Profound impoverishment of the large-tree stand in a hyper-fragmented
landscape of the Atlantic forest
M.A. Oliveira, A.M.M. Santos, M. Tabarelli
*
Departamento de Botaˆnica, Universidade Federal de Pernambuco, 50670-901 Recife, PE, Brazil
1. Introduction
Collectively, habitat loss and fragmentation represent the most pervasive and disturbing results of present-day human land use dynamics (Wright, 2005; Laurance et al., 2007). As human population continues to expand, even vast remote tracts of old-growth tropical forests are likely to be converted into severely fragmented landscapes (Aide and Grau, 2004; Wright and Muller-Landau, 2006) and force small fragments to remain embedded within open-habitat matrices (Turner and Corlett, 1996; Gascon et al., 2000). Increasing empirical evidence indicates that habitat fragmentation rapidly drives many ecological groups of tree species to decline at landscape scale, such as large-seeded trees that are gut dispersed by vertebrates (Stoner et al., 2007), desiccation-sensitive shade-tolerant species (Laurance, 2001), and tree species depending on long-distance pollen movement and animal-mediated pollination services (Laurance et al., 2006; Gira˜o et al., 2007). In contrast, some short-lived pioneers appear to
be permanently favored in truly hyper-fragmented landscapes (Tabarelli et al., 1999; Oliveira et al., 2004; Michalski et al., 2007). These shifts in the functional profile of tree assemblages, including the collapse of aboveground biomass, have been recently described as a fragmentation-induced degeneration process (Santos et al., 2008), which may impair conservation and ecosystem services, as well as the economic opportunities provided by forest remnants embedded in anthropogenic landscapes (see Nascimento and Laurance, 2004; Michalski et al., 2007).
Large-stemmed (DBH>70 cm) and very tall trees (>31 m height) represent the most conspicuous population component of the canopy/emergent tree species, and their large crowns constitute one of the building blocks of tropical forests—the emergent layer (Primack and Corlett, 2005). In the lowland forests, large canopy and emergent tree species (hereafter referred to as large tree species) usually represent less than 10% of the overall tree species richness (10 cm DBH), and their large individuals account for 1–6% of all tree stems (Clark and Clark, 1992; Oliveira and Mori, 1999; Turner, 2001; Small et al., 2004; Castilho et al., 2006). However, large trees provide a disproportional contribution regarding aboveground biomass, nutrient cycling and carbon storage (Chambers et al., 2001; Nascimento and Laurance, 2004;
A R T I C L E I N F O
Article history:
Received 22 January 2008 Received in revised form 3 July 2008 Accepted 17 July 2008
Keywords:
Anthropogenic landscapes Edge effects
Forest emergent layer Habitat fragmentation Large trees
A B S T R A C T
Large tree species have a disproportional influence on the structure and functioning of tropical forests, but the forces affecting their long-term persistence in human-dominated landscapes remain poorly understood. Here we test the hypothesis that aging forest edges and small fragments (3.4–295.7 ha) are greatly impoverished in terms of species richness and abundance of large trees in comparison to core areas of forest interior. The study was conducted in a hyper-fragmented landscape of the Atlantic forest, northeast Brazil. Large tree species were quantified by recording all trees (DBH10 cm) within fifty-eight 0.1-ha plots distributed in three forest habitats: small forest fragments (n= 28), forest edges (n= 10), and primary forest interior areas within an exceptional large forest remnant (n= 20). Large tree species and their stems10 cm DBH were reduced by half in forest edges and fragments. Moreover, these edge-affected habitats almost lacked large-stemmed trees altogether (0.240.27% of all stems sampled), and very tall trees were completely absent from forest edges. In contrast, large trees contributed to over 1.5% of the whole stand in forest interior plots (2.92.8%). Habitats also differed in terms of tree architecture: relative to their DBH trees were on average 30% shorter in small fragments and forest edges. Finally, an indicator species analysis yielded an ecological group of 12 large tree species that were significantly associated with forest interior plots, but were completely missing from edge-affected habitats. Our results suggest a persistent and substantial impoverishment of the large-tree stand, including the structural collapse of forest emergent layer, in aging, hyper-fragmented landscapes.
