w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Distribution
of
serological
screening
markers
at
a
large
hematology
and
hemotherapy
center
in
Minas
Gerais,
Southeastern
Brazil
Sônia
Mara
Nunes
da
Silva
a,
Milena
Batista
de
Oliveira
a,
Edson
Zangiacomi
Martinez
b,∗aFundac¸ãoCentrodeHematologiaeHemoterapiadeMinasGerais(Hemominas),BeloHorizonte,MG,Brazil bUniversidadedeSãoPaulo(USP),FaculdadedeMedicinadeRibeirãoPreto(FMRP),RibeirãoPreto,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received14January2016 Accepted17May2016 Availableonline11June2016
Keywords:
Bloodbanks Blooddonors Blood
Serologicaltests Screening
a
b
s
t
r
a
c
t
Objective:Toassessthedistributionofserologicalmarkersinblooddonorsatthebloodbanks oftheFundac¸ãoCentrodeHematologiaeHemoterapiadeMinasGerais(Hemominas),Brazil, betweenJanuary2006andDecember2012.
Methods:Thisisadescriptive,retrospectivestudyonblooddonorsscreenedusingserological testsformarkersoftransmitteddiseasesatthestateblood-bankingnetwork.
Results:Approximately78.9%ofthedonors wereconsidered eligibleforthestudy after clinicalscreening.Repeatdonorsrepresented68.2%ofthetotalsample,withmalesbeing predominantasblooddonors(66.8%).Totalserologicalineligibilitywas3.05%,withtotal anti-HBcbeingthemostcommonmarker(1.26%),followedbysyphilis(0.88%)andhuman immunodeficiencyvirus(0.36%).TheprevalencesofthemarkersforhepatitisC,Human T-celllymphotropicvirus,ChagasdiseaseandHBs-Agwere0.15%,0.09%,0.13%and0.18%, respectively.ThebloodbankofGovernadorValadareshadthehighestpercentageofpositive anti-HBcdonors(2.41%).Withregardtohumanimmunodeficiencyvirus,thebloodbankof AlémParaíbahadthelowestpercentageofpositivedonorswhilethebloodbanksofJuizde ForaandBetimhadthehighestpercentages.ThebloodbankinthecityofMontesClaros hadthehighestprevalenceofthemarkerforChagasdisease(0.69%).
Conclusions:DataontheprofileofserologicalineligibilitybythebloodbanksoftheFundac¸ão Hemominashighlightstheparticularitiesofeachregiontherebycontributingtomeasures forhealthsurveillanceandhelpingtheblooddonationnetworkinitsdonorselection pro-ceduresaimedatimprovingbloodtransfusionsafety.
©2016PublishedbyElsevierEditoraLtda.onbehalfofAssociac¸ ˜aoBrasileirade Hematologia,HemoterapiaeTerapiaCelular.ThisisanopenaccessarticleundertheCC BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:FaculdadedeMedicinadeRibeirãoPreto,UniversidadedeSãoPaulo(FMRP-USP),AvenidaBandeirantes3900,
14049-900RibeirãoPreto,SP,Brazil.
E-mailaddress:edson@fmrp.usp.br(E.Z.Martinez). http://dx.doi.org/10.1016/j.bjhh.2016.05.005
Introduction
InBrazil,uptothe1960s,transfusionproceduresweremostly performedbyprivate hospitalblood bankswithno govern-mentalregulation.Paradoxically,theimportanceofbloodas essentialtothehealthcaresystemwasfirstlynotedwiththe BrazilianRevolutionof1964.Thearmy,inviewofthe immi-nenceofanarmed conflict,foundthatstocksofbloodand componentswouldbeinsufficienttomeettheneedsinthe eventofarmedcombat.1Itwasatthattimethatthefirstpublic
initiativesweretakeninBraziltotrytonormalizethisactivity. Thefirstgovernmentalactwasthecreationofanational hemotherapycommissionaimedatsetting apolicyto reg-ulatetheblood collection procedure,including storageand transfusion.2 This commission formulated basic rules for
donorsandbloodtransfusionbyestablishingthemandatory serologicalscreeningtestsneededforsafebloodtransfusions. In1980,theFederalGovernmentimplementedtheNational ProgramonBloodandBloodDerivativeswiththe participa-tionofthecivilsociety,inordertodefineapolicyforblood anditscomponentsinBrazil,thusensuringtheavailability, safetyandamountoftheseproducts.3
Theregionalblood-bankingnetworkofthestateofMinas GeraiswasthusestablishedinJune1982withtheobjectiveof implementingthepoliciesproposedbytheNationalProgram onBloodandBloodDerivatives.In1985,the blood-banking network of Minas Gerais was officially inaugurated, and becameknownastheCentrodeHematologiaeHemoterapia deMinasGerais(Hemominas)andthen Fundac¸ão Hemom-inasfour years later.4 Today, Fundac¸ão Hemominasis one
ofthe largest blood screeningservices inBrazil.Itis com-prisedof21bloodbanksatthefollowingsites:AlémParaíba, Belo Horizonte, Shopping Estac¸ão (Belo Horizonte), Betim, Diamantina,JuizdeFora,Divinópolis,GovernadorValadares, PatosdeMinas,SeteLagoas,MontesClaros,Uberlândia, Itu-iutaba,SãoJoãoDel Rei, Manhuac¸u,Pouso Alegre,Hospital JúliaKubitscheck(BeloHorizonte),Uberaba,PonteNova, Pas-sos,andPoc¸osdeCaldas.5
Fundac¸ãoHemominasreceivesabout280,000blooddonors peryearandaccountsfor91%ofbloodtransfusionsinMinas Gerais.Epidemiologicalinformationonthediseasesidentified amongblooddonorcandidatesisimportanttogatherdata onthebloodbanksofFundac¸ãoHemominas,thusenablinga reductionoftherisksofdiseasetransmissionandensuringthe qualityofdonatedblood.6Withtheincreaseintransfusions,
andconsequentlyinthetransmissionofblood-bornediseases, hemotherapyservices are notonlydeveloping blood bank-ing services, but researching these diseases. Subsequently manydevelopmentsoccurredwithinafewyearsincludinga shiftfrompaidtovoluntarydonation,andautologousblood donations.Theimprovementinserologicalscreeningtests, rigoroustransfusionrequirementsandstandardizationof pro-cedureshavebeenessentialforthesafetyofblooddonation andtransfusion.7
The transmission of infectious diseases through blood transfusionischaracterizedbyahigher-riskofadverse reac-tionsinthebloodrecipient.Theidentificationofpathogens bymeans ofserologicaltestsisone wayofpreventingthe disseminationoftheseinfectiousagentsduringtransfusion.
