w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Survival
and
treatment
response
in
adults
with
acute
promyelocytic
leukemia
treated
with
a
modified
International
Consortium
on
Acute
Promyelocytic
Leukemia
protocol
Erick
Crespo-Solis
∗,
Jorge
Contreras-Cisneros,
Roberta
Demichelis-Gómez,
Adriana
Rosas-López,
Juan
Mauricio
Vera-Zertuche,
Alvaro
Aguayo,
Xavier
López-Karpovitch
InstitutoNacionaldeCienciasMédicasyNutriciónSalvadorZubirán(INCMNSZ),CiudaddeMéxico,Mexico
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received3June2015
Accepted24August2016
Availableonline21September2016
Keywords:
Acutepromyelocyticleukemia
IC-APLprotocol
Developingcountries
Survival
a
b
s
t
r
a
c
t
Acutepromyelocyticleukemiahasgoodprognosisinviewofthehighcompleteremission
andsurvivalratesachievedwiththerapiescontainingall-transretinoicacidorarsenic
tri-oxide.However,thereisasignificantriskofdeathduringinductionduetohemorrhage
secondarytodisseminatedintravascularcoagulation.Thishascontributedtoagapinthe
prognosisofpatientsbetweendevelopedanddevelopingcountries.TheInternational
Con-sortiumonAcutePromyelocyticLeukemiawascreatedin2005andproposedatreatment
protocolbasedondaunorubicinandall-transretinoicacidstratifiedbyriskgearedtoward
developingcountries.Hereinarepresentedtheresultsfromthefirstpatientcohorttreated
inasingledevelopingcountryhospitalemployingaslightlymodifiedversionofthe
Interna-tionalConsortiumprotocolinareallifesetting.Twentypatientswithacutepromyelocytic
leukemiawereenrolled:27.8%hadlow-risk,55.6%intermediateriskand16.7%high-risk.
Thecompleteremissionratewas94.4%afteramedianof42days.Bothrelapseratesand
deathrateswereonepatient(5.5%)each.Nodeathswereobservedduringconsolidation.
Afteramedianfollow-upof29months,theoverallsurvivalratewas89.1%.Efficacyand
safetyoftheInternationalConsortiumonAcutePromyelocyticLeukemiaprotocolhasbeen
reproducedinacutepromyelocyticleukemiapatientsfromadevelopingcountry.
©2016Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published
byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:InstitutoNacionaldeCienciasMédicasyNutriciónSalvadorZubirán(INCMNSZ),VascodeQuiroga15,col.
BelisarioDomínguez,Tlalpan,CP14000MexicoCity,DF,Mexico.
E-mailaddress:erick.crespo.solis@gmail.com(E.Crespo-Solis).
http://dx.doi.org/10.1016/j.bjhh.2016.08.002
1516-8484/©2016Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.PublishedbyElsevierEditoraLtda.Thisisan
Introduction
Acutepromyelocyticleukemia(APL)isaclinicaland
biolog-icalvariantofacutemyeloid leukemia (AML)characterized
byapredominanceofabnormalpromyelocytes.Accordingto
theWorldHealthOrganization(WHO),itiscurrentlyclassified
amongtheAMLwithrecurrentcytogeneticabnormalities[APL
andt(15;17)(q22;q12);PML-RARA].1Untreated,APListhemost
aggressiveformofAML,withamediansurvivalofunderone
month.2Itismainlydiagnosedinyoungadultsagedbetween
20and 59.Somestudieshavesuggestedanincreased
inci-denceinLatinos(24.3%vs.8.3%innon-Latinopopulations)
but this appears to reflect a selection and referral bias.3,4
Werecentlyreportedthatthistypeofleukemiaaccountsfor
10.5%ofallacuteleukemiasand21%ofAMLattheInstituto
NacionaldeCienciasMédicasyNutriciónSalvadorZubirán
(INCMNSZ).5
Sincetheintroductionofall-transretinoicacid(ATRA)in
1988byHuanginShangai,completeremission(CR)ratesof
85% changedthe naturalhistory ofthisdisease.6 The
Ital-ianGroupforAdultHematologicDiseases(GIMEMA)reported
their experience with regimens including ATRA in
combi-nation with anthracyclines, obtaining high CR rates and
encouragingdisease-freesurvival(DFS)andoverall survival
rates(OS).7,8
In 1999, Sanz et al. designed a risk-adapted strategy
afterobservingthat patientswithhigh white blood counts
(>10×109/L)andlowplateletcounts(<40×109/L)wereathigh
riskofdeathduringinductionandrelapse.9Theirstudyadded
ATRAtotheconsolidationregimensinintermediateand
high-riskpatients.Improvementsintreatmenthavetransformed
thisleukemiaintotheonewiththebestsurvivalratesinadults
today.