ß2008 Elsevier B.V. All rights reserved.
* Corresponding author. Tel.: +55 81 2126 8945; fax: +55 81 2126 8348. E-mail address:mtrelli@ufpe.br(M. Tabarelli).
Contents lists available atScienceDirect
Forest Ecology and Management
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / f o r e c o
Vieira et al., 2004), primary productivity (Chambers et al., 1998), and the natural disturbance regime driven by treefall gaps (Brokaw, 1982; Putz, 1983). Although the full complement of organisms inhabiting the emergent layer is yet to be documented (seeLowman and Nadkarni, 1995), it is likely that a substantial portion of tropical forest biodiversity relies on resources provided by the sun-exposed crowns of large trees as they provide an irreplaceable light environment. Through mast events of repro-duction, for example, several emergent tree species can tempora-rily offer to herbivores immense supplies of flower, fruit and seed resources (Gribel et al., 1999; Curran and Leighton, 2000; Newbery et al., 2006). Many birds and primates, in fact, are strictly dependent on large trees for nesting or food supply, leading several large tree species to be recognized as key-stone plants (see
Poonswad, 1995; Peres, 2000; Delannoy and Tossas, 2002). Unfortunately, large trees appear to be highly vulnerable to habitat fragmentation and the establishment of artificial forest edges. Long-term tree inventories in the Amazon region have documented a chronic, increased mortality of large trees near forest edges surrounded by open habitats (Laurance et al., 2000; Nascimento and Laurance, 2004). Specifically, large trees may face a 40% increase in mortality near edges (particularly in the aftermath of habitat fragmentation) and die three times faster than those inhabiting spots located over 300 m into the forest (Laurance et al., 2000) due to elevated rates of uprooting and breakage caused by increased wind turbulence near forest edges: the ‘‘wind damage hypothesis’’ (sensuD’Angelo et al., 2004). In addition to this direct, edge-induced mortality, surface forest fires and logging may constitute aggravating sources of large tree mortality in human-impacted, fragmented landscapes (Barlow et al., 2003). However, increased mortality has been documented primarily in recently created fragmented landscapes, severely limiting accurate insights about the long-term persistence of large trees in consolidated, human-dominated landscapes. Assuming that the edge-induced mortality observed in the Amazon region represents a more persistent fragmentation-related effect, one would expect a profound impoverishment or even the structural collapse of the large-tree stand in aging, hyper-fragmented landscapes, regardless of whether these landscapes have experienced expressive forest fires and logging. This prediction has not been examined thoroughly despite its far-reaching consequences for biodiversity conservation in landscapes vastly dominated by edge-affected habitats.
Therefore in this study we examine whether edge-affected habitats are able to retain the large-tree stand and the forest emergent layer in a hyper-fragmented landscape of the Atlantic forest with no detectable signs of forest fires and logging. In particular, we test the hypothesis that aging forest edges and small fragments (3.4–295.7 ha) are greatly impoverished in terms of species richness and abundance of large trees in comparison to forest interior patches of an exceptionally large fragment (the control area). First, we provide scores for the relative importance of large tree species and their stems10 cm DBH within these three habitats—forest edges, small fragments and primary forest interior areas. Second, we describe tree distribution within categories of DBH and height, and tree architecture across the habitats. Third, a set of large tree species serving as indicator species of the forest interior habitat is presented. Finally, we address plausible edge-related mechanisms driving the impoverishment of the large-tree stand and its potential implications for the persistence of biodiversity in anthropogenic, hyper-fragmented landscapes.