InBrazil,theMinistryofHealthestablishedthatserological testsmustbeperformedforeveryblooddonationregarding the following pathogens: hepatitisB virus (HBV), hepatitis C virus (HCV), humanimmunodeficiency virus (HIV) types 1 and 2, humanT lymphotropic virus (HTLV) types 1 and 2,Trypanossomacruzi,Treponemapallidum,andPlasmodiumin malaria-endemicareas(includingmolecular tests).Another importantprocedureadoptedtominimizethe risksof con-taminationistheclinicalandepidemiologicalscreeningofthe candidates’healthstatus,habits,andriskybehaviortohelp todeterminepossiblerisksofblooddonationinrespecttothe healthofdonorsandrecipients.8–10 Inordertostandardize
theserologicalscreeningproceduresthroughoutthestateof MinasGerais,Fundac¸ãoHemominascreatedaserology cen-terinMay2005toserveall21bloodbanks,thusaccounting forcomplementaryserologicalscreeningandexamsforboth donors and patients. Today, this serology center isone of thelargestdonorscreeninglaboratoriesinBrazil,performing abouttwomilliontestsperyear.5
Theobjectiveofthepresentstudyistodescribethe preva-lence ofserological screeningmarkers amongblood donor candidates ofthe Fundac¸ãoHemominasbetween2006and 2012.ThestateofMinasGeraisisoneofthe27statesofBrazil withapproximately20millioninhabitantsdistributedamong 853towns.With586,522,122km2,theareaofthestateofMinas
Gerais,thefourthlargeststateofBrazil,correspondsto6.89% ofthenationalterritory;2,525,800km2areinurbanareas.The
cityofBeloHorizonte,locatedinthecentralregion,hasa pop-ulationofabout2.4millionpeople.Thesouthernregionofthe stateismoreindustrializedandeconomicallydeveloped,thus ithasgoodsocialindicators.Ontheotherhand,thenorthern regionisoneofthepoorestregionsinBrazil,becauseofthe droughtandlackofeffectivegovernmentpolicies,with ineffi-cientenvironmentalsanitaryservicesandhighchildmortality andilliteracyrates.
Methods
Design
Thisdescriptiveretrospectivestudyincludedallblooddonors screened usingserological tests formakersof blood-borne diseasesattheFundac¸ãoHemominas.Thetestswere manda-toryduringtheperiodofthestudyfrom2006to2012,even forrepeat donorsaspriornegativeserologicaltestsdonot sparethemfrompossibleserologicalineligibility.Theblood bank atPoc¸osdeCaldas wasinaugurated in2010.All data were collected from computerizedrecords ofthe Fundac¸ão Hemominas.
The present study was approved in June 2013 by the ResearchEthicsCommitteeoftheHospitaldasClinicasofthe FaculdadedeMedicinadeRibeirãoPretodaUniversidadede SãoPaulo(FMRP-USP:#356.616).
Serologicalscreeningtests
To determine HBsAg: PRISM (Abbott, CMIA) in 2006: MonolisaTM HBsAg ULTRA (Biorad) in 2007–2012 and
ARCHITECT®HBsAgAssay(Abbott,CMIA)in2012.
To determine human immune deficiency virus (HIV) – method1:PRISM(Abbott,CMIA)in2006,GSHIV-1/HIV-2PLUS OEIA(Biorad)in2007and2008,HIVAg/AbCombination(EIE, Murex)in2008and2009andARCHITECT®HIVAg/AbCombo
(Abbott,CMIA)in2009–2012.
To determine human immune deficiency virus (HIV) – method 2: HIV-1.2.0 (EIE, Murex) in 2006 and 2007, GenscreenTM ULTRA HIVAg-Ab (EIE, Biorad) in2007–2012
andVITROSAnti-HIV1+2Assay(OrthoClinicalDiagnostics, CMIA)in2012.
TodeterminehepatitisCvirus(HCV):Anti-HCV-version4.0 (EIE,Murex)in2006and2007,HepatitisCVirusEncoded Anti-gen(Recombinantc22-3,c200andNS5)ORTHO®HCVVersion
3.0ELISATestSystemin2007–2009,HCVAg/AbCombination (EIE,Murex)in2009–2011,andMonolisaTMHCVAg-AbULTRA
Assay(EIE,Biorad)in2011and2012.
