Tânia Patrícia Martins Fernandes
Candida tropicalis biofilms response to
antifungal agents
Tânia Patrícia Martins Fernandes
Candida tropicalis biofilms response to antifungal agents
Dissertação de Mestrado
Mestrado em Bioengenharia
Trabalho Efetuado sob a orientação da
Professora Doutora Mariana Henriques
e da
Doutora Sónia Silva
Tânia Patrícia Martins Fernandes
Candida tropicalis biofilms response to
antifungal agents
Nome: Tânia Patrícia Martins Fernandes
Endereço Eletrónico: tania_fernandes3@hotmail.com Cartão de Cidadão: 13777826
Título da Tese de Mestrado:
Candida tropicalis biofilms response to antifungal agents
Resposta dos biofilmes de Candida tropicalis a agentes antifúngicos
Orientadora: Professora Doutora Mariana Henriques Co-orientadora: Doutora Sónia Silva
Ano de conclusão: 2014
Designação do Mestrado: Mestrado em Bioengenharia
É AUTORIZADA A REPRODUÇÃO INTEGRAL DESTA TESE/TRABALHO APENAS PARA EFEITOS DE INVESTIGAÇÃO, MEDIANTE DECLARAÇÃO ESCRITA DO INTERESSADO, QUE A TAL SE COMPROMETE.
Universidade do Minho, ____/____/______
Agradecimentos
Esta dissertação de mestrado, apesar de ser um trabalho académico individual, teve o contributo de vários elementos que proporcionaram a sua realização e, como tal, não podem nem devem deixar de ser destacados. Por isso, queria expressar os meus sinceros agradecimentos:
À minha orientadora, Professora Mariana Henriques, pela oportunidade de poder realizar este projeto no âmbito da dissertação de mestrado e por ter partilhado comigo os seus excelentes ensinamentos, aconselhamentos e competência científica. Agradeço ainda a sua ajuda, apoio, disponibilidade, dedicação, empenho e incentivo que sempre demonstrou ao longo deste trabalho, através das suas orientações, críticas, correções e sugestões sempre muito relevantes. Gostaria de dizer ainda que foi um enorme prazer ter tido a possibilidade de trabalhar consigo. Um muito obrigado por sempre ter acreditado nas minhas capacidades.
À minha co-orientadora, Doutora Sónia Silva, para a qual todas as palavras de agradecimento nunca serão suficientes. Durante esta extensa caminhada foste a minha mentora e contigo aprendi mais do que com qualquer outra pessoa, por isso agradeço-te todos os teus ensinamentos, a tua desmesurada paciência e disponibilidade para comigo, e ainda pelo teu sentido de humor e boa disposição, mesmo estando sempre empilhada em trabalho, porque arranjavas sempre tempo e forma de me ajudar e aconselhar. Por isso, mais do que ninguém mereces um agradecimento muito especial, não só pela melhor orientação que podia ter tido e por teres sempre acreditado no meu trabalho, mas também pela tua enorme amizade. Foi mais do que um privilégio trabalhar contigo e espero um dia voltar a fazê-lo. UM ENORME OBRIGADO POR TUDO!
Aos meus colegas dos Laboratórios de Microbiologia Aplicada I e II, especialmente aos integrantes do meu grupo (“Candidas”) e ao grupo dos “Fagos”, pelo excelente ambiente de trabalho e boa disposição, pelo apoio, ajuda e estima sempre mostradas, destacando o Leonel, pela confiança demostrada desde logo, pela desmedida disponibilidade, paciência e ajuda, e a imensurável boa disposição e amizade que sempre me presenteou, a Célia, pela ajuda e sugestões sempre prestadas ao longo desta caminhada, e ao Carlos Tiago, pela boa disposição contagiante e também pelos conselhos sempre que necessários. Queria também fazer um agradecimento muito especial ao grupo “Poderosas” (Chaiene, Eduarda, Márcia, Patrícia e Marta), pela ajuda, amizade e diversão desmedidas durante os intensos dias de trabalho.
e Soraia), pela paciência e ajuda imensuráveis, mas sobretudo pela enorme amizade. Aos meus amigos de Bioquímica com quem partilhei e irei sempre partilhar os melhores momentos da minha vida, pela vossa desmedida amizade, pelo vosso incentivo e apoio constantes, e por acreditarem sempre no meu trabalho e alegrarem sempre os meus dias.
À FCT que financiou este trabalho no âmbito do projeto PTDC/EBB-EBI/120495/2010. Ao Departamento e ao Centro de Engenharia Biológica por terem cedido as suas instalações para a realização desta tese, à Universidade do Minho pelas excelentes condições de trabalho que me proporcionou, e aos meus professores em geral, por colaborarem na minha formação e pela disponibilidade e ajuda concedidas.
Aos meus familiares, pelo apoio absoluto, em especial, aos meus pais e irmã, por terem sempre acreditado em mim e por estarem sempre ao meu lado em todos os momentos, para os quais todos os agradecimentos não serão suficientes, e à minha tia com quem resido em Braga, por tanto me ter ajudado e me ensinar que a coragem e a força de vontade são uma mais-valia na nossa vida. Também não podia deixar de agradecer, aos meus amigos, por estarem sempre disponíveis e por me ajudarem, apoiarem e incentivarem descomedidamente.
O meu muito obrigado, a todos aqueles que, de certa forma, contribuíram, ajudaram, estimularam e me apoiaram na concretização desta dissertação.
Abstract
Candidiasis is the most prevalent opportunistic fungal infection of humans and has been increasing in the last decades, namely due to Candida tropicalis species. Its pathogenicity can be strongly related to their potential virulence factors, as adhesion and biofilm formation, secretion of hydrolytic enzymes and filamentous forms development. Biofilm formation is a potent virulent factor for a number of Candida species, as it confers significant resistance to antifungal therapy, due to, among other factors, limiting the penetration of substances throughout the matrix. However, the exact mechanism of biofilm resistance to antifungals is not yet fully understood, especially for C. tropicalis, and, as such, it is essential to enhance the knowledge on its virulence factors and resistance mechanisms. Thus, the main aim of this work was to understand the mechanisms behind C. tropicalis tolerance, to fluconazole (Flu), voriconazole (Vcz) and amphotericin B (Amp B). In order to accomplish this main goal, three approaches were performed. Firstly, the effect of the Flu and Vcz on ERG genes expression was determined on both planktonic and biofilm cells; secondly, the influence of azole antifungals on C. tropicalis virulence factors expression was analyzed; and finally, the role of the C. tropicalis biofilm matrices on its resistance to Amp B was evaluated.
With this work it was possible to demonstrate that C. tropicalis resistance to Flu, Vcz and Amp B is strain dependent. Moreover, it was also observed that all the three antifungal agents were able to control moderately the biofilm formation, but were unable to eradicate C. tropicalis pre-formed biofilms. Biofilms treated with Flu presented significant damage in its overall structure in opposition to Vcz, however both lead to a slight reduction on its compact structure. The results showed that the ERG genes were more expressed in planktonic cells than in biofilm cells. Additionally, these azoles had no influence on main C. tropicalis virulence factors. Candida tropicalis
biofilms treated with Amp B lead to an increase of matrix production and, consequently, to an increase in its amount of protein and carbohydrate. This is an indicative that the C.
tropicalis biofilm matrices have an important role in its resistance to Amp B. This work
demonstrated that C. tropicalis biofilms respond to treatment with different antifungals in different forms, so its resistance mechanism to antifungals seems to be a multifactorial phenomenon, such as reported for C. albicans. Thus, by increasing our knowledge on C. tropicalis resistance to antifungals, potential targets may well be identified that can be used as adjuvant for new therapies.
