomechanical properties and high degree of hydration, these biomaterials closely resemble living tissues, justifying their use in a large variety of biomedical applications (Sagar et al. 2018). The fact that hydrogels can retain a high content of water in their structure is essentially explained by hydrophilic groups attached to the polymer chains and the assurance of cross-links between them (Cai and Gupta 2012). These polymer chains can be tied together through more or less stable bonds, according to the nature of the cross-linking forming the network.
Physical gels are governed by reversible bonds such as ionic, H-bonding and hydrophobic forces, allowing to design stimuli responsive hydrogels that undergo structural modifications based on variations of the environmental conditions such as pH, ionic strength and temperature (Fig.8).
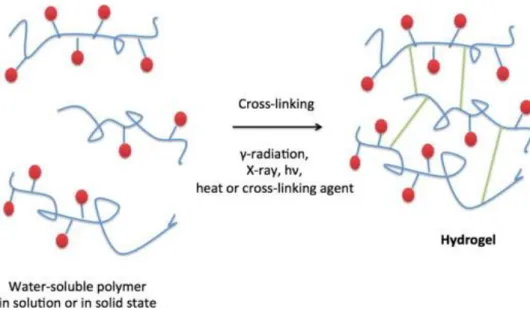
On the other hand, chemical gels are formed by permanent covalent bonds that can be produced using two common techniques: 3D-polymerization, in which water-soluble monomers react with multifunctional cross-linkers, or through direct cross-linking of water-soluble polymers. In this case, the process of polymerization can be initiated with free-radicals or using radiation (Fig.9), with the latter presenting the major advantage of being free of potential unreacted toxic compounds (Caló and Khutoryanskiy 2015).
26 – Introduction
Hydrogel design can also contemplate the incorporation of polymeric chains of synthetic and/or natural origin. This enables the production of biomaterials with a wide range of properties. While natural materials are employed to intensify biochemical properties of the scaffold, the use of synthetic materials is typically considered to provide a better control over its mechanical properties (Catoira et al. 2019). Nevertheless, numerous reports have demonstrated that natural hydrogels biomechanical features can be improved by simply adding chemical crosslinkers or functional groups (Achilli, Lagueux, and Mantovani 2010; Zhu and Marchant 2011). In the particular case of cancer research, the myriad of 3D platforms developed to date with the intention of mimicking the tumor microenvironment are based on natural or synthetic materials, either used alone or as hybrid systems (Sharma, Sharma, Lavy, Kiltie, et al. 2014).
3.1.1 Natural materials
3.1.1.1 Collagen
Collagen is the most abundant and the primary structural component of the ECM (Frantz, Stewart, and Weaver 2010). It presents low antigenicity, low inflammatory response, biocompatibility, biodegradability and excellent biological properties (Couet, Rajan, and Mantovani 2007; Marelli et al. 2012; Pankajakshan and Agrawal 2010). As so, its incorporation into 3D biomaterials has been considered for many different purposes, including tissue engineering, regenerative medicine and cancer research (George, S.R, and S.P 2018; Li and Kumacheva 2018).
It must be highlighted, however, that the mechanical strength it provides to native tissues results from its assembly structure and from its natural cross-linking capacity (Avery and Bailey 2008). Since its extraction process disrupts these interactions, the induction of exogenous
27 – Biomimetic materials for cancer research
crosslinking is often required to obtain stable biomaterials with an improved mechanical performance (Catoira et al. 2019; Gu et al. 2019). Cross-linking can be achieved by physical (e.g. UV irradiation and dehydrothermal treatment (DHT)) (Davidenko et al. 2016; Haugh, Jaasma, and O’Brien 2009), chemical (e.g. glutaraldehyde and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS)) (Gu et al. 2019; Kozłowska and Sionkowska 2015) and biological methods (e.g. lysyl oxidase, transglutaminase, etc.) (Elbjeirami et al. 2003; Orban et al. 2004). In addition, compression of collagen hydrogels has also been reported to render constructs of higher density and increased structural integrity (Sharma, Sharma, Lavy, Hamdy, et al. 2014).