TodeterminehepatitisBcoreantibody(HBc):totalanti-HBc (IgGandIgM–ELISA Test SystemOrtho-Clinical Diagnos-tics)in2006and2007,MONOLISAAnti-HBcPLUSAssay(EIE, Biorad)in2007–2012andARCHITECT® Anti-HBcII(Abbott,
CMIA)in2011and2012.
To determine Chagas disease: CHAGAS TEST ELISA BIOS CHILEin2006and2007andGOLDELISACHAGAS(REM)in 2007–2012.
TodeterminehumanT-lymphotropicretroviruses(HTLV)I/II: ELISA HTLV-I/II rp21 ELISA Ortho Diagnostics® System in
2006–2011,GOLDELISAHTLVI/II(REM)in2011and2012and ARCHITECT®rHTLV-I/II(Abbott,CMIA)in2012.
Todeterminesyphilis:ICE*Syphilis(Murex)in2006and2007; andBioelisaSyphilis3.0(Biokit)in2007–2012.
Thepresentstudydidnotusetheconfirmatorytestresults ofinitiallypositiveresults.
Results
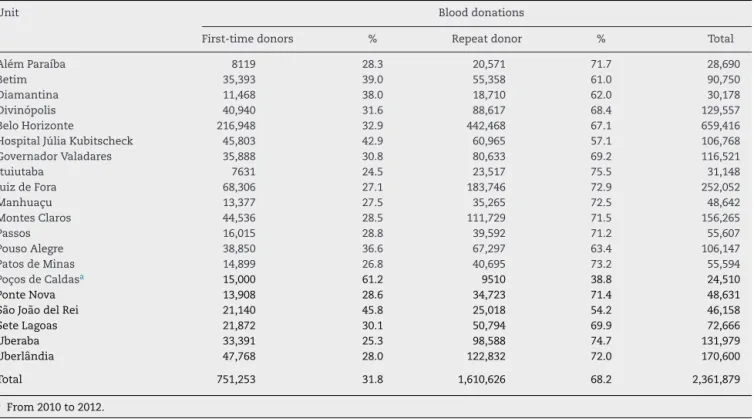
Atotalof2,361,879blooddonorcandidateswereinterviewed inFundac¸ãoHemominasbloodbanksfromJanuary2006to December 2012,with 1,864,553 donationsbeing considered eligibleafterclinicalscreening(Table1).Thisscreening con-sistsofevaluatingtheclinicalandepidemiologicalhistoryof candidates,and their healthstatus,habits andbehavior to determinewhethertheycanbeconsideredforblood dona-tionwithoutriskingtheirhealthorthatoftherecipient.11,12
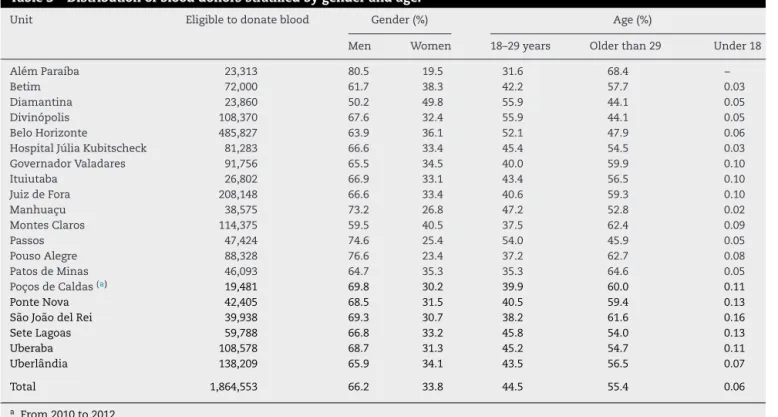
Theoverall percentageofdonationsfrom clinicallyeligible donorsofallthebloodbankswas78.9%,rangingfrom73.2% inMontesClarosto87.2%inPonteNova.Thepercentageof donationsfromrepeatdonors(68.2%)wasabout twicethat offirst-timedonors(31.8%)duringtheperiodofthestudyin allbloodbanksexceptforPoc¸osdeCaldaswhere61.2%and 38.8%werefirst-timeandrepeatdonors,respectively(Table2). ItshouldberememberedthatthePoc¸osdeCaldasbloodbank wasinauguratedin2010.Thedistributionofdonationsfrom eligibledonorsstratifiedbygenderfoundapredominanceof men(66.2%),exceptforthebloodbankofDiamantinawhere
50.2% ofthe donorsweremen(Table3). Table3shows the distributionofeligibledonorsstratifiedbyage.