Resumo
A candidíase é a infeção fúngica oportunista mais prevalente em seres humanos e tem vindo a aumentar nas últimas décadas, nomeadamente devido à espécie Candida
tropicalis. A sua patogenicidade pode estar fortemente relacionada com os seus
potenciais fatores de virulência, como adesão, formação de biofilme, e produção de enzimas hidrolíticas e formas filamentosas. A formação de biofilme é importante para espécies de Candida, conferindo-lhes significativa resistência à terapia antifúngica, devido, entre outros fatores, à limitação da penetração de substâncias pela matriz. Contudo, o mecanismo exato de resistência do biofilme aos antifúngicos não está totalmente compreendido, especialmente para C. tropicalis, e, como tal é essencial compreendê-lo melhor. Assim, o principal objetivo deste trabalho foi compreender os mecanismos inerentes à tolerância de C. tropicalis ao fluconazol (Flu), voriconazol (Vcz) e anfotericina B (Amp B). De forma a alcançá-lo, três abordagens diferentes foram realizadas. Inicialmente foi determinado o efeito do Flu e Vcz na expressão de genes ERG em células plantónicas e de biofilme; de seguida, foi analisada a influência destes azóis na expressão dos seus fatores de virulência; e, finalmente foi avaliado o papel das matrizes de biofilmes de C. tropicalis na sua resistência à Amp B.
Este trabalho demonstrou que a resistência de C. tropicalis aos antifúngicos é dependente da estirpe. Foi também observado que estes antifúngicos foram capazes de controlar moderadamente a formação de biofilme, mas incapazes de erradicar biofilmes pré-formados. Os biofilmes tratados com Flu apresentaram danos na sua estrutura em oposição aos tratados com Vcz, mas ambos levaram a uma ligeira redução da sua estrutura. Os resultados mostraram que os genes ERG foram mais expressados em células plantónicas do que em biofilme. Adicionalmente, os azóis testados não tiveram influência nos principais fatores de virulência de C. tropicalis. Foi também mostrado que os biofilmes tratados com Amp B apresentaram um aumento de produção de matriz e da quantidade de proteínas e carbohidratos. Isto é indicativo que as matrizes destes biofilme têm um papel importante na resistência à Amp B. Este trabalho demonstrou que os biofilmes de C. tropicalis responderam diferentemente ao tratamento com diferentes antifúngicos, pelo que seu mecanismo de resistência parece ser um fenómeno multifactorial, como relatado para C. albicans. Assim, aumentando o conhecimento sobre a sua resistência aos antifúngicos, melhora-se a identificação de novos potenciais alvos, que podem vir a ser usados como adjuvantes para novas terapias.
Table of contents
Agradecimentos ... III Abstract ... V Resumo ... VII Table of contents ... IX List of abbreviations ... XIII List of figures ... XV List of tables ... XIX Objectives and scope of the thesis ... XXI
Chapter I | General introduction......1
1.1. Fungal infections: Candidiasis ... 3
1.2. Candida species causing candidiasis ... 4
1.2.1. Candida species distinction ... 5
1.2.2. Candida tropicalis ... 7
1.2.2.1. Risks associated to Candida tropicalis infections ... 8
1.2.2.2. Candida tropicalis virulence factors ... 9
1.2.2.2.1. Dimorphism ability ... 9
1.2.2.2.2. Adhesion ability ... 11
1.2.2.2.3. Biofilm formation ... 12
1.2.2.2.4. Dissemination ability ... 16
1.2.2.2.5. Hydrolytic enzyme secretion ... 17
1.2.2.2.5.1. Secreted aspartyl proteinases ... 17
1.2.2.2.5.2. Phospholipases ... 18
1.2.2.2.5.3. Haemolysins ... 18
1.2.2.3. Resistance to antifungal agents ... 19
1.2.2.3.1. Amphotericin B ... 23
1.2.2.3.2. Fluconazole and Voriconazole ... 24
Chapter II | Candida tropicalis biofilms tolerance to fluconazole and voriconazole and its relationship with ERG genes expression......27
2.1. Introduction ... 29
2.2. Materials and Methods ... 30
2.2.3.1. Influence of antifungal agents in biofilm formation ... 32
2.2.3.2. Influence of antifungal agents in pre-formed biofilm ... 32
2.2.4. Biofilm analyses ... 33
2.2.4.1. Biofilm biomass ... 33
2.2.4.2. Biofilm cultivable cells ... 33
2.2.4.3. Biofilm structure ... 33
2.2.5. Gene expression analysis... 34
2.2.5.1. Gene selection and primers design for quantitative Real-Time PCR ...34
2.2.5.2. Planktonic and biofilm cells preparation ... 35
2.2.5.3. RNA extraction ... 35
2.2.5.4. Synthesis of cDNA ... 35
2.2.5.5. Quantitative Real-Time PCR ... 36
2.2.6. Statistical analysis ... 36
2.3. Results ... 36
2.3.1. Candida tropicalis fluconazole and voriconazole minimum inhibitory concentrations ... 36
2.3.2. Effect of the fluconazole and voriconazole on Candida tropicalis biofilm ...38
2.3.2.1. Influence of antifungal agents on biofilms formation ... 38
2.3.2.2. Antifungal agents effect against pre-formed biofilms ... 39
2.3.3. Effect of the fluconazole and voriconazole on Candida tropicalis biofilm structure ... 40
2.3.4. Effect of the fluconazole and voriconazole on ERG genes expression...41
2.4. Discussion ... 45
Chapter III | Effect of the fluconazole and voriconazole on Candida tropicalis virulence factors...........51
3.1. Introduction ... 53
3.2. Materials and Methods ... 54
3.2.1. Organisms ... 54
3.2.2. Growth conditions ... 54
3.2.3.1. Cultivable cells enumeration ... 56
3.2.3.2. Biofilm biomass assessment ... 56
3.2.4. Hydrolytic enzyme secretion ... 56
3.2.4.1. Proteolytic and phospholytic activity ... 56
3.2.4.2. Hemolytic activity ... 57
3.2.5. Influence of antifungal agents in the filamentous forms formation ... 57
3.2.6. Statistical analysis...60
3.3. Results ... 58
3.3.1. Fluconazole and voriconazole influence on Candida tropicalis virulence factors ... 58