Regarding cancer applications, and more specifically CRC, collagen hydrogels have been considered for the generation of 3D in vitro models (Magdeldin et al. 2017; Nyga et al. 2013). Interestingly, one of these studies aimed at developing a 3D platform comprised of a dense artificial cancer mass, created by plastic compression of collagen hydrogels containing HT29 CRC cells, inserted in a non-dense collagen hydrogel incorporating fibroblasts and/or endothelial cells. Results showed that HT-29 growth rates were in line with values observed in vivo, besides forming tumor spheroids that invaded surrounding collagen matrix. Also, authors demonstrated the ability of this system to create an oxygen depletion within the construct, resulting in a hypoxic environment and in the production of vascular endothelial growth factor (VEGF) (Nyga et al. 2013).
3.1.1.2 Fibrin
Fibrin is a fibrous protein involved in the clotting of blood. It is originated from the thrombin-catalyzed cleavage of fibrinogen, which causes it to polymerize into protofibrils and consequently fibrin fibers, which then branch to give rise to an extremely extensible and compressible tridimensional network, called fibrin clot (Litvinov and Weisel 2016). Fibrin has been used as a sealant or glue, as a matrix for cells, in scaffolds for tissue engineering or carriers/vectors for targeted drug delivery (Roura, Gálvez-Montón, and Bayes-Genis 2017). In the particular case of cancer research, its use has been suggested as a substrate for invasive cancer cells, as result of the strong ability demonstrated by metastatic tumors to invade fibrin (Knowles et al. 2013). Also, gels comprised of colon tumor-derived ECM and fibrin have been reported in studies aiming to elucidate the role of ECM in cancer cell behavior (Romero-López et al. 2017).
3.1.1.3 Matrigel
Matrigel is a commonly used cell culture matrix, consisting of a gelatinous protein mixture secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells. This solution is enriched in laminin, type IV collagen, perlecan, entactin, several growth factors and proteases, which resembles the complex environment of the BM. It is especially popular as cell culture models for evaluating angiogenesis and cellular differentiation (Gomillion and Burg 2017). In addition, Matrigel-based hydrogels have been commonly used for 3D cancer cell culture, being considered as the “gold standard” scaffold for growing multicellular cancer spheroids (MCS)
28 – Introduction
(Li and Kumacheva 2018). It has been used for MCS growth from breast cancer, colorectal cancer, prostate cancer, stomach cancer, pancreatic cancer, bladder cancer, among others (H. Xu et al. 2018) . Since it is a natural product, a particular disadvantage of its use is related to being an undefined matrix. Therefore, it introduces variability to experimental results (Gomillion and Burg 2017).
3.1.1.4 Silk
Silk is a naturally occurring biopolymer that consists of two types of proteins, silk fibroin and sericin. Silk sericin is a sticky material surrounding the filamentous core protein (fibroin), that is usually removed in tissue engineering approaches to avoid antigenic effects of silk and improve biocompatibility (Sharma, Sharma, Lavy, Kiltie, et al. 2014). In turn, the core protein (fibroin) has been widely considered as a biomaterial for several biomedical applications due to its remarkable properties such as slow biodegradability, good biocompatibility and excellent mechanical properties (Cao and Wang 2009). In cancer studies, cancer cells grown on 3D-silk scaffolds have demonstrated increased invasive and metastatic potential (Talukdar et al. 2011), besides displaying gene expression profiles more similar to in vivo tumors (Tan et al. 2011).
3.1.1.5 Chitosan/Alginate
Chitosan is a linear cationic polysaccharide composed of β-(1→4)-linked D-glucosamine and N-acetyl-D-glucosamine units. It is derived from the N-deacetylation of chitin, an abundant mucopolysaccharide found in nature, i.e., in the exoskeleton of shrimps, crabs, insects, cell walls of fungi and algae (Z. Yang, Wang, and Lu 2019). Chitosan-based scaffolds/hydrogels have been widely used in tissue engineering approaches as well as for drug delivery systems, presenting main advantages such as low cytotoxicity, good biocompatibility and biodegradability (Venkatesan, Kim, and Wong 2015). Notably, chitosan has been introduced in studies addressing the formation of tumoroids, given the biochemical similarity with ECM components, namely GAGs (Arya et al. 2012).