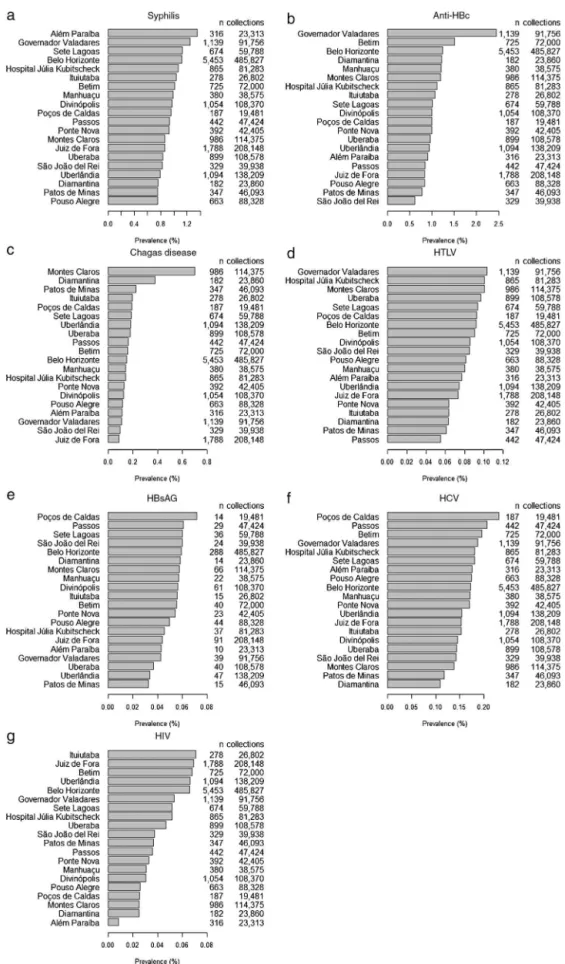
Consideringtheclinicallyeligibledonors,Figure1shows thepercentagesofdonationsfromdonorswithpositive sero-logyforsyphilis,anti-HBc,Chagasdisease,HTLV,HBsAG,HCV, andHIVateachbloodbank.ThebloodbankinGovernador Val-adareshadthehighestpercentageofpositiveanti-HBcdonors (Figure1b).PositivityfortheserologicalmarkerforChagas dis-easewasmorecommonintheMontesClarosandDiamantina bloodbanks;thecitiesaresituatedinthenorthernregionof thestateandtheJequitinhonhaValley,respectively,wherethe disease isendemic.13,14 SerologicalmarkersforHBs-Agand
HCVweremorecommonlyobservedinthePoc¸osdeCaldas bloodbank(Figure1eandf).
Discussion
Despite all the technological advancesin recent years,the productionofbloodcomponents andblood derivativesstill dependson blooddonation. However,thenumber ofblood donorsdoesnotfulfillthecurrentdemandinBrazil.15Inthis
work,themeanpercentageofclinicallyeligibledonorsinthe Hemominasbloodbankswas78.9%,avaluesimilartothat reportedbytheannualreportontheproductionofblood com-ponentsin2013asthepercentageofclinicaleligibilitywas closeto81.4%nationally.16Rohretetal.,17conductedastudy
attheHospitalSantoAngeloinSouthernBrazil,andreported aclinicaleligibilityof73.6%for2005–2015.
In this work, the percentage of donations from repeat donors(68.2%)wasapproximatelytwiceashighasfor first-timedonors(31.8%).Thesepercentagesaresimilartothose observed throughout the country,16 as the percentages of
donorsisestimatedat63.1%and36.9%forrepeatand first-time donors, respectively. However, ahigher percentageof first-timedonors(61.2%)wasobservedinthePoc¸osdeCaldas bloodbank;thisfindingcanbeexplainedasthebloodbank wasimplantedonlyin2010.
Amongtheeligibledonors,therewasapredominanceof men(66.2%).SantosandMacedo18reportedapercentageof
63.8%ofmaledonorsinastudyconductedbetween2005and 2009attheCampoMourãobloodbank,stateofParaná.Similar datawerereportedbytheAgenciaNacionaldeVigilancia San-itaria(ANVISA),whosestudyevaluatedtheprofileofdonors andnon-donorsthroughoutBrazil.19Amongthedonor
popu-lationinBrazil,menwerefoundtobepredominant(65.67%); 61.02%insoutheasternBrazil,63.97%inthesouthernregion, 70.95%intheNortheast,75.25%innorthernBraziland75.96% inthecentral-westernregion.
Inthepresentstudy,42.8%ofeligibleblooddonorswere inthe 18- to29-yearage groupand 57.1%were older than 29yearsold.Similarresultsarefoundacrossthecountry,16
with the majorityofthe blood donorsbeing older than 29 years(57.08%).Otherstudiesshowthatthenumberofblood donationsincreasesastheagedecreases.18,20
Another factor affectingthe decrease inthe number of blooddonationsistherateofserologicalineligibility.In2002, the serologicalineligibility raterangedfrom 10% to20%in Brazilianblood banks.21 Thisrateisveryhighcomparedto
Table1–Distributionofblooddonationsaccordingtoeligibilityandineligibilityofblooddonorcandidates.
Bloodbank Blooddonations
Eligible % Ineligibility % Dropouts % Total
AlémParaíba 23,313 81.3 5272 18.4 105 0.37 28,690
Betim 72,000 79.3 18,501 20.4 249 0.27 90,750
Diamantina 23,860 79.1 6151 20.4 167 0.55 30,178
Divinópolis 108,370 83.6 20,392 15.7 795 0.61 129,557
BeloHorizonte 485,827 73.7 156,701 23.8 16,888 2.56 659,416
HospitalJúliaKubitscheck 81,283 76.1 25,031 23.4 454 0.43 106,768
GovernadorValadares 91,756 78.7 22,881 19.6 1884 1.62 116,521
Ituiutaba 26,802 86.0 4231 13.6 115 0.37 31,148
JuizdeFora 208,148 82.6 42,479 16.9 1425 0.57 252,052
Manhuac¸u 38,575 79.3 9753 20.1 314 0.65 48,642
MontesClaros 114,375 73.2 40,867 26.2 1023 0.65 156,265
Passos 47,424 85.3 7983 14.4 200 0.36 55,607
PousoAlegre 88,328 83.2 17,151 16.2 668 0.63 106,147
PatosdeMinas 46,093 82.9 9347 16.8 154 0.28 55,594
Poc¸osdeCaldasa 19,481 79.5 4897 20.0 132 0.54 24,510
PonteNova 42,405 87.2 6059 12.5 167 0.34 48,631
SãoJoãodelRei 39,938 86.5 6059 13.1 161 0.35 46,158
SeteLagoas 59,788 82.3 12,561 17.3 317 0.44 72,666
Uberaba 108,578 82.3 22,435 17.0 966 0.73 131,979
Uberlândia 138,209 81.0 31,745 18.6 646 0.38 170,600
Total 1,864,553 78.9 470,496 19.9 26,830 1.14 2,361,879
a From2010to2012.
