Universidade de Lisboa
Faculdade de Farmácia
Functionalization of Silicone Catheters with
Glycolipids
Cristiana Vieira Pontes
Mestrado Integrado em Ciências Farmacêuticas
Universidade de Lisboa
Faculdade de Farmácia
Functionalization of Silicone Catheters with
Glycolipids
Cristiana Vieira Pontes
Trabalho de Campo de Mestrado Integrado em Ciências Farmacêuticas apresentado à Universidade de Lisboa através da Faculdade de Farmácia
Orientador: Doutora Isabel A. C. Ribeiro, Professora Auxiliar
Resumo
As infeções associadas aos cuidados de saúde são atualmente um grave problema clínico, provocando um aumento do tempo de hospitalização, morbilidade e mortalidade. A maioria destas infeções resulta da formação de biofilmes nos dispositivos médicos utilizados, como os cateteres de silicone, devido à hidrofobicidade das suas superfícies que favorece a colonização de microrganismos. Deste modo, novas estratégias para prevenir a formação de biofilmes nos dispositivos médicos são necessárias, entre as quais se destaca a funcionalização permanente das superfícies com compostos ativos. Os soforolípidos são glicolípidos bem estudados que apresentam a capacidade de criar superfícies com atividade antibiofilme, tornando-os assim numa abordagem promissora para prevenir a formação de biofilmes.
No presente estudo, foi investigada pela primeira vez a aplicação de uma técnica de tratamento com plasma na ligação de soforolípidos a segmentos de silicone com o objetivo de se obter uma superfície funcionalizada que mantenha as propriedades antibiofilme proporcionadas pelos soforolípidos por um longo prazo.
Os soforolípidos produzidos por Starmerella bombicola foram utilizados na funcionalização dos segmentos de silicone após estes terem sido submetidos a um tratamento com plasma de ar. A superfície obtida foi caracterizada através de medições do ângulo de contato, análises de FTIR-ATR e SEM e as propriedades antibiofilme foram avaliadas através da quantificação de unidades formadoras de colónias de Staphylococcus aureus.
Os resultados revelaram uma diminuição da hidrofobicidade do silicone, que se deve à presença dos soforolípidos na sua superfície. No entanto, não foram detetados sinais de moléculas de soforolípidos nas análises FTIR-ATR e SEM e não foi conseguida inibição da formação de biofilme.
Com este estudo concluiu-se que a técnica de funcionalização testada originou uma superfície funcionalizada com soforolípidos, em baixa quantidade, capaz de gerar uma superfície menos hidrofóbica. No entanto, essa quantidade não foi suficiente para ser detetável nem para produzir uma superfície com propriedades antibiofilme.
Palavras-chave: Soforolípidos; Tratamento com plasma; Dispositivos médicos;
Abstract
Healthcare-associated infections are a nowadays major clinical problem that lead to an increase in hospital staying time, mobility and mortality. The majority of these infections result from the biofilm formation in medical devices currently used, such as silicone catheters, due to their hydrophobic surface that favors the microorganisms colonization. Thus, new strategies to avoid the biofilm formation in medical devices are obligatory and among those is surface long-lasting functionalization with active compounds. Sophorolipids are well known glycolipids that represent a promising approach due to their ability to generate antibiofilm surfaces.
In the present study it was investigated for the first time the application of a plasma treatment technique for the linkage of sophorolipids on silicone rubber surface indeed for catheter devices in order to generate a functionalized surface that maintains the antibiofilm properties given by sophorolipids for a long term.
Sophorolipids produced by Starmerella bombicola were used to attach the silicone segments surface after their activation by an air plasma treatment. Surface characterization was evaluated through contact angle measurements, FTIR-ATR and SEM and antibiofilm properties were assessed through colony forming units quantification of Staphylococcus aureus.
Results revealed a decrease in the hydrophobicity of silicone segments that was due to the presence of sophorolipids in the surface. However, no signs of sophorolipids molecules were detected in FTIR-ATR or SEM analyzes and no inhibition of biofilm formation was achieved.
From this study it was possible to conclude that the tested functionalization technique results in a small amount of sophorolipids at the silicone surface that was capable to create a less hydrophobic surface. Nevertheless, it is not enough to be detectable neither to develop an antibiofilm surface.
Acknowledgements
I would like to thank all the people who contributed in some way to the development of this work.
I would first like to thank my advisor Professora Doutora Isabel Ribeiro for the years of working together on this research, always with an excellent orientation and dedication. I am grateful for the invitation to participate in this project, for all the confidence, encouragement and teaching that allowed me to grow up as a pharmaceutical student.
I also would like to express my very profound gratitude to my parents, for their unfailing support, encouragement and patience during all my years of study and through the process of researching and writing this work. For always being the first ones to believe in me and in my capacities to get here. This accomplishment would not have been possible without them.
Finally, to my wonderful colleagues for all the friendship, frustrations and accomplishments that we shared over the course.