3.3.1.1. Fluconazole and voriconazole effect on Candida tropicalis adhesion ability ... 58
3.3.1.2. Fluconazole and voriconazole effect on Candida tropicalis biofilm formation ... 59
3.3.1.3. Fluconazole and voriconazole effect on Candida tropicalis hydrolytic enzymes and filamentous forms formation ... 60
3.4. Discussion ... 61
Chapter IV | Candida tropicalis biofilm resistance to amphotericin B: matrix influence...65
4.1. Introduction ... 67
4.2. Materials and Methods ... 68
4.2.1. Organisms and Growth conditions ... 68
4.2.2. Antifungal susceptibility test methods ... 68
4.2.3. Biofilm formation ... 69
4.2.3.1. Influence of amphotericin B in biofilm formation ... 69
4.2.3.2. Influence of amphotericin B on pre-formed biofilms ... 70
4.2.4. Biofilm analyses ... 70
4.2.4.1. Biofilm total biomass ... 70
4.2.4.2. Biofilm cultivable cells ... 70
4.2.4.3. Biofilm structure ... 71
4.2.5. Biofilm matrix composition analysis ... 71
4.2.5.1. Matrix extraction method ... 71
4.3.2. Amphotericin B effect on Candida tropicalis biofilm ... 73
4.3.2.1. Influence of amphotericin B on biofilm formation ... 73
4.3.2.2. Amphotericin B effect against pre-formed biofilms ... 74
4.3.3. Effect of amphotericin B on Candida tropicalis biofilm structure and matrix composition ... 75
4.4. Discussion ... 77
Chapter V | General conclusions and future perspectives..............81
5.1. General conclusions ... 83
5.2. Future perspectives ... 84
References...85
Appendices........93
Appendix A – Candida tropicalis biofilm formation ... 95
List of abbreviations
ABC – ATP-binding cassette;
ACT1 – Actin gene;
AIDS – Acquired immunodeficiency syndrome; Als – Agglutinin-like sequence;
Amp B – Amphotericin B; ANOVA – Analysis of variance;
ATCC – American Type Culture Collection; ATP – Adenosine triphosphate;
BCA – Bicinchoninic acid; BSA – Bovine serum albumin; cDNA – Complementary DNA;
CDR– Candida drug resistance;
CFUs – Colony forming units; CGD – Candida Genome Database;
CHROMagar – Chromogenic agar medium; CLSI – Clinical and Laboratory Standards Institute; CV – Crystal violet;
DMSO – Dimethyl sulfoxide; DNA – Deoxyribonucleic acid; ECM – Extracellular matrix;
ERG genes – Ergosterol synthesis associated genes;
EUCAST – European Committee on Antimicrobial Susceptibility Testing; FIs – Fungal infections;
Flu – Fluconazole;
FNIs – Fungal nosocomial infections; HIV – Human immunodeficiency virus; ICUs – Intensive care units;
MDR– Multi-drug resistance;
MFCs – Minimal fungicidal concentrations; MFS – Major facilitator superfamily;
MRS – Major repeat sequence;
NCAC – Non-Candida albicans Candida; NRT – Non-transcriptase reverse;
PBS – Phosphate buffer saline;
PCR – Polymerase chain reaction; PIA – Polysaccharide intercellular adhesin; Pls – Phospholipases;
Pz – Enzymatic activity;
qRT-PCR – Quantitative Real-time PCR; RHE – Reconstituted human epithelium; RNA – Ribonucleic acid;
RPMI – Roswell Park Memorial Institute; Saps – Secreted aspartyl proteinases; SDA – Sabouraud dextrose agar; SDB – Sabouraud dextrose broth; SEM – Scanning electron microscopy; Tm – Temperature of melting;
List of figures
Chapter I
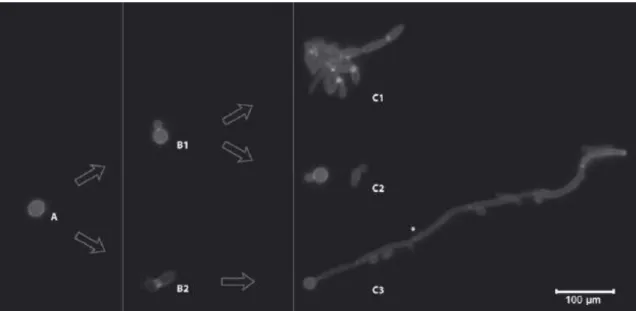
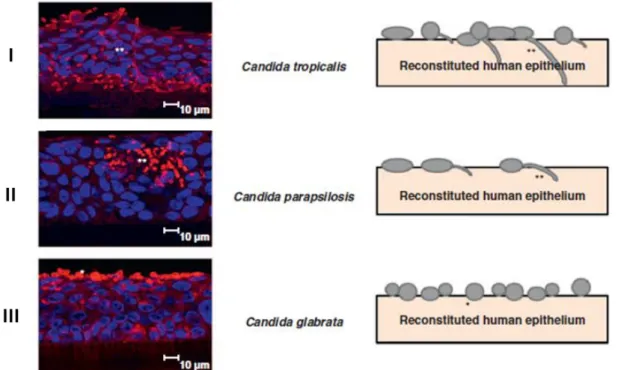
Figure 1.1. Differences morphological growth forms of Candida by epifluorescence photocomposition. A) Blastoconidia; B1) Reproduction by budding; B2) Germ tube formation; C1) Pseudohyphae formation; C2) Yeast form; C3) Hyphae formation. *Septa. Retrieved from: Silva et al. 1. ... 7 Figure 1.2. Confocal laser scanning microscopy images of the reconstituted human oral epithelium infected with Candida tropicalis (I), Candida parapsilosis (II) and Candida
glabrata (III) during 12 h. *Yeast; ** Filamentous forms. Adapted from: Silva et al. 24.
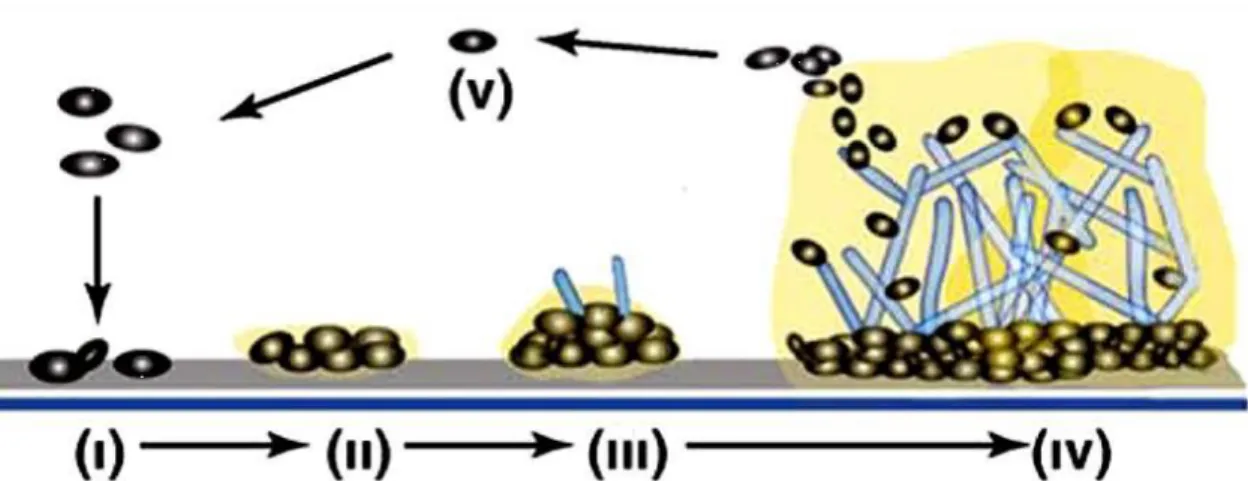
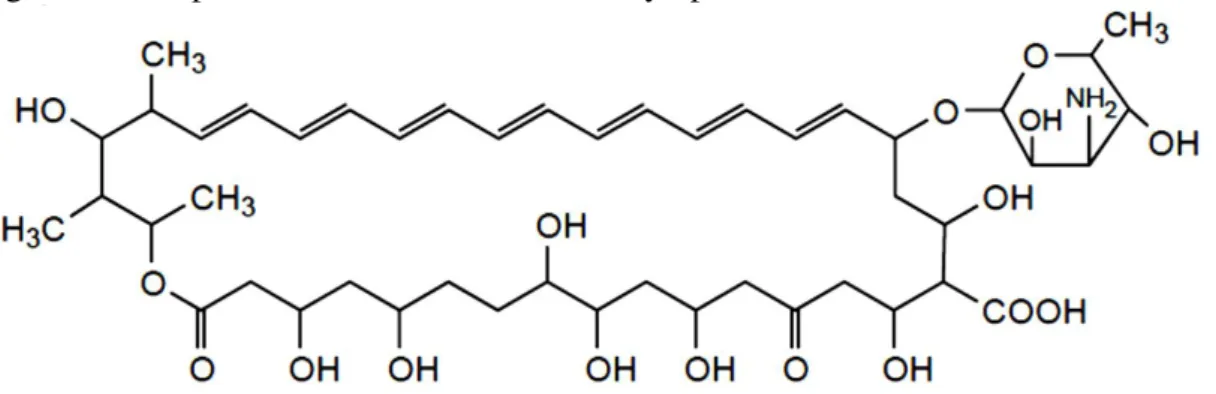
... 10 Figure 1.3. Biofilm formation development of Candida tropicalis. Adapted from: Harding et al. 75. ... 13 Figure 1.4. Scanning electron microscopy images of non-Candida albicans Candida species biofilm grown in Sabouraud dextrose broth for 48 h. The bar in the images corresponds to 20 µm. Magnification x 1000. Adapted from: Silva et al. 24. ... 16 Figure 1.5. Mechanisms of action of the main classes of antifungals. Adapted from: Terézhalmy et al. 94. ... 21 Figure 1.6. Chemical structural of the amphotericin B. Adapted from: Odds et al. 102. 23 Figure 1.7. Chemical structural of the fluconazole (A) and voriconazole (B). Adapted from: Odds et al. 102. ... 25
Chapter II
Figure 2.1. Effect of fluconazole (I) and voriconazole (II) on Candida tropicalis biofilm formation after 48 h. (A) Mean values of the logarithm of CFUs normalized by unit of area (log10 CFU/cm2); (B) Mean values of the absorbance at 570 nm normalized
by unit of area (Abs570nm/cm2). Error bars indicate the standard deviation. **, *** and
**** correspond to p < 0.01, p < 0.001 and p < 0.0001, respectively. ... 38 Figure 2.2. Effect of fluconazole (I) and voriconazole (II) against 24 h pre-formed
Candida tropicalis biofilms during 48 h. (A) Mean values of the logarithm of CFUs
normalized by unit of area (log10 CFU/cm2); (B) Mean values of the absorbance at 570
nm normalized by unit of area (Abs570nm/cm2). Error bars indicate the standard
deviation. *, **, *** and **** correspond to p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively. ... 39 Figure 2.3. Scanning electron microscopy images of 24 h pre-formed Candida
tropicalis AG1 (A), Candida tropicalis 12 (B) and Candida tropicalis 519468 (C)
biofilms grown in RPMI medium for 48 h in (I) absence of antifungal agents, and in the presence of (II) 500 mg/L of the fluconazole and (III) 100 mg/L of the voriconazole.