Table2–Distributionofblooddonorsasfirst-timeorrepeatdonors.
Unit Blooddonations
First-timedonors % Repeatdonor % Total
AlémParaíba 8119 28.3 20,571 71.7 28,690
Betim 35,393 39.0 55,358 61.0 90,750
Diamantina 11,468 38.0 18,710 62.0 30,178
Divinópolis 40,940 31.6 88,617 68.4 129,557
BeloHorizonte 216,948 32.9 442,468 67.1 659,416
HospitalJúliaKubitscheck 45,803 42.9 60,965 57.1 106,768
GovernadorValadares 35,888 30.8 80,633 69.2 116,521
Ituiutaba 7631 24.5 23,517 75.5 31,148
JuizdeFora 68,306 27.1 183,746 72.9 252,052
Manhuac¸u 13,377 27.5 35,265 72.5 48,642
MontesClaros 44,536 28.5 111,729 71.5 156,265
Passos 16,015 28.8 39,592 71.2 55,607
PousoAlegre 38,850 36.6 67,297 63.4 106,147
PatosdeMinas 14,899 26.8 40,695 73.2 55,594
Poc¸osdeCaldasa 15,000 61.2 9510 38.8 24,510
PonteNova 13,908 28.6 34,723 71.4 48,631
SãoJoãodelRei 21,140 45.8 25,018 54.2 46,158
SeteLagoas 21,872 30.1 50,794 69.9 72,666
Uberaba 33,391 25.3 98,588 74.7 131,979
Uberlândia 47,768 28.0 122,832 72.0 170,600
Total 751,253 31.8 1,610,626 68.2 2,361,879
a From2010to2012.
studied,themeanrateofserologicalineligibilityin2013was 3.43%.14Similardatawerefoundinthepresentwork,asthe
rateofserologicalineligibility atthe Fundac¸ãoHemominas was3.05%.Thefrequentchangesinthebrandsofscreening testkitsduetothebiddingnormsthathavetobefollowedby governmentblood bankstopurchaseproducts,contributed totheserologicalineligibility.Thisphenomenonoccurseven
whenhigh-sensitivityandspecificitytestsareused.Different testkitbrandsproducedifferentfalse-positiveratesand, con-sequently,itismorelikelythattheresultsofahealthyblood donorare positiveto atleast oneofthe brands used.This wouldnothappenifthesamebrandofkitwerealwaysused.21
Table3–Distributionofblooddonorsstratifiedbygenderandage.
Unit Eligibletodonateblood Gender(%) Age(%)
Men Women 18–29years Olderthan29 Under18
AlémParaíba 23,313 80.5 19.5 31.6 68.4 –
Betim 72,000 61.7 38.3 42.2 57.7 0.03
Diamantina 23,860 50.2 49.8 55.9 44.1 0.05
Divinópolis 108,370 67.6 32.4 55.9 44.1 0.05
BeloHorizonte 485,827 63.9 36.1 52.1 47.9 0.06
HospitalJúliaKubitscheck 81,283 66.6 33.4 45.4 54.5 0.03
GovernadorValadares 91,756 65.5 34.5 40.0 59.9 0.10
Ituiutaba 26,802 66.9 33.1 43.4 56.5 0.10
JuizdeFora 208,148 66.6 33.4 40.6 59.3 0.10
Manhuac¸u 38,575 73.2 26.8 47.2 52.8 0.02
MontesClaros 114,375 59.5 40.5 37.5 62.4 0.09
Passos 47,424 74.6 25.4 54.0 45.9 0.05
PousoAlegre 88,328 76.6 23.4 37.2 62.7 0.08
PatosdeMinas 46,093 64.7 35.3 35.3 64.6 0.05
Poc¸osdeCaldas(a) 19,481 69.8 30.2 39.9 60.0 0.11
PonteNova 42,405 68.5 31.5 40.5 59.4 0.13
SãoJoãodelRei 39,938 69.3 30.7 38.2 61.6 0.16
SeteLagoas 59,788 66.8 33.2 45.8 54.0 0.13
Uberaba 108,578 68.7 31.3 45.2 54.7 0.11
Uberlândia 138,209 65.9 34.1 43.5 56.5 0.07
Total 1,864,553 66.2 33.8 44.5 55.4 0.06
a From2010to2012.