Abbreviations
ATCC American Type Culture Collection
BHI Brain heart infusion broth
CBS Centraalbureau voor schmieel cultures
CFU Colony forming units
ECDC European Centre for Disease Control and Prevention
EPS Extracellular polysaccharide
FTIR-ATR Fourier transform infrared spectroscopy with attenuated total reflectance
GYPA Glucose yeast peptone agar
HAIs Healthcare-associated infections
ICU Intensive care unit
MTPs Microtiter plates
OD600 Optical density at 600 nm
SEM Scanning electron microscopy
SLs Sophorolipids
TSA Tryptone-casein soy agar
List of Contents:
1 Introduction ... 8
2 Materials and Methods ... 12
2.1 Chemicals ... 12
2.2 Preparation of silicone rubber specimens ... 12
2.3 Microorganisms and culture conditions ... 12
2.4 Sophorolipids production ... 13
2.5 Surface functionalization of silicone rubber specimens with sophorolipids 13 2.6 Surface characterization ... 14
2.6.1 Contact angle measurements ... 14
2.6.2 Fourier transform infrared spectroscopy with attenuated total reflectance (FTIR-ATR) ... 14
2.6.3 Scanning electron microscopy (SEM) ... 15
2.7 Evaluation of the modified surface antibiofilm properties ... 15
2.7.1 Inoculum adjustment ... 15
2.7.2 Biofilm formation ... 15
2.7.3 Biofilm viability ... 16
2.7.3.1 Colony forming units (CFU) quantification ... 16
3 Results ... 17
3.1 Surface characterization ... 17
3.1.1 Contact angle measurements ... 17
3.1.2 FTIR-ATR and SEM analysis ... 21
3.2 Evaluation of the modified surface antibiofilm properties ... 21
3.2.1 Inoculum adjustment ... 21
3.2.2 Biofilm viability ... 22
3.2.2.1 Colony forming units quantification ... 22
4 Discussion ... 24
5 Conclusions ... 26
References ... 27
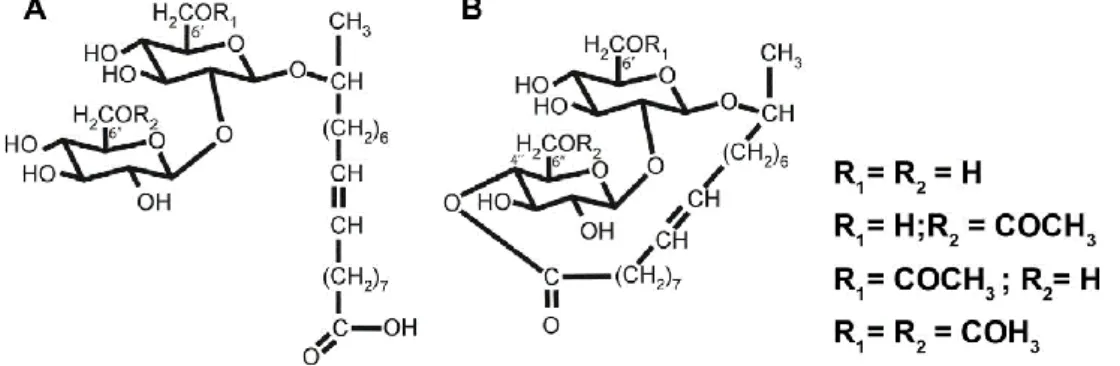
List of Figures: Figure 1. Sophorolipids produced by S. bombicola in the acidic (A) and lactonic (B) form. ... 10
Figure 2. Contact angle (Ɵ°) measurements at time = 0 s in Silicone-SLs specimens with different washing times (0.5, 1, 1.5 and 24 h). ... 17
Figure 3. Contact angle (Ɵ°) measurements along 10 min in Silicone-SLs specimens with 24 h of washing time. ... 18
Figure 4. Contact angle (Ɵ°) measurements along 10 min in Silicone-SLs specimens with different plasma treatment times (5, 10, 15, 20 and 30 min). ... 19
Figure 5. Surface wettability of silicone specimens. ... 20
Figure 6. Calibration curve obtained with spectrophotometer Hitachi U 2000. ... 21
Figure 7. Biofilm CFU counts. ... 22
Figure 8. Biofilm CFU counts in Silicone-SLs specimens with different plasma treatment times (5, 10, 15, 20 and 30 min). ... 23
1 Introduction
Healthcare-associated infections (HAIs) are infections acquired by patients while receiving treatment for an unrelated condition in any healthcare setting. Associated with an increase in healthcare costs, such infections are a major problem for patients, leading to high rates of morbidity and mortality (1,2). According to the European Centre for Disease Control and Prevention (ECDC), in 2014, 8% of the patients who stayed in an intensive care unit (ICU) for more than two days acquired at least one HAI. Among these HAIs the most frequents were pneumonia (5.3%), urinary tract infections (3.1%) and bloodstream infections (3.0%) (3).
The majority of HAIs are associated with a predisposition to infection caused by invasive medical devices used in many procedures such as the insertion of intravascular and urinary catheters (4). Indeed, in 2014, bloodstream and urinary infections acquired in ICU were reported as catheter-related in 43.3% and 96.7% of the cases, respectively (3). Bacteria commonly isolated from infected catheters include Candida species, coagulase-negative staphylococci, Staphylococcus aureus and Staphylococcus epidermidis in case of bloodstream catheters and Escherichia coli, Klebsiella pneumoniae, Klebsiella oxytoca and Enterococcus species in urinary catheters (2). The contamination of the medical device initiates with a small number of microorganisms, which are present in the skin of the patient or healthcare personnel, contaminated water or other sources in the environment (5). Moreover, physicochemical properties of the surfaces of these devices, such as hydrophobicity, provide ideal environment for initial microorganisms to attach and colonize, leading to biofilm formation and growth (5,6). Biofilms are communities of microorganisms attached into surfaces and surrounded in an organic polymeric substance. Biofilm formation can be described in three stages: attachment, maturation and dispersion. In the first stage, the cells adhere to the surface through increased shear forces, bacterial motility, electrostatic interactions and features of the microbial cell surface including flagella, pili, fimbriae and glycocalyx (5). Then, adhered cells start the production of extracellular polysaccharide (EPS) which leads to the bacterial irreversible attachment and initiation of biofilm formation (7). During the maturation phase, the attached microbes multiply and form several layered cell clusters that are enclosed by the EPS matrix. Within the EPS matrix, intercellular signaling or quorum sensing takes place,
cells get specialized functions and more biofilm scaffolds, such as proteins, DNA and polysaccharides are secreted into the biofilm structure (7,8). Once the biofilm is matured, some bacteria get detached and start dispersing in response to several factors such as lack of nutrients, intense competition and outgrown population. The release of planktonic bacteria can cause a systemic infection or restart the biofilm process at other locations (5,8). Growth within biofilm state provides important advantages to microorganisms. Bacteria can easily interact to each other leading to dissemination of nutrients, chemical signals and genes mutations. Furthermore, due to the protection offered by EPS matrix, it is difficult for the immune system and antibiotics to penetrate and eradicate the cells in biofilm community, and therefore, the biofilm infection becomes persistent (7,8).