Indevelopedcountries,CRratesinAPLpatientsareclose
to95%withDFScurvesattwoyearsbetween87%and97%.9,10
However,somestudiesindevelopingcountriesreportedCR
ratesof67.9%,deathduringinductionin32%ofcasesandan
OSbelow60%in2005.11Themaincauseofdeathduring
induc-tionwashemorrhagefollowedbydifferentiationsyndrome.
ThecurrentbasisoftherapyofAPLpatientshingesonthe
useofspecificagentsthatinducedifferentiation,suchasATRA
and arsenictrioxide(ATO) inconjunction with
chemother-apeutic agentssuchasanthracyclines.12 ATRA and arsenic
trioxidearedrugswhosecostsareprohibitivetoalargegroup
ofpatientsindevelopingcountries.
The International Consortium on Acute Promyelocytic
Leukemia (IC-APL) was created in 2005in order tonarrow
the gap betweenthe response rates reportedin developed
countriesand those insomedeveloping countries. Its
pur-pose was to integrate an inter-institutional web designed
programto generate and implementdiagnostic and
thera-peutic strategies for APL patients in developing countries.
This endeavor was supported by expert North American
andEuropeanworkgroups.Thetargetedcountriesincluded
Brazil,Mexico,ChileandUruguay.TheIC-APLimplemented
the regimen designed bythe Programa para el Estudiode
la Terapéutica en Hemopatía Maligna
(PETHEMA)/Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) LPA
2005, in which idarubicin was replaced by daunorubicin
(equivalence:daunorubicin5mg=idarubicin1mg) in
induc-tionandconsolidationtherapy.Thissubstitutionwasmainly
duetoeconomiclimitations.Theregimenanditsresultswere
recentlypublished.13CRrateswere85%witha15%mortality
duringinductionand5%duringconsolidation.Median
follow-upwas28monthswitharelapserateof4.5%,OS80%andDFS
91%.13
The Department of Hematology and Oncology of the
INCMNSZparticipatedintheConsortium’soriginalserieswith
theinclusionoftwopatients.Becauseoflogisticaspects,we
couldnotincludemorepatients.Duetotheencouraging
pre-liminarydataatthetime,thisregimenwaslateradoptedas
first-linetherapyinAPLpatientstreatedattheINCMNSZasof
January2007.ByJune2013,18morepatientshadbeentreated
withthisregimen,butnotincludedintotheoriginal
Consor-tiumstudy.Patientcharacteristics,responsetotreatmentand
survivalaredescribedinthisretrospectivestudyofareal-life
scenario.
Methods
Aretrospectivecohortstudywascarriedoutofadultpatients
with APL t(15;17)(q22;q12);PML-RARA, treated in the Acute
LeukemiaClinic,DepartmentofHematologyandOncologyof
the INCMNSZ, betweenJanuary2007and June1,2013.The
diagnosis of APL t(15;17) was established according to the
WHO criteria.1 Morphological andcytogenetic studies were
conductedinallpatients(conventionalkaryotype andFISH
withaprobeforPML/RARA)inabonemarrowsampleobtained
at diagnosis. Disseminated intravascular coagulation (DIC)
wasdefinedinaccordancewiththeInternationalSocietyfor
Thrombosisand Hemostasiscriteria(ISTH)ifscoreswere5
orabove.14Coagulopathywasdefinedastheprolongationof
coagulationtimes(PTorPTT)oradecreaseinfibrinogenthat
didnotfulfillDICcriteria(Table1).