markersforsyphilis,anti-HBc,Chagasdisease,HTLV,HBsAG, HCV, and HIV. Of the 1,864,553 clinically eligible donors referredforserological testingbetween 2006and 2012,the total anti-HBc (IgM/IgG) was the commonest serological markerfound (1.12% ofblood donors)followed by syphilis (0.98% ofthe study population). Data from a national sur-veyin2012onthedistributionofserologicalineligibilitydue to blood-borne disease markers reported a predominance of anti-HBc (1.47%), followed by syphilis (0.67%), and HIV (0.36%).16Inthenorthernregion,approximately5.16%ofblood
donorsareineligibilitybecauseofserologicalresults,withthe prevalenceoftheanti-HBcmarkerbeing3.77%.16Ontheother
hand,ineligibilityduetosyphilisinthisregionofBrazil(0.26%) isfarlowerthanthenationalaverage(0.67%).Thehighestrates inBrazilforHIVandHTLVmarkersare inthenortheastern region(0.66%and0.28%ofblooddonors,respectively). Over-all ineligibility inthis region is4.39%.Thecentral-western regionhasaprevalenceofHIVmarkersof0.26%,thelowest ratefoundinBrazil.Totalserologicalineligibilityinthisregion is2.76%.SoutheasternBrazilhasthelowestrateof serologi-calineligibility(2.68%),whereasthesouthernregionhasthe second-highestprevalenceofanti-HBcmarkers(1.94%).16
InthestudybySallesetal.21whoevaluatedblooddonors
atthe Fundac¸ão Pro-Sangue, the blood bank in SãoPaulo, the prevalence of markers for syphilis was 1.1% between 1992and2001.Theauthorsusedtwosimultaneousteststo investigate syphilis infections [enzyme immunoassay(EIA) andvenerealdiseaseresearchlaboratory(VDRL)].Using dif-ferentscreeningalgorithms,Baião23identifieddifferentrates
attheblood-bankingnetworkofSanta Catarina(HEMOSC). From January 2009 to July 2011, using VDRL for screening andELISAandFTA-ABSasconfirmatorytests,0.28%of pos-itiveresultswerefoundforsyphilis.BetweenJuly2011and
September 2012,0.68% ofpositiveresultswerereportedfor syphilisusingchemiluminescentmicroparticleimmunoassay (CMIA)toscreenandVDRLandFTA-ABStoconfirmpositive test results. Soussumi,24 usingnon-treponemal anti-bodies
(Reaginas)toscreenforsyphilis,foundaprevalenceof1.3% amongfirst-timeblood donorsattendingthe bloodbankof RibeirãoPretobetween1996and2001.However,Rodrigues25
reported aprevalenceof0.61%forsyphilisinblooddonors ofthe bloodbank ofGoiásbetween2002and 2011usinga non-treponemaltest(VDRL).Inanotherstudy performedat the Fundac¸ãodeHematologiaeHemoterapiadoAmazonas (HEMOAM)between2000and2004,Ferreiraetal.26alsoused
aVDRLtestandfoundaprevalenceof1.98%.
There issignificant variability inthe prevalenceofHCV amongblooddonors(rangingfrom0.34%to1.8%)reportedby Brazilianstudies.27–29Comparisonsbetweentheprevalenceof
markersindifferentservicesandregionsshouldbecautious, astheycanvaryduetotheuseofdifferentserological tech-niquesaswellasbytheuse ornotofconfirmatorytests.28
TheprevalenceofHCVmarkersintheFundac¸ãoHemominas was0.15%,withthebloodbankatPoc¸osdeCaldashavingthe highestrate(0.24%)andthebloodbanksofDiamantinaand PatosdeMinashavingthelowestfrequencies.Accordingto theannualreportontheproductionofbloodcomponents pub-lishedin2013,16theprevalencesofHCVmarkersinthestates
ofTocantins(0.09%)andSantaCatarina(0.14%)weresimilar totheratefoundbytheFundac¸ãoHemominas(0.15%).
oftheuseofnon-intravenousillicitdrugs.Thishighlightsthe importanceofmonitoringandrefining theprocessofblood donorselection.30
There were significant differencesin the prevalencesof anti-HBcmarkersbetweensomebloodbanks.Thebloodbank ofGovernadorValadareshadthehighestrateofanti-HBc pos-itivedonors(2.41%);theprevalencewassignificantlyhigher thantheothersixbloodbanks.ThebloodbankofSãoJoão DelReihadthelowestrateofpositiveresultsforthismarker (0.61%).Onehypothesisforthehighprevalenceofanti-HBcin themiddleregionoftheValedoRioDocemaybethe prox-imitytothestatesofBahiaandEspíritoSantothatreported positiveratesforanti-HBc of2.97%and 1.81%,respectively. Accordingtothe annual reportonthe productionofblood componentsfrom2013,16theprevalenceoftheHBs-Agmarker
inBrazilrangedfrom0.07%inthestateofEspíritoSantoto 0.43%inthestateofParaíba.ThestateofSãoPaulohadthe lowestrate foranti-HBc (0.76%),whereas the highestrates werereportedinRondônia(4.38%)andAcre(4.33%).Despite evidence that immunization against hepatitis B virus can producean immune response, the anti-HBc marker is not consideredaneutralizingantibody,anditspresencedoesnot indicate recoveryfrom hepatitisBinfection.31 Even so,the
exclusionofanti-HBc-positivedonorsisacontroversialissue asahighnumberofthesedonorsarenotallowedtodonate bloodand thereisevidenceoffalse-positiveresults.Inany case,itshouldbestressedthatdonorswhoarepositiveonly forthehepatitisBmarkeraredefinitelyconsideredineligible fordonation.28Ontheotherhand,anti-HBcisanimportant