Control the formation and development of biofilms becomes mandatory in the fight against catheters-related infections. Due to biofilms resistance against antimicrobials, new strategies for the prevention of biofilm formation are necessary instead of strategies that focus on disruption of the formed biofilm (9,10). In this context, many efforts have been made to find strategies to modify the surface of medical devices, in order to prevent the bacterial adhesion and subsequent biofilm formation (11).
A common approach is the impregnation of catheters surface with antimicrobial substances capable of interfering with the attachment and expansion of immature biofilms. Some recent examples are the use of several antibiotics such as azithromycin (12), rifampin (13), gentamicin (14) and dicloxacillin (15), antiseptics like chlorhexidine (16,17), antimicrobial peptides (18,19), silver particles (20,21) and quaternary ammonium (22). Nevertheless, the use of antimicrobials in medical devices has been debated due to the risk of bacterial resistance and potential toxicity (23). Modification of the chemical or physical surface properties of medical devices creating antiadhesive surfaces is another valuable strategy that can help to overcome the limitations associated with the use of antimicrobial coatings (5,23). Common strategies include coating catheters surface with antiadhesive polymers (24–26) or hydrophilic hydrogels (27,28).
Surface-active agents (biosurfactants), such as glycolipids, represent a promising approach for the formation of antiadhesive surfaces. These amphiphilic compounds are capable of changing properties like surface tension and hydrophobicity and therefore
interfering with the microbial adhesion (29). Sophorolipids (SLs) are one of the most promising glycolipid biosurfactants due to their low toxicity, high biodegradability, stability at extremes of pH and temperature, antimicrobial activity and the possibility of large-scale industrial production (30). SLs are produced as a mixture by non-pathogenic yeast species, including Candida apicola, Rhodotorula bogoriensis, Starmerella bombicola, Wickerhamiella domercqiae and Candida batistae. Their structure consists of a hydrophilic dimer of sophorose suger linked through a glycosidic bond to a hydroxy fatty acid hydrophobic tail (Figure 1). The sophorose moiety can present different acetylation degrees and be esterified with the carboxylic end. Therefore, SLs can occur in the lactonic (esterified) or in the acidic (not esterified) form (30–32).
Figure 1. Sophorolipids produced by S. bombicola in the acidic (A) and lactonic (B) form.
The antibiofilm potential of these glycolipids was already assessed in a previous study (33). Adsorption of SLs onto silicone rubber intended for medical catheters generates simultaneously antiadhesive and antimicrobial surfaces that were able to reduce the biofilm charge of S. aureus and E. coli while revealed to be biocompatible to HaCaT cell line. Thus, functionalizing catheters surface with SLs represents a very promissory strategy to prevent biofilm infections on these medical devices (33).
Nevertheless, additional research is needed to find strategies to coat catheters surfaces with SLs in order to achieve long-lasting functionalization avoiding their washout from the surface and thus maintain the antibiofilm properties of the modified surface for a long time (33). In the last years, plasma treatment has been pointed out as a versatile and biocompatible technique for surface modification of materials. This approach can be used for permanent functionalization of surfaces, since plasma-treated surfaces act as interfacial bonding layers for the subsequent immobilization of diverse molecules (22).
The present study aimed to investigate a plasma-based approach for SLs immobilization onto silicone rubber surfaces designed for medical catheters application and then evaluate if the previously revealed antibiofilm properties of SLs-functionalized silicone surfaces are maintained when SLs are immobilized instead of adsorbed.
2 Materials and Methods
2.1 Chemicals
The following chemicals and solvents were used: glucose, sodium sulphate 99%, sodium chloride, potassium chloride, sodium phosphate dibasic and potassium phosphate monobasic from Sigma-Aldrich (St. Louis, USA); barium chloride dehydrate from Riedel-de Haën (Seelze, Germany); sulfuric acid 95-97%, n-hexane and oleic acid (extra pure) from Merck (Darmstadt, Germany); ethyl acetate from Thermo Fisher Scientific (Massachusetts, USA); agar bacteriological and peptone from Oxoid (Hampshire, UK); yeast extract and tryptone-casein soy broth (TSB) from Biokar Diagnostics (Allonne, France); brain heart infusion broth (BHI) from Liofilchem (Roseto degli Abruzzi, Italy); liquid silicone rubber and catalyst from Wacker (Munich, Germany); Milli-Q water (Millipore).
