ISSN 1678-992X
ABSTRACT: A significant number of bacterial species, particularly in the rhizosphere, may ben-efit plant growth and development. This group of bacteria is known as plant growth-promoting rhizobacteria (PGPR). This study identified genetically isolates of common bean nodules used to trap bacteria from Amazon pastureland and investigated their capacity of nodulating and promoting growth of common bean when inoculated or co-inoculated with CIAT899 strain ( Rhi-zobium tropici). Two experiments were carried out in a greenhouse, in axenic conditions, using the common bean cultivar Talismã. In the first experiment, 56 PGPR strains were evaluated individually regarding growth promotion and nodulation. In the second experiment, inoculation of seven PGPR strains previously selected in the first experiment was evaluated in three forms of N supply: Co-inoculation with CIAT 899 in the presence of low N-mineral concentration; individual inoculation in the presence of high N-mineral concentration; and individual inoculation in the pres-ence of low N-mineral concentration. The 16S rRNA gene sequencing showed predominance of
Pseudomonas genus, identified in 35 % of the sequenced strains. Other genera were identified:
Rhizobium, Burkholderia, Xanthomonas and Bacillus. Inoculation of the seven strains with CIAT 899 promoted distinct plant growth in different forms of N supply. In addition, N-mineral supply can be replaced by co-inoculation with strains of Pseudomonas sp. (UFLA 281 and UFLA 02-293) and Bacillus sp. (UFLA 02-298) identified in this study.
Keywords: Phaseolus vulgaris, co-inoculation, endophytic bacteria, rhizobia
Growth promotion of common bean and genetic diversity of bacteria from
Linnajara de Vasconcelos Martins Ferreira1, Fernanda de Carvalho2, Júlia Fonseca Colombo Andrade2, Fatima Maria de Souza Moreira2*
1Federal Institute of Pará – Campus Marabá Rural, BR 155 km 25, PA 26 de Março, C.P. 41 – 68508-979 – Marabá, PA – Brazil.
2Federal University of Lavras – Dept. of Soil Science – Sector of Biology, Microbiology and Biological Processes, C.P. 3037 – 37200-000 – Lavras, MG – Brazil.
*Corresponding author <fmoreira@dcs.ufla.br>
Edited by: Fernando Dini Andreote
Received February 07, 2017 Accepted July 24, 2017
Introduction
In the search for promising isolates that promote plant growth, the Amazon region stands out for its high diversity of organisms in the soil, including microorgan-isms. This fact was observed in the diversity of rhizo-bia genera and strains found in western Amazon (Gui-marães et al., 2012; Jaramillo et al., 2013). Besides, in the Amazon region, expressive area of the soil is used for pastureland, where recent studies have found a high bacterial diversity (Carvalho et al., 2016; Soares et al., 2016).
Studies show that bacterial strains isolated from this region have the potential to act as plant growth pro-moters in the biological nitrogen fixing (BNF) process (Ferreira et al., 2012) or in other processes, such as inhi-bition of phytopathogenic fungal growth, solubilization of phosphates (Marra et al., 2012), production of indole-3-acetic acid (IAA) (Silva et al., 2012; Oliveira-Longatti et al., 2013) and having adaptation to abiotic stresses (Medeiros et al., 2011; Soares et al., 2014).
Co-inoculation of Rhizobium and plant growth-pro-moting rhizobacteria (PGPR) in legumes have received more attention in recent years (Tilak et al., 2006; Mishra et al., 2011; Samavat et al., 2012). This combination brings positive effects on the cultivation of legume spe-cies, since it provides growth and nutrient absorption, as it happens in lentil plants with the co-inoculation of
Pseudomonas sp. with Rhizobium leguminosarum (Mishra et al., 2011), increases of nodulation in some crops, such as in pigeon pea [(Cajanus cajan (L.) Mill sp.)], with the
co-inoculation of Pseudomonas fluorescens with Rhizo-bium sp. (Tilak et al., 2006). Besides, this combination presents positive effects on grain yield, as it happens in bean crop, with the co-inoculation of Pseudomonas and
Rhizobium (Samavat et al., 2012).
In Brazil, three Rhizobium strains are authorized as inoculants for the common bean: CIAT 899, PRF 81, and H 12. Results show that bean plants can benefit from BNF in the field, with no need of N fertilizers applica-tion. The challenge is to apply appropriate management of this symbiosis to increase its efficiency in N supply to plants. Co-inoculation of Rhizobium and PGPR may be a strategy to improve efficiency of these strains.
The objective of this study was to genetically iden-tify isolates from nodules of common bean inoculated with Amazon pasture land soil, and to verify their abil-ity to nodulate and promote growth of bean plants co-inoculated or not with CIAT 899.
Materials and Methods
Strains origin
The strains used in this study (56) were isolated from inside the nodules of the common bean inoculated with soils from the Amazon region under pasture sys-tems (Moreira et al., 2009).
16S rRNA gene sequencing of bacterial strains
Bacterial strains were grown in medium 79 (Fred and Waksman, 1928). DNA of 56 bacterial strains was extracted by the alkaline lysis method (Niemann et al.,
Agricultural Micr
obiology
|
Resear
ch Ar
ticle
1997). The 16S rRNA gene was partially amplified with final reaction volume of 50 µL. The following concentra-tions were: 5 µL DNA, 5 µL 10X buffer for each PCR, 5 µL dNTP Mix (0.2 mM of each dNTP), 5 µL MgCl2 (2.5 mM), 1 µL of each primer (10 mmol L–1) (27F primer (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R primer (5’-GGTTACCTTGTTACGACTT-3’) (Lane, 1991), 0.4 µL Taq DNA polymerase (5 U µL–1), and sterile. Amplifi-cation reaction occurred following initial denaturation (94 °C for 5 min), 40 denaturation cycles (94 °C for 40 s), annealing (55 °C for 40 s), extension (72 °C for 1.5 min) and final extension (72 °C for 7 min). Reaction was per-formed in thermocycler. PCR products were separated in 1 % agarose gel and visualized under UV light.
PCR products were sequenced using the oligonu-cleotide primer 27F and 1492R. Sequence quality was evaluated using the BioNumerics software (version 7.1). Additionally, the sequences were subjected to BLAST to compare with similar sequences deposited in the Gen-Bank database (National Center for Biotechnology Infor-mation - NCBI) (http://www.ncbi.nlm.nih.gov) and were deposited under the accession numbers KU613374 to KU613408.