2. Study area
This study took place at Usina Serra Grande, a private sugar-cane landholding located in the State of Alagoas, northeastern
Brazil (88300S, 35
8500W). We selected an aging, large (667 km2) hyper-fragmented landscape containing 109 forest fragments, all of which are entirely surrounded by a uniform, stable and inhospitable matrix of sugar-cane monoculture (Fig. 1). Sugar-cane cultivation in this landscape dates back at least to the 19th century, and the remaining forest has been protected against wildfires and logging to provide watershed protection and water supply for sugar-cane irrigation (Santos et al., 2008). This has ensured the positional stability of forest fragment borders and the subsequent establishment of reproductive pioneer and shade-tolerant trees along post-closure forest edges (Melo et al., 2006). Currently, 9.2% of forest cover is left, including the 3500-ha Coimbra Forest—the largest and best preserved forest patch in the region (Grillo et al., 2006). Coimbra still retains a full complement of plant and vertebrate groups typical of vast undisturbed tracts of Atlantic forest, including large-seeded trees, large frugivorous birds, and large populations of valuable timber trees such as the softwood Ucuuba (Virola gardneri) and the hardwoods Sapucaia (Lecythis pisonis) andJatoba(Hymenaea courbaril); seePoˆrto et al. (2006) for a comprehensive mutitaxa checklist of plant and vertebrate species inhabiting the Serra Grande landscape. The predominantly lowlandterra firmeforest (sensuWhitmore, 1998) includes two physiognomic subtypes: evergreen and semi-deciduous forests (Veloso et al., 1991). Leguminosae, Lauraceae, Sapotaceae, Moraceae, Chrysobalanaceae and Euphorbiaceae account for half of tree species richness (Grillo et al., 2006).
3. Methods
3.1. Tree surveys and large tree species
In 2003–2004, all live trees10 cm in diameter at breast height (DBH) were inventoried within fifty-eight georeferenced 0.1-ha plots (10 m100 m) in three habitats (as described inSantos et al., 2008)—(1) forest edge: 10 plots located in the peripheral areas within 100 m of the border of the largest fragment (the Coimbra Forest, 3500 ha); (2)core area: 20 plots located in the old-growth forest interior of Coimbra Forest beyond 200 m of any border and showing no detectable edge influence; (3)small forest fragments: one plot per fragment located at the geometric center of 28 forest fragments ranging between 3.4 and 295.7 ha in size and being entirely surrounded by sugar-cane fields. The distance between plots and the nearest forest edge was 0 m for edge plots, 200– 1012.7 m for forest interior plots, and 60.4–502.7 m for plots in forest fragments. The average distance between all 58 plots exceeded 1000 m. All trees10 cm DBH within the 58 plots had their diameter at breast height measured and total height visually estimated (upper crown height). Height estimates were provided exclusively by Oliveira, who initially calibrated his estimates against previously height-measured poles. In order to reduce the influence of any error in these estimates, tree heights were assigned within seven broad classes of height. Trees were identified to species level and plant vouchers are available at the Federal University of Pernambuco (UFP) Herbarium, Brazil (voucher nos. 34,445–36,120).
We defined large trees as those species that usually occur with individuals>70 cm DBH or taller than 31 m as adopted byClark
emergent tree species, those with large adults reaching the highest forest layer; and (2) canopy species, those with the largest adults reaching intermediate canopy layers (Tabarelli et al., 1999).
