w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Telomere
length
correlates
with
disease
severity
and
inflammation
in
sickle
cell
disease
Marina
Pereira
Colella
a,
Barbara
A.
Santana
b,c,
Nicola
Conran
a,
Vinicius
Tomazini
a,
Fernando
F.
Costa
a,
Rodrigo
T.
Calado
b,c,
Sara
T.
Olalla
Saad
a,∗aUniversidadeEstadualdeCampinas/Hemocentro(UNICAMP),Campinas,SP,Brazil
bUniversidadedeSãoPaulo(USP),RibeirãoPreto,SP,Brazil
cFundac¸ãodeAmparoàPesquisadoEstadodeSãoPaulo(FAPESP),RibeirãoPreto,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received17January2017 Accepted15February2017 Availableonline11March2017
Keywords:
Sicklecelldisease Telomerelength Inflammation
a
b
s
t
r
a
c
t
Background:Telomeres,theendsoflinearchromosomes,shortenduringmitoticcelldivision
anderosionmaybeaggravatedbyinflammationorproliferativeandoxidativestress.Asthe bonemarrowisunderhyperproliferativepressureinsicklecelldiseaseandseveraltissues aresubmittedtochronicinflammation,thisstudysoughttodeterminethetelomerelength ofpatientswithsicklecelldisease.
Methods:Themeantelomerelengthwasmeasuredinperipheralbloodleukocytesby
quan-titativepolymerasechainreaction.Theage-adjustedtelomeretosinglecopygeneratiowas comparedbetween91adultsicklecelldiseasepatientsand188controls.
Results:Sicklecelldiseasepatientshadsignificantlyshortertelomeresthanthecontrols
(p-value<0.0001).Moreover,amongsicklecelldiseasegenotypes,HbSSpatientshad sig-nificantlyshorter telomeres compared to Hb SC and Hb S patients (p-value<0.0001). Patientsonhydroxyureaalsohadshortertelomeresincomparisontothoseoffthedrug(p -value=0.02).Apositivecorrelationwasobservedbetweentelomerelengthandhemoglobin level(r=0.3;p-value=0.004),whereasnegativecorrelationsweredetectedbetween telo-merelengthandlymphocytecount(r=−0.3;p-value=0.005)andinterleukin-8serumlevels
(r=−0.4;p-value=0.02).
Conclusions:Thefindingsofthisstudyindicatethattelomeresareshortinsicklecelldisease
patientsandthattelomereerosiondirectlycorrelateswithdiseasegenotype,inflammation markers,andtheuseofhydroxyurea.
©2017Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:HematologyandHemotherapyCenter,UniversidadeEstadualdeCampinas(UNICAMP),RuaCarlosChagas,
480,13083-878Campinas,SP,Brazil.
E-mailaddress:sara@unicamp.br(S.T.Saad).
