Contents lists available atScienceDirect
Industrial Crops and Products
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / i n d c r o p
Application of cellulose-immobilized riboflavin as a redox mediator
for anaerobic degradation of a model azo dye Remazol Golden Yellow
RNL
Luide Rodrigo Martins
a, Bruno Eduardo Lobo Baêta
b, Leandro Vinícius Alves Gurgel
b,
Sérgio Francisco de Aquino
b, Laurent Frédéric Gil
b,∗aEnvironmental Engineering Post-Graduate Program, Federal University of Ouro Preto, Ouro Preto, MG, Brazil
bDepartment of Chemistry, Institute of Biological and Exact Sciences, Federal University of Ouro Preto, Ouro Preto, MG, Brazil
a r t i c l e
i n f o
Article history:
Received 29 July 2014
Received in revised form 17 October 2014 Accepted 27 October 2014
Available online 13 November 2014
Keywords:
Riboflavin
Succinylated cellulose Immobilized redox mediator Remazol Golden Yellow RNL Anaerobic treatment
a b s t r a c t
This study described the anaerobic degradation of the azo dye Remazol Golden Yellow RNL (RGY-RNL) using cellulose-immobilized riboflavin (MC 3) as the redox mediator. This new solid support containing immobilized riboflavin was synthesized from succinylated mercerized cellulose, and was characterized by elemental analysis, FTIR, and solid-state13C NMR. MC 3 was resistant to pH 2–9, and anaerobic degra-dation of RGY-RNL using MC 3 in the presence of anaerobic sludge yielded a zero order degradegra-dation rate constant (k0,obs) equal to 0.189 mg/L h, which was 56% better than experiments carried out without a redox mediator. Color removal efficiency after 48 h of degradation averaged 89.4% in experiments with MC 3 and 72% without the addition of a redox mediator. These results showed that MC 3 can be used to immobilize redox mediators, allowing reduction of wastewater treatment costs.
© 2014 Elsevier B.V. All rights reserved.
1. Introduction
Highly-colored effluents generated by different types of industries, especially the textile sector, are a matter of great
envi-ronmental interest (Field and Brady, 2003; Méndez-Paz et al.,
2005). The textile industry employs several steps to give the
fab-rics the properties and characteristics desired in the final product, of which dyeing is one of the most important, and it is estimated that 10–15% of dyes used in the dyeing process do not adhere to the
fibers and end up in the textile effluent (Corrêa et al., 2009). Apart
from their potential toxicity, dyes and pigments are recalcitrant (resistant to degradation) and may remain in the environment for long periods, accumulating in water and soil if not suitably treated
before being discharged (dos Santos et al., 2007).
Most dyes used for textile industries (60–70%) are aromatic azo
compounds, which bear the functional group R N N R′, in which
R and R′are usually substituted aromatic compounds (van der Zee
et al., 2003). Azo dyes are removed by a non-specific reduction mechanism under anaerobic conditions, but the low rate of degra-dation is the primary issue when adopting anaerobic reactors to
∗Corresponding author. Tel.: +55 31 3559 1717; fax: +55 31 3551 1707. E-mail address:laurent@iceb.ufop.br(L.F. Gil).
remove these compounds from industrial wastewater (van der Zee
et al., 2001a).
Studies have indicated the main limitation for azo dye reduction under anaerobic conditions is transferring the reducing equivalents generated by the cells during the metabolic process to the azo dye (Field and Brady, 2003). Redox mediators (RM) such as riboflavin and quinone groups, can improve this electron transfer, and stud-ies have been performed to find cheap sources of such catalysts
or for immobilizing them onto solid supports (Alvarez et al., 2010;
Cervantes et al., 2011; dos Santos et al., 2004; Field and Brady, 2003; Martínez et al., 2013). Riboflavin is soluble in water and normally lost in the treated wastewater, while immobilization onto an insol-uble solid support would reduce costs by allowing the recovery and reuse of riboflavin.
Cellulose is the most abundant renewable biopolymer in nature.
This homopolymer of-d-glucopiranose units can undergo
chemi-cal modifications through the reaction of its primary and secondary hydroxyl functional groups, and such chemical modifications have yielded new materials with specific physicochemical properties (Alvarez et al., 2010).Gurgel et al. (2008)andGurgel and Gil (2009) prepared modified cellulose containing internal carboxylic acid anhydride functional groups through the reaction of succinylated cellulose (containing carboxylic acid groups) with acetic anhy-dride. Such functional groups are excellent electrophiles that can react with nucleophilic compounds containing amine or hydroxyl
http://dx.doi.org/10.1016/j.indcrop.2014.10.059
functional groups, resulting in the formation of amide or ester linkages on the modified cellulose.
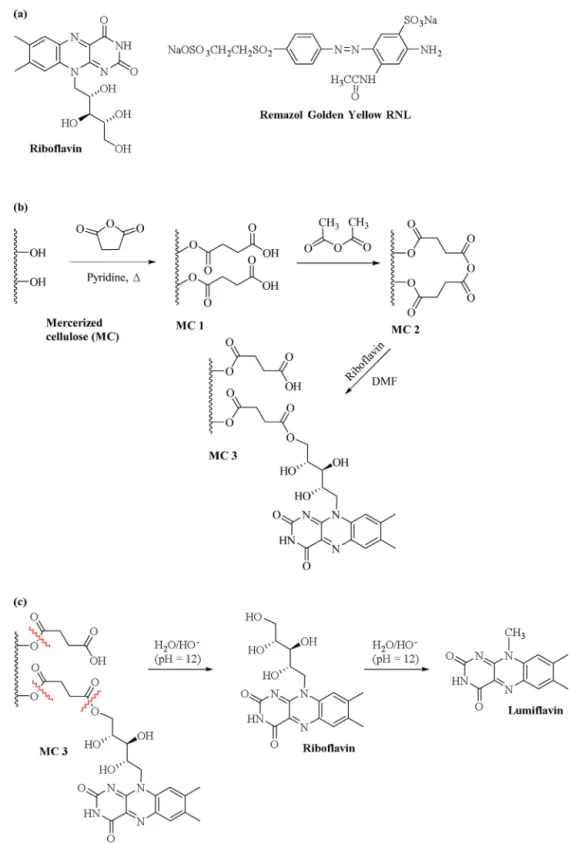
In this study, succinylated mercerized cellulose was reacted with acetic anhydride to yield internal carboxylic acid anhydride functional groups used to incorporate riboflavin (Rib) onto the solid support through the formation of ester linkages. The efficiency of this new solid support as a redox mediator was evaluated during anaerobic degradation of the model azo dye Remazol Golden Yellow RNL (RGY-RNL).