Experiment 1: Authentication and promotion of vegetative growth of common bean cultivar Talismã plants: in vitro assay
The experiment was carried out in a greenhouse from June to July 2014 and was conducted for 40 days. Plants were cultivated in sterilized 500 mL long neck bot-tles. The experimental design was completely randomized, with three replications and 59 treatments. Treatments consisted of inoculations with 56 bacterial strains isolated from bean nodules (Table 1); one reference strain approved by MAPA (Brazilian Ministry of Livestock Agriculture and Supply) as inoculum for bean crops; and two negative con-trols without inoculation, with high and low N-mineral concentrations. The experiment used the following stock solutions of N-mineral sources: Ca(NO3)2.4H2O mol L–l : 236.6 g L–1; KNO
3 mol L
–l : 101.111 g L–1 and NH 4H2PO4 mol L–l: 132.14 g L–1. From these stock solutions, high N concentration solution received respectively: 4; 0.6 and 0.1 mL L–1, while low N concentration solution received: 0.1; 0.125 and 0.25 mL L–1.
In the inoculated treatments, and in the control without inoculation and with low N concentration, Hoa-gland (HoaHoa-gland and Arnon, 1950) nutrient solution with low N concentration (5.25 mg L–1) was applied. In the control with high N-mineral concentrations, com-plete Hoagland solution was used, with 52.5 mg L–1 ni-trogen. Subsequently, all bottles were autoclaved for 60 min at a pressure of 1.5 kg cm2, at 121 °C.
Long neck bottles containing 500 mL Hoagland nutrient solution were covered with aluminum foil, and two filter paper strips (2 cm in width and length equal to the bottle height) were put inside each bottle to support roots and promote contact of the plant with the nutrient solution. Before sowing, bean seeds (cv. Talismã) were
surface disinfected using 98 % ethanol (30 s), 2 % so-dium hypochlorite (2 min) and then subjected to succes-sive rinses in autoclaved distilled water (Guimarães et al., 2012). After disinfection, seeds were placed in sterile Petri dishes containing moistened filter paper and cot-ton, where they remained for 48 h in a growth chamber at 28 °C, for rootlets emergence.
For inoculum preparation, bacterial strains were cultivated in liquid culture medium 79 (Fred and Waks-man, 1928), stirred at 110 rpm, at 28 °C, for 3 days. At sowing, 1 mL of the inoculant (109 Cells mL–1) was add-ed to the pre-germinatadd-ed seadd-eds in the inoculatadd-ed treat-ments. In the controls without inoculation, 1 mL of the culture medium without inoculum was applied.
Thirty-five days (pre-flowering stage) after the ex-periment beginning, plants were harvested to determine the following traits: number of nodules (NN); nodule dry matter (NDM); shoot dry matter (SDM); root dry matter (RDM); and total dry matter (TDM). To determine NN, nodules were detached from roots and counted. To de-termine NDM, SDM and RDM, nodules, shoot and roots were placed in paper bags and allowed to dry in forced air circulation oven at 60 °C until constant weight. Data were subjected to analysis of variance, using the statisti-cal software SISVAR version 5.1 (Ferreira, 2011). Treat-ments were compared by the Scott-Knott test at 5 % probability. NN and NDM values had been previously transformed into square root of (Y + 0.5).
Experiment 2: Inoculation and co-inoculation of bean with plant growth-promoting bacteria
To evaluate plant growth promotion potential sub-jected to inoculation and co-inoculation with bean in-oculant rhizobia strain (CIAT 899), seven strains were selected (UFLA 02-274, UFLA 02-276, UFLA 02-281, UFLA 282, UFLA 298, UFLA 290, UFLA 02-293) from those that stood out for shoot and root dry matter, as observed in experiment 1, as well as, from those that had already been identified by sequencing, at the time the experiment was carried out.
The experiment was carried out in Oct and Nov, 2014, in autoclaved Leonard jars (Vincent, 1970), con-taining 700 mL Hoagland solution (Hoagland and Arnon, 1950) in the bottom and a mixture of sand and vermicu-lite (1:2) in the upper part. The experiment was conduct-ed for 50 days. The experimental design was completely randomized in a factorial design (8 × 3) with three repli-cations. Factor 1 was the individual inoculation of seven strains, plus one treatment without inoculation. Factor 2 was three forms of N supply: low (5.25 mg L–1) and high (52.5 mg L–1) N-mineral concentration in the nutri-ent solution and co-inoculation with CIAT899 with low N-mineral concentration.
without inoculation, only 1 mL autoclaved liquid cul-ture medium 79 was added.
After sowing and inoculating, jars were covered with a layer of sand paraffin (10 kg sand, 1 L chloroform, and 10 g paraffin) to prevent contamination. Thinning was carried out five days after emergence, leaving one plant per jar. During the experiment, the nutrient solu-tion was prepared, autoclaved, and periodically replaced in jars, according to the plant absorption rate.
At 45 days after sowing, corresponding to the flowering stage, plants were harvested to determine the following traits: number of nodules (NN); nodules dry matter (NDM); shoot dry matter (SDM); root dry mat-ter (RDM); total dry matmat-ter (TDM); and N accumulation in the shoots (NAS). To determine NDM and SDM, the same procedures described for the authentication exper-iment were used. After weighing, N accumulated in the shoots (NAS) was calculated by multiplying the weight of dry shoots by the N content, and measured by the semi micro-Kjeldahl method described by Liao (1981).
Data were subjected to analysis of variance, us-ing the statistical program SISVAR, version 5.1 (Ferreira, 2011). Treatments were compared by the Scott-Knott test at 5 % probability. NDM and NN values had been previously transformed into square root of (Y + 0.5).
In order to confirm the cultural and genetic char-acteristics of the inoculated strains, 10 nodules were selected from each co-inoculation treatment for re-iso-lation. For surface disinfection, nodules were first im-mersed in 95 % ethanol. Next, in H2O2 for 3 min, and after that, they were rinsed 10 times with sterile distilled water (Guimarães et al., 2012). Subsequently, nodules were macerated in plates containing culture medium 79 (Fred and Waksman, 1928) and the material was spread to obtain isolated colonies. Afterward, sequencing was carried out as described before.