markertodetect occult hepatitisB,which ischaracterized bythepresenceofHBVDNAinthebloodserumof HBs-Ag-negativeindividuals.32
Inthiswork,theprevalenceofthemarkerofChagas dis-easeforresearchpurposeswas0.18%.Datafromtheannual report on the production of blood components in 201314
reportedthattheprevalenceofChagasdiseaseinBrazilranged from0.02%inthestateofEspíritoSantoto0.54%inthestate ofRiodeJaneiro.AccordingtoLacaz,33inthestateofMinas
Gerais,whereChagasdisease wasdiscovered,the epidemic is high as the parasite has domestic habits, and is found in virtually all the state. However, there has been a great decreaseinthe occurrenceofnewcasesofChagas disease inrecentdecades.Thiswaspossiblythankstothe epidemio-logicalsurveillancepracticedincommunitiestocontrolvector transmission,alliedwiththeNationalProgramforChagas Dis-easeControlandruralsocioeconomicfactors.Infact,notonly wasaruralexodusobserved,butalsoimprovementinincome andhousingconditions,thesupplyofelectricity,andaccess toeducationandhealthcare.34However,theseimprovements
were notassignificantinthe northernsemi-arid regionof thestate ofMinasGerais,wherethe socioeconomic condi-tionsofthelocalpopulationarestillprecariousandworrying, mainlyintheruralzone,thusitisconsideredoneofthe poo-restregionsofBrazil.Theseconditionsmayaccountforthe re-emergenceofChagasdiseaseinthisregion,asitsspatial distributioniscoincidentwiththatofotherpoorpopulations asthediseaseisdirectlyrelatedtosocioeconomicconditions. TheprevalenceofHIVmarkersforresearchpurposeswas 0.052%inthisstudy.From2003to2013,thedistributionof inel-igibleblooddonorswhowerepositiveforHIV(types1and2)
inthefiveregionsofBrazilwere0.02%and10.14%inRibeirão Preto35andBelém,respectively.36TheprevalenceofHIV
mark-ersforresearchpurposeswas0.36%inBrazil,rangingfrom 0.18%inthestateofPiauíto1.32%inthestateofParaíba.16
Conclusion
The constant assessment of epidemiological data is of paramountimportanceforthecontrolandevaluationofboth blood donorrecruitment strategiesand public health poli-cies as a whole. It is fundamental to implement policies thatuseinformationasmanagementtoolstoplan interven-tionprojectsandfollow-upactionsintheareaoftransfusion medicine,particularlyfortherecruitmentofblooddonors.The presentationofdataonserologicalineligibilityfoundinthe bloodbanksofthestateofMinasGeraisshowsusthe peculiar-itiesofeachregion,thuscontributingtohealthsurveillance measuresandassistingtotheblood-bankingnetworkandits donorselectionproceduresaimingatimprovingtransfusion safety.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
This work was supported by FAEPA (process number 1449/2014)andConselhoNacionaldeDesenvolvimento Cien-tíficoeTecnológico(CNPq,Processnumber307767/2015-9).
r
e
f
e
r
e
n
c
e
s
1.SouzaCA,CovasDT,AddasCM.SangueeHemoderivados: DesafiosaindanãoconcretizadosnoâmbitodoSistema ÚnicodeSaúde(SUS).In:LenirSantos(Org.),editor.Direitoda SaúdenoBrasil,vol.1,1sted.Campinas:SaberesEditora; 2010.p.311–41.
2.Brasil.MinistériodaSaúde.DOU.Decreton◦54.494,de16de
Outubrode1964–CriaGrupodeTrabalhoparaestudantese proporalegislac¸ãodisciplinadoradahemoterapianoBrasile dáoutrasprovidências.DiarioOficialdaUnião,9517.Brasília, DF,1964.
3.Brasil.MinistériodaSaúde.Pró-Sangue.Umarealidade Nacional,vol.1.Brasília,DF:ProgramaNacionaldeSanguee Hemoderivados;1985.p.1.
4.Fundac¸ãoHemominas:1985–2007.Fundac¸ãoCentrode HematologiaeHemoterapiadeMinasGerais.Horizonte: Fundac¸ãoHemominas;2007,202pp.
5.Fundac¸ãoHemominas.ManualdaQualidadedaCentral Sorológica,3;2014.BeloHorizonte,MG.
6.Fundac¸ãoHemominas.Fundac¸ãoHemominas–Centrode HematologiaeHemoterapiadoEstadodeMinasGerais;2011. BeloHorizonte.
7.AlmeidaNetoC[Doctoralthesis]Perfilepidemiológicode doadoresdesanguecomdiagnósticodesífiliseHIV. FaculdadedeMedicinadaUniversidadedeSãoPaulo;2008. 8.Brasil.MinistériodaSaúde.DOU.Portarian◦2.712,de12de
9. Brasil.MinistériodaSaúde.Anvisa.DOU.Resoluc¸ãoRDCN◦
51,07denovembrode2013–AlteraaResoluc¸ão–RDCn◦57,
de16dedezembrode2010.DeterminaoRegulamento SanitárioparaServic¸osquedesenvolvematividades relacionadasaocicloprodutivodosanguehumanoe componentes,125;2013.Brasília,DF.
10.Brasil.MinistériodaSaúde.Resoluc¸ãoRDCn◦34de11de
junhode2014.Dispõesobreasboaspráticasnociclodo sangue;2014.Brasília,DF.
11.BrenerS,CaiaffaWT,SakuraiE,ProiettiFA.Factorsassociated toclinicalaptnessforblooddonation–demographicand socioeconomicdeterminants.RevBrasHematolHemoter. 2008;30(2):108–13.
12.PinhoAM,LopesMI,LimaMJ,CastroV,MarteletoMA. TriagemClínicadeDoadoresdeSangue.Brasília:Ministério daSaúde,DF;2001.Availableat:http://bvsms.saude.gov. br/bvs/publicacoes/cd0720.pdf
13.Oliveira-CamposM,CerqueiraMB,RodriguesNetoJF. Dinâmicapopulacionaleoperfildemortalidadeno municípiodeMontesClaros(MG).CienSaudeColet.2011;16 Suppl.1:1303–10.