2.2 Preparation of silicone rubber specimens
Silicone rubber segments were made in the laboratory to simulate the surface of catheters tubes. Liquid silicone rubber was blended with 10%(w/w) of catalyst using an agate mortar. The mixture was cured on aluminum molds for 24 h under room temperature and atmospheric pressure. Solid silicone was then cut into segments with 0.8 x 0.8 cm2 or 2 x 2 cm2 of area. The obtained squares were washed twice with deionized water and sterilized at 121 °C for 15 min in a vertical autoclave (Medline Italy).
2.3 Microorganisms and culture conditions
Starmerella bombicola CBS 6009 was obtained from the Fungal Biodiversity Centre, CBS (Centraalbureau voor Schimmelcultures) and stored at 4 °C on glucose yeast peptone agar (GYPA) slants with monthly transfers.
Staphylococcus aureus ATCC 25923 was obtained from the American Type Culture Collection (ATCC) and stored at 4 °C on tryptone-casein soy agar (TSA) plates with
weekly transfers. Cultures growth were performed in an incubator (Thermo Scientific Revco Ultima) for 24 h at 37 °C.
Culture mediums used in the present work were sterilized at 121 °C for 15 min in a vertical autoclave (Medline Italy) and prepared under aseptic conditions in a vertical laminar flow chamber (PBI mini flow chamber).
2.4 Sophorolipids production
SLs were produced by non-pathogenic yeast Starmerella bombicola according to previous studies about SLs biosynthesis (31,34). The procedure started with a preculture in GPY medium (four colonies in 20 mL of medium in 100 mL shake flask) that grown for 24 h at 31 °C and 120 rpm on an orbital incubator (Gallenkamp Cooled). Subculture was prepared by transferring 20 mL of preculture into 1L shake flask with 200 mL GPY medium supplemented with 2%(V/V) of oleic acid as lipidic carbon source. This culture was incubated at 31 °C and 120 rpm on the orbital incubator for 144 h. After the time of growth, the produced SLs were extracted twice with ethyl acetate (1:1) and centrifuged (Allegra 6R Beckman Coultar) at 4000 rpm for 5 min. Afterward, the extracts were dehydrated with sodium sulphate and the solvent was evaporated on a rotatory-evaporator (heidolph rotary evaporator vv 2000) at 40 °C. To remove the residual oleic acid on the resulted extracts, it was added n-hexane (1:3) twice following by centrifugation at the same conditions mentioned before. Residual n-hexane was then removed under nitrogen stream.
2.5 Surface functionalization of silicone rubber specimens with
sophorolipids
The surface functionalization of silicone rubber specimens was carried out in two steps. At first, silicone rubber specimens were treated with air plasma to allow the occurrence of reactive groups on their surface. This step was performed in a High Power Expanded Plasma Cleaner (Harrick Plasma, USA). Silicone specimens were introduced inside the plasma cleaner and exposed to 5 min of reduced pressure vacuum following by air plasma discharge. Different plasma treatment times ranging from 5 to 30 min were experienced to determine the influence of plasma treatment duration on surface
functionalization. The second step consisted in the attachment of SLs molecules to the reactive groups formed on silicone surface. After plasma treatment, the activated silicone specimens were immediately immersed in SLs aqueous solutions ranging from 0.375 to 1.5 mg mL-1 and maintained for approximately 3 h under agitation at 200 rpm (Grant bio PMS-1000 microplate shaker). Silicone specimens were then recovered and dried in a desiccator with a vacuum pump for at least 24 h. Before surface characterization, silicone specimens were washed with Milli-Q water under agitation at 200 rpm (ika-MS 3 digital shaker) and dried in the desiccator for 24 h. Different washing times ranging from 30 min to 24 h were tested to determine the ideal washing time for the removal of non-immobilized molecules.
Obtained silicones will be further designated as Silicone-SLsconcentration according to the SLs concentration solution from which the modified silicone was obtained. Untreated silicone samples and silicone specimens only treated with air plasma (Silicone-SLs0) were used as controls.
2.6 Surface characterization
2.6.1 Contact angle measurements
Contact angle measurements were carried out with the sessile drop method. Using a micropipette, 2 µL of Milli-Q water were deposited on the surface of the vacuum-dried silicone specimens after surface modification. The images of the obtained water drops were photographed along 10 min through a digital microscope (Rohs Usb Digital Microscope) which was connected to the computer. Contact angles were evaluated by image analysis using the ImageJ software. All measurements were done at room temperature (22 ºC). Experiments were done in duplicate and it was used no less than four drops per specimen.
2.6.2 Fourier transform infrared spectroscopy with attenuated total reflectance (FTIR-ATR)
FTIR-ATR (Thermo Scientific, Class 1 Laser Product Nicolet 6700) was used to evaluate SLs immobilization onto silicone segments surface by detecting characteristics bands of the SLs and silicone chemical groups. Samples (untreated silicones, SLs
powder and silicone segments functionalized with SLs) were placed on the ATR diamond crystal with an incidence angle of 42°. Spectra ranging from 4000 to 8000 cm -1 resulting from the average of 32 scans collected with a resolution of 8 cm-1 were obtained. OMNIC Software was used for spectra acquisition.
2.6.3 Scanning electron microscopy (SEM)
SEM analysis was used for the observation of silicone specimens surface allowing to compare their roughness. Modified silicone segments and untreated silicones were analyzed through a field emission gun scanning electron microscope FEG-SEM model JSM7001F (JEOL, Japan) operated at 10 Kv. Samples were covered with a thin layer of conductive gold film, under vacuum in an argon atmosphere, to increase their conductivity (Quorum Technologies, Polaron E5100).