Results
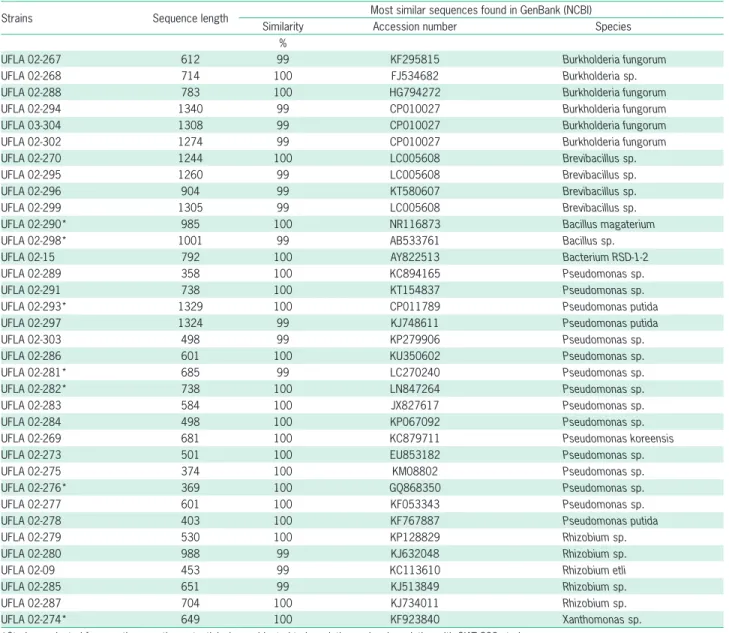
Of the 56 strains studied, 35 were successfully identified. A comparison of the 16S rRNA gene partial sequencing of strains evaluated with sequences depos-ited in the GenBank revealed that strains belong to Rhi-zobium, Burkholderia, Xanthomonas, Brevibacillus, and
Bacillus genera, with predominance of Pseudomonas
(Table 2).
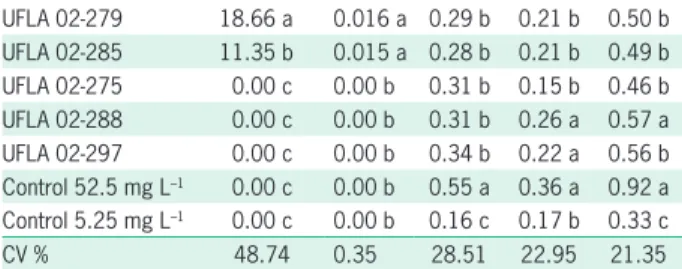
Table 1 – Number of nodules (NN), nodules dry matter (NDM), shoot dry matter (SDM), root dry matter (RDM), and total dry matter (TDM) of bean plants inoculated with strains isolated from the Amazon pastureland.
Treatment NN NDM SDM RDM TDM
NN per plant g per plant ---UFLA 02-305 0.00 c 0.00 b 0.10 c 0.09 c 0.19 c UFLA 02-306 0.00 c 0.00 b 0.12 c 0.16 b 0.28 c UFLA 02-307 0.00 c 0.00 b 0.15 c 0.19 b 0.34 c UFLA 02-308 0.00 c 0.00 b 0.16 c 0.15 b 0.31 c UFLA 02-309 0.00 c 0.00 b 0.16 c 0.19 b 0.36 c UFLA 02-310 0.00 c 0.00 b 0.16 c 0.18 b 0.35 c UFLA 02-311 0.00 c 0.00 b 0.16 c 0.12 b 0.29 c UFLA 02-272 0.00 c 0.00 b 0.16 c 0.23 a 0.40 c UFLA 02-295 0.00 c 0.00 b 0.16 c 0.23 a 0.40 c UFLA 02-312 0.00 c 0.00 b 0.17 c 0.17 b 0.34 c UFLA 02-313 0.00 c 0.00 b 0.17 c 0.22 a 0.38 c UFLA 02-314 0.00 c 0.00 b 0.17 c 0.23 a 0.40 c UFLA 02-315 0.00 c 0.00 b 0.18 c 0.20 b 0.38 c UFLA 02-316 0.00 c 0.00 b 0.18 c 0.16 b 0.33 c UFLA 02-273 0.00 c 0.00 b 0.19 c 0.23 a 0.42 c UFLA 02- 267 0.00 c 0.00 b 0.19 c 0.21 b 0.39 c UFLA 02-317 0.00 c 0.00 b 0.19 c 0.20 b 0.40 c UFLA 02-318 0.00 c 0.00 b 0.19 c 0.28 a 0.48 b UFLA 02-281 0.00 c 0.00 b 0.20 c 0.23 a 0.43 c UFLA 02-289 0.00 c 0.00 b 0.20 c 0.18 b 0.38 c UFLA 02-269 0.00 c 0.00 b 0.20 c 0.20 b 0.40 c UFLA 02-319 0.00 c 0.00 b 0.21 c 0.25 a 0.45 b UFLA 02-290 0.00 c 0.00 b 0.21 c 0.23 a 0.43 c UFLA 02-272 0.00 c 0.00 b 0.21 c 0.21 b 0.41 c UFLA 02-320 0.00 c 0.00 b 0.21 c 0.14 b 0.35 c UFLA 02-283 0.00 c 0.00 b 0.21 c 0.31 a 0.52 b UFLA 02-293 0.00 c 0.00 b 0.21 c 0.22 a 0.41 c UFLA 02-271 0.00 c 0.00 b 0.21 c 0.19 b 0.40 c UFLA 02-284 0.00 c 0.00 b 0.21 c 0.26 a 0.47 b UFLA 02-277 0.00 c 0.00 b 0.21 c 0.25 a 0.46 b UFLA 02-295 0.00 c 0.00 b 0.22 c 0.19 b 0.42 c UFLA 02-280 15.21 b 0.014 a 0.22 c 0.19 b 0.46 b UFLA 02-270 0.00 c 0.00 b 0.22 c 0.24 a 0.46 b UFLA 02-278 0.00 c 0.00 b 0.22 c 0.24 a 0.47 b UFLA 02-287 12.04 b 0.013 a 0.23 c 0.19 b 0.42 c UFLA 02-302 0.00 c 0.00 b 0.23 c 0.21 b 0.44 b UFLA 02-211 0.00 c 0.00 b 0.23 c 0.27 a 0.50 b UFLA 02-303 0.00 c 0.00 b 0.24 c 0.22 a 0.46 b UFLA 02-276 0.00 c 0.00 b 0.24 c 0.23 a 0.48 b UFLA 02-292 0.00 c 0.00 b 0.23 c 0.24 a 0.50 b UFLA 02-301 0.00 c 0.00 b 0.25 b 0.25 a 0.50 b UFLA 02-15 0.00 c 0.00 b 0.25 b 0.23 a 0.49 b UFLA 02-296 0.00 c 0.00 b 0.25 b 0.28 a 0.51 b UFLA 02-274 0.00 c 0.00 b 0.25 b 0.23 a 0.47 b UFLA 02-299 0.00 c 0.00 b 0.26 b 0.21 a 0.47 b UFLA 02-298 0.00 c 0.00 b 0.26 b 0.23 a 0.50 b UFLA 02-300 0.00 c 0.00 b 0.27 b 0.29 a 0.56 b UFLA 02-09 11.45 b 0.017 a 0.21 c 0.21 b 0.42 c UFLA 02-286 0.00 c 0.00 b 0.27 b 0.22 a 0.49 b UFLA 02-282 0.00 c 0.00 b 0.28 b 0.24 a 0.51 b UFLA 02-268 0.00 c 0.00 b 0.20 c 0.23 a 0.51 b
UFLA 02-279 18.66 a 0.016 a 0.29 b 0.21 b 0.50 b UFLA 02-285 11.35 b 0.015 a 0.28 b 0.21 b 0.49 b UFLA 02-275 0.00 c 0.00 b 0.31 b 0.15 b 0.46 b UFLA 02-288 0.00 c 0.00 b 0.31 b 0.26 a 0.57 a UFLA 02-297 0.00 c 0.00 b 0.34 b 0.22 a 0.56 b Control 52.5 mg L–1 0.00 c 0.00 b 0.55 a 0.36 a 0.92 a Control 5.25 mg L–1 0.00 c 0.00 b 0.16 c 0.17 b 0.33 c
CV % 48.74 0.35 28.51 22.95 21.35
In the individual inoculation experiment (experi-ment 1), treat(experi-ments influenced all the evaluated param-eters (p ≤ 0.05) (Table 1). There was no nodulation in the control groups, indicating no contamination in the experiment.