http://dx.doi.org/10.1016/j.bjhh.2017.02.007
Introduction
Sicklecelldisease(SCD)ischaracterizedbythepresenceof apathologicalhemoglobin,denominatedhemoglobin(Hb)S. TheformationofHbSiscausedbyamutationinthe-globin gene atthe 17th nucleotide,in which thymine is changed toadenine, resultinginthesubstitutionofthesixth amino acidofthe-globinchainfromglutamicacidtovaline.The termSCDincludesseveralgenotypeswiththepresenceofS allele,including thehomozygousform(HbSSor sicklecell anemia)andheterozygousformswithco-inheritanceofother mutationssuchas C allele (Hb SC or Hb SC disease) and the-thalassemiaallele(HbSorSThalassemia).The clini-calhallmarksofSCDarechronicintravascularhemolysisand acutevaso-occlusiveevents,whichinvolveendothelial dys-function,increasedcellularadhesion,chronicinflammation, leukocytosis, coagulation activation and oxidative stress.1
Hemolysis plays a central role in the disease mechanism due to the constant release of free hemoglobin and free heme from red blood cells (RBC) into plasma, leading to nitric oxide (NO) depletion causing potent immediate sys-temic and vascular inflammation.2 NOdepletion may lead
toahighly adhesiveendothelium,and platelet and coagu-lation activation.3 Unbound extracellular heme causes the
generation of reactive oxygen species (ROS) and activa-tionofinnateimmunitypathwaysthroughToll-likereceptor 4(TLR4).4,5
Telomeres are hexameric T2AG3 tandem repeats coated by specialized proteins covering the ends of lin-ear chromosomes.6 Telomeres confer protection against
chromosomeinstabilityand activation ofthe DNA-damage response(DDR)machinery.Telomereshorteningisa chrono-logicalmarkerofaging, andits acceleratedshorteningrate hasbeeninvolvedinthedevelopmentofseveraldiseases,the telomeropathies.7 In addition, cumulative events resulting
from replicative stress, such as exposure to biochemical stressors,excessiveoxidation,andinflammation,maycause excessive telomere erosion.8,9 Maintenance of telomere
integrityrequirestelomerasereversetranscriptase(TERT),its RNAtemplate(TERC),andotherproteins,whichcomposethe telomerasecomplex.
Inviewoftheinflammatoryandoxidativestressfeatures observedinSCD,thetelomerelengthwasdeterminedinSCD patientsandhealthycontrolsinordertocorrelatethiswith diseasegenotype,severity,andinflammationmarkers.
Methods
Patientsandcontrols
ThisstudywasperformedinacohortofadultSCDpatients seen at the Outpatient Clinic of the Hematology and Hemotherapy Center, Universidade Estadual de Campinas (UNICAMP).PatientsthatwerehomozygousforHbS(HbSS), heterozygous with Hb C and S (Hb SC), and heterozygous with-thalassemiaand S(HbS)were included.All ofthe patientswereinsteadystatethereforenonehadhadpainful crises,hospitalizationsorbloodtransfusionsduringthethree
months precedingblood sample collection. Agroup of188 age-matchedhealthysubjects(HbAA)wereanalyzedas con-trols for telomere lengthmeasurement.10 Anadditional 70
age-andgender-matchedhealthysubjects(HbAA)were eval-uated forlymphocytecountsandinterleukin-8(IL-8)levels. Venousbloodsamplesforallstudyanalyses,including periph-eralbloodcountsandhemolysismarkers,wereobtained dur-ingclinicvisits.TheUniversity’sEthicsCommitteeapproved thestudyandallpatientsgavetheirwritteninformedconsent.
DNAextraction
GenomicDNAwasextractedfromthebuffycoatofhealthy controls’peripheralbloodleukocytesupto48hafter collec-tion, usingthe GentraPurege Blood Kit(Qiagen, Maryland, USA).DNAsampleswerequantified,dilutedto50ng/Land storedat−20◦C.GenomicDNAfromSCDpatientswas
iso-lated from frozen white blood cells (WBC). Briefly, whole peripheralbloodsampleswerewashedinphosphatebuffered saline(PBS)with0.1%bovineserumalbumin(BSA)upto16h after collection and incubated on ice coldammonia chlo-ride (NH4Cl) for osmotic red blood cell lyses. WBCs were
counted,aliquoted,andfrozenat−80◦Cin10%dimethyl
sulf-oxide(DMSO)untildefrostingandDNAextraction,performed usingtheGentraPuregeBloodKit(Qiagen,Maryland,USA). Genomic DNA (50ng/L) was checked forintegrity in0.8% agarose gelat80Vfor40min. For quantitativepolymerase chainreaction(qPCR),DNAdilutionsof5ng/Lwereprepared andkeptat4◦Cuptosevendays,whenthesubsequent
dilu-tionsof0.2ng/Lwereusedforeveryrunpreparedjustbefore experiments.