(III), ERG9 (IV) and ERG11 (V) genes in Candida tropicalis strains grown in planktonic form (A) or biofilm (B), without or with 10 and 500 mg/L of fluconazole. Comparisons were made with planktonic and biofilm cells growth in the absence of fluconazole. Error bars indicate the standard deviation. *, **, *** and **** correspond to p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively. ... 42 Figure 2.5. Mean values of n-fold expression levels of ERG1 (I), ERG3 (II), ERG6 (III), ERG9 (IV) and ERG11 (V) genes in Candida tropicalis strains grown in planktonic form (A) or biofilm (B), without or with 0.75 and 100 mg/L of voriconazole. Comparisons were made with planktonic and biofilm cells growth in the absence of voriconazole. Error bars indicate the standard deviation. *, **, *** and **** correspond to p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively. ... 44
Chapter III
Figure 3.1. Adhesion ability of Candida tropicalis cells after grown in the presence of fluconazole (A) and voriconazole (B). Mean values of the logarithm of CFUs normalized by unit of area (log10 CFU/cm2). Error bars indicate the standard deviation.
* Correspond to p < 0.05. ... 58 Figure 3.2. Biofilm formation ability of Candida tropicalis cells after previously grown in the presence of fluconazole (I) and voriconazole (II). (A) Mean values of the logarithm of CFUs normalized by unit of area (log10 CFU/cm2); (B) Mean values of the
absorbance at 570 nm normalized by unit of area (Abs570nm/cm2). Error bars indicate the
standard deviation. *, **, *** and **** correspond to p < 0.05, p < 0.01, p < 0.001 and
p < 0.0001, respectively. ... 59
Chapter IV
Figure 4.1. Effect of amphotericin B on Candida tropicalis biofilm formation after 48 h. (A) Mean values of the logarithm of CFUs normalized by unit of area (log10
CFU/cm2); (B) Mean values of the absorbance at 570 nm normalized by unit of area
(Abs570nm/cm2). Error bars indicate the standard deviation. *, ** and **** correspond to
p < 0.05, p < 0.01 and p < 0.0001, respectively. ... 74
Figure 4.2. Effect of amphotericin B against 24 h pre-formed Candida tropicalis biofilms during additional 48 h. (A) Mean values of the logarithm of CFUs normalized by unit of area (log10 CFU/cm2); (B) Mean values of the absorbance at 570 nm
normalized by unit of area (Abs570nm/cm2). Error bars indicate the standard deviation. *,
**, *** and **** correspond to p < 0.05, p < 0.01, p < 0.001 and p < 0.0001, respectively. ... 75 Figure 4.3. Scanning electron microscopy images of 24 h pre-formed Candida
tropicalis AG1 (A), Candida tropicalis 12 (B) and Candida tropicalis 519468 (C)
and (II) in the presence of 2 mg/L of the amphotericin B. The bar in the images corresponds to 20 µm. Magnification x 1000. ... 76
Appendices
Figure A.1. Candida tropicalis biofilm formation during 24, 48 and 72 h. (I) Mean values of the logarithm of CFUs normalized by unit of area (log10 CFU/cm2); (II) Mean
values of the absorbance at 570 nm normalized by unit of area (Abs570nm/cm2).
Comparisons were made with biofilm formed during 48 h. Error bars indicate the standard deviation. **, *** and **** correspond to p < 0.01, p < 0.001 and p < 0.0001, respectively. ... 95 Figure B.1. Calibration curve of the bovine serum albumin. The equation of standard curve is Abs 562 nm = (0.0010 ± 0.0001) BSA concentration + (0.0218 ± 0.0536) and
presents a R2 = 0.9955. ... 96 Figure B.2. Calibration curve of the glucose. The equation of standard curve is Abs 490 nm = (0.3035 ± 0.0187) glucose concentration + (0.0230 ± 0.0459) and presents a R2 =
List of tables
Chapter II
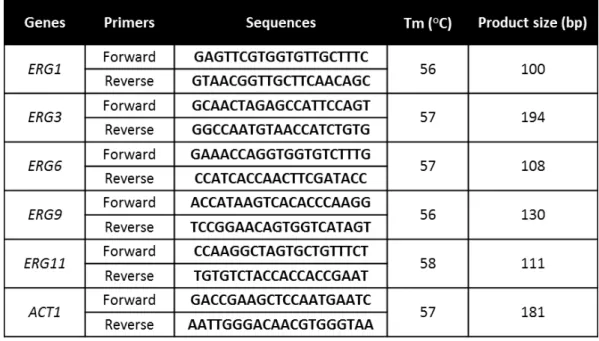
Table 2.1. Primers used for qRT-PCR, the respective melting temperature and product size ... 34 Table 2.2. Candida tropicalis minimum inhibitory concentrations and minimal fungicidal concentrations obtained with fluconazole and voriconazole ... 37
Chapter III
Table 3.1. Proteolytic, phospholytic and hemolytic activity and percentage (%) of filamentous forms formation of Candida tropicalis strains after grown in the absence and presence of fluconazole and voriconazole ... 60
Chapter IV
Table 4.1. Candida tropicalis minimum inhibitory concentrations and minimal fungicidal concentrations obtained with amphotericin B ... 73 Table 4.2. Effect of the amphotericin B in the Candida tropicalis pre-formed biofilm matrix composition in terms of proteins and carbohydrates quantity. Mean ± standard deviation values of the proteins (mg protein/g dry weight biofilm) and carbohydrates (mg
Objectives and scope of the thesis
The main aim of this thesis was the evaluation of the C. tropicalis biofilm resistance to antifungal agents. In order to achieve this aim, three different steps were followed: firstly, the influence of the fluconazole (Flu) and voriconazole (Vcz) on ERG genes expression in planktonic and biofilm cells; secondly, the effect of azole antifungals on C. tropicalis virulence factors expression; and finally, the role of the C.
tropicalis biofilm matrices on its resistance to amphotericin B (Amp B). Therefore, this
dissertation is organized in five chapters.
Chapter I is a general introduction, in which the state of the art concerning the biology and epidemiology of the C. tropicalis is presented, as well as their virulence factors and resistance to antifungal agents.
Chapter II reports C. tropicalis biofilms tolerance to Flu and Vcz and its relationship with ERG genes expression. In a first stage the determination of the planktonic cells tolerance of several C. tropicalis clinical isolates to Flu and Vcz was performed, and the ones with the most different results were selected for the evaluation of the role of the antifungal agents in their biofilm formation and on pre-formed biofilms. Moreover, the effect of Flu and Vcz on ERG genes expression and its involvement in C. tropicalis resistance was also evaluated.
In the Chapter III it is described the influence of the same azole agents on C.
tropicalis virulence factors, such as adhesion and biofilm formation ability, secretion of
hydrolytic enzymes and development of filamentous forms.
Chapter IV shows the evaluation of the role of the matrix compounds on C.
tropicalis biofilms resistance to Amp B. Thus, it was determined the planktonic cells
tolerance to Amp B, its effect on C. tropicalis biofilms and analysis of the C. tropicalis biofilm matrices treated with Amp B in terms of total protein and carbohydrate.
The last chapter includes the main conclusions of this thesis and the suggestions for future work.
Chapter I
1.1. Fungal infections: Candidiasis
In the last decades, there has been a significant increase of fungal infections (FIs) in humans, particularly of fungal nosocomial infections (FNIs) 1–3. The FNIs are now a serious public health problem, since these infections are among the main cause of morbidity and mortality, leading to an increase in the hospitalization time and, consequently, high costs associated to patients’ treatment 2,4,5. These infections may
either be superficial, affecting the skin, hair, nails and mucosal membranes, or systemic, involving major body organs 1,6. Furthermore, the FNIs have been particularly prominent in intensive care units (ICUs), where the incidence is much greater than in the general population of hospitalized patients 2,7,8. The causes for the increase risk of FNIs in ICUs have been associated to several factors, but is generally accepted that the increase and widespread use of certain medical practices, as the long length of stay in ICU, the augment of invasive surgical procedures and in the number of patients with compromised immune systems, as well as the multiple exposure to antibiotics are significant 1–3,9–13. Beyond of the hospital unit and disease involving patient, factors related to microorganism are of major importance to the progression of hospital-acquired infections, once most of the nosocomial infections are caused by microorganisms of the normal microbiota that cause infection in special situations, such as under immunosuppression. In these patients considered at risk, invasive FIs are often severe with a rapid progression and difficultly to diagnose and/or treat 2–4,14.
One of the most prevalent invasive FIs is the candidiasis, which it is caused by members of the Candida genus. These infections correspond to approximately 80 % of FNIs, being the fourth most common cause of nosocomial bloodstream infection and responsible for the overwhelming majority of urinary tract infections 2,3,12,15–17. The
Candida genus contains over 150 heterogeneous species, however only a minority has
been implicated in human candidiasis. Moreover, Candida species normally exist as commensals, but they are also opportunistic pathogens with ability to cause a variety of superficial and systemic infections. Additionally, it is known that approximately 65 % of Candida species are unable to grow at a temperature of 37 °C, which precludes these species from being successful pathogens or indeed commensals to humans. It is important to note that Candida species are most frequently isolated from the oral cavity,
and vulvovaginal and urinary tracts, and are detected in approximately 31 – 55 % of healthy individuals 1,13.
Candidiasis has been considered the most prevalent opportunistic fungal infection in humans with particular significance in patients undergoing treatment for cancer, organ transplantation, receiving broad spectrum antibiotics, human immunodeficiency virus (HIV)-infected and with acquired immunodeficiency syndrome (AIDS). This infection has merging due to increase of the elderly population base, immunocompromised patients number and a more widespread use of indwelling medical devices 2,11,13,18,19. Furthermore, new extracorporeal reservoirs colonized by
Candida species has increased greatly in recent years, as natural environments,
inanimate surfaces, health professionals’ hands and hospitals, clarifying the epidemiological cycle of hospital infections caused by Candida species of exogenous origin. In fact, the hands of health professionals may be colonized by yeasts and also serve as reservoirs for NIs. The use of catheters and other medical devices is also related to development of NIs with Candida species via exogenous routes, because the high prevalence of Candida isolates in samples from catheter tips may have an important role in the spread, progression and persistence of NIs 20–22.However, the Candida species are not related exclusively to hospital candidemia, because hematogenous infections also occur in the community 20.
1.2. Candida species causing candidiasis
The main cause of candidiasis is Candida albicans, once it is the most commonly isolated and responsible for the majority of superficial and systemic FIs, hence receiving major clinical attention 1,2,18. However, in parallel with the overall increase of FIs, it has been observed that infections caused by non-Candida albicans
Candida (NCAC) species are also emerging, particularly when caused by Candida tropicalis, Candida parapsilosis and Candida glabrata 2,3,13,15,18,23,24. In fact, the
prevalence of Candida species in human infections has been changing in recent years, once twenty years ago, C. albicans has accounted for 70 – 80 % of clinical isolates, with other NCAC species only rarely encountered, however, in the last two decades, the number of infections due to NCAC species has increased significantly 1,13,24,25. In a study on the epidemiology of invasive candidiasis, Pfaller & Diekema 26 demonstrated
that C. albicans, C. glabrata, C. tropicalis and C. parapsilosis collectively accounted for about 95 % of identifiable Candida infections. Furthermore, recently, Bassetti et al.
7 reported that 60 % of the fungemia cases were due to NCAC species. Regardless to the
basis of this change, recent epidemiological data reveal a mycological shift and, while
C. albicans remains the most common causative agent, its relative incidence in infection
is declining with the increasing prevalence of other species, such as C. glabrata, C.
tropicalis and C. parapsilosis 1,7,27,28.
Indeed, the reasons for this alteration in the pattern of Candida species distribution has not yet been completely understood, but it is believed that could be attributed to several factors. The apparent increasing involvement of NCAC species in human candidiasis could be related in part to improvements in diagnostic methods, as the use of chromogenic media with ability to differentiate between Candida species and introduction of molecular diagnostic techniques in the routine diagnosis of fungemia
1,2,24,29. But this emergence can also be associated with the degree of diseases of the
patients, and a higher introduction and more widespread use of certain medical practices, as immunosuppressive therapy, the use of broad spectrum antibiotics and an increase in the number of invasive surgical procedures, such as organ transplantations
1,11,24,30. Furthermore, the increasing number of Candida species as causative of
candidiasis could also be a reflection of species selection in the presence of certain antifungals, given the high level of resistance demonstrated by the NCAC species to antifungal agents 1,2,17,31. However, it is important to emphasize that there are significant variations in the Candida species isolation depending on the geographical region and patient group, with some NCAC species more prevalent even than C. albicans in certain countries 1,32.
1.2.1. Candida species distinction
Such as referred, the Candida genus comprises an extremely heterogeneous group of fungal organisms and, as such, present some different characteristics, which may also be involved in the distribution of Candida species 1. The several Candida species may be distinguished by some factors, as yeast size, colony color on chromogenic agar medium (CHROMagar), and morphological, biochemical and genetic characteristics 1. Regarding colonies’ morphology, when grown on Sabouraud dextrose
border, C. parapsilosis produces white, creamy, shiny and smooth/wrinkled colonies, while C. glabrata forms glistening, smooth and cream-coloured colonies, which are largely indistinguishable of the other Candida species, except from their relative size, once it is smaller 1,2. Indeed, C. albicans, C. tropicalis and C. parapsilosis form blastospores with about 4 – 6 x 6 – 10, 4 – 8 x 5 – 11 and 2.5 – 4 x 2.5 – 9 µm of size, respectively, while the C. glabrata cells have a size of 1 – 4 µm, approximately, therefore they are noticeably smaller than the other Candida species 1. Regarding the CHROMagar medium, it can present blue-green, dark-blue, white, white or pink-purple colonies for C. albicans, C. tropicalis, C. parapsilosis and C. glabrata, respectively 1.
Moreover, certain Candida species can exhibit morphological alternation from yeast, pseudohyphae and hyphae forms, depending on the environmental conditions. This ability to morphological alternation is designated by dimorphism (Figure 1.1), which has also revealed to be an important virulence factor of Candida species and will be involved in their pathogenicity 1,24,33. The distinction between hyphae and pseudohyphae is related to the way in which they are formed. The true hyphae are formed from yeast cells or even as branches of existing hyphae, and their development is initiated by a “germ tube” projection, which elongates and then branches with defined septa that divide in hyphae into separate fungal units 1. In comparison, the pseudohyphae are formed from yeast cells or hyphae by budding, but the new growth remains attached to the parent cell and elongates, resulting in filaments with constrictions at the cell-cell junctions, and there are no internal cross walls (septa) associated with pseudohyphae 1. So, the morphological transition can be observed in some Candida species, whereas other species grow only in the yeast form. Candida
albicans is a true polymorphic organism, because it is able to grow as hyphae and/or
pseudohyphae, and as blastospores 1,2,24. Concerning C. tropicalis, it produces oval blastospores and pseudohyphae, and according to some reports, true hyphae 1,24,34. In the case of C. parapsilosis, the blastospore growth is prevalent, and although this species does not produce true hyphae, it can occasionally generate pseudohyphae 1,24,35. In contrast, C. glabrata is not polymorphic, growing only as blastospores 1,24.
Figure 1.1. Differences morphological growth forms of Candida by epifluorescence photocomposition. A) Blastoconidia; B1) Reproduction by budding; B2) Germ tube formation; C1) Pseudohyphae formation; C2) Yeast form; C3) Hyphae formation. *Septa. Retrieved from: Silva et
al. 1.
Regarding the biochemistry of the several Candida species, it involves the fermentation and/or assimilation of different sugars, C. albicans ferments and/or assimilates a large number of sugars, such as C. tropicalis. These two species are then similar between them, but differs from the other Candida species, that do not ferment, neither assimilate so many sugars 1,36. There are also other differences between the
Candida species, as their genome. Candida glabrata presents a main distinctive genetic
characteristic, once it has a haploid genome, in contrast to diploid genome of C.
albicans and the other NCAC species 1,25. Genetically, C. tropicalis has the highest and
least similarity to C. albicans and C. glabrata, respectively, since it contains more major repeat sequence (MRS) elements, than C. parapsilosis and C. glabrata. The discovery of MRS elements in C. tropicalis suggests that these repeats play a similar role in karyotypic variation in this species, although the contribution of these changes to pathogenesis is even not known 1,2,37.
1.2.2. Candida tropicalis
As referred, in the recent decades, the increase of candidiasis has been accompanied by an augment of infections caused by NCAC species, particularly by C.
tropicalis, which it has been reported as one of the Candida species that is most likely
and, besides it has been associated with a propensity for dissemination and high mortality and morbidity 2,16,24,38–41. Unfortunately, the pathogenic mechanisms of C.
tropicalis have not yet been fully elucidated 1,2,38,39.
1.2.2.1. Risks associated to Candida tropicalis infections
Already since 1960 that C. tropicalis has been recognized as responsible for serious invasive candidiasis 2,42. Candida tropicalis is a diploid ascomycete yeast and an opportunistic human pathogen, which colonizes several anatomically distinct sites, including the skin, gastrointestinal and genitourinary tracts, and may also be evidenced in the respiratory tract. Beyond these anatomical sites, C. tropicalis can also be recovered from the environment, particularly from surfaces in medical settings 2,16,43–45. Indeed, the infections caused by C. tropicalis can be acquired by an exogenous or endogenous form. The exogenous infection can occur through contact of the hands of health professionals with patients or through catheters, implantable prostheses, as well as parenteral solutions, which were previously contaminated, among others, while the endogenous infection is acquired when the individual is already colonized by the microorganism as part of the normal flora, but under altered conditions yeasts may be spread through the gastrointestinal tract to different anatomic sites, causing infection
2,14,16,17,46,47. In fact, colonization by C. tropicalis, especially from a specific body site
can be highly predictive of the development of invasive disease with this organism. Moreover, C. tropicalis infections involve a broad spectrum of invasive diseases, affecting patients exposed to a wide variety of risk factors 2,14,48.
Among the C. tropicalis invasive infections, the most common are candiduria and candidemia. Usually, C. tropicalis is considered the first or the second NCAC species most frequently isolated from candidiasis, particularly from bloodstream and from urinary tract infections 2,31,46,49,50.
However, the particularities of each Candida species may be influenced by specific risk factors and affect different types of persons 2. Studies have been shown that, in opposition to C. parapsilosis, C. tropicalis was less likely to occur among children of less than one year of age, but more likely to occur in patients with cancer or neutropenia, and is strongly associated with the presence of biofilms in urinary catheters. Thus, C. tropicalis appears to display a higher potential for dissemination in neutropenic and malignancy individuals compared with C. albicans and other NCAC
species 1,2,11,46,49. According to Kontoyiannis et al. 41, there are distinct differences in the presentation and risk factors of C. tropicalis and C. albicans fungemia, with the former being more persistent and leading to longer ICU stays during the course of infection. This may imply a higher virulence and greater resistance to the commonly used antifungals by C. tropicalis when compared with C. albicans, despite the proportion of candiduria and candidemia caused by C. tropicalis vary widely with geographical area and patient group, as already mentioned. Actually, in certain countries, C. tropicalis is more prevalent, even compared with C. albicans or other NCAC species. The propensity for dissemination in some way may explain the reported relatively high mortality associated with C. tropicalis 1,2,40,46.
1.2.2.2. Candida tropicalis virulence factors
Despite C. tropicalis have emerged in the last decades associated to severe candidiasis, there is still a lack of information about their pathogenicity 24. This C.
tropicalis pathogenicity has been strongly attributed to several virulence factors. It can
be argued that the virulence factors are all the traits required for establishing disease. However, strictly speaking, virulence factors are those that interact directly with host cells causing damage 1,2,51. In fact, some epidemiologic studies documented that the fact of C. tropicalis be associated with higher mortality than other NCAC species and, even than C. albicans 1,2,14,41, it can be due to its pathogenicity that seems to be strongly related with several virulence factors exhibited by this species, such as adhesion ability to different host surfaces (host cells and medical devices) and, consequently, biofilm formation, that is considered the first step to initiate C. tropicalis infection. Furthermore, the secretion of certain hydrolytic enzymes, and dissemination and dimorphism capacity are recognized as important virulence factors in tissue invasion by
C. tropicalis 2,16,45,52–55, increasing their persistence within the host, as well as it can
cause host cell damage 1,24. Thus, it is known that C. tropicalis is able to adhere, colonize and infect host tissues and further disseminate in vivo and in vitro 2,16,38,56.
1.2.2.2.1. Dimorphism ability
An important virulence factor of some Candida species is dimorphism, which is the ability to perform morphological transition, which can increase the ability of invade
the host tissues. However, not all the Candida species have the dimorphism ability and, as such, the ability to invade host tissues differs between Candida species 1,2,24,33.
The pathogenesis of mucosal candidiasis has mainly been investigated using animal models, but recently, the reconstituted human epithelium (RHE) was successfully used to study in vitro invasion by Candida species 16,24. Jayatilake et al. 57 showed increased ability of C. tropicalis to colonize and invade RHE, similarly to C.
albicans, compared with C. parapsilosis and C. glabrata, what it was also verified by
Silva et al. 24, which has reported that C. tropicalis showed extensive colonization and invasion (Figure 1.2.I), while C. parapsilosis showed moderate colonization and invasion (Figure 1.2.II) and C. glabrata showed colonization but no invasion (Figure 1.2.III). However, Silva et al. 56 demonstrated recently that only filamentous forms of C.
tropicalis were able to invade an oral epithelium, because in the case of C. parapsilosis,
it was verified that hyphae transition occurs in a strain-dependent manner, and contrarily to C. albicans and C. tropicalis, the ability of C. parapsilosis to invade an oral epithelium reconstituted model did not correlate with pseudohyphae production.
Figure 1.2. Confocal laser scanning microscopy images of the reconstituted human oral epithelium infected with Candida tropicalis (I), Candida parapsilosis (II) and Candida glabrata (III) during 12 h. *Yeast; ** Filamentous forms. Adapted from: Silva et al. 24.
In fact, the dimorphism ability can be considered as a C. tropicalis virulence factor, because it was found that the morphological forms exhibited by C. tropicalis are similar to those shown by C. albicans, but despite these few studies there is no more
evidence on the importance of C. tropicalis morphology in the virulence 1,2.However, it is believed that the filamentous forms have an important role in tissue and biomaterial invasion, and in fact, in vitro research showed that C. albicans hyphae mutants and NCAC strains with lacking of filamentous forms exhibit have lower ability to invade tissue compared with wild-type C. albicans strains 1,33,57. Moreover, the filamentous forms (hyphae and/or pseudohyphae) of Candida species demonstrated also increase in the adhesion properties and resistance to phagocytosis, when compared with yeast forms. Thus, the filamentous forms formation due to dimorphism ability is considered to be significant to the pathogenicity of some Candida species, in this case of C.
tropicalis 1,33.
1.2.2.2.2. Adhesion ability
Usually, the first event in the C. tropicalis infection is its adhesion to host and/or medical devices surfaces, which often leads to biofilms formation. This yeast adheres to several host cell types, including epithelial, endothelial and phagocytic cells 24,33. Its adhesion to host surfaces is required for initial colonization and contributes also to microorganism persistence within the host, being considered essential in the establishment of disease, since high-density colonization is indicative of high risk factor for host 1,2,33,40,58. Thus, the adhesion ability is an extremely important step in the infection process and its extent is dependent of the microbial, host and abiotic surface properties, such as cell wall composition, mainly the profile of cell wall proteins, and cell surface physicochemical properties, particularly its hydrophobicity 1,24,59–61.
An important factor that has correlated with the adhesion ability of Candida species is the presence of specific cell wall proteins, which are responsible for Candida interaction with animate and inanimate surfaces and play a key role in morphogenesis and pathogenesis 2,24,56,62. These Candida cell wall surface proteins that are involved in specific adhesion are often referred to as adhesins, which allows adhesion to specific substrates 1,2,24. Among the many adhesins expressed by Candida, agglutinin-like sequence (Als) proteins have been implicated in pathogenesis and biofilm formation, representing the most prevalent growth form of microorganisms 33,63. Candida Als is considered an important protein family during the process of adhesion, mediating attachment to different epithelial cells, functioning as adhesins 2. Concerning C.
tropicalis cell wall proteins, at least three ALS-encoding genes were identified, but to
our knowledge, no further work has been undertaken in this area 1,24,64.
Moreover, other factors as cell wall surface physicochemical properties and environmental factors can influence the C. tropicalis initial adhesion and another
Candida species. It is known that, the yeast cell wall surface is the site of
physicochemical interactions between yeast cells with host tissues or medical devices surface, leading to its adhesion 1,2,24,38,56,62. According to Panagoda et al. 65, the initial adhesion of C. tropicalis cells, and also of C. parapsilosis, was associated with surface hydrophobicity, as reported for C. glabrata.
Candida tropicalis has the ability to colonize urinary epithelial cells, however,
the extent of adhesion is strain dependent 2. Furthermore, according to some researchers, C. tropicalis strains showed intermediate levels of adhesion to oral epithelial cells and to human epithelial cell monolayers, whereas C. albicans strains showed high in vitro adhesion 2,44. However, in other studies, C. tropicalis showed similar or higher extent of adhesion than C. albicans when in contact with human epithelial cell monolayers and endothelium from porcine vascular tissues. Therefore, it is possible to verify that Candida species do not adhere in the same manner to the different mucosal types of cells, and also that there is distinct interaction between epithelium morphology and molecular events during Candida adhesion 2,66–68.
1.2.2.2.3. Biofilm formation
One of the major contributions to Candida virulence is its versatility in adapting to a variety of different habitats and the formation of surface-attached microbial communities known as biofilms, an important feature that promotes both infection and persistence in the host 13,18,69. The biofilm formation can be explained in five steps (Figure 1.3), including the initial adsorption of yeast cells (Figure 1.3.I) and its adhesion on host surfaces or/and on biomaterials used in indwelling medical devices (Figure 1.3.II), where cell division occurs. After cells have attached to each other, biofilm proliferation begins through the formation of complex yeast colonies basal layers intertwined with pseudohyphae and hyphae pre-formed, embedded in an extracellular matrix (ECM) (Figure 1.3.III). After that biofilm maturation occurs, forming a highly complex structure, comprising an association between several microcolonies with also water channels that will allow circulation of nutrients and other substances within the
biofilm (Figure 1.3.IV), leading even more to its grown and, consequently to dispersion of biofilm cells (Figure 1.3.V) 1,2,18,70–72. As such, the biofilms have been considered the most prevalent growth form of microorganisms and its ECM is one of the most distinctive features of a microbial biofilm, because it is a complex extracellular material that can function as a defense against phagocytic cells, serve as a scaffold for maintaining biofilm integrity and limit the diffusion of toxic substances into the biofilm
1,2,13,24,69,71.
Figure 1.3. Biofilm formation development of Candida tropicalis. Adapted from: Harding et al. 72.
The biofilm formation is, therefore, an important and potent virulence factor for
Candida species, once the biofilm cells are phenotypically distinct from their planktonic
forms, having important clinical repercussions with higher mortality rates, due to their increased resistance to antifungal therapy. Primarily, by limiting the penetration of substances through the biofilm matrix, that acts as a barrier to diffusion of antifungal agents, but also by conferring protection to yeast cells within of the biofilms against host immune responses 1,13,18,24,73. Some studies indicate that C. tropicalis biofilms exhibit large amounts of matrix material completely resistant to antifungals, whereby those data can explain why the major risk factor of C. tropicalis in candidiasis development is related to the difficulty of treatment and prolonged catheterization 2,52,74.
The biofilm formation impact as the main virulence factor in different Candida species is not always clear and, as such, several studies have compared the prevalence of biofilm formation in clinical isolates from different Candida species 15,75,76. Such as
C. albicans, all NCAC species were able to form biofilms, although more extensive for C. tropicalis and C. parapsilosis. In fact, previous studies have demonstrated that C. tropicalis and C. parapsilosis have produced in Sabouraud dextrose broth medium
(SDB) a higher biofilm biomass 13,24. These results are also in accordance with Shin et al. 76, who reported that biofilm positivity occurred most frequently in C. tropicalis,
when cultured in nutritionally rich media.
It is thought that the mature biofilm formation, subsequent matrix production, both composition and structure, are strongly dependent on species, strain and environmental conditions, as medium composition, pH, oxygen and growth conditions (static or flow) 1,2,13,24,77. Interestingly, the biofilm matrix composition is highly strain dependent, but is a phenomenon that has not been observed from all NCAC species 1,13. According to Silva et al. 13, the C. parapsilosis biofilm forming ability was highly strain dependent, but such it was less evident in C. tropicalis. Undoubtedly, all such factors reflect the inherent physiological differences and could have significance for their pathogenic potential 24.
Regarding biofilm matrix composition, little is known in NCAC species, but about the C. albicans biofilm matrix, it is known that is mainly composed by carbohydrates, proteins, hexosamines and phosphorus 1,13,73. More lately, Al-Fattani & Douglas 74 showed that matrix material extracted from C. tropicalis and C. albicans biofilms contained also uronic acid, beyond of the other components previously mentioned. Besides this notable difference, the C. tropicalis biofilms matrix has lower amounts of proteins and carbohydrates, compared with other NCAC species. Furthermore, the major component in the C. tropicalis biofilm matrix is hexosamine, whereas in C. albicans is glucose 1,2,13,24. It is important to emphasize that hexosamine is sometimes referred as a polysaccharide intercellular adhesin (PIA) and is known to mediate cell-cell interaction within of the biofilm 2. Silva et al. 13 reported that C.
tropicalis biofilms could be partially detached after treatment with lipase type VII and
chitinase, while the C. albicans biofilms are detachable after treatment with proteinase K, chitinase, DNase I or β-N-acetylglucosamidase. Furthermore, in the same study 13, C.
albicans biofilms were more easily detached from plastic surfaces by treatment with the
enzyme lyticase than were the of C. tropicalis. These findings highlight that detachment and destruction of Candida species biofilms should depend on matrix composition, which opens the possibility that some medical devices could have be coated with specific types of hydrolytic enzymes as a means of preventing biofilm formation by NCAC species 1,13,24.
The deoxyribonucleic acid (DNA) may also be one of the components that may cooperate in the C. tropicalis biofilm formation, since the DNA has been described as a component of the bacteria biofilm ECM 1,78,79. In Candida, the knowledge concerning extracellular DNA contribution in the biofilm matrix and overall structure is scarce, although recently, Martins et al. 80 have highlighted the DNA importance in the C.
albicans biofilm formation, integrity and structure. Also Fonseca et al. 81 tried to show
the importance of DNA in C. glabrata biofilms matrices, however were unable to reach any conclusion. Therefore, there is a knowledge lack concerning NCAC species and extracellular DNA role on biofilm composition 1.
Moreover, several genes and proteins have been reported as essential for
Candida biofilm formation and matrix composition. While extensive work has been
performed on the C. albicans genes involved in adhesion/colonization and biofilm formation, little is known about the genes involved in biofilm formation in C. glabrata,
C. parapsilosis and C. tropicalis 1,24.
Concerning the biofilm structure it is usually analyzed by scanning electron microscopy (SEM), notwithstanding the inherent destructive nature of the technique by the possible loss of some cells and matrix during the dehydration process. This revealed structural differences in Candida species biofilms and their strains, in respect to differences in the cell morphology and structure, and spatial arrangement 13. The C.
albicans biofilm formation is associated with the dimorphic switch between yeast and
hyphae growth, and their biofilms generally have two distinct layers, a thin basal yeast layer and a thicker less compact hyphae layer 24. The same has been observed in C.
tropicalis and C. parapsilosis mature biofilms that consist in a cells dense network with
a variety of morphologies, while all C. glabrata strains biofilms were comprised only by yeast (Figure 1.4) 13. Several studies have demonstrated that the biofilms formed by
C. tropicalis strains, exhibited only yeast morphology, with exception of some strains
that presented filamentous forms, being that some of them appear as especially long filaments 13,24. Bizerra et al. 52 also reported that C. tropicalis biofilms formed in SDB medium, contained only blastoconidia or generated a multilayer heterogeneous structure covering the entire surface as a thick biofilm. Other study 13 reinforces and emphasizes a previous study 52, where a C. tropicalis strain formed a thin layer of filamentous forms compared with other strains that only presented blastoconidia. Furthermore, Parahitiyawa et al. 82 reported that on polystyrene surfaces, C. tropicalis biofilms
consisted of large coaggregated microcolonies of blastoconidia with a thick extracellular polymeric layer. In fact, almost all microorganisms exhibited structural heterogeneity within their biofilm architecture, which indicates that this heterogeneity appears to be common in biofilms formed by strains of NCAC species, revealing new important aspects in the NCAC species biofilm ultrastructure 13,24.
Figure 1.4. Scanning electron microscopy images of non-Candida albicans Candida species biofilm grown in Sabouraud dextrose broth for 48 h. The bar in the images corresponds to 20 µm. Magnification x 1000. Adapted from: Silva et al. 24.
1.2.2.2.4. Dissemination ability
The biofilm life cycle is related to dispersion/detachment or dissolution of biofilm cells, namely the dissemination ability. From the biofilm are released cells that sow new surfaces with consequent establishment of disseminated candidiasis at distal organs 2. Additionally, there are indications that dispersed cells from biofilms are more virulent than the planktonic counterparts 2,71. Moreover, it is thought that these cells may also have the ability to evade host defenses, because in order to establish infection, opportunistic pathogens have to evade the immune system, survive, reproduce in the host environment, and in the case of systemic infection, disseminate to new tissues and organs 1. However, little is still known about C. tropicalis cells detachment from biofilms and more studies are necessary to better understand this process.
1.2.2.2.5. Hydrolytic enzyme secretion
Candida tropicalis is able to produce and secrete several hydrolytic enzymes
frequently associated to pathogenicity, including proteases (as secreted aspartyl proteinases (Saps)), phospholipases (Pls) and haemolysins. The release of these enzymes in a local environment has been associated to Candida adhesion, cell damage and host tissue invasion 1,24.
1.2.2.2.5.1. Secreted aspartyl proteinases
Of all secreted hydrolytic enzymes by Candida and among the various potential virulence factors proposed, Saps are the ones that have attracted most interest and that have been more intensively investigated, being widely considered central to the development of the Candida infection 2,33. Saps are recognized as an important virulence determinant for this species, because it facilitates the colonization and invasion of host tissues through of the disruption of the host mucosal membranes and degradation of important immunological and structural defense proteins 24,83. However, the Candida ability to adhere to mucosae, invade deep organs and resist to phagocytic cells, apparently requires the use of several different proteinases suitable to each particular condition during the infection 2.
Saps are only produced by certain Candida species, as for example C. albicans that secretes nine distinct Saps, often at much higher levels compared with other species
33. Such as in C. albicans, in vitro studies reveal that C. tropicalis secretes high levels of
Saps in culture media containing bovine serum albumin (BSA) as sole source of nitrogen 1,2,16,24,54. Furthermore, C. tropicalis has at least four genes encoding Saps, designated by SAPT1, SAPT2, SAPT3 and SAPT4, however up to now, the Sapt1 is the only enzyme that was purified from culture supernatant, being biochemically characterized and crystallized 1,2,24,54. The presence of Saps secreted by C. tropicalis has also been reported on the surface of fungal elements penetrating tissues during the disseminated infection and invading macrophages after phagocytosis of yeast cells
1,24,54. In contrast with other types of proteinases, Saps showed proteolytic activity only
under acid conditions (pH < 4.0) and so it is important for the oral infection, because the environment under a removable denture is acidic, which provides conditions suitable for both production and activity of Saps 33. One the other hand, there is no conclusive evidence of that proteinase activity is always associated to infection and this probably
reflects the multifactorial nature of Candida infections 33, such as demonstrated recently by Silva et al. 62, which reported that the Saps expression during C. tropicalis colonization of an oral epithelium is not associated with invasion and tissue damage, a finding similar to that was reported for C. albicans.
It is important to highlight that although several studies have emphasized the differential expression and potential roles of several SAP genes during host colonization and infection by Candida species, there were discrepancies in the results obtained. The reasons for such discrepancies could relate to differences in the sensitivities of the methods used, conditions of cells cultivation, intrinsic differences even in apparently similar infection models (as RHE and murine), and interspecies and strain variability, as its state and origin 1,24,56,84,85.
1.2.2.2.5.2. Phospholipases
In addition to Saps, other hydrolytic enzymes are also secreted by Candida species, as Pls, which are frequently considered as a factor associated to Candida pathogenicity 24. Pls are enzymes that hydrolyze phospholipids to fatty acids. Depending on the different and specific ester bonds cleaved, these enzymes have been classified into Pls A, B, C and D, and their presence could contribute to host cell membrane damage, which could promote the cell damage and/or expose receptors to facilitate the Candida adhesion 2,24,86.
Several recent studies indicated that NCAC species, particularly C. tropicalis 55, are able of produce in vitro extracellular Pls, but at significantly lower levels when compared with C. albicans 86, whereby its production is highly species and strain dependent 1,2,24,43. In fact, recent studies reported that C. tropicalis seems to have even a reduced ability to produce extracellular Pls and that the production is also strongly strain dependent 1,2,16,24,55. It has also been reported that the Pls activity is enhanced when hyphae are in direct contact with host tissue 33. Moreover, although some studies of Pls activity have already been undertaken for some Candida species, were still however only few concern C. tropicalis 1,24,87.
1.2.2.2.5.3. Haemolysins
Other important virulence factor of Candida recently described in the literature is reported as haemolytic activity 2,53. These pathogenic microorganisms can grow in the