Of the 56 strains that were inoculated, only strains UFLA 02-09, UFLA 02-287, UFLA 02-279, UFLA 02-280 and UFLA 02-285 nodulated bean plants and belonged to the Rhizobium genus (Table 2). All strains showed SDM production lower (p≥ 0.05) when compared to the control with high N-mineral concentration (Table 1). Among the evaluated strains, 14 stood out for presenting SDM pro-duction superior to that of the control with low N concen-tration (p≤ 0.05) (Table 1). Of these strains, only two are nodulating strains (UFLA 02-279 and UFLA 02-285).
In relation to RDM, 55 % of the strains had better performance, with gains similar (p≤ 0.05) to that of the control with high N concentration (Table 1).
Seven strains studied in the inoculation and co-inoculation tests (experiment 2) are non-nodulating species. However, they showed good results for plant growth promotion, as observed in experiment 1. These strains belong to the genera: Pseudomonas (UFLA 02-276, UFLA 02-281, UFLA 02-282, UFLA 02-293), Bacil-lus (UFLA 02-290 and UFLA 02-298) and Xanthomonas
(UFLA 02-274).
There was significant interaction between inocula-tion of the seven strains and forms of N supply for all variables studied, except for NDM (Tables 3 and 4), since only the forms of N supply presented effects.
Table 2 – Identification of strains isolated from nodules of common bean grown in soil of pasture land from Amazon based on the most similar sequences found in GenBank (NCBI).
Strains Sequence length Most similar sequences found in GenBank (NCBI)
Similarity Accession number Species
%
UFLA 02-267 612 99 KF295815 Burkholderia fungorum
UFLA 02-268 714 100 FJ534682 Burkholderia sp.
UFLA 02-288 783 100 HG794272 Burkholderia fungorum
UFLA 02-294 1340 99 CP010027 Burkholderia fungorum
UFLA 03-304 1308 99 CP010027 Burkholderia fungorum
UFLA 02-302 1274 99 CP010027 Burkholderia fungorum
UFLA 02-270 1244 100 LC005608 Brevibacillus sp.
UFLA 02-295 1260 99 LC005608 Brevibacillus sp.
UFLA 02-296 904 99 KT580607 Brevibacillus sp.
UFLA 02-299 1305 99 LC005608 Brevibacillus sp.
UFLA 02-290* 985 100 NR116873 Bacillus magaterium
UFLA 02-298* 1001 99 AB533761 Bacillus sp.
UFLA 02-15 792 100 AY822513 Bacterium RSD-1-2
UFLA 02-289 358 100 KC894165 Pseudomonas sp.
UFLA 02-291 738 100 KT154837 Pseudomonas sp.
UFLA 02-293* 1329 100 CP011789 Pseudomonas putida
UFLA 02-297 1324 99 KJ748611 Pseudomonas putida
UFLA 02-303 498 99 KP279906 Pseudomonas sp.
UFLA 02-286 601 100 KU350602 Pseudomonas sp.
UFLA 02-281* 685 99 LC270240 Pseudomonas sp.
UFLA 02-282* 738 100 LN847264 Pseudomonas sp.
UFLA 02-283 584 100 JX827617 Pseudomonas sp.
UFLA 02-284 498 100 KP067092 Pseudomonas sp.
UFLA 02-269 681 100 KC879711 Pseudomonas koreensis
UFLA 02-273 501 100 EU853182 Pseudomonas sp.
UFLA 02-275 374 100 KM08802 Pseudomonas sp.
UFLA 02-276* 369 100 GQ868350 Pseudomonas sp.
UFLA 02-277 601 100 KF053343 Pseudomonas sp.
UFLA 02-278 403 100 KF767887 Pseudomonas putida
UFLA 02-279 530 100 KP128829 Rhizobium sp.
UFLA 02-280 988 99 KJ632048 Rhizobium sp.
UFLA 02-09 453 99 KC113610 Rhizobium etli
UFLA 02-285 651 99 KJ513849 Rhizobium sp.
UFLA 02-287 704 100 KJ734011 Rhizobium sp.
UFLA 02-274* 649 100 KF923840 Xanthomonas sp.
Bean inoculant strain (CIAT 899) efficiently nodu-lated this species, and the uninocunodu-lated controls showed no nodulation, indicating that the experiment was not contaminated and that the experimental conditions were favorable to nodulation. In the treatments individually inoculated with the seven previously selected strains, no
bean nodulation was observed in the presence of low and high N-mineral concentration in the nutrient solu-tion, as expected.
Co-inoculation of UFLA 02-293 (Pseudomonas sp.), UFLA 02-290 and UFLA 02-298 (Bacillus sp.) with CIAT 899 resulted in higher NN (176, 120 and 187 nodules per
Table 3 – Number of nodules, nodules dry matter, shoot dry matter, root dry matter, and total dry matter of bean plants inoculated with strains isolated from Amazon pastureland in different forms of N supply.