Leukocytetelomerelengthmeasurementbyquantitative polymerasechainreaction
The mean leukocyte telomere length was determined by qPCR as hasbeen described previously.11–13 All qPCR
reac-tions were prepared on a QIAgility automated pipettor (Qiagen, California, USA), amplification was conducted in triplicate inthe Rotor-Gene Q5plex HRMInstrument (Qia-gen), and analysis was completed using Rotor-Gene Q Software Version 2.2.3. The 24L final volume reactions included: 8L of genomic DNA (0.2ng/L), 2× Rotor-Gene
SYBR Green PCR kit (Qiagen, Hilden, Germany), RNase-free water (Qiagen), and the primers Telomere Fw (CGGT-TTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT – 300nM) andRv(GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT –300nM)orsinglegene(36B4)Fw (CAGCAAGTGGGAAGGTG-TAATCC–300nM)andRv(CCCATTCTATCATCAACGGGTACAA – 500nM). Telomere reactions were performed as follows: denaturationat95◦Cfor5minfollowedby25cyclesof7sat
98◦Cand 10s at60◦C,whereas singlegenereactionswere
denaturedat95◦Cfor5minfollowedby35 cycles of7sat
98◦Cand10sat58◦C.Thetelomerelengthforeachsample
wasdeterminedusingthetelomeretosinglecopygeneratio (T/Sratio)withthecalculationofCt[Ct(telomere)/Ct(singlegene)].
TheT/Sratioforeachsample(x)wasnormalizedtothemean T/Sratioofthereferencesample[2−(Ctx−Ctr)=2−Ct],which
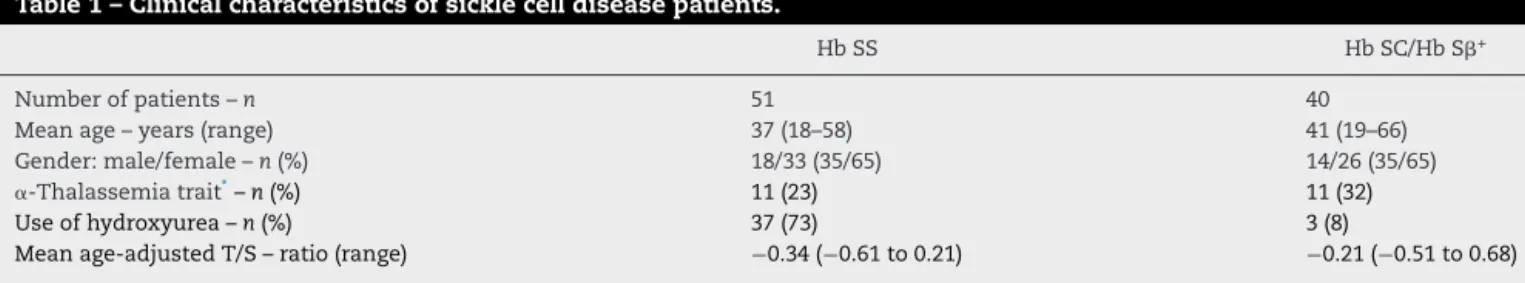
Table1–Clinicalcharacteristicsofsicklecelldiseasepatients.
HbSS HbSC/HbS+
Numberofpatients–n 51 40
Meanage–years(range) 37(18–58) 41(19–66)
Gender:male/female–n(%) 18/33(35/65) 14/26(35/65)
␣-Thalassemiatrait*–n(%) 11(23) 11(32)
Useofhydroxyurea–n(%) 37(73) 3(8)
Meanage-adjustedT/S–ratio(range) −0.34(−0.61to0.21) −0.21(−0.51to0.68)
T/S:telomeretosinglecopygeneratio. ∗ ␣-Thalassemiatrait:−3.7kbdeletion(−␣3.7).