2. Materials and methods
2.1. Materials
Microcrystalline cellulose and sulfanilic acid (≥99.0%) were
purchased from Sigma-Aldrich (cat. No. 31,069-7 and S5263,
respectively, Brazil). Riboflavin (Fig. 1a), succinic anhydride,
acetic anhydride, acetic acid, pyridine, sodium hydroxide, ethanol, acetone, and methylene chloride were purchased
from Vetec (Brazil). Hydrochloric acid, diethyl ether, N,N′ -dimethylformamide (DMF), and dimethylsulfoxide (DMSO) were purchased from Synth (Brazil). Remazol Golden Yellow RNL
(RGY-RNL, C16H18N4O10S3Na2,Fig. 1a) azo dye was kindly provided by a
local textile industry and used without further purification. Yeast
extract containing 50g/g of riboflavin was purchased from
Hime-dia.
2.2. Apparatus and operational conditions
Flat-bottom amber glass bottles (250 mL) with polypropylene caps were used as reactors and inoculated with anaerobic sludge from a small-scale UASB reactor at the Center of Research and Train-ing on Sanitation (CePTS) UFMG/COPASA, located at the Arrudas Wastewater Treatment Plant (WWTP) in Belo Horizonte, Brazil. The nutrient solution contained glucose (except in the experiments 5, 10, and 11), RGY-RNL (except in the experiments 1–3), yeast extract (except in the experiments 1–4 and 6–11), riboflavin (except in the experiments 1, 2, 4, and 8–11) and a macronutrient solution
(com-position is described in SupplementaryTable 1). Redox mediators
tested were MC 3 (cellulose-immobilized riboflavin), riboflavin and
yeast extract (containing∼50g riboflavin per g of yeast extract).
The efficiency of color removal following Remazol Golden Yel-low RNL degradation was assessed by measuring the absorbance
(max= 410 nm) of the centrifuged suspension with a UV–vis
spec-trophotometer (model HP 8453).
2.2.1. Methods
The experiments (except the modification of microcrystalline cellulose) were performed in triplicate. Pyridine was refluxed with
NaOH pellets overnight and distilled. N,N′-Dimethylformamide
(DMF) was treated with 4 ˚A molecular sieves (Merck) overnight and distilled under reduced pressure. Succinic anhydride, acetic anhy-dride, and dimethyl sulfoxide (DMSO) were used without further purification.
2.2.2. Chemical modification of microcrystalline cellulose
2.2.2.1. Mercerization. Microcrystalline cellulose (15 g) was added
to a polyethylene beaker containing 400 mL of 20% (w/v) NaOH and
the suspension stirred for 18 h at 25◦C. The suspension was filtered
(sintered disk filter funnel, porosity 3) and the retentate washed with distilled water until pH 7 was reached in the wash. The mercer-ized microcrystalline cellulose (MC) was washed with 95% ethanol
and dried at 85◦C for 90 min.
2.2.2.2. Preparation of MC 1. MC (11.66 g) and succinic anhydride (34.8 g) were placed in a round-bottom flask, anhydrous pyridine
(233 mL) added and the suspension heated at 120◦C with
con-stant stirring for 6 h. The succinylated mercerized cellulose (MC 1) was separated by filtration (sintered glass funnel, porosity 3) and washed successively with a solution of 1 mol/L acetic acid in methy-lene chloride, 95% ethanol, distilled water, 0.01 mol/L HCl, distilled
water, and acetone. The MC 1 was dried at 80◦C for 1 h.
2.2.2.3. Preparation of MC 2. MC 1 (9.4 g) and acetic anhydride
(265 mL) were added to a round-bottom flask and heated at 100◦C
for 24 h with constant stirring. The suspension was separated by filtration (sintered glass funnel, porosity 3), washed with diethyl ether (previously treated with a 4 ˚A molecular sieve) and dried at
100◦C for 20 min.
2.2.2.4. Preparation of MC 3. MC 2 (produced from 9.4 g of MC 1) and riboflavin (2.35 g) were placed in a round-bottom flask,
anhy-drous DMF (140 mL) added and the suspension heated at 75◦C for
24 h under constant magnetic stirring. MC 3 was separated by filtra-tion (sintered glass funnel, porosity 3), washed with DMF, DMSO, an excess of distilled water, 95% ethanol, and acetone, and dried at
80◦C for 30 min. The water wash was monitored at 267 nm using an
UV–vis spectrophotometer, which corresponded to the maximum absorption of riboflavin in aqueous medium. The synthetic route
used to prepare MC 3 is shown inFig. 1.
2.2.3. Characterization of the materials
The synthetized materials (previously dried in an oven at 90◦C)
were characterized by weight gain, elemental analysis, FTIR and
13C NMR. For FTIR characterization, 1 mg of the material was mixed
with 100 mg of KBr (spectroscopy grade) and spectra recorded from
400 to 4000 cm−1with 32 scans at a resolution of 4 cm−1. Elemental
analysis was performed on a CHNS/O Perkin Elmer Series II, model
2400 analyzer.13C NMR (solid state) of MC 3 was recorded on a
Bruker Avance III-400 spectrometer at room temperature and mea-surements obtained at frequencies of 100 MHz with magic angle spinning of 5 kHz using the CPTOSS technique.
2.2.4. Evaluation of chemical stability of MC 3 as a function of pH
As ester functional groups (used to incorporate riboflavin onto MC 3) can be hydrolyzed at pH values below 2 and greater than 9, the chemical stability of MC 3 was assessed in aqueous solu-tions as a function of pH. For this, 20.0 mg porsolu-tions of MC 3 were added to 250 mL Erlenmeyer flasks, 100.0 mL of HCl or NaOH aqueous solutions at pH 2, 9, 12, or 14 were added to
individ-ual flasks and stirred (100 rpm) for 24 h at 25◦C. The suspensions
were separated by filtration (sintered glass funnel, porosity 3) and thoroughly washed with distilled water. The absorbance of the
Table 1
Experimental conditions used to evaluate the stability of MC 3, the adsorption of RGY-RNL on anaerobic sludge, the influence of the nutritional medium and the use of different redox mediators on color removal.