Number of nodules (NN per plant)
Strains Species CIAT 899 High N concentration (52.5 mg L–1) Low N concentration (5.25 mg L–1)
UFLA 02-282 Pseudomonas sp. 42.33 dA 0.00 aB 0.00 aB
UFLA 02-276 Pseudomonas sp. 44.33 dA 0.00 aB 0.00 aB
UFLA 02-290 Bacillus magaterium 120.33 bA 0.00 aB 0.00 aB
UFLA 02-281 Pseudomonas sp. 90.00 cA 0.00 aB 0.00 aB
UFLA 02-298 Bacillus sp. 187.00 aA 0.00 aB 0.00 aB
UFLA 02-293 Pseudomonas putida 176.33 aA 0.00 aB 0.00 aB
UFLA 02-274 Xanthomonas sp. 85.66 cA 0.00 aB 0.00 aB
Control 86.00 cA 0.00 aB 0.00 aB
Shoot dry matter (g per plant)
UFLA 02-282 Pseudomonas sp. 0.34 bB 2.48 aA 0.26 bB
UFLA 02-276 Pseudomonas sp. 0.39 bA 0.48 cA 0.27 bA
UFLA 02-290 Bacillus magaterium 0.45 bB 1.41 bA 0.33 bB
UFLA 02-281 Pseudomonas sp. 1.06 aA 1.17 bA 0.53 bB
UFLA 02-298 Bacillus sp. 1.37 aB 2.08 aA 0.87 aB
UFLA 02-293 Pseudomonas putida 1.67 aA 2.13 aA 0.49 bB
UFLA 02-274 Xanthomonas sp. 0.40 bB 1.50 bA 0.29 bB
Control 0.19 bB 0.92 bA 0.17 bB
Root dry matter (g per plant)
UFLA 02-282 Pseudomonas sp. 0.26 aB 1.35 aA 0.32 aB
UFLA 02-276 Pseudomonas sp. 0.32 aA 0.48 cA 0.34 aA
UFLA 02-290 Bacillus magaterium 0.44 aA 0.57 cA 0.41 aA
UFLA 02-281 Pseudomonas sp. 0.42 aB 1.43 aA 0.58 aB
UFLA 02-298 Bacillus sp. 0.49 aB 1.05 bA 0.64 aB
UFLA 02-293 Pseudomonas putida 0.59 aB 1.05 bA 0.50 aB
UFLA 02-274 Xanthomonas sp. 0.34 aB 0.80 cA 0.38 aB
Control 0.50 aA 0.36 cB 0.37 aB
Total dry matter (g per plant)
UFLA 02-282 Pseudomonas sp. 0.60 cB 3.84 aA 0.58 bB
UFLA 02-276 Pseudomonas sp. 0.71 cA 0.96 cA 0.61 bA
UFLA 02-290 Bacillus magaterium 0.77 cA 0.87 cA 1.99 aA
UFLA 02-281 Pseudomonas sp. 1.48 bB 2.61 bA 1.11 bB
UFLA 02-298 Bacillus sp. 1.87 bB 3.13 bA 1.51 aB
UFLA 02-293 Pseudomonas putida 2.75 aA 2.95 bA 1.00 bB
UFLA 02-274 Xanthomonas sp. 0.75 cB 2.30 bA 0.67 bB
Control 0.69 cB 1.30 bA 0.54 bB
Nitrogen accumulation in shoots (mg per plant)
UFLA 02-282 Pseudomonas sp. 11.52 bB 96.57 aA 8.00 aC
UFLA 02-276 Pseudomonas sp. 18.13 bB 26.98 dA 6.79 aC
UFLA 02-290 Bacillus magaterium 16.86 bB 73.26 bA 6.28 aC
UFLA 02-281 Pseudomonas sp. 18.86 bB 47.63 cA 13.31 aC
UFLA 02-298 Bacillus sp. 41.77 aB 59.52 bA 12.96 aC
UFLA 02-293 Pseudomonas putida 50.35 aB 78.20 bA 17.81 aC
UFLA 02-274 Xanthomonas sp. 10.44 bB 46.11 cA 6.55 aB
Control 15.80 bB 26.86 dA 7. 95 aC
plant, respectively) in relation (p ≤ 0.05) to individual inoculation with CIAT 899 (86 nodules per plant) (Table 3). Co-inoculation of CIAT 899 with UFLA 02-276 and UFLA 02-282 reduced NN (Table 3).
For NDM, effects were observed only for N supply (p≥ 0.05) (Table 4). Co-inoculation of the seven strains with CIAT 899 provided the best results for this variable. The best results for SDM production (p ≤ 0.05) were observed in the treatments inoculated with the UFLA 02-293, UFLA 02-298 and UFLA 02-282 in the presence of high N-mineral concentration (Table 3), in the co-inoculation of CIAT 899 with these strains along together with the UFLA 02-281, and in the individual inoculation of UFLA 02-298 in the presence of low N concentration (Table 3). The co-inoculation of CIAT 899 with UFLA 02-281 and UFLA 02-293 showed that the production of shoot dry matter was higher than in treat-ments cultivated with low N concentration (p ≤ 0.05), and similar to treatments cultivated with high N concen-tration (p≥ 0.05) (Table 3).
Inoculation of seven strains in the presence of low N concentration and co-inoculation of these strains with CIAT 899 did not affect root dry matter production (p
≥ 0.05) (Table 3). However, in the presence of high N concentration, inoculation with UFLA 282, UFLA 02-281, UFLA 02-298 and UFLA 02-293 promoted higher RDM production (p ≤ 0.05) and these strains were al-located in groups superior to the control, which was not inoculated with any strain (Table 3). Forms of N supply in the treatments inoculated with UFLA 276 and 02-290 UFLA did not influence RDM production. Higher RDM production was observed for the other strains in-oculated in the presence of high N concentration (Table 3).
Co-inoculation of UFLA 02-281, UFLA 02-298 and UFLA 02-293 strains with CIAT 899 provided the
high-est TDM production (p ≤ 0.05) (Table 3). However, in the presence of high N concentration, only UFLA 02-282 stood out (p≤ 0.05) (Table 3). The same form of N supply showed inhibitory effect (p≤ 0.05) (Table 3) for inocula-tion of UFLA 02-276 and UFLA 02-290 strains. The high-est TDM production in the presence of low N concentra-tion was verified by the inoculaconcentra-tion with UFLA 02-290 and UFLA 02-298 (p≤ 0.05) (Table 3). Treatments with high N concentration were superior to the other forms of N supply, except for the treatments with co-inoculation of CIAT 899 with UFLA 02-276, UFLA 02-290 and UFLA 02-293, with no difference between them (p≤ 0.05) (Ta-ble 3). Therefore, when inoculated with CIAT 899, these strains were effective in promoting bean growth, since inoculation with only CIAT 899 promoted TDM similar to the control.