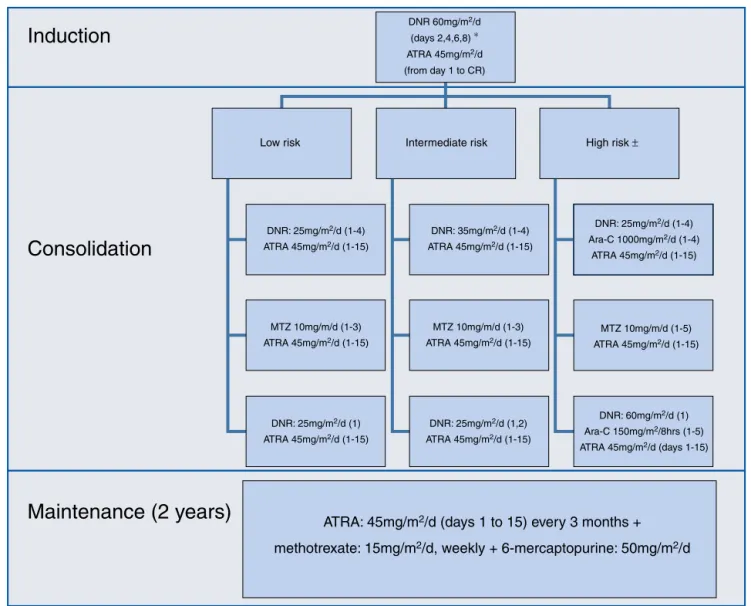
Theinduction,consolidationandmaintenanceregimens
werethesameasthoseusedbytheIC-APL200613;theywere
appliedwithoutanymodification(Figure1).
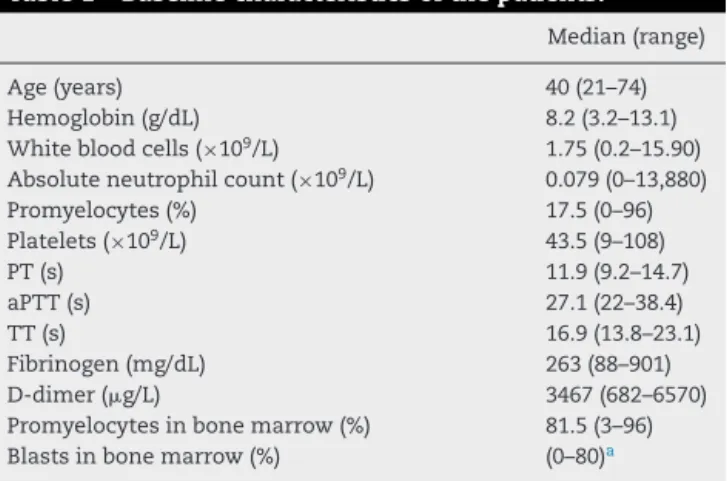
Table1–Baselinecharacteristicsofthepatients.
Median(range)
Age(years) 40(21–74)
Hemoglobin(g/dL) 8.2(3.2–13.1)
Whitebloodcells(×109/L) 1.75(0.2–15.90)
Absoluteneutrophilcount(×109/L) 0.079(0–13,880)
Promyelocytes(%) 17.5(0–96)
Platelets(×109/L) 43.5(9–108)
PT(s) 11.9(9.2–14.7)
aPTT(s) 27.1(22–38.4)
TT(s) 16.9(13.8–23.1)
Fibrinogen(mg/dL) 263(88–901)
D-dimer(g/L) 3467(682–6570)
Promyelocytesinbonemarrow(%) 81.5(3–96)
Blastsinbonemarrow(%) (0–80)a
PT:prothrombintime;aPTT:activatedpartialthromboplastintime; TT:thrombintime.