Theresultswereavailablefor47patientsintheHbSSgroupand34patientsintheHbSC/HbSgroup.
variations(CV)oftriplicatemeasurementswere2%and1%or less,fortelomereandsinglegenereactions,respectively.The consideredinter-assayCVwasupto5%.
LeukocytetelomerelengthbySouthernblotanalysis
To validate the qPCR terminal restriction fragment (TRF), analysiswasperformedbySouthernblotof17samples accord-ing tothe manufacturer’sinstructions withminorchanges (TeloTAGGGTelomereLengthAssay–RocheAppliedScience, Mannheim,Germany). Briefly, 800ngof genomic DNA was digestedbyanoptimized mixtureofHinfI andRsaI FastDi-gestrestriction enzymes (ThermoScientific,Waltham, MA, USA)at37◦Cfor2h.FollowingDNAdigestion,DNAfragments
were separated byelectrophoresisin 0.8%agarosegel dur-ing four hoursat80V. Gel wasdenaturedand neutralized, samplesweretransferredtoanylonmembranebySouthern blottingandprobed,andthe terminalrestrictionfragments weredetectedbychemiluminescence.MeanTRFlengthwas determinedaccordingtotheformulaTRF=(ODi)/(ODi/Li), whereODiisthechemiluminescentsignalandLiisthelength ofthefragmentatagivenposition.
Inflammationmarkers
Tumornecrosisfactor-alpha(TNF-␣)andIL-8wereassessedas pro-inflammatorymarkers.TNF-␣andIL-8levelswere mea-sured,in duplicate,inserum samplesusing ultra-sensitive enzyme-linkedimmunosorbentassay(ELISA)kitsaccording tothemanufacturer’sinstructions(TNF-␣USandIL-8US, Invi-trogen,Camarillo,CA,USA).
Statisticalanalyses
The relationship between telomere length and age was estimated using linear regression. Estimated regression coefficients were used to calculate the observed minus expected(O-E),orage-adjustedtelomerelengthforeach sub-ject. Age-adjusted T/S ratios were used for all the study analysesexceptforthecorrelationwithage.Spearman’srank correlationcoefficientwasusedtoanalyzebivariate associa-tionsbetweentelomerelengths,hemolysisandinflammation markers.T/Sratioswerecomparedaccordingtothediagnosis, useofhydroxyureaandgenderusingWilcoxonranksumtest. Linearregressionwasusedtoanalyzethecorrelationbetween
telomerelengthmeasuredbyqPCRandTRFbySouthernblot. Allp-values≤0.5wereconsideredsignificant.Statistical
anal-yses wereperformedusingtheRstatisticsprogramversion 3.1.3.
Results
Patients’characteristics
Ninety-one adultSCDpatients were includedin thestudy: 51 Hb SS, 38 Hb SC,and 2 Hb S+. Forty were on hydrox-yureawithamediandoseof1g/day(range:500–1750mg/day). The dose was titrated according to clinical improvement or to the maximum tolerated dose. The clinical indica-tions for hydroxyurea were frequent painful crises (n=14; 35%), acute chest syndrome (n=5;13%), stroke (n=6; 16%), and severehemolyticanemia(n=15;37%).Patients’ clinical characteristics are described in Table 1. Thecontrol group consisted of188healthy individuals (Hb AA),witha mean ageof38years(range:18–88years),including97womenand 91men.
Telomeresareshortinsicklecelldiseasepatients
Telomeres were significantly shorterin SCD patients com-pared toage-matched healthy controls[T/S ratio: −0.28 in
SCD vs.−0.01incontrols;standard deviation(SD)=0.20 vs.