Alginate is a natural polymer consisting of linear copolymers of β-(1-4) linked d-mannuronic acid and β-(1-4)-linked l-guluronic acid units (Alihosseini 2016). This biomaterial forms hydrogels in the presence of divalent ions. In particular, calcium alginate hydrogels have been widely used in biomedical studies. The porous structure and high degree of hydration make these hydrogels optimal for the development of wound dressings (Aderibigbe and Buyana 2018), artificial matrices for cell encapsulation and controlled drug delivery systems (Mumper et al. 1994; Tariverdian et al. 2019). Moreover, alginate presents limited immunogenicity and excellent biological properties (Moradali, Ghods, and Rehm 2018).
Finally, polyelectrolyte complexes comprised of opposite charged polymeric networks (e.g. interconnected layers of alginate/chitosan) are other strategies employed in tissue engineering and cancer studies (Jiang et al. 2014). For the purpose of cancer research, it has been considered the creation of chitosan-alginate porous scaffolds for co-culture of cancer cells and immune cells (Florczyk et al. 2012), as well as the development of hybrid hydrogels with the
29 – Biomimetic materials for cancer research
intention of investigating the effects of changing stiffness, microstructure and porosimetry on tumor progression (C. Liu et al. 2018).
3.1.2 Synthetic polymers
Synthetic polymers have many advantages over naturally occurring biomaterials, as they usually present a controlled structure, a higher degree of processing flexibility and no concerns of immunological reactions (Magnusson et al. 2011). For tissue engineering applications, these aspects are particularly relevant to produce scaffolds of high reproducibility in terms of mechanical and physical properties, such as tensile strength, elastic modulus and degradation rate (Seal, Otero, and Panitch 2001).
Aliphatic polyesters are amongst the most frequently used synthetic polymers for tissue engineering applications, due to their biocompatible and biodegradable nature (Guo and Ma 2014; Song et al. 2018). Polylactide (PLA), polyglycolide (PGA) and poly(lactide-co-glycolide) (PLGA) are some common polyesters used for this purpose. For applications in the cancer field, it has been reported the use of PLGA 3D-scaffolds (Zhang et al. 2010) and microspheres (Kang and Bae 2009) for culture of melanoma and breast cancer cells, respectively.
3.2 Decellularized extracellular matrix -based biomaterials
Decellularization is the process of removing cellular components from tissues/organs in order to produce a natural ECM scaffold (Taylor and Sampaio 2015). This approach has essentially attracted a lot of interest in the fields of tissue engineering and regenerative medicine since one of the biggest challenges for these areas is to engineer scaffolds that mimic the complex morphology and diverse composition of native ECM (Mendibil et al. 2020). More than supporting cellular organization, ECM helps to regulate many cellular processes including cell growth, migration, proliferation and differentiation (Rozario and DeSimone 2010). It represents a highly dynamic structure that provides instructive signals to cells and the dysregulation of its normal composition and organization is often associated with pathologies (Bonnans, Chou, and Werb 2014). The fact that decellularization can retain major ECM components, while preserving the structural integrity of the tissue, is the reason why it is considered a promising tool to develop cell-instructive scaffolds that may provide cell- or tissue-specific support (Maeda, Suuronen, and Ruel 2017). Furthermore, they can lead to new insights into the role of the microenvironment in the initiation and evolution of certain pathological conditions. As so, decellularized matrices have been recently proposed to be used in cancer research in an attempt of fully understand the mechanisms underlying this complex disease (Hoshiba 2019).