Experiment MC 3 concentration
(mg/L) Riboflavinconcentration (mg/L) Yeast extract(mg/L) Glucose concentration(mg/L) Biomass concentration(mg/L) Nutrient solution(mL)
1 (Control)a – – – 250 650 10
2a 50 – – 250 650 10
3a – 3.56 – 250 650 10
4 (Control)b – – – 250 650 10
5b 0.245 – – 250 – 10
6b 0.245 – – – – –
7a,b 0.245 – – – 650c 10
8 (Control)b – – – 250 650 10
9b – – 350 – 650 10
10b 0.245 – – 250 650 10
11b – 0.0175 – 250 650 10
aConcentration of biomass (expressed as volatile suspended solids—VSS) in the stock anaerobic sludge = 12.66 g/L. b Remazol Golden Yellow RNL concentration was 50 mg/L in all reaction flasks.
washes was measured on a UV–vis spectrophotometer (model HP 8453) at 267 nm and 354 nm, which corresponded to the maximum absorbance wavelengths of riboflavin in acidic and basic aqueous media, respectively.
2.2.5. Determination of riboflavin concentration on MC 3
Riboflavin in aqueous solutions pH 9 and above, is
predomi-nantly in the form of lumiflavin (Fig. 1c) (Penzkofer, 2012). The
amount of riboflavin incorporated on MC 3 was assessed by hydrolyzing the ester bond used to attach riboflavin onto the suc-cinylated mercerized cellulose at basic pH. MC 3 (31.0 mg) was placed in a 250 mL Erlenmeyer flask, 50.0 mL of a 0.1 mol/L NaOH
solution (pH 12) added and shaken (100 rpm) for 24 h at 25◦C.
The suspension was centrifuged and the absorbance of the yel-lowish supernatant was measured on a UV–vis spectrophotometer
at 354 nm, the maximum absorption wavelength (max) for
lumi-flavin at pH 12, and the concentration of lumilumi-flavin estimated by comparison to a calibration curve.
2.2.6. Evaluation of the stability of MC 3 in the reaction flaks
To verify the chemical stability of MC 3 under the experimental conditions used, experiments were carried out in the presence of all components of the reaction medium (with exception of
RGY-RNL), andTable 1shows the experimental conditions used. After the
incubation period (24 or 48 h), samples were removed, centrifuged and the absorbance measured on a UV–vis spectrophotometer at
267 and 354 nm, the maximum absorption wavelengths (max) for
riboflavin in acidic and basic aqueous media, respectively.
2.2.7. Influence of the reaction medium on the degradation of RGY-RNL dye
Experiments were performed to determine whether color removal occurred by adsorption of RGY-RNL onto the anaerobic
sludge by inoculating flasks with autoclaved (121◦C for 20 min)
anaerobic sludge. The influence of reaction medium components (e.g. nutrient solution) and whether their adsorption onto MC 3 affected color removal efficiency were also evaluated. Experimental
conditions are described inTable 1.
2.2.8. Anaerobic degradation of Remazol Golden Yellow RNL (RGY-RNL)
Experiments were performed to (1) evaluate the influence of MC 3 on anaerobic degradation of Remazol Golden Yellow RNL, (2) compare the performance of MC 3 in relation to well-known redox mediators (riboflavin and yeast extract), and (3) investigate the contribution of RGY-RNL adsorption onto MC 3 and/or anaerobic sludge. All experiments were carried out in triplicate and the degra-dation of Remazol Golden Yellow RNL was monitored by measuring the absorbance of the supernatants at 410 nm after centrifugation at 3600 rpm for 20 min.
Batch experiments were performed in inoculated reaction flasks with a total volume of 200 mL (nutritional conditions shown in
Supplementary Table 1, Chernicharo (2007)) and experimental
conditions set so the COD:N:P ratio was∼350:5:1 (concentration
of macro- and micronutrients in the solution according toAquino
et al., 2007). The concentration of anaerobic biomass (collected from a small-scale UASB reactor fed with sewage) was estimated by analysis of volatile suspended solids (VSS) according to APHA (Clesceri et al., 1998). The initial concentration of biomass, RGY-RNL and yeast extract in the reaction flasks were fixed at 650 mg/L, 50 mg/L, and 350 mg/L, respectively. Concentrations of glucose, RGY-RNL, yeast extract, nutrient solution, and microorganisms in the reaction flasks were determined from optimization studies realized in our research group. In experiments performed with riboflavin or MC 3 as the redox mediators, the amount of riboflavin was proportional to that contained in reaction flasks in which yeast
extract was used as the redox mediator. Control flasks were inocu-lated with active biomass without the redox mediator (RM).
All reaction flasks were purged with nitrogen (White Martins,
99.999% purity), sealed, incubated at 25◦C with mechanic stirring
(150 rpm) and monitored for 48 h by sampling (sampling frequency varied with the type of experiment) 3 mL of the content by using
plastic syringes.Table 1shows the conditions used in batch
exper-iments for the anaerobic degradation of Remazol Golden Yellow RNL assisted by redox mediators (riboflavin, yeast extract, and MC 3).
2.2.9. Aromatic amines analysis
Aromatic amines generated in the anaerobic degradation of Remazol Yellow Gold RNL were analyzed by high performance liquid chromatography (HPLC) using Shimadzu chromatography equipped with a diode array UV–vis detector set at 191 nm and
an ion exchange column Aminex HPX-87H (300×7.8 mm,
Bio-Rad) at 55◦C with 0.01 mol/L H
2SO4 as eluent and a flow rate of
0.6 mL/min according to Baêta et al. (2013). Samples were
cen-trifuged before injection (30L). The separated aromatic amines
generated as byproducts of sulfonated azo dye degradation (e.g. sulfanilic acid derivatives) can be detected at 191 nm according to Pinheiro et al. (2004).
3. Results and discussion
3.1. Synthesis and characterization of solid supports
3.1.1. Synthesis and characterization of MC 1
Microcrystalline cellulose was mercerized to convert cellulose I into cellulose II. This rearrangement of crystal packing increases the separation of chains, providing easier access to the hydroxyl groups of cellulose, reducing packing efficiency, facilitating penetration of succinic anhydride, promoting a higher degree of substitution and more uniform succinylation due to a greater number of available
hydroxyl groups (Gurgel et al., 2008). Mercerized microcrystalline
cellulose was reacted with succinic anhydride using pyridine as the solvent and catalyst, which yielded succinylated mercerized
cellulose (MC 1) (Gurgel et al., 2008). The weight gain percentage
after succinylation was calculated as follows:
wgp (%)=
mf−mi mi×100 (1)
wheremf(g) is the weight of modified mercerized cellulose andmi
is the weight of mercerized cellulose.
The weight gain percentage (wgp) obtained after succinylation was 105.2%, due to the incorporation of succinyl functional groups through the formation of ester linkages with the primary and sec-ondary hydroxyl groups of cellulose, which released carboxylic acid
groups (Fig. 1b). The number of carboxylic groups (nCOOH) released
after succinylation reaction was estimated at 7.1 mmol/g by
back-titration (Karnitz et al., 2007).
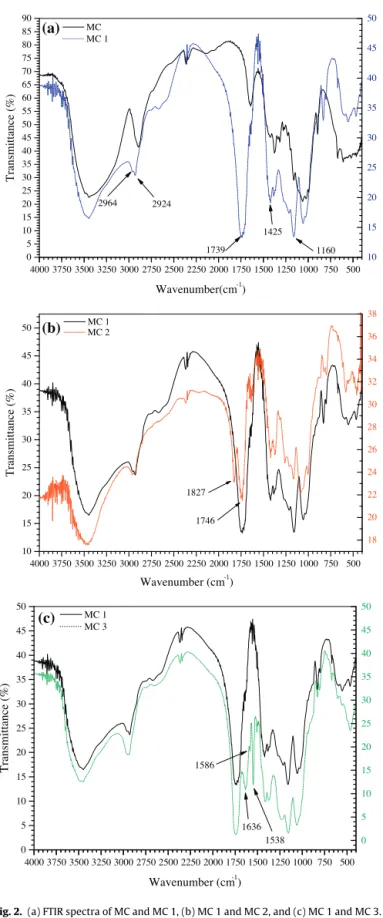
MC 1 was characterized by FTIR, and the spectra of MC and
MC 1 are shown inFig. 2a. When comparing the FTIR spectrum
of MC 1 with the spectrum of unmodified mercerized cellulose
(MC), the appearance of bands at 2964 and 2924 cm−1 can be
attributed to asymmetric and symmetric stretching of methylene
groups ( CH2 ), while the bands at 1425 and 1160 cm−1 were
attributed to deformation vibration of hydroxyl groups and the stretching of C O groups from the dimer in carboxylic acid. A strong
band at 1739 cm−1 was attributed to asymmetric and symmetric
stretching of the ester bond carbonyl group ( O C O) introduced
by succinylation (Gurgel et al., 2008). These bands confirmed the
750 1250 1750 2250 2750 3250 3750 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 1160 1425 1739 2964 2924 Transmittance (%)
Wavenumber(cm-1)
MC MC 1 500 1000 1500 2000 2500 3000 3500 4000 10 15 20 25 30 35 40 45 50 500 750 1000 1250 1500 1750 2000 2250 2500 2750 3000 3250 3500 3750 4000 10 15 20 25 30 35 40 45
50 MC 1
1746 1827
Transmittance (%)
Wavenumber (cm-1)
18 20 22 24 26 28 30 32 34 36 38 MC 2 0 5 10 15 20 25 30 35 40 45 50
(c)
1586 1538 1636 Transmittance (%)Wavenumber (cm-1)
MC 1 MC 3 500 750 1000 1250 1500 1750 2000 2250 2500 2750 3000 3250 3500 3750 4000 0 5 10 15 20 25 30 35 40 45 50
(a)
(b)
Fig. 2.(a) FTIR spectra of MC and MC 1, (b) MC 1 and MC 2, and (c) MC 1 and MC 3.
3.1.2. Synthesis and characterization of MC 2
Succinylated mercerized cellulose (MC 1) was reacted with acetic anhydride (solvent and dehydrating agent) to promote the formation of internal anhydride functional groups from carboxylic acid groups, yielding MC 2. These internal anhydride groups were excellent electrophiles capable of reacting with nucleophilic groups such as the primary hydroxyl groups of riboflavin, which were
bet-ter nucleophiles than secondary hydroxyl groups (Fig. 1b). In this
study, the nucleophile was the primary hydroxyl group riboflavin and the reaction of riboflavin with MC 2 allowed incorporation of riboflavin onto the solid support through formation of an ester bond.
The synthesis of MC 2 was confirmed through FTIR analysis and
the spectra of MC 2 and MC 1 are shown inFig. 2b. When comparing
the spectrum of MC 2 to the spectrum of MC 1, the appearance of
bands at 1827 and 1746 cm−1were attributed to the formation of
carboxylic acid anhydride functional groups. In general, these two
bands were separated by a maximum wavelength range of 81 cm−1
which is characteristic of the presence of a functional anhydride
group (Nakanishi and Solomon, 1977). The changes seen in the
spectrum of MC 2 (with respect to MC 1) confirmed the formation of internal anhydride functional groups on the solid support.
3.1.3. Synthesis and characterization of MC 3
MC 3 was obtained by the reaction of MC 2 with riboflavin using DMF as the solvent. The concentration of riboflavin chem-ically bonded to MC 3 was assessed by hydrolyzing the ester
bond in an aqueous solution at pH 12 (Fig. 1c) and quantifying
lumiflavin (a riboflavin byproduct at pH 12,Fig. 1c). Analysis
indi-cated a lumiflavin concentration in the supernatant of 44.19 mg/L, and considering all lumiflavin in the supernatant was released by hydrolysis of MC 3, riboflavin content was 71.3 mg/g.
MC 3 was characterized by infrared spectroscopy with FTIR,13C
NMR and elemental analysis. The FTIR spectra of MC 1 and MC 3
are shown inFig. 2c, where the three bands at 1548; 1586 and
1636 cm−1were due to the incorporation of riboflavin.Abe et al.
(1986)studied the infrared spectra of riboflavin and its
deriva-tives, indicating the bands at 1552, 1580 and 1583 cm−1could be
attributed to stretching vibrations of C24 N25and C19 N20bonds in
the isoalloxazin unit rings (seeFig. 3), while the band at 1636 cm−1
was due to vibrations in the frequency of the isoalloxazin ring
car-bonyl group (C23 O).
The elemental analysis for MC 1 and MC 3 revealed carbon, hydrogen, and nitrogen contents of 43.71 and 35.04%, 5.20 and 3.99%, and 0.27 and 2.25%, respectively. These results showed an increase in nitrogen content after modification of MC 1 with riboflavin to produce MC 3, which confirmed the incorporation of
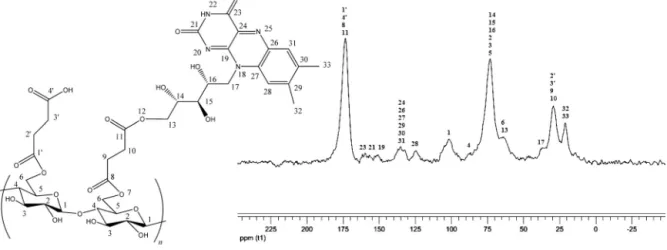
riboflavin into cellulosic matrix. The solid-state13C NMR (CP/MAS)
spectrum of MC 3 is shown inFig. 3. The13C NMR spectra of MC
3 exhibited chemical shifts attributed to the presence of cellulose
and riboflavin in the chemical structure of this material (Keller et al.,
1983; Melo et al., 2011). The signals at 105 and 90 ppm were related to the involvement of the C-1 and C-4 carbons (respectively) in the
acetal bond of the(1→4)-d-glucopiranose units of cellulose. The
signal at 75 ppm was attributed to secondary carbons bonded to the hydroxyl groups of riboflavin (C-14, C-15, and C-16), cellulose (C-2 and C-3) and to the tertiary carbon of the cellulose structure (C-5). As a consequence of succinylation, the modified cellulose contained ester bonds formed after esterification of the primary hydroxyl groups in the glucopiranose units of cellulose. Thus, the signal at 66 ppm was related to the C-6 of cellulose, whereas the
sig-nal at 175 ppm was attributed to carbonyl groups at C-1′, C-4′, C-8,
and C-11. Additionally, the signal at 66 ppm indicated the C-13 from riboflavin, which was involved in the ester bond linking riboflavin to the succinylated cellulose through the primary hydroxyl groups of riboflavin at C-13. The carbons in the riboflavin aromatic rings appeared as broad signals from 110 to 150 ppm, while the chemical shifts at 125, 137, 155, and 160 ppm were related to the presence of aromatic rings in the chemical structure of MC 3, which indicated the chemical modification of MC 2 to bind riboflavin. These data and
published studies evaluating succinylated cellulose (Gurgel and Gil,
Fig. 3.Solid-state13C CP/MAS NMR spectrum of MC 3.
3.2. Evaluation of the stability of MC 3 as a function of pH
MC 3 was treated with an aqueous HCl solution (pH = 2) for 24 h, and no signal was seen at 267 nm for the washes, which confirmed the ester bond used to attach riboflavin onto MC 3 was not cleaved
at pH 2 (MC 3 was stable at pH≥2). Treatment of MC 3 with an
aqueous NaOH solution (pH = 9) for 24 h also showed no signal at 354 nm in the washes. However, at pH 11 and 12, the ester bond was hydrolyzed, which could be verified by the appearance of peaks at 354 nm in the washings. These observations indicated the ester bond used to attach riboflavin onto MC 3 was stable at pH 2–9.
3.3. Influence of the reaction medium on the stability of MC 3
Experiments were carried out to evaluate the influence of the reaction medium on MC 3 stability, and were performed in the presence of all components of the reaction medium with exception of RGY-RNL. No riboflavin was released from MC 3 (by hydrolysis
of ester bond) under the experimental conditions used (Table 2).
If a release had occurred, there would have been an increase in absorbance over time for the supernatants of experiment 2 (MC 3 was added). The absorbance of supernatants from experiment 3 was twice as high as those observed in experiment 2, which indi-cated that if riboflavin were released, it would have been detected.
3.4. Influence of the reaction medium on degradation of RGY-RNL dye
These experiments were carried out to evaluate RGY-RNL removal by adsorption onto anaerobic sludge and/or reaction with medium components. Adsorption of the RGY-RNL dye onto anaer-obic sludge (experiment 7), MC 3 (experiment 6) or medium components (experiment 5) did not result in significant decoloriza-tion. Reasonable dye (color) removal only occurred in experiment
Table 2
Influence of reaction medium on the stability of MC 3.
Experimenta Absorbance (267 nm)
t= 0 h t= 24 h t= 48 h
1b 0.224 0.217 0.215
2c 0.252 0.216 0.238
3d 0.504 0.470 0.492
aAll experiments were carried out with anaerobic sludge and without RGY-RNL. bControl flasks, incubated without MC 3.
c Experiments carried out with MC 3.
d Experiments carried out with riboflavin instead of MC 3.
4 due to RGY-RNL reduction by anaerobic microorganisms in the absence of a redox mediator.
3.5. Anaerobic degradation of RGY-RNL dye using MC 3 as the redox mediator
In addition to the experiments evaluating anaerobic degrada-tion of RGY-RNL dye using MC 3 as redox mediator (experiment 10), experiments using redox mediators such as soluble riboflavin (experiment 11) and yeast extract (experiment 9) were performed. The effect of each redox mediator on the decolorization of RGY-RNL
in an anaerobic system is shown inFig. 4.
According todos Santos (2005), color removal of azo dyes in
anaerobic systems without the addition of redox mediators varies
from 60 to 80%.Corrêa et al. (2009)reported that color removal
efficiencies of the azo dye blue Drimarem HF-RL in anaerobic sys-tems varies 39–45% within the first 24 h, with a final degradation
efficiency of 91% after 150 h.van der Zee et al. (2001a)reported a
30% color removal efficiency in a bench-scale UASB reactor kept at
30◦C without redox mediators and fed with the azo dye reactive
red (RR2), while a 95% color removal efficiency was seen for
differ-ent azo dyes in anaerobic systems in less than 6 days (van der Zee
et al., 2001b).
In this study, removal efficiency of RGY-RNL by anaerobic biomass in the absence of RM was 30.7% within the first 24 h and
50 45 40 35 30 25 20 15 10 5 0 0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0 1.1
Riboflavin Control Yeast extract MC 3
A
f/A
i
Time (h)
72.1% after 48 h in batch mode at 25◦C, which were in agreement
with results reported bydos Santos (2005). It should be noted that
the maximum removal efficiency in this study was seen after 48 h, while over 144 h of degradation time was required in the study of Corrêa et al. (2009).
The use of redox mediators such as anthraquinone sulfonate (AQS), disulfonated anthraquinone (AQDS), yeast extract, and riboflavin (among others) have led to significant improvements in
azo dye removal kinetics under anaerobic conditions (Baêta et al.,
2012; Corrêa et al., 2009; Costa et al., 2010; Field and Brady, 2003; dos Santos, 2005).Costa et al. (2010)reported a 2.27-fold increase in the degradation kinetic rate constant for reactive red 2 (RR2)
azo dye in anaerobic reactors fed with AQDS.van der Zee et al.
(2001a)demonstrated an increase in color removal from 30% to 88% by adding AQDS as the catalyst in an anaerobic system used for the
degradation of RR2 dye.dos Santos (2005)found a 2.9-fold increase
in the RR2 color removal rate in reaction flasks that received AQS when compared to flasks without. When yeast extract was used as the redox mediator (riboflavin) in the degradation of blue azo
dye Drimaren HF-RL,Corrêa et al. (2009)andBaêta et al. (2012)
report color removal efficiencies up to 87% within the first 24 h of degradation, and a final removal efficiency higher than 90% after
150 h.Field and Brady (2003)found the degradation rate of Yellow
mordant 10 dye increases 61% with the addition of riboflavin in anaerobic systems operating in batch mode.
RGY-RNL color removal efficiency increased from 30.7 to 50.1% within the first 24 h after addition of yeast extract (source of the redox mediator riboflavin), which increased to 82.6% after 48 h. When riboflavin was added, degradation of RGY-RNL dye was 26.7% within the first 24 h, which increased to 84.3% after 48 h. These
results were similar to those reported byCorrêa et al. (2009)for
the same times of dye degradation. Possible explanations for the differences between the results of this study and those reported in literature may due to factors such as the chemical structure of the dyes used in the experiments, the ratio of dye to redox mediator (can cause differences in the transfer rate of electrons between RM and dye) and even the electron donors used.
The degradation rate constant for RGY-RNL dye using MC3 was improved by 1.56-fold when compared with experiments without addition of redox mediators and followed the zero-order kinetic model. This result is similar to those reported in the literature. Several authors evaluated the use of immobilized redox media-tors to improve the kinetics of azo dye decolorization in anaerobic treatment systems. Three factors are important to compare var-ious reducing systems using immobilized redox mediators such as type of solid support, redox mediator for immobilization, and
the structure of dyes.Cervantes et al. (2010) has improved the
dye removal rate constant of methyl orange by 8.8-fold using NQS immobilized onto ion exchange R1 type resin and by 1.9-fold using NQS immobilized onto ion exchange R2 type resin. AQDS
immobi-lized to nanoparticles of Al(OH)3also improved the dye degradation
rate constant for reactive red 2 (RR2) by 7.5-fold, while AQDS immobilized onto ion exchange resin improved the dye
degra-dation constant 1.9-fold (Alvarez et al., 2010). Humic substances
(HS) attached to ion exchange resin (AER) increase degradation
rates for RR2 by 2-fold (Cervantes et al., 2011).Martínez et al.
(2013)reported the degradation of RR2 using immobilized HS sup-ported on an AER in an upflow anaerobic sludge reactor (UASB).
The decolorization efficiency of RR2 increased∼90% in
compari-son with the control UASB reactor operated without immobilized HS.Amezquita-Garcia et al. (2014)investigated the anchorage of two anthraquinones (AAQ and AQDS) on surface of polyacriloni-trile activated carbon fibers (ACFs) to obtain materials with redox functional groups on their surface to be applied for the treatment of contaminants with electron withdrawing groups. These authors reported that only AQDS anchored on ACFs was an effective redox
mediator improving the reductive conversion of 4-nitrophenol (4NP) to 4-aminophenol. The catalytic properties of AQDS-ACFs improved the reduction of 4NP by 49% in comparison with ACFs alone. Many studies have shown the influence of the structure of dyes in the anaerobic reduction process using immobilized redox
mediators.Yuan et al. (2012)have immobilized AQS on ceramsites
and found an increase of degradation rate constant for Acid dye yellow 36 (AY36) by 8.0-fold, while for RR2, Acid red 27 (AR27), and Acid orange 7 (AO7), the increases in degradation rate were 2.3, 2.7, and 2.8-fold, respectively, when compared to experiments
carried out without redox mediator.Guo et al. (2007)reported
that anthraquinone immobilized on calcium alginate beads had an increase in decolorization rates for reactive brilliant red K-2BP, reactive brilliant red X-3B, acid black 10B, acid scarlet GR, acid red B and Acid red G of 1.5–2, 1.57, 1.88, 2.1, 1.65, and 1.48-fold,
respectively.Lu et al. (2010)also reported AQS immobilization on
polyurethane foam (PUF) and found an increase in degradation
rates for RR2 by 4-fold, whileZhou et al. (2014)investigated
decol-orization of Reactive red K-2G using AQS immobilized on PUF in an upflow anaerobic bioreactor. These authors reported improve-ments in decolorization efficiencies by 1.47-fold in comparison with the control.
The results obtained in this study indicated a removal efficiency of 51.8% for anaerobic degradation of RGY-RNL dye in the presence of riboflavin immobilized onto MC 3 after 24 h of incubation. This result was similar to those experiments using yeast extract as the source of redox mediators (50.1%), twice as high as when soluble riboflavin was used (26.7%), and higher than those which did not receive any redox mediator (control flasks, 30.7%). After 48 h, the color removal efficiency in the flasks without redox mediator was
72.1±0.1 and 89.4±0.0% for those incubated with MC 3, which
was higher than when yeast extract (82.6%) and riboflavin (84.3%) were used as redox mediators.
In comparison to other studies on azo dyes such as RR2 (van
der Zee et al., 2001a) and Blue Drimaren HF-RL (Corrêa et al., 2009) which were degraded anaerobically for 24 h without redox medi-ators, the percentages of color removal obtained in this study in the presence of MC 3 were significantly higher. This study indi-cated MC 3 was as efficient as riboflavin and yeast extract in the anaerobic decolorization of RGY-RNL, and could be employed for the anaerobic degradation of other azo dyes.
In order to provide evidence of the reduction process of the RGY-RNL dye mediated by immobilized riboflavin derivative (MC 3) through the azo cleavage, the released aromatic amines were
analyzed by HPLC. According toPinheiro et al. (2004), the
degra-dation of azo dyes containing sulfonic acid groups such as the model dye used in this study generates as byproducts aromatic amines with sulfonic groups such as analogous of sulfanilic acid. Baêta et al. (2015, 2013)have studied the anaerobic degradation of RGY-RNL dye with powdered activated carbon and evaluated the byproducts released. The possible degradation byproducts of
RGY-RNL dye were evaluated as sulfanilic acid derivatives (Baêta
et al., 2013).Fig. 5shows the chromatograms of samples collected from anaerobic degradation experiments, which were carried out in
the same conditions described in Section2.2.8. These experiments
were accomplished with and without (control) addition of MC 3. Standards solutions of sulfanilic acid and volatile fatty acids (VFA)
(intermediates in anaerobic digestion) (Baêta et al., 2013) were also
36 34 32 30 28 26 24 22 20 18 16 14 12 10 8 6 4 2 0
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34
0 510000 1020000 1530000 2040000
1
2
3
4
5
Sulfanilic acid MC3 Control 48h Control 0h VFA Retention time (min)
Intensi
ty
(mV
)
Retention time (min)
Intensit
y
(mV)
Sam ple
nam e
Fig. 5.Chromatogram (signal at= 191 nm) of standard solutions and supernatant of anaerobic degradation of RGY-RNL dye with MC 3 in batch mode. Standard VFA solution (5); control 0 h (4); control 48 h (3); MC 3 (2), and standard sulfanilic acid solution (1).