0.23; p-value<0.0001 – Figure 1A]. When genotypes were compared, the telomeres were significantly shorter in Hb SS patients than in the Hb SC and Hb S genotypes (T/S ratio: −0.34 vs. −0.21, respectively; SD=0.17 vs. 0.21; p
-value<0.0001 – Figure 1B). Telomereswere also shorterin patientsonhydroxyurea(T/Sratio:−0.34vs.−0.25;SD=0.20
vs.0.20;p-value=0.02)(Figure1C).Althoughtelomeres short-enedwithageinhealthycontrols(r=−0.5;p-value<0.0001),
age did not affect telomere length in SCD patients (r=−0.02;p-value=0.8).Telomerelengthwasnotinfluenced
by genderin either SCD patients (p-value=0.2) or controls (p-value=0.9).
Inordertovalidatethesefindings,telomerelengthswere also measured by Southern blot for 17 patients showing thatT/SratiosandTRFswerehighlycorrelated(R2=0.86;p
A
B
C
1.00.5
0.0
–0.5
–1.0
1.0
0.5
0.0
–0.5
–1.0
1.0
0.5
0.0
–0.5
–1.0 Controls
Age-adjusted T/S
ratio
Age-adjusted T/S
ratio
Age-adjusted T/S
ratio
SCD HbSS HbSC/HbSβ HbSS/HbSC HU HbSS/HbSC/HbSβ
P<0.0001
P<0.0001
P<0.02
Figure1–Age-adjustedT/Sratioincontrolsandsicklecelldisease(SCD)patients.(A)SCDpatientspresentedshortened telomerelengthcomparedtonormalcontrols.(B)HbSSpatientspresentedshortenedtelomerelengthcomparedtoHbSC andHbSpatients.(C)HbSSandHbSCpatientstreatedwithhydroxyurea(HbSS/HbSCHU)presentedshortenedtelomere lengthswhencomparedtopatientsnottreatedwithhydroxyurea(HbSS/HbSC/HbS+).T/Sratioswerecompared
accordingtothediagnosisanduseofhydroxyureausingWilcoxonranksumtest.Meantelomerelengthwasmeasuredin peripheralbloodleukocytesbyquantitativepolymerasechainreaction(qPCR).
Telomerelengthcorrelateswithhemolysisand inflammationinsicklecelldisease
Theassociationbetweentelomerelengthandmarkerlevels for hemolysis (hemoglobin, hematocrit, lactate dehydroge-nase,indirectbilirubin,reticulocytecounts)andinflammation (IL-8, TNF-␣, total leukocyte, neutrophil, lymphocyte and monocytecounts)wereanalyzed(Table2).Therewasaweak positivecorrelationbetweentelomerelengthandhemoglobin concentration(r=0.3;p-value=0.004),butnocorrelationwith other hemolysis markers. Among inflammatory markers, there was a weak negative correlation of telomere length withlymphocytecounts(r=−0.3;p-value=0.005)andwith
IL-8serumlevels(r=−0.4;p-value=0.02;Table2).Ofnote,overall,
SCDpatientshadhigherlymphocytecounts(SCD:2.9×109/L
15.0
12.5
10.0
7.5
5.0
2.5
0.0
0.0 0.2 0.4 0.6
T/S ratio (qPCR)
TRF (Kb)
0.8 1.0
Figure2–Correlationbetweentelomerelengthmeasured byquantitativepolymerasechainreaction(qPCR)and terminalrestrictionfragment(TRF)bySouthernBlot. Analysisof17sampleswasperformedtovalidateqPCR TRF.TelomerelengthsmeasuredbyqPCRpresentedan adequatecorrelationwithmeasurementsofTRFby Southernblot,analyzedbylinearregression(R2=0.86;
p-value<0.0001).
vs.controls:1.9×109/L;SD=1.4vs.0.51;p-value<0.0001)and
IL-8levelscomparedtocontrols(SCD:3.3pg/mLvs.controls: 2.3pg/mL;SD=1.9vs. 0.8; p-value=0.009). Telomerelength wasnotassociatedwiththeplasmalevels ofother inflam-matorymarkers.