Tree species assignment into these functional groups was carried out based on (1) an intensive tree survey conducted in Serra Grande, including seventy-five 0.1-ha plots located in forest fragments, forest edges, second-growth stands and forest interior areas (Oliveira et al., 2004; Grillo et al., 2006; Santos et al., 2008); (2) a comprehensive literature review, including books, papers, plant monographs, and MSc and PhD dissertations (e.g., Roosma-len, 1985; Lorenzi, 1998; Barroso et al., 1999; Ribeiro et al., 1999; several issues of Flora Neotropica); and (3) the examination of voucher descriptions housed in the eight major herbaria of northeastern Brazil. These sources were further supplemented by our own personal knowledge of the life-history traits of Atlantic forest tree species (seeTabarelli et al., 1999; Silva and Tabarelli, 2000; Tabarelli and Mantovani, 2000; Tabarelli and Peres, 2002; Oliveira et al., 2004; Melo et al., 2006, 2007; Santos et al., 2008). Based on this collective information, we calculated the number and proportion of large tree species and their stems (DBH10 cm) for all plots within the three habitats (i.e. forest edge, forest fragments and forest interior). As mentioned elsewhere (Santos et al., 2008), we are aware that the study design was limited by the landscape configuration available to us; the Coimbra Forest does not fully represent a ‘continuous forest’ and consists of a single, unrepli-cated tract of forest at the landscape and regional scales. Moreover, reduced forest cover at landscape scale greatly limited plot size and number, as only small plots could be accommodated in small forest fragments. Our sampling limitation contrasts with the large-scale approach (plots 1–50 ha in size), which is frequently adopted to examine demographic aspects of tropical trees. However, the Serra Grande landscape is the best available contemporary scenario to assess the long-term effects of habitat fragmentation on the
structure of Atlantic forest tree assemblages, particularly because tree assemblages may be approaching near-equilibrium conditions (Santos et al., 2008).
3.2. Explanatory variables
Because a number of patch and landscape-scale environmental variables may contribute to differences in the structure of tree assemblages (Laurance et al., 2007; Santos et al., 2008), we explicitly considered fragment size, distance to the nearest forest edge, the amount of core area (percentage of forest fragment areas considering an edge effect of 50 m), and the amount of forest cover retained in the surrounding landscape as explanatory variables for the proportion of large tree species and their stems in forest fragments (n= 28). Additionally, landscape-scale forest cover is believed to be positively correlated with both overall connectivity between patches (Gorresen and Willig, 2004) and amelioration of edge effects (Laurance et al., 2002); therefore, it was quantified as the percentage of forest within a 1-km external buffer set from the perimeter of each fragment. To examine the effects of habitat type, vegetation type and soil type on between-plot species similarity, these baseline variables were considered as factors in ANOSIM tests (Clarke and Gorley, 2001). We also performed a Mantel test with weighted Spearman rank correlation to address the effect of plot location on levels of taxonomic similarity. Straight-line distances between plots were ln-transformed, as suggested by
Condit et al. (2002) and Jones et al. (2006), and postulated by
by the Usina Serra Grande Agriculture Office. It should be noted that possible effects of selective logging on the size structure of tree assemblages across habitats were not explicitly examined here. Although this human-induced disturbance is an impacting source of large tree mortality in tropical forests (Brown and Gurevitch, 2004), the Serra Grande landscape has long been protected against logging (Santos et al., 2008). This current status of unlogged forest was confirmed by the complete lack of stumps across both the habitats and plots inventoried in this study, via interviews with long-term residents and company employees who had long been in charge of forest protection, and by our own experience in Serra Grande landscape since 2000.
3.3. Data analysis
Between-habitat differences in the proportion (i.e. relative importance) and absolute number of large tree species and their stems10 cm DBH were compared via one-way ANOVA (followed by Tukey post hoc comparisons). Stepwise multi-linear regressions (sensuSokal and Rohlf, 1995) were used to detect any influence of the explanatory variables on relative importance of large tree species and their stems within the 28 forest fragment plots. Forest interior and forest edge plots were excluded from regressions because they were unavoidably placed within the same large fragment (Coimbra Forest). Explanatory variables were trans-formed as follows: fragment size (square root transtrans-formed), plot distance to the nearest forest edge (log transformed), amount of forest cover (arcsine transformed), and amount of core area within fragments (square root transformed).
In order to compare the distribution of trees (considering all tree species) within size classes across the three habitats, we adopted a planned comparison approach sensuSokal and Rohlf (1995). First, cross-habitat distributions were compared via two-way ANOVAs assigning habitat and size classes (DBH and height) as independent factors; i.e. one ANOVA for DBH and another for tree height. ANOVAs were then followed by contrast analyses which tested for differences between forest edge and forest fragments (first comparison) and between these two habitats and forest interior (second comparison). Percentage of trees within size classes (dependent variables) were arcsine transformed in order to stabilize variance, improve normality of data and consequently increase the explanatory power of models. To infer the proximate mechanisms of large tree erosion in the edge-affected habitats (i.e. edges and small fragments), we also examined stem-specific DBH– height relationships across habitats via an ANCOVA; tree-height log was fitted to stem-DBH log (covariate) and habitat type (factor). Finally, we performed an indicator species analysis (sensuDufreˆne and Legendre, 1997) based on two groups of tree plots: one consisting exclusively of forest interior plots (n= 20) and the other group comprised of forest edge and small fragment plots (n= 38). Analyses were carried out using Statistica 6.0 and PC-Ord 4.36 (McCune and Mefford, 1999).
4. Results
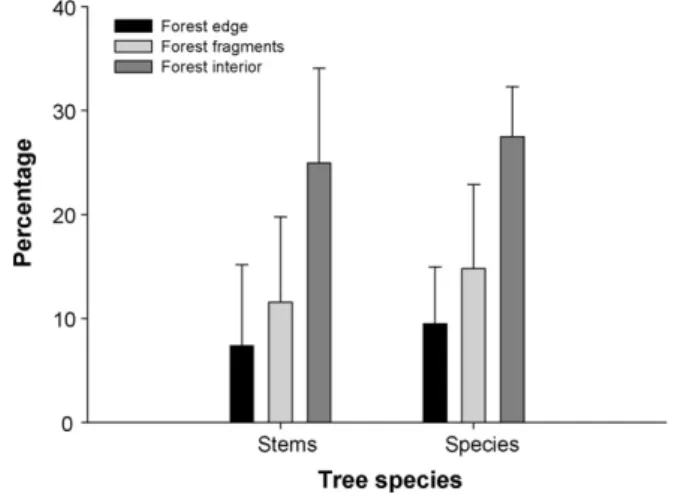
A total of 4799 living trees belonging to 198 tree species were recorded within the 58 plots across the 3 habitats; 34 species (17.1%) and 794 stems (16.9%) were assigned to the functional group of large tree species, most of them as long-lived emergent species. Edge-affected habitats were greatly impoverished in terms of tree species: 18.44.5 species per plot (meanS.D.) in forest edges and 22.58.5 species in forest fragments vs. 34.87.7 species in forest interior plots (F= 20.61, d.f. = 2,p<0.0001). Edge-affected habitats were far more impoverished in terms of large tree species as this functional group accounted for less than 15% of all stems and tree
species in these habitats, whereas they reached nearly 25% in forest interior plots (Fig. 2). This50% decline was highly significant for both species and stems (F= 20.02, d.f. = 2, p<0.0001; F= 30.5, d.f. = 2,p<0.0001) and resulted from the low occurrence of large tree species in forest edges (1.81.1 large tree species per plot) and forest fragments (3.52.8 species) as compared to forest interior plots (9.52.4 species; F= 45.5, d.f. = 2, p<0.0001). Collectively, these scores revealed a robust decline on large tree species richness in edge-affected habitats regardless of measurement in absolute or relative terms. Finally, stepwise regressions detected a small but rather significant effect of forest cover, which ranged from 0% up to 31.1%, on the proportion of large tree species (adjustedR2= 15.3%,
F= 5.87,p= 0.023) and their stems10 cm DBH (adjustedR2= 14.7%,
F= 5.65,p= 0.025,n= 28).
Although large-stemmed and very tall trees were consistently rare across habitats (seeTable 1), the distribution of trees within size classes greatly differed among habitats, and it clearly revealed the current status of the large-tree stand in the study landscape. As expected, forest edges and forest fragments did not differ (p= 0.84, contrast analysis) since they were completely dominated by small and medium-sized stems (Fig. 3) and almost completely lacked large-stemmed trees (DBH>70 cm); 0.240.27% of all stems. In forest interior plots, stem distribution was less asymmetric and significantly differed from edge-affected habitats (F= 17.6, d.f. = 1,
p<0.0001). Large-stemmed trees reached 1.682.16% of the whole plot stand in this habitat, a five-fold increase in comparison to edges and small fragments. The tree distribution across height classes in forest edges and fragments showed a drastic and similar shift towards shorter trees; these habitats were largely dominated by those shorter than 21 m (Fig. 4). Moreover, very tall trees (31 m height) were completely absent from all forest edge plots and represented only 0.220.7% of the stems sampled in the 28 fragment plots. Conversely, this group of trees accounted for 2.92.8% of all stems recorded in forest interior plots, where distribution was more symmetric and substantially differed from those in forest edges and fragments (F= 4.58, d.f. = 1, p= 0.032). These remarkable differences resulted from the dramatic rarity of large-stemmed trees in edge-affected habitats—7 stems (0.25% of all stems,Table 1) within 2 plots (5.2%) vs. 27 large-stemmed trees distributed over 50% of forest interior plots (G= 10.7, d.f. = 1,p= 0.002). Relative to their DBH, forest edges and fragments supported relatively shorter trees as well (adjustedR2= 39.7%,F= 1046.69,p<0.0001). Precisely, trees10 cm in DBH were approximately 30% shorter in comparison to forest interior trees (Fig. 5), which implies a depressed height/diameter
ratio (a drastic change in tree architecture) and an evident simplification/loss of forest vertical structure in edge-affected habitats.
Therefore, forest edges exhibited the largest differences compared to forest interior plots in terms of the size structure of tree assemblages despite the fact that these two habitats were embedded within the same large remnant (Coimbra Forest) and separated by shorter distances; edge and forest interior plots were on average 2480.4 m960.2 m apart (n= 200 random pairs), whereas forest fragment and forest interior plots were 20,373.9 m3836.28 m apart (n= 200 random pairs). This finding reinforces our assumption that both historical and contemporary logging has not structured tree assemblages; it is unreasonable to suppose that logging completely eroded the vertical structure of Coimbra forest edges, but saved large trees only 200 m apart in forest interior areas.
ANOSIM uncovered no significant correlation between soil type and degree of taxonomic similarity between plots (R= 0.102;
p= 0.987), but detected a small effect of vegetation type (R= 0.38;
p= 0.01) and a stronger effect of habitat type (R= 0.706;p= 0.01). Tree community structure in forest interior plots was significantly different from that along edge (R= 0.934;p= 0.01) and those in small fragments (R= 0.79; p= 0.01). This was confirmed by a Mantel test, which failed to uncover any large-scale spatial effect on the taxonomic similarity across the 58 plots (Rho = 0.329;
p= 0.99). Finally, an indicator species analysis yielded a set of 12 large tree species, including emergent, long-lived pioneer, hard and softwood timber species (e.g. Virola, Lecythis, Manilkara,
Aspidospermagenera), which were significantly associated with forest interior plots (Table 2). In contrast, forest edges and fragments (edge-affected habitats) lacked any large tree species serving as indicator of these altered habitats.