30 – Introduction
3.2.1 Sources of decellularized ECM (dECM)
Decellularized ECM can be tissue/organ or cultured cell-derived. Tissue-derived dECM presents the major advantage of more accurately reflecting the original ECM composition and microstructure since it is originated from native tissue (Hoshiba et al. 2010). However, the limited availability of samples and the existing intertumoral heterogeneity, even in patients diagnosed with the same type of cancer, are some relevant limitations of its use (Hoshiba 2019; Hoshiba et al. 2010). In contrast, ECM derived from in vitro cultured cells can have its composition and organization tuned to have the desired properties, by controlling the culture conditions (initial substrates (Hoshiba and Tanaka 2015), cell source (Hoshiba et al. 2011), culture stage (Hoshiba et al. 2012), culture media (Soucy et al. 2011), etc.). Moreover, this method may represent an advantageous strategy to recreate specific regions of the ECM, such as stem cell niches (Hoshiba et al. 2010). The main drawback of this in vitro dECM type is the difficulty to prepare dECM with the biological complexity that only native dECM can provide.
3.2.2 Decellularization methods
There are three types of methods used to decellularize tissues: chemical, physical and enzymatic (Srokowski and Woodhouse 2017). Chemical methods relate to the use of detergents, such as sodium dodecyl sulfate (SDS), Triton X-100 and sodium deoxycholate (SDC), to disrupt cytoplasmatic and nuclear membranes, but also acids and bases to cause nucleic acid decomposition and solubilization of cytoplasmatic components (G. Chen and Kawazoe 2019). Physical treatments include freeze-thawing cycles, high hydrostatic pressure or supercritical CO2 (Gilpin and Yang 2017). Enzymatic agents are referred to the use of trypsin, dispase, phospholipases as well as nucleases such as DNAse, to promote a more efficient removal of DNA fragments (Fernández-Pérez and Ahearne 2019). All these methods present advantages and disadvantages, a reason to employ a combination of methods to produce a dECM as unaltered as possible, both in terms of original composition and structural integrity. As so, an accurate selection of the treatments to be used is key to cause minimal disruption of the ECM. Indeed, the main objective is to produce a counterbalanced result between efficiency of decellularization and ECM preservation (White et al. 2017). The use of more concentrated detergent solutions and longer exposure times enhance the removal of cellular component, but important ECM molecules are removed and disruption of the microstructure occurs. On the other hand, if very mild conditions are used, the efficiency of decellularization is compromised. In addition to this, the existing variability between different sources and types of tissue should be considered because protocols that are effective in some situations may not be in others. Tissue’s cellularity, density, lipid content and thickness are some critical determinants of the success of a decellularization protocol (Crapo, Gilbert, and Badylak 2011).
31 – Biomimetic materials for cancer research
3.2.3 Characterization of the decellularization efficacy
The most frequently used characterization methods include confirmation of cell removal, compositional analysis and structural analysis (Hoshiba 2019). The confirmation of cell removal, usually proceeded by the detection of cell nuclei/DNA, is critical to minimize immunological responses (Gilpin and Yang 2017). Additionally, the use of histochemical methods to detect the presence of intracellular components such as fibrous actin and cytosolic proteins can also be considered to complement the results obtained (Hoshiba 2019).
Regarding the compositional characterization of decellularized matrices, histological analyses have been employed to evaluate the preservation of major ECM components such as collagens and GAGs. For that, Sirius red and Alcian blue stainings are commonly used to detect their presence, respectively. In the case of a more detailed examination, immunohistochemical methods with specific antibodies can also be applied to detect specific ECM proteins. Ultimately, mass spectrometry analysis may be performed to characterize the ECM proteome (Hoshiba 2019).
For the purpose of analyzing the structural organization of the dECM, electron microscopy-based techniques are often used. While scanning electron microscopy (SEM) provides information of the structural properties of the ECM, transmission electron microscopy (TEM) can be used to detect the ultrastructure of collagen fibrils or the formation of the basement membrane (Hoshiba and Yamaoka 2019). Moreover, the use of the Fast Fourier transform analysis can provide information on the matrix fibril alignment (Harris, Raitman, and Schwarzbauer 2018).