Treatments with low N concentration showed that inoculation of the seven strains did not influence NAS. However, NAS increased when plants were cultivated in high N concentrations inoculated with UFLA 02-282, UFLA 02-290, UFLA 02-281, UFLA 02-298 UFLA 02-274 and UFLA 02-293, or with the co-inoculation of CIAT 899 with UFLA 02-298 and UFLA 02-293. Treatments inoculated in the presence of high N concentration pro-moted higher NAS. Co-inoculation results were higher than in treatments with low N concentration (Table 3).
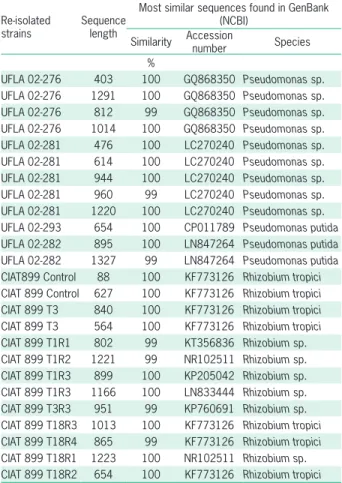
Results of sequencing re-isolated strains (Table 5) suggested that four are endophytes nodules. Re-isolation of UFLA 02-274 (Xanthomonas sp.) and UFLA 02-290 (Bacillus sp.) (Table 2) was not successful. Thus, their identities were not possible to confirm. UFLA 02-298 was not successfully amplified and was only evaluat-ed by phenotypic characterization in culture mevaluat-edium. CIAT 899 was also observed, confirming that nodulation was promoted by the bean inoculant strain.
Discussion
The 16S rRNA gene sequencing, with the identi-fication of six distinct genera, demonstrate a significant incidence of possible endophytes nodules, since most genera are not recognized as legume symbiont.
Among the studied strains, bacteria of the genus
Rhizobium were the only ones to nodulate bean plants. Other studies report nodulation of this bean species by
R. tropici (Martínez-Romero et al., 1991), R. etli (Sego-via et al.,1993; Wang et al., 1999), R. gallicum (Amarger et al., 1997), R. giardinii (Amarger et al., 1997), R. yan-glingense (Tan et al., 2001) and by other genera, such as
Sinorhizobium sp. (Toledo et al., 2003), Mesorhizobium sp. (Chen et al., 1991) and Burkholderia sp. (Ferreira et al., 2012).
PGPR can be free-living, associative or endophytic. Endophytic PGPR are able to colonize plant roots and, in the case of legume species, it is capable of cohabiting with BNF within the nodules. According to Kan et al. (2007), these bacteria possibly penetrate into the plant tissue, together with nodulating strains, during infection
Table 4 – Nodules dry matter (NDM) of bean plants inoculated with strains isolated from Amazon pastureland in different forms of N supply.
Strains Species Dry matter of nodules
mg per plant UFLA 02-282 Pseudomonas sp. 16NS
UFLA 02-276 Pseudomonas sp. 12
UFLA 02-290 Bacillus magaterium 55
UFLA 02-281 Pseudomonas sp. 23
UFLA 02-298 Bacillus sp. 27
UFLA 02-293 Pseudomonas putida 35
UFLA 02-274 Xanthomonas sp. 16
Control 25
Forms of N supply
CIAT 899 79 a
and nodules formation. The genera Agrobacterium, Pseu-domonas, Enterobacter, Pantoea, Bacillus and Paenibacil-lus are more frequently reported as nodule endophytes (Kan et al., 2007; Li et al., 2008; Shiraishi et al., 2010; Costa et al., 2016). In this study, Bacillus (UFLA 02-298) and Paenibacillus (UFLA 02-276, UFLA 02-293, UFLA 02-281 and UFLA 02-282) were detected as possible en-dophytes nodules.
Similar to pasturelands, a significant genetic di-versity of nodulating and non nodulating PGPR genera were also found in soils under agricultural systems in western Amazon by Guimarães et al. (2012) ( Bradyrhizo-bium, Rhizobium, Burkholderia and Achromobacter) and agroforestry systems by Jaramillo et al. (2013) ( Bradyrhi-zobium, Rhizobium, Ochrobactrum, Paenibacillus, Bosea,
Bacillus, Enterobacter, Stenotrophomonas) using Vigna un-guiculata (L.) Walp as trap plant. These results show that high symbiotic and genetic diversity of bacterial strains from different land use systems in the western Amazon as a potential PGPR source.
In the first experiment, although strains belonging to the genera Pseudomonas, Xanthomonas, Burkholderia, and Bacillus (Table 2) did not nodulate common bean
plants, they promoted plant growth in low N concentra-tion. This result was probably caused due to phytoes-timulation by other biological processes, such as phyto-hormones production (Costa et al., 2016). Azospirillum,
Bacillus, Enterobacter, Herbaspirillum, Paenibacillus, Pseu-domonas and Burkholderia strains are often described as potential plant growth promoters due to the action in different biological processes, especially in phosphate solubilization and phytohormones synthesis (particu-larly IAA) (Samavat et al., 2012; Oliveira-Longatti et al., 2013; 2014; Costa et al., 2016).
The ability to fix N may be affected by several bi-otic and abibi-otic factors (Tsai, 1993; Ali et al., 2009). The interaction of Rhizobium with other microorganisms in the soil is one of the factors that affects this process and both stimulation and inhibition of nodulation and plant growth may occur, depending on the interaction between symbionts and growth-promoting bacterial strains. In the present study, this fact was presented by nodulation stimulation by Pseudomonas sp. (UFLA 02-293), Bacillus
sp. (UFLA 02-290) and Pseudomonas sp. (UFLA 02-298) strains, and nodulation inhibition by Pseudomonas sp. (UFLA 02-282 and UFLA 276) strains. However, not all strains that stimulated nodulation in co-inoculated treat-ments with CIAT 899 promoted growth or increased SDM, RDM, and TDM production.
In RDM, SDM and TDM production, NAS was different for strains depending on N supply, except for RDM in the co-inoculated treatments and in the pres-ence of low N concentration. Notably, the highest in-crease in SDM was obtained by the combination of
Bacillus sp. (UFLA 02-298) and Paenibacillus sp. (UFLA 02-293, UFLA 02-281 and UFLA 02-282) co-inoculated with Rhizobium tropici CIAT 899. Individual inoculations of PGPR in the presence of low N concentration did not influence NAS. This result was expected since they are not nodulating strains, nor N2-fixing strains; however, they influence plant growth by other processes (Costa et al., 2016). Nevertheless, co-inoculation of PGPR with
Bacillus sp. (UFLA 02-298) and Paenibacillus sp.(02-293) together with Rhizobium tropici (CIAT 899) increased NAS.