a 80%myeloidblastcountwasreportedintheinitialbonemarrow
Induction
DNR 60mg/m 2/d(days 2,4,6,8) ∗ ATRA 45mg/m2/d
(from day 1 to CR)
Low risk
DNR: 25mg/m2/d (1-4)
ATRA 45mg/m2/d (1-15)
DNR: 35mg/m2/d (1-4)
ATRA 45mg/m2/d (1-15)
DNR: 25mg/m2/d (1-4)
Ara-C 1000mg/m2/d (1-4)
ATRA 45mg/m2/d (1-15)
DNR: 60mg/m2/d (1)
Ara-C 150mg/m2/8hrs (1-5)
ATRA 45mg/m2/d (days 1-15)
ATRA: 45mg/m
2/d (days 1 to 15) every 3 months +
methotrexate: 15mg/m
2/d, weekly + 6-mercaptopurine: 50mg/m
2/d
DNR: 25mg/m2/d (1,2)ATRA 45mg/m2/d (1-15)
DNR: 25mg/m2/d (1)
ATRA 45mg/m2/d (1-15)
MTZ 10mg/m/d (1-5) ATRA 45mg/m2/d (1-15)
MTZ 10mg/m/d (1-3)
ATRA 45mg/m2/d (1-15)
MTZ 10mg/m/d (1-3)
ATRA 45mg/m2/d (1-15)
Intermediate risk High risk ±
Consolidation
Maintenance (2 years)
Figure1–Induction,consolidationandmaintenanceregimens.
During induction, differentiation syndrome prophylaxis
was administered with dexamethasone 2.5mg/m2q 12h
for two weeks, in patients with a white blood cell count
≥5×109/L.Patientsathigh-riskandoverage60weretreatedas
intermediate-riskpatients.Maintenancewasinitiatedwhen
hematologicalrecoverywasachieved,usuallyonemonthafter
the last consolidation. Support measures during induction
andconsolidationstrictlyadheredtotherecommendations
oftheEuropeanLeukemiaNet.15
ToxicitywasevaluatedaccordingtotheNationalCancer
Institutecommontoxicitycriteriascale(version4.03).16The
onlymodificationtotheoriginalprotocol wasthe
adminis-trationofprophylacticintrathecalchemotherapytopatients
considered tobe athigh-risk; it consistedof methotrexate
(12.5mg)and cytarabine (60mg),every month,sixdosages,
onceallthreesystemicconsolidationshadbeenconcluded.
Modificationswere madein monitoringduringfollow-up –
evaluationofminimalresidualdiseasewasnotperformed.At
thattime,PML/RARAbreakpointswerenotidentifiedinour
center.
Statisticsandethics
This study was approved by our local Ethics Committee,
governed by the principles established in the Declaration
of Helsinki for research in humans. Due to the
retrospec-tivenatureofthis report,aconsentformwasnotrequired;
howeveratourcenter,weroutinelyrequestwritten
autho-rizationforchemotherapyadministrationandthepatientis
informedontherisksandbenefits.Continuousvariablesare
describedasmediansandintervalswhilecategoricalvariables
arepresentedasfrequenciesandproportions.Between-group
differences were establishedwith Mann–Whitney Uor Chi
squaretestsfornumericalandcategoricalvariables,
respec-tively. Survivalcurveswere createdusingtheKaplan–Meier
method.TheSPSSv15.0statisticalpackagewasused.
Results
Twentypatientswere includedbut tworequestedtransfers
APL: n=20
Transfered to other institutions: n=2
Induction with modified IC-APL: n=18
Induction mortality: n=1
Consolidations (3): n=17
Maintenance: n=17
Relapse and death in progresion:
n=1
Lost to follow-up: n=1
Alive in CR: n=15
Figure2–Studydesign.
underwentinductiontherapywiththemodifiedIC-APL
proto-col(Figure2).
Elevenofthe18evaluatedpatients(61.1%)werefemaleand
seven(38.9%)weremale,aratioof1.6:1.Medianageat
diagno-siswas40witharangeof21–74years;notethatthreepatients
(16.6%)were over65 yearsofageatdiagnosis.Ofthetotal
numberofpatients,five(27.8%)wereclassifiedaslow-risk,10
(55.6%)wereintermediateandthree(16.6%)werehigh-risk.
Atdiagnosis,fivepatients(27.8%)fulfilledDICcriteriaand
38.9%fulfilledthose forcoagulopathy.None ofthepatients
hadabnormalliverorkidneyfunctiontestresults.Ofthe18
analyzedpatients,nine(50%)hadoneormorecomorbidities:
four(22%)hadhypothyroidism,two(11%)hadtype2diabetes
mellitus,two(11%)wereobese,two(11%)hadahistoryof
can-cer(onepatienthadhadbreastandbasalcellcarcinomaand
theotherbreastcancer),two(11%)hadcardiovasculardisease
(onehadsystemichypertensionandtheotherhadatrial
fibril-lation),one(5.5%)hadrheumatoid arthritis,one(5.5%)had
chronicobstructivepulmonarydisease(COPD)andone(5.5%)
hadrelapsingAPLandwasadmittedtotheprotocol.Oneof
thepatientshadmultiplecomorbiditiesatthetimeof
diag-nosis:COPD,obesity,pulmonarytuberculosis,pastbreastand
basalcellcarcinoma,post-radiationpulmonaryfibrosis,type
2diabetesmellitusandhypothyroidism;thiswasthepatient
thatdiedduringinduction.
The morphologic characteristics of the bone marrow
aspirate were analyzed in 17 cases: 16 (94.1%) had a
clas-sic morphology and one case (5.9%) was considered a
variant. The diagnosis of AML was initially established
in this patient because the variant promyelocytes were
countedasblasts. Immunophenotypes wereobtainedin16
patients: 100% had a classic immunophenotype: CD34(−),
HLA-DR(−), CD13(+), CD33(+), and myeloperoxidase(+). The
t(15;17)(q22;q21) translocation was found by karyotype or
FISHin17patients(94.4%)andonlyinonecase(5.5%)was
thedemonstrationofthePML/RARArearrangementby
poly-merasechainreactionnecessary.
Ofthe18patientstreatedwiththemodifiedIC-APL2006
regimen,17(94.4%)achievedCRinamedianof42days(range:
34–158 days).Onepatientdied (5.5%)duringinduction, the
aforementionedpatientwithmultiplecomorbidities.Twelve
patients (66.7%) developed grade 3–4 infectious
complica-tionsandtwo(11.1%)developedgrade3–4non-hematological
complications(subarachnoidhemorrhageand
leukocytoclas-ticvasculitis).Therateofseverefebrile neutropeniaduring
inductionwas61.1%(11/18)andwasmorefrequentinpatients
overtheageof65:100%vs.53.3%,althoughthisdifferencewas
notstatisticallysignificant(p-value=0.2).Differentiation
syn-dromewasidentifiedinfourpatients(22.2%).Interestingly,a
groupoffourpatients(22%)didnotdevelopsevere
complica-tionsduringinduction.
Among the 17 patients in hematologic CR, cytogenetic
remissionwascorroboratedbyfluorescenceinsitu
hybridiza-tion(FISH)in15;ofthese,100%wereincompletecytogenetic
remission.Molecularremissionswerenotanalyzed.
Ofthe 17 patientsthatachieved CR,14 (82.3%)received
threeprogrammedconsolidationsandthreepatients(17.6%)
receivedoneconsolidationonemonthlate;inonecaseitwas
due toeconomichardship and intwocases, asaresultof
severe treatment-related complications(severe febrile
neu-tropeniaandlivertoxicityduringthesecondconsolidation).
Therateofsevere febrileneutropeniaduringconsolidation
was29.4%(5/17).Onepatientwaslosttofollow-upafterthe
thirdconsolidationbutwecorroboratedthathewasaliveand
inremissionafterwhichwewereunabletocontacthim.
During follow-up, one bone marrow relapse was
doc-umented (5.9%). The patient was administered rescue
chemotherapy and underwent autologous hematopoietic
stem cell transplantation;however, the disease progressed
and led toherdeath.Ofall the studiedpatients, two died
(11.1%):one,duetopost-chemotherapyaplasiaduring
induc-tion(5.5%)andtheotherasaresultofgraftversushostdisease
(5.5%).Atthetimeofanalysis,thetreatmentprotocolhadbeen
completedby10ofthe16patientsinremission(62.5%)andthe
medianoverallsurvivalhadyettobereached(Figure3).After
afollow-upof29months,OSwas89.1%andonlyonepatient
hadrelapsed(5.8%).
Discussion
Todate,this isthefirstseries ofAPL patientstreatedwith
the IC-APL protocol after its publication in 2013.Although
the cohortissmall,weconfirmedthepreviouslypublished
responseratesandsurvival.
As in the IC-APL and European reports, patients at an
intermediate-risk prevail (55.5% in the current cohort vs.
0.0
0.0 25.0 50.0 75.0
Time in months
100.0 125.0 0.2
0.4 0.6 0.8 1.0
Figure3–Overallsurvival.
(16.7%inthiscohortvs.28.17–32%)andagreaterincidenceof
low-riskpatients(37.8%inthiscohortvs.16–19.1%).13,17
Ourdatacannotformallybecomparedwiththeprevious
reportoftheIC-APLinterms ofsuperiorityorinferiorityof
resultsbecausethisstudywasnotdesignedwiththatgoal.
Moreover,thisstudyhadsomelimitationssuchasthesmall
samplesizeandshortfollow-up.However,wethinkthatsafety
aspectsand responsetotreatmentwere reproduced.Thus,
someinterestingnumbersareworthnoting:theCRratewas
94.5%andthosedescribedbytheoriginalConsortiumwere
85%andEuropeancenters91–94.3%.Intermsofoverall
sur-vival,itisslightlyabovethatreportedbytheconsortium(89.1%
vs. 80% attwo years); both were determined after a short
follow-up when compared with other reports.13,18,19 Death
ratesduringinductionandconsolidationwere5.5%and0%,
respectivelycomparedtoratesof15%and5%reportedbythe
originalConsortium.13Theseratesaresuperiortothose
pre-sented byaBrazilianreportbeforethe IC-APL initiative, in
whichdeathratesduringinductionandconsolidationwere
32%and10%,respectively.11Also,incomparisonwith
devel-opedcountries,themortalityinthecurrentstudyiswithinthe
previouslydescribedrange:mortalityduringinduction5–10%
and<5%duringconsolidation.9,10,17–19
Hemorrhageisthemaincauseofdeathdescribedinthe
literaturefollowedbydeathduetocoagulopathy.13,19 Inthis
study,theratesforbothDIC(27.8%)andcoagulopathy(38.9%)
were low; there were no deathsdirectly related to
hemor-rhages.
TherearepeculiaritiestoclottingabnormalitiesandDICin
patientswithAPL.TheincidenceofDICmaybepossibly
over-estimatedifthediagnosisisestablishedaccordingtotheISTH
criteria,sincethrombocytopeniaand increasesinD-dimers
scoremanypointsinthisclassificationsystemandtheyare
alsocommonfindingsinleukemiapatientswithnoother
clot-tingabnormalities.5,14,20Thepathophysiologyoffibrinolysisin
patientswithAPLisalsodifferenttothatofotherDICcauses,
sothesecriteriashouldnotnecessarilybeappliedtopatients
withAPL.20Webelievethatthevalueofserumfibrinogenis
perhapsthemostrelevantparameterwhendefiningDICand
fortherapeuticdecision-makinginAPLpatients;inanycase,
closedynamicmonitoringofthesepatientsisveryimportant
duringinduction.
To date,nocentral nervous system(CNS) relapses have
beenobserved;however, wecannotreachanysolid
conclu-sionsonprophylactictreatmentofthesepatientssincethe
samplesizewassmall.CNSprophylaxiswithchemotherapy
iscurrentlyrecommendedinpatientsathigh-riskofrelapse.21
TherearemanyreportsonthepresenceoftheFLT3-ITD
mutation in APL patients. The frequency of the mutation
isvariable(12–38%)anditsprognosticvalueisquestionable
sinceit has notaffected survivalcurves in all reports.22–25
Arecentmeta-analysiscorroboratedthatAPLpatientswith
the FLT3-ITDmutationhavesimilarCRrates;however, this
mutationdoesleadtoshortenedOSandDFSduetoagreater
incidenceofrelapse.25TheFLT3-ITDmutationhasalsobeen
correlatedtoCNSrelapse.26However,todatethepresenceof
theFLT3-ITDmutationhasnotbeenincorporatedinto
thera-peuticalgorithmsnorshoulditbeconsideredintherapeutic
decision-makingunlessitisinthecontextofaclinicaltrial.