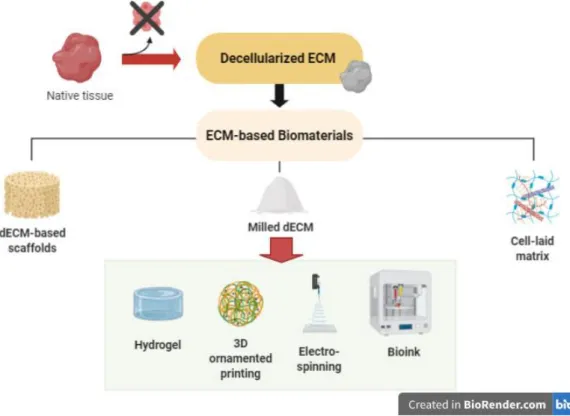
3.2.4 Decellularized extracellular matrix (dECM)-based biomaterials
The possibility of manipulating dECM offers the opportunity to design improved dECM-based biomaterials with customized properties to be used in a range of applications (Fig.10) (Heath 2019; Y. S. Kim et al. 2019). Currently, there is a large array of biomaterials in which decellularized matrices have been incorporated. Some examples are available throughout most recent research focused on tissue engineering and more specifically, on the basis of organ transplantation, injury repair, tissue regeneration (Hussey, Dziki, and Badylak 2018) and in vitro tumor modeling (Ferreira, Gaspar, and Mano 2020). Besides, the extensive amount of tissues targeted by this approach has made researchers process dECM in different ways, thus leading to the development of a variety of dECM-based platforms. The more simplistic approaches are based on tissue decellularization, followed by recellularization (Hillebrandt et al. 2019). However, several other and more complex strategies are reported in the literature. Major examples are hydrogels developed as dECM particles (Beachley et al. 2018; Townsend et al. 2017) or as solubilized dECM (Freytes et al. 2008; Koh et al. 2018; Seif-Naraghi et al. 2010).
The fragmentation of dECM has been particularly employed in studies directed to regenerative medicine in hope that preserved biochemical cues in the dECM can guide stem cell proliferation and proper differentiation (Brown et al. 2015). Some authors have even demonstrated the possibility of using thermally- or UV-initiated crosslinking, prospecting hydrogel polymerization (Cheung et al. 2014). On the other hand, the solubilization of dECM has started
32 – Introduction
to be approached in cancer studies, with the development of 3D hydrogels composed of solubilized glioblastoma multiforme-derived ECM. Importantly, glioblastoma cells cultured on these hydrogels showed a prompt invasion capacity and an increased expression of MMP9 and hyaluronan synthases (Koh et al. 2018). Apart from hydrogels, the formation of porous (Gawlitta et al. 2015) and microsphere-based scaffolds (V. Gupta et al. 2016) are as well reported in literature, with results showing the presence of dECM to beneficially impact cell behavior.
3D printable bioinks are also being considered for the development of dECM-based scaffolds (Pati et al. 2014), as a matter of fact confirming its recent wide use in tissue engineering approaches due to the ability of this technology to fabricate scaffolds of complex architecture and with precisely positioned biological materials (Rider et al. 2018). Regarding cancer field, there are numerous studies published to date focused on 3D bioprinted tumor models, using different scaffold materials and bioprinting methods (i.e. droplet-based, extrusion-based and laser-based) (Bae, Han, and Park 2020). One related study from X. Ma et al. proposes the use of 3D bioprinted liver dECM scaffolds, with different stiffness values, as platforms for studying hepatocellular carcinoma (HCC) progression. Interestingly, authors found that HepG2 cells encapsulated in stiffer constructs displayed an upregulation of invasion markers in comparison to healthy controls. (X. Ma et al. 2018).
Ultimately, an alternative to tissue-derived ECM has been proposed by using previously cultured scaffolds to allow in vitro secretion of matrix components, followed by its decellularization (Pati et al. 2015). Although this strategy was reported to be advantageous in decorating complex surfaces, it has been shown that the ECM produced in vitro does not reproduce the one found in vivo (Y. S. Kim et al. 2019). Thus, the use of native ECM in engineered platforms proves to be a more suitable option, particularly when the main objective is to model the ECM of a desired tissue.
Fig. 10 - A schematic representation of extracellular matrix-based biomaterials to be used in a variety of applications.