14.BorgesJD,AssisGF,GomesLV,DiasJC,PintoID,Martins-Filho OA,etal.SeroprevalenceofChagasdiseaseinschoolchildren fromtwomunicipalitiesofJequitinhonhaValley,Minas Gerais,Brazil;sixyearsfollowingtheonsetofepidemiological surveillance.RevInstMedTropSaoPaulo.2006;48(2):81–6. 15.Brasil.AgênciaNacionaldeVigilânciaSanitária.
Hemovigilância:manualtécnicoparainvestigac¸ãodas reac¸õestransfusionaisimediatasetardiasnãoinfecciosas, 124;2007.Brasília,DF.
16.Brasil.AgênciaNacionaldeVigilânciaSanitária.Boletim AnualdeProduc¸ãoHemoterápica;2013.p.1–10.Brasília,DF. 17.RohrJI,BoffD,LunkesDS.Perfildoscandidatosinaptospara
doac¸ãodesanguenoservic¸odehemoterapiadoHospital SantoAngelo.RevPatolTrop.2012;41:27–35.
18.SantosMC,MacedoLC.Prevalênciaeperfildedoadoresde sanguerealizadaspeloHemonúcleodeCampoMourão-PR. SaudPesq.2013;6(1):8–12.
19.Brasil.AgênciaNacionaldeVigilânciaSanitária.Pesquisa revelaoperfildedoadoresenãodoadoresdesangue;2006. Brasília,DF.Availablefrom:http://www.anvisa.gov.br/ DIVULGA/NOTICIAS/2006/1101061.htm[cited11.08.14]. 20.ZagoA,daSilveiraMF,DumithSC.Blooddonationprevalence
andassociatedfactorsinPelotas,SouthernBrazil.RevSaude Publica.2010;44(1):112–20.
21.SallesNA,SabinoEC,BarretoCC,BarretoAM,OtaniMM, ChamoneDF.Thediscardingofbloodunitsandthe prevalenceofinfectiousdiseasesindonorsatthePro-Blood Foundation/BloodCenterofSãoPaulo,SãoPaulo,Brazil.Rev PanamSaludPublica.2003;13(2–3):111–6.
22.GlynnSA,KleinmanSH,SchreiberGB,BuschMP,WrightDJ, SmithJW,etal.Trendsinincidenceandprevalenceofmajor transfusion-transmissibleviralinfectionsinUSblooddonors, 1991to1996.RetrovirusEpidemiologyDonorStudy(REDS). JAMA.2000;284(2):229–35.
23.BaiaoAM[Master’sthesis]Avaliac¸ãodedesempenho diagnósticodostesteslaboratoriaisparasífilisemdoadores desanguedeSantaCatarinaem2009a2012.Universidade FederaldeSantaCatarina;2013.
24.SoussumiLM[Master’sthesis]Estudodadistribuic¸ãode doadoresreativosparadoenc¸adeChagasnoHemocentrode RibeirãoPreto,SP.RibeirãoPretoMedicalSchool,Universityof SãoPaulo;2004.
25.RodriguesMA.Soroprevalênciadesífilisemdoadoresde sanguedoHemocentrodeGoiásnoperíodode2002a2011. Bachelor’sdegreecompletion.UniversidadeEstadualde Goiás;2012.
26.FerreiraC,FerreiraW,MottaC,VasquezFG,PintoAF. ReactivityofVDRLtestinbloodbagsoftheAmazon HematologyandHemotherapyFoundation–HEMOAM,the decurrentcostsofdischargeandestimativeofsyphilis prevalenceinblooddonorsoftheAmazonState.DSTJBras Doenc¸asSexTransm.2006;18(1):14–7.
27.RosiniN,MousseD,SpadaC,TreitingerA.Seroprevalenceof HbsAg,Anti-HBcandanti-HCVinSouthernBrazil,1999–2001. BrazJInfectDis.2003;7(4):262–7.
28.ValenteVB,CovasDT,PassosAD.HepatitisBandCserologic markersinblooddonorsoftheRibeirãoPretoBloodCenter. RevSocBrasMedTrop.2005;38(6):488–92.
29.Brasil.MinistériodaSaúde.Seguranc¸aTransfusional:Um olharsobreosservic¸osdehemoterapiadasregiõesNortee CentroOestedoBrasil.IIICursodeEspecializac¸ãoem Seguranc¸aTransfusional.Brasília,DF:Resumodas monografiafinais;2012.
30.SoaresBC,ProiettiAB,ProiettiFA.InterdisciplinaryHTLV-I/II ResearchGroup.HTLV-I/IIandblooddonors:determinants associatedwithseropositivityinalowriskpopulation.Rev SaudePublica.2003;37(4):470–6.
31.MilichDR.ImmuneresponsetohepatitisBvirusproteins: relevanceofthemurinemodel.SeminLiverDis.
1991;11(2):93–112.
32.vanHemertFJ,ZaaijerHL,BerkhoutB,LukashovVV.Occult hepatitisBinfection:anevolutionaryscenario.VirolJ. 2008;5:146.
33.LacazCS.Introduc¸ãoàGeografiaMédicadoBrasil.SãoPaulo: EdgardBlucher,UniversidadedeSãoPaulo;1972,568pp. 34.MendesPC,LimaSC.Influênciadoclimanaocorrênciade
TriatomíneosdomunicípiodeUberlândia-MG.Cad PrudentinoGeogr.2011;33(2):5–20.
35.FerreiraO[Master’sthesis]Estudodedoadoresdesangue comsorologiareagenteparahepatitesBe22C.HIVeSífilis noHemocentrodeRibeirãoPreto.RibeirãoPretoMedical School,UniversityofSãoPaulo;2007.