Discussion
Inthepresentstudy,wefoundthattelomeresofperipheral bloodleukocytesofpatientswithSCDareshortindependent ofageandtelomereattritionwasmorepronouncedinpatients withHb SScomparedtoHb SCorHb S+ genotypes. Telo-mere lengthcorrelated withhemoglobinconcentrationand inverselycorrelatedwithIL-8levelandabsolutelymphocyte count.Takentogether,thesefindingssuggest thattelomere lengthisassociatedwithdiseaseseverityandchronic inflam-mationinSCD.
Ourresultsareinsharpcontrastwithonesingleprevious reportbyDrasaret al.,whofoundSCDpatientshad longer telomeres in comparison to age-matched controls.14
How-ever,theirstudypresentedsometechnicalissuesprecluding appropriate interpretation oftheir findings. First, it is not clearintheirwork whethertheyusedfreshorfrozen sam-plesforDNAextraction.Second,telomerelengthwashighly heterogeneousamongtheirpatients,andasignificant propor-tionofolderpatientshadtelomereslongerthanexpectedfor healthycordbloods.Finally,theirqPCRresultswerenot vali-datedwithasecondmethod.Takentogether,theseconcerns mightsuggestthattheDNAusedforanalysiscouldhavebeen degraded. During qPCR,DNAdegradation causes T/S ratios tobehigherandconsequentlytelomeres tobeerroneously interpretedaslonger.10Thiseffectisexplainedbythe
Table2–Correlationsoftelomerelengthswithhemolysisandinflammationmarkersinsicklecelldiseasepatients.
Mean;median(range) Correlationwithtelomerelength
(T/Sratio)r(p-value)a
Hemoglobin(g/dL) 9.8;9.6(6.0–16.0) 0.3(0.004)
Hematocrit(%) 29.2;29.1(16.7–48.4) 0.2(0.2)
Lactatedehydrogenase(U/L) 716;615(257–1680) −0.2(0.08)
Indirectbilirubin(mg/dL) 2.2;1.3(0.6–9.0) −0.2(0.3)
Absolutereticulocytecount(×109/L) 274;261(80–667) −0.1(0.3)
Absoluteleukocytecount(×109/L) 8.7;8.6(3.6–16.5) −0.1(0.3)
Absoluteneutrophilcount(×109/L) 4.5;4.6(1.5–9.5) 0.04(0.7)
Absolutemonocytecount(×109/L) 0.5;0.4(0.1–1.4) −0.03(0.7)
Absolutelymphocytecount(×109/L) 3.1;2.9(0.7–7.3) −0.3(0.005)
Absoluteplateletcount(×109/L) 406;407(81–1164) −0.2(0.1)
TNF-␣(pg/mL) 2.6;2.6(0–6.9) 0.07(0.7)
IL-8(pg/mL) 3.5;3.3(0.8–11.6) −0.4(0.02)
IL-8:interleukin8;TNF-␣:tumornecrosisfactor-alpha.
a Spearman’srankcorrelationcoefficient(r)wasusedtoanalyzebivariateassociationsandallp-valuesaretwo-tailed.p-Values<0.05are
consideredsignificant.
andSouthernblotting.Toavoidthisproblem,allsamplesin ourstudywerecollectedandprocessedwithin16hfromblood drawingforthepurposeoftelomerelengthmeasurementover athree-monthperiodunderrigorousqualitycontrol.To vali-date the qPCR findings ofthis study,telomere lengthwas alsoperformedbySouthernblottingfor17patients,withhigh correlationbetweenbothmethods(R2=0.86).Analyzingthe
distributionofourpatientssimultaneously withonesfrom theEnglishstudy,the agesofourpatientsweredistributed morebetween20and60 yearsold whereasthe patientsof theEnglishstudyweremainlyconcentratedbetween20and 40yearsold.Infact,themedianageofoursampleisalmost ten years higher than the English sample (median age of Campinas vs. England – Hb SS: 39 vs. 32 years; Hb SC/Hb S+: 43 vs. 34 years). Thus,we believe thatdivergences in sample number, disease severity, co-morbidities, socioeco-nomicstatus,ethnicityand agedistributionofthepatients and possibly DNA collection might be responsible for the conflictingresultsandencouragefurtherstudiestoclarifythis issue.