In the literature, PGPR of the genus Pseudomonas
are reported as growth promoters, as verified by Samata-va et al. (2012). This genus also showed releSamata-vant perfor-mance when co-inoculated with Rhizobium tropici CIAT 899. In other studies, the contribution of the co-inocula-tion of Paenibacillus strains (Rodrigues et al., 2012) and
Enterobacter (Costa et al., 2016) with Bradyrhizobium was observed on the dry matter yield of cowpea and soybean plants, respectively.
Inoculation of CIAT 899 with Pseudomonas sp. and Bacillus sp. can be an effective strategy to produce bio-fertilizers for beans. The results help establish an inoculum or a combination of inoculum for bean yield improvement, as well as the understanding of their per-formance under low and high N-minerals concentra-tions.
Table 5 – Genetic identification of Re-isolated strains from nodules of common bean obtained from co-inoculation experiment based on the most similar sequences found in GenBank (NCBI).
Re-isolated strains
Sequence length
Most similar sequences found in GenBank (NCBI)
Similarity Accession number Species
%
UFLA 02-276 403 100 GQ868350 Pseudomonas sp.
UFLA 02-276 1291 100 GQ868350 Pseudomonas sp. UFLA 02-276 812 99 GQ868350 Pseudomonas sp. UFLA 02-276 1014 100 GQ868350 Pseudomonas sp. UFLA 02-281 476 100 LC270240 Pseudomonas sp.
UFLA 02-281 614 100 LC270240 Pseudomonas sp.
UFLA 02-281 944 100 LC270240 Pseudomonas sp.
UFLA 02-281 960 99 LC270240 Pseudomonas sp. UFLA 02-281 1220 100 LC270240 Pseudomonas sp. UFLA 02-293 654 100 CP011789 Pseudomonas putida
UFLA 02-282 895 100 LN847264 Pseudomonas putida
UFLA 02-282 1327 99 LN847264 Pseudomonas putida
CIAT899 Control 88 100 KF773126 Rhizobium tropici
CIAT 899 Control 627 100 KF773126 Rhizobium tropici
CIAT 899 T3 840 100 KF773126 Rhizobium tropici
CIAT 899 T3 564 100 KF773126 Rhizobium tropici
CIAT 899 T1R1 802 99 KT356836 Rhizobium sp. CIAT 899 T1R2 1221 99 NR102511 Rhizobium sp. CIAT 899 T1R3 899 100 KP205042 Rhizobium sp. CIAT 899 T1R3 1166 100 LN833444 Rhizobium sp. CIAT 899 T3R3 951 99 KP760691 Rhizobium sp. CIAT 899 T18R3 1013 100 KF773126 Rhizobium tropici
CIAT 899 T18R4 865 99 KF773126 Rhizobium tropici
CIAT 899 T18R1 1223 100 NR102511 Rhizobium sp.
Conclusions
16S rRNA gene sequencing showed the predomi-nance of Pseudomonas genus as bean plants nodules endophytic strains. Other genera were identified: Rhi-zobium, Burkholderia, Xanthomonas and Bacillus. Thus, high incidence of possible endophytic strains was found in the nodules, since most of these genera are not known as legume plants symbiont.
This result also demonstrates a significant genetic diversity of bacterial strains under pasture system by the presence of strains of Bacillus and Pseudomonas
genera, showing potential to be used as plant growth promoters. Inoculation of seven strains with CIAT 899 promoted varied plant growth in the different forms of N supply. N-mineral supply may be replaced by the co-inoculation of CIAT 899 with UFLA 281, UFLA 02-286 and UFLA 02-293, which are plant growth promot-ing strains. Tests under field conditions are necessary to validate the promising results on a large scale.
Acknowledgments
The authors thank the Brazilian National Coun-cil for Scientific and Technological Development (CNPq) 304527/2016-5; 560551/2010-0; 106350/2015-3; 141672/2014-5; Coordination for the Improvement of Higher Level Personnel (CAPES) - PROEX AUXPE 5902014 and Minas Gerais State Foundation for Research Support Support (FAPEMIG) – PACCSS (Programme for supporting graduate courses with grades six and seven) AUXPE 26182012 for financial support and for granting and fellowships
References
Ali, S.F.; Rawat, L.S.; Meghvansi, M.K.; Mahna, S.K. 2009. Selection of stress-tolerant rhizobial isolates of wild legumes growing in dry regions of Rajasthan, India. Journal of Agriculture and Biological Sciences4: 13-18.
Amarger, N.; Macheret, V.; Laguerre, G. 1997. Rhizobium gallicum
sp. nov. and Rhizobium giardinii sp. nov. from Phaseolus vulgaris
nodules. International Journal of Systematic Bacteriology 47: 996-1006.
Carvalho, T.S.; Jesus, E.C.; Barlow, J.; Gardner, T.A.; Soares, I.C.; Tiedje, J.M.; Moreira, F.M.S. 2016. Land use intensification in the humid tropics increased both alpha and beta diversity of soil bacteria. Ecology 97: 2760-2771.
Chen, W.X.; Li, Y.L.; Wang, E.T.; Yuan, H.L.; Li, J.L. 1991.
Rhizobium huahuii sp. nov. isolated from root nodules
of Astragalus sinicus. International Journal of Systematic
Bacteriology 41: 275-280.
Costa, E.M.; Carvalho, F.; Nóbrega, R.S.A.; Silva J.S.; Moreira, F.M.S. 2016. Bacterial strains from floodplain soils perform different plant-growth promoting processes and enhance cowpea growth. Scientia Agricola 73: 301-310.
Ferreira, D.F. 2011. Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia 35: 1039-1042.
Ferreira, P.A.A.; Bomfeti, C.A.; Soares, B.L.; Moreira, F.M.S. 2012. Efficient nitrogen-fixing Rhizobium strains isolated from Amazonian soils are highly tolerant to acidity and aluminium. World Journal of Microbiology and Biotechnology 28: 1947-1959.
Fred, E.B.; Waksman, S.A. 1928. Laboratory Manual of General Microbiology: with Special Reference to the Microorganisms of the Soil. McGraw-Hill, New York, NY, USA.
Hoagland, D.R.; Arnon, D.T. 1950. The Water Culture Method for Growing Plants without Soil. California Agriculture Experiment Station, Berkeley, CA, USA.
Guimarães, A.A.; Jaramillo, P.M.D.; Nóbrega, R.S.A.; Florestino, L.A.; Silva, K.; Moreira, F.M.S. 2012. Genetic and symbiotic diversity of nitrogen fixing bacteria isolated from agricultural soils in the western Amazon by using cowpea as the trap plant. Applied and Environmental Microbiology 78: 6726-6733. Jaramillo, P.M.D.; Guimarães, A.A.; Florentino, L.A.; Silva,
K.B.; Nóbrega, R.S.A.; Moreira, F.M.S. 2013. Symbiotic nitrogen-fixing bacterial populations trapped from soils under agroforestry systems in the western Amazon. Scientia Agricola 70: 397-404.