2.7 Evaluation of the modified surface antibiofilm properties
2.7.1 Inoculum adjustment
McFarland standard was used as a reference to adjust the turbidity of bacterial suspensions in order to standardize the number of bacteria for biofilm assays. McFarland scale was prepared by mixing different volumes of 1%(V/V) sulphuric acid and 1%(w/V) barium chloride aqueous solutions to obtain barium sulphate precipitate standards with different turbidity representing a range of bacteria concentrations from 0 to 21 x 108 cells mL-1. The optical density was determined at 600 nm (OD600) in a spectrophotometer (Hitachi U 2000) and a calibration curve was obtained.
2.7.2 Biofilm formation
For biofilm formation onto modified silicones surface, the specimens were fixed on 24-well microtiter plates (MTPs). The inoculum, adjusted to 1.0 McFarland units, was prepared by suspending bacterial colonies from 24 h-cultured TSA plates in BHI medium. The obtained bacterial suspension was diluted in BHI medium with glucose at 1%(w/V) and 1 mL was added to the microtiter wells in a way that the final bacterial concentration in each well was approximately 106 CFU mL-1. MTPs were incubated at
37 °C for 24 h under static conditions. Assays were carried out at least in three independent experiments.
Biofilm formation onto silicone surfaces with SLs absorbed instead of immobilized was used as positive control. The adsorption assay was carried out according to the previous study (33). SLs solutions with a concentration of 0.75 and 1.5 mg mL-1 were used to promote SLs adsorption onto silicone surface through overnight incubation in MTPs under agitation at 120 rpm (ika-KS 130 basic orbital shaker) at 25 °C.
2.7.3 Biofilm viability
2.7.3.1 Colony forming units (CFU) quantification
After biofilm growth, the planktonic suspension was removed from the wells and the attached cells were washed two times with 1 mL of phosphate buffered saline (PBS). Silicone specimens with the attached biofilm were transferred to microcentrifuge tubes filled with BHI medium and vigorously shaken (vortex) for 4 min to resuspend the biofilm. Biofilm resuspended solution was diluted (1:20000) and an aliquot was spread into TSA plates for CFU counting. The plates were incubated at 37 °C for 24 h and the CFU cm-2 in silicone segments were calculated.
3 Results
3.1 Surface characterization
3.1.1 Contact angle measurements
The first contact angle evaluations were performed with the purpose of determining the ideal washing time for the removal of non-immobilized molecules on silicone specimens after the surface modification process, so it could be assured that the results obtained would be only due to the SLs immobilized on the surface. For this evaluation, the same silicones specimens were washed four times (making a total of 24 h) and their contact angles at time = 0 s (immediately after the deposition of the drop) were measured between each of the washes. As it can be seen in Figure 2, the contact angle values after 1.5 h of washing were identical with the values obtained after 24 h in all the tested samples, which indicates that there was no difference in the amount of SLs on silicone surface after these two washing times. Thus, non-immobilized molecules were fully removed after 1.5 h of washing.
Figure 2. Contact angle (Ɵ°) measurements at time = 0 s in Silicone-SLs specimens with different washing times (0.5, 1, 1.5 and 24 h).
All Silicone-SLs specimens were submitted to 5 min of plasma treatment. The contact angle values of silicone specimens with 1.5 and 24 h of washing time were
identical.
The wettability properties of Silicone-SLs specimens with 24 h of washing time were also assessed along 10 min (Figure 3) and it was verified differences in contact angle values between the various Silicone-SLs specimens after stabilization (10 min).
Figure 3. Contact angle (Ɵ°) measurements along 10 min in Silicone-SLs specimens with 24 h of washing time.
All Silicone-SLs specimens were submitted to 5 min of plasma treatment. Silicone-SLs0.9 and Silicone-SLs0.75 specimens achieved a notable reduction in
contact angle values after 10 min of stabilization.
Contact angle measurements were also performed to study the influence of plasma treatment duration on surface functionalization. For this purpose, the wettability properties of silicone specimens submitted to different plasma treatment times ranging from 5 to 30 min were assessed (Figure 4). Three points of Silicone-SLs concentrations were selected for this evaluation, 0, 0.75 and 1.5 mg mL-1. Only slight differences were observed between the various plasma times in the Silicone-SLs specimens that were tested and so no correlation was found between the plasma treatment time and the decrease in contact angle. Thus, the shortest plasma treatment time (5 min) was selected for all functionalization experiments.
Figure 4. Contact angle (Ɵ°) measurements along 10 min in Silicone-SLs specimens with different plasma treatment times (5, 10, 15, 20 and 30 min).
(A) Silicone-SLs0. (B) Silicone-SLs0.75. (C) Silicone-SLs1.5. All Silicone-SLs
specimens were submitted to the same washing time (1.5 h). No big differences were observed in contact angle values between the various times of plasma
treatment tested in each Silicone-SLs specimen.
The wettability properties of silicone specimens modified with different SLs concentrations were assessed through contact angle measurements during 10 min of Silicone-SLs specimens submitted to the selected washing time of 1.5 h (3 x 0.5 h) and plasma treatment time of 5 min (Figure 5 A). The wettability properties of Silicone-SLs0.375 specimens were very similar to those of Silicone-SLs0 specimens (82 ± 3° and 81 ± 9°, respectively) and in both specimens the wettability properties were slightly lower than in silicone without any treatment (83 ± 3°). Differences between the contact angles of silicone specimens with and without SLs functionalization could be verified for SLs concentrations higher than 0.375 mg mL-1. However, the decrease in contact angle was not directly proportional to the SLs concentration. The higher decrease in
contact angle was observed with Silicone-SLs0.75 which was in order of 50 ± 7° (after stabilization). The contact angles of Silicone-SLs0.9 and Silicone-SLs1.25 were identical (around 60°) and Silicone-SLs1.5 experienced a lower decrease (68 ± 16°). An additional issue is that according to the results error bars, this method of silicone functionalization seems to be not very reproducible. In Figure 5 B is present an image of water drops on silicone specimens surface at initial time (0 s) and after 10 min, which illustrates the wettability changes in silicone surfaces modified with SLs.
Figure 5. Surface wettability of silicone specimens.
(A) Contact angle (Ɵ°) measurements along 10 min in Silicone-SLs specimens. Differences in contact angle values were observed only in silicone specimens with
SLs concentrations higher than 0.375 mg mL-1. (B) Images of water drops onto
Silicone-SLs specimens surface (Silicone-SLs0; Silicone-SLs0.75; Silicone-SLs0.9;
Silicone-SLs1.5) at t = 0 s and t = 10 min. All Silicone-SLs specimens were
3.1.2 FTIR-ATR and SEM analysis
The spectrum of silicones modified with SLs exhibited the same peak positions as untreated silicone spectrum. Characteristic bands of SLs powder were not detected in any of the silicone specimens. Regarding the SEM analysis, no differences were detected in surface roughness between modified silicones and untreated silicones.
3.2 Evaluation of the modified surface antibiofilm properties
3.2.1 Inoculum adjustment
Through the OD600 of McFarland scale measured in a spectrophotometer (Hitachi U 2000) it was made a graphic (Figure 6) from which a calibration curve (1) that correlates the concentrations of bacterial cells with the absorbance of the suspensions was obtained.
𝐴𝑏𝑠𝑜𝑟𝑏𝑎𝑛𝑐𝑒 = 0.0586 × 𝑐𝑒𝑙𝑙𝑠 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 (× 108) + 0.0222 (1) The correlation factor of 0.993 indicates that the curve is representative of the linear relationship between the variables. This calibration curve was used along the experimental work to adjust the inoculum value for biofilm formation.
3.2.2 Biofilm viability
3.2.2.1 Colony forming units quantification
The antibiofilm properties of silicone specimens were evaluated through CFU quantification (Figure 7 A). No differences were observed in CFUs between modified and non-modified silicones, as the plate count remained around 108 CFU cm-2 in all the tested samples. The CFU counts of biofilm formed on silicones with SLs adsorbed onto their surface were also assessed to assure that the SLs used in the modification process of silicone specimens were intact and that the lack of differences observed in the results was not due to the degradation of SLs used. In Figure 7 B it is possible to verify a pronounced reduction in CFU of silicone specimens with SLs adsorbed onto their surface when compared with silicone without any treatment which agrees with the previous study on SLs antibiofilm proprieties (33).
Figure 7. Biofilm CFU counts.
(A) Silicone specimens modified with SLs functionalization process (5 min of plasma treatment and 1.5 h of washing time). US – Untreated silicone. No differences were observed in the CFUs between the tested specimens. (B) Silicone
specimens with adsorbed SLs. Two different SLs solution concentrations (0.75 and 1.5 mg mL-1) were used to promote adsorption. In both concentrations, it
was observed a reduction in the CFUs relatively to the untreated silicone.
Silicone-SLs specimens with different plasma treatment times were also submitted to plate count assay (Figure 8). The plate count units of each Silicone-SLs concentration were identical with the various times of plasma treatment experienced.
Figure 8. Biofilm CFU counts in Silicone-SLs specimens with different plasma treatment times (5, 10, 15, 20 and 30 min).
(A) Silicone-SLs0. (B) Silicone-SLs0.75. (C) Silicone-SLs1.5. All Silicone-SLs
specimens were submitted to the same washing time (1.5 h). No differences were observed in the CFUs between the various times of plasma treatment tested in
4 Discussion
This study aimed to explore, for the first time, a plasma treatment technique for silicone rubber surface functionalization with SLs as a strategy to link the glycolipids more strongly with the silicone surfaces intended for catheter medical devices and thereby maintain the antibiofilm properties of SLs-modified surfaces for a long-term. For this purpose, silicone segments were submitted to an air plasma discharge, in which occurs the creation of reactive groups on their surface, and then immersed in SLs aqueous solutions allowing the created reactive groups to interact with SLs by chemical interfacial reactions. Simultaneously, some plasma-treated SLs-free (Silicone-SLs0) were also tested to allow to understand the effect of plasma treatment alone on silicone surface.
Contact angle measurements with Milli-Q water were used to characterize and to compare the wettability properties of the different modified silicone specimens. Surface wettability experiments showed that plasma treatment by itself has no effect on surface wettability, since contact angles of Silicone-SLs0 specimens were similar to those of untreated silicones. This means that the observed decrease in hydrophobicity is due to the surfactant effect of SLs on silicone specimens surface. When a biosurfactant, such as SLs, contacts with a solid surface, the apolar portion interacts with the hydrophobic surface, while the polar portion is exposed to the aqueous environment, resulting in a decrease in the hydrophobicity of the surface. This change interferes with the microbial adhesion on the surface (35). Nevertheless, the modification process of silicone specimens with SLs resulted in a decrease in hydrophobicity only when using SLs concentrations higher than 0.375 mg mL-1. The SLs concentration which achieved the higher decrease in hydrophobicity was 0.75 mg mL-1. Probably, the interactions that occur between the reactive groups on the activated silicone and SLs saturate at this point, not allowing the bonding of more SLs molecules.
FTIR-ATR and SEM analyzes were used with the intention of proving the immobilization of SLs on the silicone specimens surface. However, the presence of SLs on silicone surfaces was not confirmed, since it was not observed any difference in the FTIR-ATR spectrum or in the surface roughness between modified and non-modified silicones. On the other hand, the changes observed in the surface wettability assays suggest that SLs are present on silicone specimens surface, so it is possible that SLs are
indeed present but in a small amount that is lower than their limit of detection through these analyzes. In CFU counting assays, even increasing the SLs concentration and the plasma treatment time, the silicone specimens modified with the SLs functionalization process were always unable to achieve the inhibition of biofilm growth that was obtained with the silicone specimens with adsorbed SLs. Thus, the low amount of SLs present on the modified silicones were insufficient to influence the biofilm growth. It is well known that the antibiofilm properties of SLs are related to their ability to create antiadhesive surfaces due to the changes in hydrophobicity effect and also to their antimicrobial activity (36). The functionalization process of silicone surfaces investigated in this work promoted a decrease in hydrophobicity. Nevertheless, the expected antibiofilm action of SLs was not verified. A possible explanation might be that this functionalization process results in the modification of silicone surface with SLs in a small amount that is not enough to exhibit their antimicrobial activity and the decrease in hydrophobicity by itself is unable to inhibit the biofilm formation.
The lack of studies involving plasma-based approaches to functionalize surfaces with biosurfactants limits the conclusion about their applicability for this purpose. More studies on this approach, specially to understand the type of reactions and connections that occur between the SLs, the reactive groups created by the plasma and the silicone surface, might be useful to modulate and optimize this technique. Additionally, the use of linkers to immobilize the SLs to the silicone surface through a covalent linkage in the plasma treatment technique could be a valuable approach in future works to achieve the pretended permanent functionalization.
5 Conclusions
This study investigated for the first time the application of a plasma treatment technique for the linkage of SLs with silicone segments surface aimed for medical catheters in order to achieve a more lasting functionalization and so prolong the antibiofilm properties of SLs-modified surfaces. Silicone rubber segments were submitted to an air plasma discharge and then immersed in SLs aqueous solutions for the attachment of SLs molecules to the reactive groups formed on the surface.
In surface wettability assays, the silicone specimens submitted to the plasma treatment technique with SLs concentrations higher than 0.375 mg mL-1 showed a less hydrophobic surface. Despite this, the decrease in the hydrophobicity was not directedly proportional to the SLs concentration, as it was obtained the higher decrease with the SLs concentration of 0.75 mg mL-1. These assays also revealed that the tested technique is not very reproducible. Although the presence of SLs on silicone specimens surface was manifested in the changes observed in the hydrophobicity, no signs of SLs molecules were detected in FTIR-ATR or SEM analyzes and no inhibition of biofilm formation was achieved in CFU counting assays. Moreover, the increase in plasma treatment time had no correlation with the decrease in hydrophobicity or with the biofilm inhibition.
The results obtained in the present study showed that the tested modification technique of silicone segments with SLs is unable to develop an antibiofilm surface. The amount of SLs that is exposed at the silicone surface with this technique can in fact generate a less hydrophobic surface, but is not enough to be detectable in the analyzes made or to inhibit the biofilm formation.
Future work could be focus on the investigation of strategies to immobilize SLs to the silicone surface through a covalent linkage with the use of linkers in the plasma treatment technique.
References
1. World Health Organization (Who). Report on the Burden of Endemic Health Care-Associated Infection Worldwide. WHO Library Cataloguing-in-Publication Data. 2011.
2. Babady NE. Hospital-Associated Infections. Microbiol Spectr. 2016;4(3):1–22. 3. European Centre for Disease Prevention and Control (ECDC). Annual epidemiological report 2014. Antimicrobial resistance and healthcare-associated infections. Stockholm; 2015.
4. Revelas A. Healthcare-associated infections: A public health problem. Niger Med J. 2012;53(2):59–64.
5. Percival SL, Suleman L, Donelli G. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol. 2015;64(4):323–34.
6. Shunmugaperumal T. Microbial colonization of medical devices and novel preventive strategies. Recent Pat Drug Deliv Formul. 2010;4(2):153–73. 7. Veerachamy S, Yarlagadda T, Manivasagam G, Yarlagadda PK. Bacterial
adherence and biofilm formation on medical implants: A review. Proc Inst Mech Eng Part H J Eng Med. 2014;228(10):1083–99.
8. N. Rabin et al. Biofilm formation mechanisms and targets for developing antibiofilm agents. Med Chem (Los Angeles). 2007;7(4):493–512.
9. Gupta P, Sarkar S, Das B, Bhattacharjee S, Tribedi P. Biofilm, pathogenesis and prevention - a journey to break the wall: a review. Arch Microbiol. 2016;198(1):1–15.
10. Abdel-Aziz SM, A A. Bacterial Biofilm: Dispersal and Inhibition Strategies. Sch J Biotechnol. 2014;1(1).
11. Tenke P, Köves B, Johansen TEB. An update on prevention and treatment of catheter-associated urinary tract infections. Curr Opin Infect Dis. 2014;27(1):102–7.
impregnated urinary catheters avert bacterial colonization, biofilm formation, and inflammation in a murine model of foreign-body associated urinary tract infections caused by Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61(3).
13. Rose WE, Otto DP, Aucamp ME, Miller Z, De Villiers MM. Prevention of biofilm formation by methacrylate-based copolymer films loaded with rifampin, clarithromycin, doxycycline alone or in combination. Pharm Res. 2015;32(1):61–73.
14. Rafienia M, Zarinmehr B, Poursamar SA, Bonakdar S, Ghavami M, Janmaleki M. Coated urinary catheter by PEG/PVA/gentamicin with drug delivery capability against hospital infection. Iran Polym J (English Ed. 2013;22(2):75– 83.
15. Stenger M, Klein K, Grønnemose RB, Klitgaard JK, Kolmos HJ, Lindholt JS, et al. Co-release of dicloxacillin and thioridazine from catheter material containing an interpenetrating polymer network for inhibiting device-associated Staphylococcus aureus infection. J Control Release. 2016;241:125–34.
16. Shapur NK, Duvdevani M, Friedman M, Zaks B, Gati I, Lavy E, et al. Sustained release varnish containing chlorhexidine for prevention of biofilm formation on urinary catheter surface: in vitro study. J Endourol. 2012;26(1):26–31.
17. Phuengkham H, Nasongkla N. Development of antibacterial coating on silicone surface via chlorhexidine-loaded nanospheres. J Mater Sci Mater Med. 2015;26(2):78.
18. Mishra B, Basu A, Chua RRY, Saravanan R, Tambyah PA, Ho B, et al. Site specific immobilization of a potent antimicrobial peptide onto silicone catheters: evaluation against urinary tract infection pathogens. J Mater Chem B. 2014;2(12):1706–16.
19. Lim K, Chua RRY, Ho B, Tambyah PA, Hadinoto K, Leong SSJ. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015;15:127–38. 20. Cooper IR, Pollini M, Paladini F. The potential of photo-deposited silver
2016;69:414–20.
21. Ogilvie AT, Brisson BA, Singh A, Weese JS. In vitro evaluation of the impact of silver coating on Escherichia coli adherence to urinary catheters. Can Vet J. 2015;56(5):490–4.
22. Zanini S, Polissi A, Maccagni EA, Dell’Orto EC, Liberatore C, Riccardi C. Development of antibacterial quaternary ammonium silane coatings on polyurethane catheters. J Colloid Interface Sci. 2015;451:78–84.
23. Desrousseaux C, Sautou V, Descamps S, Traoré O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J Hosp Infect. 2013;85(2):87–93.
24. Keum H, Kim JY, Yu B, Yu SJ, Kim J, Jeon H, et al. Prevention of Bacterial Colonization on Catheters by a One-Step Coating Process Involving an Antibiofouling Polymer in Water. ACS Appl Mater Interfaces. 2017;9(23):19736–45.
25. Ding X, Yang C, Lim TP, Hsu LY, Engler AC, Hedrick JL, et al. Antibacterial and antifouling catheter coatings using surface grafted PEG-b-cationic polycarbonate diblock copolymers. Biomaterials. 2012;33(28):6593–603. 26. Singha P, Pant J, Goudie MJ, Workman CD, Handa H. Enhanced antibacterial
efficacy of nitric oxide releasing thermoplastic polyurethanes with antifouling hydrophilic topcoats. Biomater Sci. 2017;5(7):1246–55.
27. Silva-Dias A, Palmeira-De-Oliveira A, Miranda IM, Branco J, Cobrado L, Monteiro-Soares M, et al. Anti-biofilm activity of low-molecular weight chitosan hydrogel against Candida species. Med Microbiol Immunol. 2014;203(1):25–33.
28. Kazmierska KA, Thompson R, Morris N, Long A, Ciach T. In vitro multicompartmental bladder model for assessing blockage of urinary catheters: Effect of hydrogel coating on dynamics of proteus mirabilis growth. Urology. 2010;76(2):515.e15-515.e20.
29. Banat IM, De Rienzo MAD, Quinn GA. Microbial biofilms: biosurfactants as antibiofilm agents. Appl Microbiol Biotechnol. 2014;98(24):9915–29.
Characterization and Biologic Activity. In: Gupta VK, Schmoll M, Tuohy M, Maki M, Mazutti MA, editors. Applications of Microbial Engineering. 1st ed. CRC Press; 2013. p. 367–407.
31. Ribeiro IA, Bronze MR, Castro MF, Ribeiro MHL. Sophorolipids: Improvement of the selective production by Starmerella bombicola through the design of nutritional requirements. Appl Microbiol Biotechnol. 2013;97(5):1875–87. 32. Ribeiro IA, Bronze MR, Castro MF, Ribeiro MHL. Optimization and correlation
of HPLC-ELSD and HPLC-MS/MS methods for identification and characterization of sophorolipids. J Chromatogr B Anal Technol Biomed Life Sci. 2012;899:72–80.
33. Pontes C, Alves M, Santos C, Ribeiro MH, Gonçalves L, Bettencourt AF, et al. Can Sophorolipids prevent biofilm formation on silicone catheter tubes? Int J Pharm. 2016;513(1–2):697–708.
34. Ribeiro IAC, Faustino CMC, Guerreiro PS, Frade RFM, Bronze MR, Castro MF, et al. Development of novel sophorolipids with improved cytotoxic activity toward MDA-MB-231 breast cancer cells. J Mol Recognit. 2015;28(3):155–65. 35. Korenblum E, de Araujo L, Guimarães C, de Souza LM, Sassaki G, Abreu F, et al. Purification and characterization of a surfactin-like molecule produced by Bacillus sp. H2O-1 and its antagonistic effect against sulfate reducing bacteria. BMC Microbiol. 2012;12(1):252.
36. Fracchia L, J. Banat J, Cavallo M, Ceresa C, M. Banat I. Potential therapeutic applications of microbial surface-active compounds. AIMS Bioeng. 2015;2(3):144–62.