Theinvolvementofwhitebloodcellsintheseverityof clin-icalmanifestationsofSCDpatientsiswellknown.15–18Indeed,
thenumberofbloodcellsincreasesinSCDformanyreasons, includinghighlevelsofcirculatinggranulocytemacrophage colony-stimulatingfactorandincreasedcellsurvival.19–22
Thisstudyalsoobservedthattelomeres wereshorterin patients using hydroxyurea. Hydroxyurea has a cytostatic effectandiscapableofreducingthenumberofhighturnover cellssuchasplatelets,neutrophils,andreticulocytes. Hydrox-yurea may have modulatedblood counts, producing more prominentlymphocytecounts.Inaddition,mostpatientswith moreseverediseasewereonhydroxyurea,whichmayexplain theassociation.
TelomerelengthdidnotcorrelatewithageinSCDpatients. Asinflammationischronicandstartsatayoungage,itmay provokeexcessive telomere shortening early inlife, induc-ingearly“aging”ofthehematopoietictissueinSCDpatients. Thisfindingmaycontributetoearlymultipleorganfailurein SCDandmayexplaintheshorterlifeexpectancyrelatedto
the disease.Thishypothesis shouldbeaddressedinfuture studies.
Our study has some limitations. More significantly, we recruitedasmallnumberofpatientswiththeHbS+ geno-type.Inaddition,wedidnotprospectivelyevaluatetheeffects oftelomerelengthondiseasecomplicationevents,suchas acutechestsyndromeandstroke.Finally,hydroxyureamay haveinterferedwithtelomerelengthbymodulatingleukocyte subsets.
In conclusion, our data confirm that telomere lengths aregreatlyinfluencedbyinflammationandreactiveoxygen speciesinSCD,awell-knownphenomenaofthisdisorder.
Authorship
Contribution:M.P.C.participatedintheselectionofpatients, clinicalfollowupofpatients,performedexperimentalwork, analyzedtheresultsandwrotethemanuscript;B.A.S. partic-ipatedintheselectionofcontrols,performedexperimental workandwrotethemanuscript;N.C.andF.F.C.participated intheanalysisoftheresultsandwritingofthemanuscript; R.T.C.conceivedthestudy,designedexperiments,analyzed resultsandwrotethemanuscript;S.T.O.S.conceivedthestudy, participatedintheselectionofpatients,clinicalfollowupof patients,analyzedtheresultsandwrotethemanuscript.All authorsapprovedthefinalversionofthemanuscript.
Conflict
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
r
e
f
e
r
e
n
c
e
s
1. ColellaMP,DePaulaEV,ConranN,Machado-NetoJA, Annicchino-BizzacchiJM,CostaFF,etal.Hydroxyureais associatedwithreductionsinhypercoagulabilitymarkersin sicklecellanemia.JThrombHaemost.2012;10(9):1967–70.
2. AlmeidaCB,SouzaLE,LeonardoFC,CostaFT,WerneckCC, CovasDT,etal.Acutehemolyticvascularinflammatory processesarepreventedbynitricoxidereplacementora singledoseofhydroxyurea.Blood.2015;126(6):711–20.
3. ConranN,Franco-PenteadoCF,CostaFF.Neweraspectsofthe pathophysiologyofsicklecelldiseasevaso-occlusion. Hemoglobin.2009;33(1):1–16.
4. DutraFF,BozzaMT.Hemeoninnateimmunityand inflammation.FrontPharmacol.2014;5:115.
5. FigueiredoRT,FernandezPL,Mourao-SaDS,PortoBN,Dutra FF,AlvesLS,etal.Characterizationofhemeasactivatorof Toll-likereceptor4.JBiolChem.2007;282(28):20221–9.