Kan, F.L.; Chen, Z.Y.; Wang, E.T.; Tian, C.F.; Sui, X.H.; Chen, W.X. 2007. Characterization of symbiotic and endophytic bacteria isolated from root nodules of herbaceous legumes cultivated in Qinghai-Tibet plateau and in other zones of China. Archives of Microbiology 188: 103-115.
Li, J.H.; Wang, E.T.; Chena, W.F.; Chena, W.X. 2008. Genetic diversity and potential for promotion of plant growth detected in nodule endophytic bacteria of soybean cultivated in Heilongjiang province of China. Soil Biology and Biochemistry 40: 238-246.
Liao, C.F.H. 1981. Devarda’s allow methods for total nitrogen determination. Soil Science Society of America Journal 45: 852-855.
Marra, L.M.; Soares, C.R.F.S.; Oliveira, S.M.; Ferreira, P.A.A.; Soares, B.L.; Carvalho, R.F.; Lima, J.M.; Moreira, F.M.S. 2012. Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant and Soil 357: 289-307.
Martínez-Romero, E.; Segovia, E.; Mercante, F.M.; Franco, A.A.; Graham, P.H.; Pardo, M.A. 1991. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena
sp. trees. International Journal of Systematic Bacteriology 41: 417-426.
Medeiros, F.H.V.; Souza, R.M.; Medeiros, F.C.L.; Zhang, H.; Wheeler, T.; Payton, P.; Ferro, H.M.; Paré, P.W. 2011. Transcriptional profiling in cotton associated with Bacillus
subtilis (UFLA285) induced biotic-stress tolerance. Plant and
Soil 1-11.
Mishra, P.K.; Bisht, S.C.; Ruwari, P.; Joshi, G.K.; Singh, G.; Bisht, J.K.; Bhatt, J.C. 2011. Bioassociative effect of cold tolerant
Pseudomonas spp. and Rhizobiu leguminosarum-PR1 on iron
acquisition, nutrient uptake and growth of lentil (Lens culinaris L.). European Journal of Soil Biology 47: 35-43.
Niemann, S.; Puehler, A.; Tichy, H.V.; Simon, R.; Selbitshka, W. 1977. Evaluation of the resolving power of three different DNA fingerprinting methods to discriminate among isolates of a natural Rhizobium meliloti population. Journal of Applied Microbiology 82: 477-484.
Oliveira-Longatti, S.M.; Marra, L.M.; Moreira, F.M.S. 2013. Evaluation of plant growth-promoting traits of Burkholderia
and Rhizobium strains isolated from Amazon soils for their
co-inoculation in common bean. African Journal of Microbiology Research 7: 948-959.
Oliveira-Longatti, S.M.; Marra, L.M.; Soares, B.L.; Bomfeti, C.A.; Silva, K.; Ferreira, P.A.V.; Moreira, F.M.S. 2014. Bacteria isolated from soils of the western Amazon and from rehabilitated bauxite-mining areas have potential as plant growth promoters. World Journal of Microbiology Biotechnology 30: 1239-1250. Samavat, S.; Samavat, S.; Mafakheri, S.; Shakouri, M.J. 2012.
Promoting common bean growth and nitrogen fixation by the co-inoculation of Rhizobium and Pseudomonas fluorescens
isolates. Bulgarian Journal of Agricultural Science18: 387-395. Segovia, L.; Young, J.P.W.; Martínez-Romero, E. 1993.
Reclassification of American Rhizobium leguminosarum biovar
phaseoli type I strains as Rhizobium etli sp. Internatinal Journal
of Systematic Bacteriology 43: 374-377.
Shiraishi, A.; Matsushita, N.; Hougetsu, T. 2010. Nodulation in black locust by the Gammaproteobacteria Pseudomonas sp. and the Betaproteobacteria Burkholderia sp. Systematic and Applied Microbiology. 33: 269-274.
Silva, K.; Cassetari, A.S.; Lima, A.S.; Brandt, E.; Pinnock, E.; Vandammec, P.; Moreira, F.M.S. 2012. Diazotrophic
Burkholderia species isolated from the Amazon region exhibit
phenotypical, functional and genetic diversity. Systematic and Applied Microbiology 35: 253-262.
Soares, B.L.; Ademar, P.A.; Oliveira-Longatti, S.M.; Marra, L.M.; Rufini, M. 2014. Cowpea symbiotic efficiency, pH and aluminum tolerance in nitrogen-fixing bacteria. Scientia Agricola 71: 171-180.
Soares, B.L.; Ferreira, P.A.A.; Rufini, M.; Martins, F.A.D.; Oliveira, D.P.; Reis, R.P.; Andrade, M.J.B.; Moreira, F.M.S.
2016. Agronomic and economic efficiency of
common-bean inoculation with rhizobia and mineral nitrogen fertilization. Revista Brasileira de Ciência do Solo 40: 1-13. Tan, Z.Y.; Kan, F.L.; Peng, G.X.; Wang, E.T.; Reinhald-Hurek, B.;
Chen, W.X. 2001. Rhizobium yanglingense sp. nov. isolated from arid and semiarid regions in China. International Journal of Systematic Evolutionary Microbiology 51: 901-914.
Tilak, K.V.B.; Ranganayaki, R.N.; Manoharachari, C. 2006. Synergistic effects of plant-growth promoting rhizobacteria
and Rhizobium on nodulation and nitrogen fixation by pigeon
pea. European Journal of Soil Science 57: 67-71.
Toledo, I.; Lloret, L.; Martínez-Romero, E. 2003. Sinorhizobium
americanum sp. nov., a new Sinorhizobium species nodulating
native Acacia spp. in Mexico. Systematic Applied Microbiology 26: 54- 64.
Tsai, S.M.; Bonetti, R.; Agbala, S.M.; Rossetto, R. 1993. Minimizing the effect of mineral nitrogen on biological nitrogen fixation in common bean by increasing nutrient levels. Plant and Soil 152: 131-138.
Vincent, J.M. 1970. Manual for the Practical Study of Root Nodule Bacteria. Blackwell, Oxford, England.
Wang, E.T.; Rogel, M.A.; García-de los Santos, A.; Martínez-Romero, J.; Cevallos, M.A.; Martínez-Martínez-Romero, E. 1999.
Rhizobium etli bv. mimosae, a novel biovar isolated from Mimosa
affinis. International Journal of Systematic Bacteriology 49: