A R C H I V E S
o f
F O U N D R Y E N G I N E E R I N G
Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences
ISSN (1897-3310) Volume 11 Issue 4/2011
51 – 54
10/4
A R C H I V E S o f F O U N D R Y E N G I N E E R I N G V o l u m e 1 1 , I s s u e 4 / 2 0 1 1 , 5 1 - 5 4 51
Modification of water glass with colloidal
slurries of metal oxides
B. Hute ra
a*
, B. Stypuła
bA. Kmita
a, P. Nowicki
aa
AGH University of Science and Technology, Faculty of Foundry Engineering, Department of Moulding Materials, Mould Technology and Foundry of Non-Ferrous Metals, 30-059 Cracow, Reymonta 23, Poland
b
AGH University of Science and Technology, Faculty of Foundry Engineering, Department of Chemistry and Metals Corrosion 30-059 Cracow, Reymonta 23, Poland
*Corresponding author. E-mail address: hutera@agh.edu.pl
Received 28.06.2011; accepted in revised form 27.07.2011
Abstract
There were taken attempts to modify the properties of water glass with suspensions of zinc oxide (ZnO) nanoparticles and boeh mite
AlOH(O) nanoparticles dissolved in various solvents. It has been shown that the wetting properties of quartz (SiO2) can be effected by:
• the amount of modifier,
• the type of solvent,
• nanoparticles size,
The best wetting properties of water glass has been achieved with the modification of ZnO nanoparticles suspended in propanol and butyl acetate ester.
Keywords: Nanoparticles, Water glass, M odification, Binder, M oulding sand
1. Introduction
Water glass is an alternative binder for moulding and core sands with organic binders. It is inexp ensive, easily available and non-toxic. Besides the advantages of the sands combined with this binder, there are also some negative characteristics, such as: brittleness, a worse knock-out property and a more difficult recovery. On the other hand, it is known that the abilities of water glass as a binder of moulding and core sands are not fully utilized. This leads to the necessity of a deeper understanding of its physico-chemical properties, such as: wettability, viscosity, as well as cohesive and adhesive strength, which enable a modification of the binder in the aspect of the improvement of the utilitarian parameters of the sand. The analysis of the technological properties of the sands with water glass shows that it is advisable to improve the strength p roperties of the sands by improving the cohesive and adhesive characteristics of the binder.
In the last years, we can see an interest in the ceramic
nano-materials (SiO2, Al2O3, CaSiO3, M gO, ZnO [1-5]) and their
application, among others, in the modification of moulding sands. These nanoparticles introduced into the systems of a multi-particle matrix (binder) can change their properties by way of a physico-chemical or chemical reaction [6, 7].
The subject of the paper is the modification of water glass by a colloidal slurries of ZnO and boehmite AlOH(O) nanoparticles, in alcohol or butyl acetate ester.
2. Materials and measurement
methodology
52 A R C H I V E S o f F O U N D R Y E N G I N E E R I N G V o l u m e 1 1 , I s s u e 4 / 2 0 1 1 , 5 1 - 5 4
• water glass R „145”; M = 2,5; d 20 = 1470 kg/m3 and
modifying colloidal solutions of oxide nanoparticles (0,3 M )
of the sizes of 10 – 100 nm,
• modifier I – suspension of ZnO nanoparticles in propanol,
• modifier II – suspension of ZnO nanoparticles in butyl
acetate ester,
• modifier III – suspension of AlOH(O) (boehmite) in butyl
acetate ester,
• optically pure quartz.
The evaluation of the wettability of quartz by the binder was performed by way of measuring the change of the value of the contact angle in time, in the system: quartz-binder, on the samples
thermostated at 20 oC. In the measurement, a prototype device for
measuring wettability was applied [8].
For the measurement of wettability, 4x8 mm quartz plates were used, whose surfaces were prepared in an identical way. This aimed at ensuring a constant free surface energy value (FSE) and the arising repeatability of the results. Each of the quartz samples was used once. The modification of water glass was performed by means of adding and homogenizing 3% or 5% mass of modifiers.
3. Test results and discussion
Figures 1 - 4 illustrate the changes of the contact angle value in time
Θ = f(τ), for the system: quartz-binder, at 20 OC. Figure 1 refers to the
non-modified water glass, and Figures 2-4 – to the water glass modified
by the suspension of ZnO nanoparticles in propanol (Fig. 2) or butyl acetate ester (Fig. 3). Figure 4 presents the changes of the contact angle
value Θ in time, for the water glass modified with the suspension of
AlOH(O) (boehmite) in butyl acetate ester.
Fig. 1. Time-related changes of contact angle Θ in a quartz-water
glass, temperature of measurement: 20o
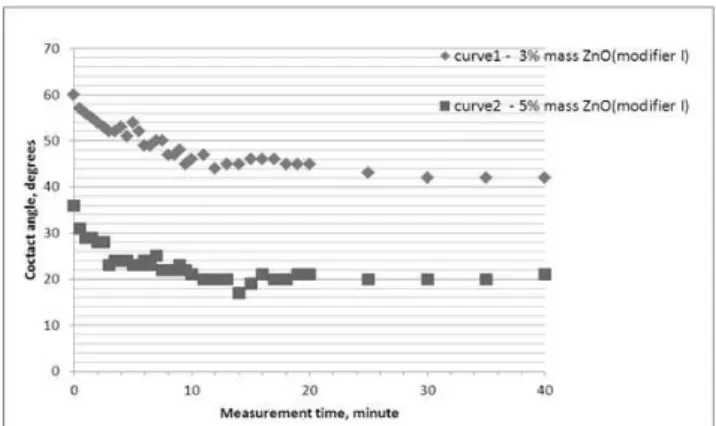
Fig. 2. Time-related changes of contact angle Θ in the quartz-binder
system; water glass + 3% mass ZnO (modifier I) suspension (curve 1), water glass + 5% mass ZnO (modifier I) suspension (curve 2), modifier concentration: calculated in relation to water glass, temperature
of measurement: 20oC
Figure 3 illustrates the effect of the addition of modifier II in the form of a suspension of ZnO nanoparticles in butyl acetate ester. Similarly to the case of modifier I, better wettability results are obtained with the application of the addition of 5% mass of
modifier II. The latter influences both the value of the initial Θ0 ,
the balance contact angle Θr and time τr. The values of the
mentioned parameters with the addition of 5% mass of modifier II equal, respectively, 46 deg and 27 deg, with the time of 11 min.
Fig. 3. Time-related changes of contact angle Θ in the quartz-binder
system; water glass + 3% mass ZnO (modifier II) suspension (curve 1), water glass + 5% mass ZnO (modifier II) suspension (curve 2), modifier concentration: calculated in relation to water glass, temperature
of measurement: 20oC
Figure 4 shows the changes of the wetting angle value of quartz by water glass modified by 3 and 5% mass of the suspension of boehmite nanoparticles in butyl acetate ester (modifier III). From
the comparison of the courses Θ = f(τ) for the binder modified by
different amounts of modifier III, it can be inferred that this
A R C H I V E S o f F O U N D R Y E N G I N E E R I N G V o l u m e 1 1 , I s s u e 4 / 2 0 1 1 , 5 1 - 5 4 53
Fig. 4. Time-related changes of contact angle Θ in a quartz-binder
system; water glass + 3% mass boehmite AlOH(O) (modifier III) suspension (curve 1), water glass + 5% mass boehmite AlOH(O)
(modifier III) suspension (curve 2), modifier concentration: calculated in relation to water glass, temperature of measurement:
20OC
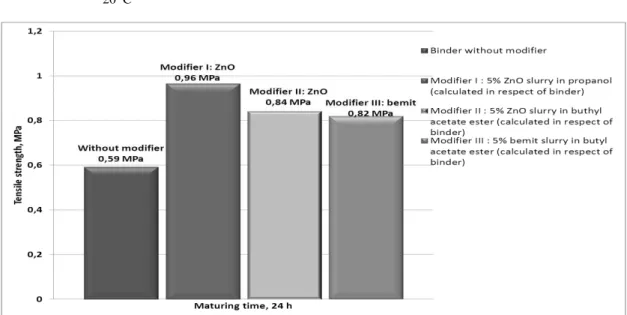
Figure 5 presents the results of the tests of the tensile strength
Rmu (after 24 h of hardening) of loose self-hardening masses with
non-modified water glass, as well as that modified by 5% mass (in proportion to the binder) of the modifiers: ZnO in propanol
(modifier I),ZnO in butyl acetate ester (modifier II), boehmite
AlOH(O) in butyl acetate ester (modifier III).
The tests show that the applied modifiers similarly influence the
increase of the strength Rmu from 0,59 M Pa up to ca. 0,96 M Pa
for modifier I and, 0.84 M Pa and 0.82 M Pa,, for modifier II and III, respectively. Thus, the percentage increase equals, respectively, ca. 25% (modifier I), ca. 22% (modifier II), and ca. 19 % (modifier III).
Fig. 5. Effect of modifier on the tensile strength Rmu of loose self-setting sands (SM S) with sodium silicate. The sands composition (in
parts by mass): ‘Szczakowa’ silica sand-100, ‘145’ water glass- 3, flodur ‘3’ –10 % mass calculated in relation to water glass
1) modifier was added –5 % mass of ZnO (modifier I) suspension, calculated in relation to binder, 2) modifier was added – 5 % mass of
ZnO (modifier II) suspension calculated in relation to binder 3) modifier was added – 5 % mass of boehmite (modifier III) suspension
calculated in relation to binder, hardening conditions: t ot≈ 23 0C; Wwzg≈ 36%
4. Conclusions
The performed tests of the effect of the modification of water glass showed that:
the additive modifier has a positive influence the
wettability of quartz by the binder,
the wettability of quartz depends on the type of the
nanoparticle and the, solvent, as well as on the mass fraction (in reference to the binder) of the applied modifier.
The best wettability of quartz by the binder was achieved with the application of 5% mass of the suspension of ZnO in propanol (36 degrees).
The verification of the effect of the modifier Rmu of loose
54 A R C H I V E S o f F O U N D R Y E N G I N E E R I N G V o l u m e 1 1 , I s s u e 4 / 2 0 1 1 , 5 1 - 5 4
Acknowledgements
Studies have been carried out under Research Project No. N N508 47 5538
This article was presented at the 8th International PhD Foundry Conference 7th-8th 06.2011 Faculty of M echanical Engineering,
Technická 2, Brno, Czech Republic.
References
[1] Kacperski M.: Wstępne badania nad wpływem rodzaju
modyfikatora na właściwości nanokompozytów
epoksydowych. Kompozyty 4 (2004)9, str. 28-32.
[2] Haiying Wang, Yilong Bai, Sheng Liu, Jiali Wu, C.P.
Wong:Combined effects of silica filler and its interface in epoxy resin. Acta M aterialia 50(2002), pp. 4369-4377.
[3] Stypuła B., Banaś J., Habdank-Wojewódzki T., Krawiec
H., Starowicz M .: PATENT: P-369 320 "Sposób
otrzymywania mikro- i nanocząstek tlenków metali",
reported: 28.07.2004, grantem: 07.10.2009.
[4] Wanwilai Chaisan, Rattikorn Yimnirun, Supon Ananta:
Preparation and characterization of ceramic
nanocomposites in the PZT-BT system. Ceramics International(2008), pp. 1-4.
[5] Avella M ., Bondioli F., Cannillo V., Errico M . E., Ferrari
A. M ., Focher B., M alinconico M ., M anfredini T. and
M ontorsi M .: Preparation, characterisation and
computational study of poly(ε-caprolactone) based
nanocomposites. M aterials Science and Technology 2004
vol.20 pp.1340 – 1344.
[6] Odegard G. M ., Clancy T. C., Gates T. S.: M odeling of the
mechanical properties of nanoparticle\polymer composites. Polymer 46 (2005), pp. 553-562.
[7] Wetzel B., Haupert F., M ing Qiu Zhang: Epoxy
nanocomposites with high mechanical and tribological perrormance. Composites Science and Technology 63 (2003) pp. 2055-2067.
[8] Hutera B., Smyksy K., Lewandowski J.L., Drożyński D.:
Wybrane aspekty oznaczania zwilżalności osnowy przez
materiały wiążące stosowane w masach formierskich,
Archiwum Technologii i Automatyzacji (2003), vol. 23,