6. BlackburnEH.Switchingandsignalingatthetelomere.Cell. 2001;106(6):661–73.
7. CaladoRT,YoungNS.Telomerediseases.NEnglJMed. 2009;361(24):2353–65.
8. MasiS,SalpeaKD,LiK,ParkarM,NibaliL,DonosN,etal. Oxidativestress,chronicinflammation,andtelomerelength inpatientswithperiodontitis.FreeRadicBiolMed.
2011;50(6):730–5.
9. WolkowitzOM,MellonSH,EpelES,LinJ,DhabharFS,SuY, etal.Leukocytetelomerelengthinmajordepression: correlationswithchronicity,inflammationandoxidative stress–preliminaryfindings.KiechlS,editor.PLoSOne. 2011;6(3):e17837.
10.Gutierrez-RodriguesF,Santana-LemosBA,ScheucherPS, Alves-PaivaRM,CaladoRT.Directcomparisonofflow-FISH andqPCRasdiagnostictestsfortelomerelength
measurementinhumans.PLoSOne.2014;9(11):e113747.
11.CawthonRM.TelomeremeasurementbyquantitativePCR. NucleicAcidsRes.2002;30(10):e47.
12.BrouiletteSW,MooreJS,McMahonAD,ThompsonJR,FordI, ShepherdJ,etal.Telomerelength,riskofcoronaryheart
disease,andstatintreatmentintheWestofScotlandPrimary PreventionStudy:anestedcase–controlstudy.Lancet. 2007;369(9556):107–14.
13.CaladoRT,CooperJN,Padilla-NashHM,SloandEM,WuCO, ScheinbergP,etal.Shorttelomeresresultinchromosomal instabilityinhematopoieticcellsandprecedemalignant evolutioninhumanaplasticanemia.Leukemia. 2012;26(4):700–7.
14.DraˇsarER,JiangJ,GardnerK,HowardJ,VulliamyT,VasavdaN, etal.Leucocytetelomerelengthinpatientswithsicklecell disease.BrJHaematol.2014;165(5):725–7.
15.OkpalaI.Theintriguingcontributionofwhitebloodcellsto sicklecelldisease–aredcelldisorder.BloodRev.
2004;18(1):65–73.
16.LitosM,SarrisI,BewleyS,SeedP,OkpalaI,Oteng-NtimE. Whitebloodcellcountasapredictoroftheseverityofsickle celldiseaseduringpregnancy.EurJObstetGynecolReprod Biol.2007;133(2):169–72.
17.KinneyTR,SleeperLA,WangWC,ZimmermanRA,Pegelow CH,Ohene-FrempongK,etal.Silentcerebralinfarctsinsickle cellanemia:ariskfactoranalysis.TheCooperativeStudyof SickleCellDisease.Pediatrics.1999;103(3):640–5.
18.PlattOS,BrambillaDJ,RosseWF,MilnerPF,CastroO, SteinbergMH,etal.Mortalityinsicklecelldisease.Life expectancyandriskfactorsforearlydeath.NEnglJMed. 1994;330(23):1639–44.
19.OpdenakkerG,FibbeWE,VanDammeJ.Themolecularbasis ofleukocytosis.ImmunolToday.1998;19(4):182–9.
20.ConranN,SaadST,CostaFF,IkutaT.Leukocytenumbers correlatewithplasmalevelsofgranulocyte-macrophage colony-stimulatingfactorinsicklecelldisease.AnnHematol. 2007;86(4):255–61.
21.AlmeidaCB,FaveroME,Pereira-CunhaFG,Lorand-MetzeI, SaadST,CostaFF,etal.Alterationsincellmaturityandserum survivalfactorsmaymodulateneutrophilnumbersinsickle celldisease.ExpBiolMed(Maywood).2011;236(11):1239–46.