Table 3
Parameters obtained from the linear fit for zero-order and first-order kinetic models for anaerobic degradation of RGY-RNL.
Experiment Kinetic model R2 k
0,obs(mg/L h) k1,obs(h−1) t1/2(h)
Control Zero-order 0.9291 0.0121 – 41.3
MC 3 Zero-order 0.9826 0.0189 – 26.4
Riboflavin Zero-order 0.9369 0.0158 – 31.6
Yeast extract First-order 0.9822 – 0.0364 19.0
upflow UASB reactor using yeast extract and powdered activated carbon as sources of redox mediators. The azo cleavage of RGY-RNL using MC 3 resulted in the formation of a large amount of sulfanilic acid derivatives than those experiments performed without MC 3 (Fig. 5). This observation proves the efficiency of MC 3 to enhance the electron transfer rate in the reduction of azo bond.
The chemical stability is an important characteristic of an immobilized redox mediator. Most of the studies reported in the literature are related to the immobilization of quinone-based redox mediators on solid supports such as ion exchange resins, calcium alginate, and nanoparticles of metal oxides through adsorption, electrostatic interaction and entrapment techniques and yielded
good mechanical strengths during the removal process (Alvarez
et al., 2010; Cervantes et al., 2010, 2011; Guo et al., 2007; Martínez et al., 2013). In this study, the mediator redox riboflavin was immobilized on cellulose by a covalent bond. A chemical bond is considered a stronger bond for incorporation of a redox mediator on solid support than immobilization by adsorption, for example. Therefore, the attachment of riboflavin on cellulose can provide good mechanical and chemical properties to this solid support to
resist for the anaerobic degradation process.Lu et al. (2010)and
Yuan et al. (2012)adopted a similar strategy for the immobilization of redox mediators on polyurethane foam and ceramsite, respec-tively.
Anaerobic degradation of RGY-RNL dye as a function of time was evaluated using three kinetic models: zero-order, first-order and second-order. Experiments performed without redox media-tors (control) and with MC 3 as the redox mediator followed the
zero-order kinetic model. Similar results were reported byField
and Brady (2003), where riboflavin was used as the redox mediator in the anaerobic reduction of azo dye mordant yellow 10, where the
zero-order kinetic model explained the experimental data.Table 3
summarizes the kinetic results.
The use of MC 3 as the redox mediator increased the zero-order
degradation rate constant (k0,obs) of RGY-RNL 1.56-fold compared
to control (Table 3), which was similar to thek0,obs reported by
Field and Brady (2003)for anaerobic degradation of Mordant yel-low 10 in the presence of riboflavin. Despite this increase, the color removal rate was lower than those reported in the literature for
immobilized redox mediators (Alvarez et al., 2010; Cervantes et al.,
2010; Lu et al., 2010). However, the comparison was not
straightfor-ward, sinceCervantes et al. (2010)andLu et al. (2010)immobilized
different redox mediators (AQDS, humic substances and NQS) on different supports to degrade a different azo dye (RR2).
Summarizing the results obtained in this study, MC 3 was as effi-cient as riboflavin and yeast extract in the anaerobic decolorization of RGY-RNL dye, and therefore, it could be employed for the anaer-obic degradation of other azo dyes. An advantage of MC 3 in relation to riboflavin and yeast extract is that MC 3 can be recovered and reused in the anaerobic degradation process.
4. Conclusions
in the presence of MC 3. The use of immobilized redox mediators as MC 3 could allow reduction of wastewater treatment costs.
Acknowledgements
The authors are grateful to the Universidade Federal de Ouro Preto (UFOP), Fundac¸ão de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, grant number CEX APQ-01356/09), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number 471863/2009-2), and Coordenac¸ão de Aperfeic¸oamento de Pessoal de Nível Superior (CAPES) for funding this research.
Appendix A. Supplementary data
Supplementary data associated with this article can be
found, in the online version, at http://dx.doi.org/10.1016/j.
indcrop.2014.10.059.
References
Abe, M., Kyogoku, Y., Kitagawa, T., Kawano, K., Ohishi, N., Takai-Suzuki, A., Yagi, K., 1986. Infrared spectra and molecular association of lumiflavin and riboflavin derivatives. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 42, 1059–1068. Alvarez, L.H., Perez-Cruz, M.A., Rangel-Mendez, J.R., Cervantes, F.J., 2010.
Immobi-lized redox mediator on metal-oxides nanoparticles and its catalytic effect in a reductive decolorization process. J. Hazard. Mater. 184, 268–272.
Amezquita-Garcia, H.J., Razo-Flores, E., Cervantes, F.J., Rangel-Mendez, J.R., 2014. Anchorage of anthraquinone molecules onto activated carbon fibers to enhance the reduction of 4-nitrophenol. J. Chem. Technol. Biotechnol.,
http://dx.doi.org/10.1002/jctb.4478(in press).
Aquino, S.F., Chernicharo, C.A.L., Foresti, E., Santos, M.L.F, Monteggia, L.O., 2007. Metodologias para determinac¸ão da atividade metanogênica específica (AME) em lodos anaeróbios. Eng. Sanit. Ambient. 12, 192–201.
Baêta, B.E., Aquino, S.F., Silva, S.Q., Rabelo, C.A., 2012. Anaerobic degradation of azo dye Drimaren blue HFRL in UASB reactor in the presence of yeast extract a source of carbon and redox mediator. Biodegradation 23, 199–208.
Baêta, B.E.L., Lima, D.R.S., Silva, S.Q., Aquino, S.F., 2015. Evaluation of soluble microbial products and aromatic amines accumulation during a com-bined anaerobic/aerobic treatment of a model azo dye. Chem. Eng. J. 259, 936–944.
Baêta, B.E.L., Luna, H.J., Sanson, A.L., Silva, S.Q., Aquino, S.F., 2013. Degradation of a model azo dye in submerged anaerobic membrane bioreactor (SAMBR) operated with powdered activated carbon (PAC). J. Environ. Manage. 128, 462–470.
Cervantes, F.J., Garcia-Espinosa, A., Moreno-Reynosa, M.A., Rangel-Mendez, J.R., 2010. Immobilized redox mediators on anion exchange resins and their role on the reductive decolorization of azo dyes. Environ. Sci. Technol. 44, 1747–1753. Cervantes, F.J., Gonzalez-Estrella, J., Márquez, A., Alvarez, L.H., Arriaga, S., 2011. Immobilized humic substances on an anion exchange resin and their role on the redox biotransformation of contaminants. Bioresour. Technol. 102, 2097–2100. Chernicharo, C.A.L., 2007. Princípios do Tratamento Biológico de Águas Residuárias Reatores anaeróbicos, second ed. Departamento de Engenharia Sanitária e Ambi-ental, Universidade Federal de Minas Gerais, Minas Gerais.
Clesceri, L.S., Eaton, A.D., Greenberg, A.E., Association, A.P.H., Association, A.W.W., Federation, W.E., 1998. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC.
Corrêa, C.A.R, Aquino, S.F., Caldas, P.C.P., Silva, S.Q., 2009. Uso de extrato de levedura como fonte de carbono e de mediadores redox, para a degradac¸ão anaeróbia de corante azo. Eng. Sanit. Ambient. 14, 559–568.
Costa, M.C., Mota, S., Nascimento, R.F., Dos Santos, A.B., 2010. Anthraquinone-2,6-disulfonate (AQDS) as a catalyst to enhance the reductive decolourisation of the
azo dyes Reactive Red 2 and Congo Red under anaerobic conditions. Bioresour. Technol. 101, 105–110.
dos Santos, A.B., 2005. Aplicac¸ão conjunta de tratamento anaeróbio termofílico por lodo granular e de mediadores redox na remoc¸ão de cor de águas residuárias têxteis. Eng. Sanit. Ambient. 10, 253–259.
dos Santos, A.B., Bisschops, I.A., Cervantes, F.J., van Lier, J.B., 2004. Effect of different redox mediators during thermophilic azo dye reduction by anaerobic granular sludge and comparative study between mesophilic (30 degrees C) and ther-mophilic (55 degrees C) treatments for decolourisation of textile wastewaters. Chemosphere 55, 1149–1157.
dos Santos, A.B., Cervantes, F.J., van Lier, J.B., 2007. Review paper on current tech-nologies for decolourisation of textile wastewaters: perspectives for anaerobic biotechnology. Bioresour. Technol. 98, 2369–2385.
Field, J.A., Brady, J., 2003. Riboflavin as a redox mediator accelerating the reduction of the azo dye mordant yellow 10 by anaerobic granular sludge. Water Sci. Tech-nol. 48, 187–193 (A Journal of the International Association on Water Pollution Research).
Guo, J., Zhou, J., Wang, D., Tian, C., Wang, P., Salah Uddin, M., Yu, H., 2007. Biocalalyst effects of immobilized anthraquinone on the anaerobic reduction of azo dyes by the salt-tolerant bacteria. Water Res. 41, 426–432.
Gurgel, L.V.A., Gil, L.F., 2009. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by succinylated mercerized cellulose modified with tri-ethylenetetramine. Carbohydr. Polym. 77, 142–149.
Gurgel, L.V.A., Junior, O.K., Gil, R.P.D.F., Gil, L.F., 2008. Adsorption of Cu(II), Cd(II), and Pb(II) from aqueous single metal solutions by cellulose and mercerized cellulose chemically modified with succinic anhydride. Bioresour. Technol. 99, 3077–3083.
Karnitz, O., Gurgel, L.V.A., de Melo, J.C.P., Botaro, V.R., Melo, T.M.S., Gil, R.P.D.F., Gil, L.F., 2007. Adsorption of heavy metal ion from aqueous single metal solution by chemically modified sugarcane bagasse. Bioresour. Technol. 98, 1291–1297. Keller, P.J., Le Van, Q., Bacher, A., Floss, H.G., 1983. Biosynthesis of riboflavin:13
C-NMR techniques for the analysis of multiply13C-labeled riboflavins. Tetrahedron
39, 3471–3481.
Lu, H., Zhou, J., Wang, J., Si, W., Teng, H., Liu, G., 2010. Enhanced biodecolorization of azo dyes by anthraquinone-2-sulfonate immobilized covalently in polyurethane foam. Bioresour. Technol. 101, 7196–7199.
Martínez, C., Celis, L., Cervantes, F., 2013. Immobilized humic substances as redox mediator for the simultaneous removal of phenol and Reactive Red 2 in a UASB reactor. Appl. Microbiol. Biotechnol. 97, 9897–9905.
Melo, J.C.P., Silva Filho, E.C., Santana, S.A.A., Airoldi, C., 2011. Synthesized cellu-lose/succinic anhydride as an ion exchanger. Calorimetry of divalent cations in aqueous suspension. Thermochim. Acta 524, 29–34.
Méndez-Paz, D., Omil, F., Lema, J.M., 2005. Anaerobic treatment of azo dye Acid Orange 7 under batch conditions. Enzyme Microb. Technol. 36, 264–272. Nakanishi, K., Solomon, P.H., 1977. Infrared Absorption Spectroscopy, second ed.
Holden-Day, Inc., San Francisco, CA.
Penzkofer, A., 2012. Photoluminescence behavior of riboflavin and lumiflavin in liquid solutions and solid films. Chem. Phys. 400, 142–153.
Pinheiro, H.M., Touraud, E., Thomas, O., 2004. Aromatic amines from azo dye reduc-tion: status review with emphasis on direct UV spectrophotometric detection in textile industry wastewaters. Dyes Pigm. 61, 121–139.
van der Zee, F.P., Bisschops, I.A., Blanchard, V.G., Bouwman, R.H., Lettinga, G., Field, J.A., 2003. The contribution of biotic and abiotic processes during azo dye reduc-tion in anaerobic sludge. Water Res. 37, 3098–3109.
van der Zee, F.P., Bouwman, R.H., Strik, D.P., Lettinga, G., Field, J.A., 2001a. Applica-tion of redox mediators to accelerate the transformaApplica-tion of reactive azo dyes in anaerobic bioreactors. Biotechnol. Bioeng. 75, 691–701.
van der Zee, F.P., Lettinga, G., Field, J.A., 2001b. Azo dye decolourisation by anaerobic granular sludge. Chemosphere 44, 1169–1176.
Yuan, S.-Z., Lu, H., Wang, J., Zhou, J.-T., Wang, Y., Liu, G.-F., 2012. Enhanced bio-decolorization of azo dyes by quinone-functionalized ceramsites under saline conditions. Process Biochem. 47, 312–318.