Althoughthenumberofover65-year-oldpatientsinthis
cohortissmall,theyallareneverthelessaliveandinCRwhich
coincideswiththedatareportedbyothergroupsthathave
describedlongOSintheelderly.27Itisimportanttomention
thattheover65-yearoldsinthisstudyhadsevereneutropenia
and infectiouscomplicationsduringinduction. This
under-scores the importance of supportive care in this group of
patients withAPL and inwhom curativetreatment should
alwaysbethegoal.
PatientswithAPLmustbetreatedinspecializedcenters.
Mortalityrisks,particularlyduringinduction,makethemvery
vulnerable. Althoughno deathsoccurred during
consolida-tion,strictsurveillanceisalsomandatoryduringthisphase.
Effortsshouldfocusonthetimelypreventionandtreatment
ofcomplicationsduringchemotherapyfollowingthe
recom-mendationsofexpertgroups.15
Conclusions
ThisisthefirstcohortofadultpatientswithAPLtreatedin
onecenterthatreproducestheresultsoftheoriginalIC-APL
protocol.TheCRratesandsurvivalcurvesobservedinthis
reportconfirmtheeffectivenessandsafetyofthistreatment
protocolinareal-lifescenario.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
2. HillestadLK.Acutepromyelocyticleukemia.ActaMedScand. 1957;159:189–94.
3. Lo-CocoF,AmmatunaE,MontesinosP,SanzMA.Acute promyelocyticleukemia:recentadvancesindiagnosisand management.SeminOncol.2008;35:401–9.
4. Mejia-ArangureJM,BonillaM,LorenzanaR,Juárez-Oca ˜naS, deReyesG,Pérez-SaldivarML,etal.Incidenceofleukemiasin childrenfromElSalvadorandMexicoCitybetween1996and 2000:population-baseddata.BMCCancer.2005;5:33.
5. Guzmán-UribeP,Rosas-LópezA,Zepeda-LeónJ,Crespo-Solís E.Incidenceofthrombosisinadultswithacuteleukemia:a singlecenterexperienceinMexico.RevInvClin.
2013;65:130–40.
6. HuangME,YeYC,ChenSR,ChaiJR,LuJX,ZhoaL,etal.Useof all-transretinoicacidinthetreatmentofacutepromyelocytic leukemia.Blood.1988;72:567–72.
7. MandelliF,DiverioD,AvvisatiG,LucianoA,BarbuiT, BernasconiC,etal.Molecularremissionin
PML/RAR␣-positiveacutepromyelocyticleukemiaby combinedall-transretinoicacidandidarubicin(AIDA) therapy.GruppoItaliano-MalattieEmatologicheMaligne dell’AdultoandAssociazioneItalianadiEmatologiaed OncologiaPediatricaCooperativeGroups.Blood. 1997;90:1014–21.
8. SanzMA,MartínG,RayónC,EsteveJ,GonzálezM, Díaz-MediavillaJ,etal.AmodifiedAIDAprotocolwith anthracycline-basedconsolidationresultsinhigh antileukemicefficacyandreducedtoxicityinnewly diagnosedPML/RARalpha-positiveacutepromyelocytic leukemia.PETHEMAgroup.Blood.1999;94:3015–21.
9. SanzMA,MartínG,GonzálezM,Díaz-MediavillaJ,BoluferP, BarragánE,etal.Risk-adaptedtreatmentofacute
promyelocyticleukemiawithall-trans-retinoicacidand anthracyclinemonochemotherapy:amulticenterstudyby thePETHEMAgroup.Blood.1999;103:1237–43.
10.Lo-CocoF,AvvisatiG,VignettiM,BrecciaM,GalloE,Rambaldi A,etal.Front-linetreatmentofacutepromyelocyticleukemia withAIDAinductionfollowedbyrisk-adaptedconsolidation foradultsyoungerthan61years:resultsoftheAIDA-2000 trialoftheGIMEMAGroup.Blood.2010;116:3171–9.
11.JacomoRH,MeloRA,SoutoFR,deMattosER,deOliveiraCT, FagundesEM,etal.Clinicalfeaturesandoutcomesof134 Brazilianswithacutepromyelocyticleukemiawhoreceived ATRAandanthracyclines.Haematologica.2007;92:1431–2.
12.SanzMA,TallmanMS,Lo-CocoF.Practicepoints,consensus, andcontroversialissuesinthemanagementofpatientswith newlydiagnosedacutepromyelocyticleukemia.Oncologist. 2005;10:806–14.
13.RegoEM,KimHT,Ruiz-ArgüellesGJ,UndurragaMS,Uriarte MdelR,JacomoRH,etal.Improvingacutepromyelocytic leukemia(APL)outcomeindevelopingonAPLcountries throughnetworking,resultsoftheInternationalConsortium. Blood.2013;121:1935–43.
14.LeviM,TohCH,ThachilJ,WatsonHG.Guidelinesforthe diagnosisandmanagementofdisseminatedintravascular coagulation.BritishCommitteeforStandardsin
Haematology.BrJHaematol.2009;145:24–33.
15.SanzMA,GirmwadeD,TallmanMD,LowembergB,FenauxP, EsteyEH,etal.Managementofacutepromyelocyticleukemia: recommendationsfromanexpertpanelformbehalfofthe EuropenaLeukemiaNet.Blood.2009;113:1875–91.
16.CommonTerminologyCriteriaforAdverseEventsv4.03
(CTCAE).Bethesda,MD:NationalCancerInstitute;2010,June
[cited03.06.15].Availablefrom:http://evs.nci.nih.gov/ftp1/
CTCAE/CTCAE4.032010-06-14QuickReference8.5x11.pdf
17.DelaSernaJ,MontesinosP,VellengaE,RayónC,ParodyR, LeónA,etal.Causesandprognosticfactorsofremission inductionfailureinpatientswithacutepromyelocytic leukemiatreatedwithall-transretinoicacidandidarubicin. Blood.2008;111:3395–402.
18.AvisatiG,Lo-CocoF,PaoloniFP,PettiMC,DiverioD,Vignetti M,etal.AIDA0493protocolfornewlydiagnosedacute promyelocyticleukemia:verylong-termresultsandroleof maintenance.Blood.2011;117:4716–25.
19.AdesL,GuerciA,RaffouxE,SanzM,ChevallierP,LapusanS, etal.Verylong-termoutcomeofacutepromyelocytic leukemiaaftertreatmentwithall-transretinoicacidand chemotherapy:theEuropeanAPLGroupexperience.Blood. 2010;115:1690–6.
20.KwaanHC,KullEH.Thecoagulopathyinacutepromyelocytic leukaemia–whathavewelearnedinthepasttwentyyears. BestPractResClinHaematol.2014;27:11–8.
21.DeBottonS,SanzMA,ChevretS,DombretH,MartinG, ThomasX,etal.Extramedullaryrelapseinacute
promyelocyticleukemiatreatedwithall-transretinoicacid andchemotherapy.Leukemia.2006;20:35–41.
22.Lucena-AraujoAR,KimHT,JacomoRH,MeloRA,Bittencourt R,PasquiniR,etal.InternaltandemduplicationoftheFLT3 geneconferspooroverallsurvivalinpatientswithacute promyelocyticleukemiatreatedwithall-transretinoicacid andanthracycline-basedchemotherapy:anInternational ConsortiumonAcutePromyelocyticLeukemiastudy.Ann Hematol.2014;93:2001–10.
23.BarragánE,MontesinosP,CamosM,GonzálezM,CalasanzMJ, Román-GómezJ,etal.PrognosticvalueofFLT3mutationsin patientswithacutepromyelocyticleukemiatreatedwith all-transretinoicacidandanthracyclinemonochemotherapy. Haematologica.2011;96:1470–7.
24.SouzaMeloCP,CamposCB,DutraÁP,NetoJC,FenelonAJ, NetoAH,etal.CorrelationbetweenFLT3-ITDstatusand clinical,cellularandmolecularprofilesinpromyelocytic acuteleukemias.LeukRes.2015;39:131–7.
25.BeitinjanehA,JangS,RoukozH,MajhailNS.Prognostic significanceofFLT3internaltandemduplicationandtyrosine kinasedomainmutationsinacutepromyelocyticleukemia:a systematicreview.LeukRes.2010;34:831–6.
26.GillH,IpHW,PangAW,SumJ,LeungAY,KwongYL.FLT3 internaltandemduplicationinacutepromyelocytic leukemia:centralnervoussystemrelapse.AnnHematol. 2015;94:1049–51.