5. Discussion
Our findings revealed a substantial shift in the size structure of tree assemblages and the impoverishment of the large-tree stand in an aging, hyper-fragmented landscape. Such erosion refers to a robust decline of large tree species richness (in relative and absolute terms), reduced abundance of large-stemmed and very tall trees (a distorted size structure), and the complete elimination of indicator emergent species in edge-affected habitats. In fact, the abundance of large trees in the edge-affected habitats of the Serra Grande landscape was far lower than the scores reported for undisturbed tracts of lowland tropical forests (including Coimbra Forest), where this group usually represents 1–6% of all tree stems 10 cm DBH (Clark and Clark, 1996). Increased mortality of large trees resulting from forest fragmentation has been reported, particularly close to forest edges (Laurance et al., 2000). A recent study (Santos et al., 2008) also reported a sharp decrease in the richness of emergent tree species in edge-affected habitats of the Serra Grande landscape. However, by examining tree distribution within size classes we were able to accurately document the floristic erosion faced by the large-tree stand in these habitats, Table 1
Number of tree species and stems recorded within fifty-eight 0.1-ha plots in forest edges, forest fragments, and forest interior plots in Serra Grande, Brazil
Habitats No. of tree species
No. of stems 10 cm in DBH
No. of stems
>70 cm in DBH
No. of trees
>31 m height
Forest edge 59 601 2 0
Forest fragments 138 2125 5 6
Forest interior 154 2073 27 56
Total sampled 198 4799 34 62
Fig. 3.Average percentage (S.D.) of trees (10 cm in DBH) within seven classes of DBH in fifty-eight 0.1-ha plots in forest edges (n= 10), forest fragments (n= 28) and forest interior plots (n= 20) in Serra Grande, Brazil.
Fig. 4.Average percentage (S.D.) of trees (10 cm in DBH) within seven classes of height in fifty-eight 0.1-ha plots in forest edges (n= 10), forest fragments (n= 28) and forest interior plots (n= 20) in Serra Grande, Brazil.
including the structural collapse of the forest emergent layer in the edge of the largest and best preserved forest remnant of northeast Brazil. This marked pattern emerged despite our relatively reduced sample effort and cannot be explained by either baseline variables (e.g. vegetation and soil type) or plot spatial location.
Therefore, our findings represent a consistent set of empirical evidence for a persistent impoverishment of the large-tree stand associated with the establishment of aging, hyper-fragmented landscapes, in which edge-affected habitats prevail. They also indicate that many floristic and structural trends documented in more recently fragmented forest landscapes, such as those in the central Brazilian Amazonia (seeLaurance et al., 2000), represent the first steps towards a more drastic and pervasive simplification of forest vertical structure. However, the forces promoting the shifts documented here are not completely understood. Some investiga-tions have observed elevated mortality of large trees due to uprooting driven by increased windshear and turbulence near forest edges, i.e. a mechanical force as an agent of tree death (D’Angelo et al., 2004). Physiological stress imposed by edge-related micro-climatic changes has also been proposed as an additional source of increased mortality among large trees (Laurance et al., 2000; Nascimento and Laurance, 2004), but this mechanism still lacks empirical support. In addition to these edge-induced sources, large canopy and emergent trees are particularly susceptible to selective logging (Brown and Gurevitch, 2004), droughts (Nepstad et al., 2007), and delayed mortality induced by surface fires (Barlow et al., 2003; Barlow and Peres, 2008). Logging and fire, in fact, are likely to rival fragmentation-induced processes as the major forces leading large tree populations to collapse (extinction filters) in intensively human exploited landscapes (seeNepstad et al., 1999).
Our study site consisted of an aging, hyper-fragmented landscape in which the impoverishment/collapse of large-tree stands had probably occurred a long time ago. Although this scenario provides an interesting opportunity to investigate long-term effects of habitat fragmentation, it does not permit a proper investigation of the proximate causes of large tree mortality, as most of these trees have already succumbed in edge-affected habitats; standing-dead large trees were inexpressive across habitats. However, some findings in this landscape apparently support the wind damage hypothesis. Large tree species in Serra Grande positively correlated with the amount of forest cover around fragments. Furthermore, trees inhabiting edge-affected habitats were drastically shorter (relative to their DBH) than those in forest interior areas. Depressed height/diameter ratio has long been accepted as resulting from plant acclimation to better withstand increased wind loading and its impacts (Ennos, 1997; Read and Stokes, 2006; Zenner, 2008), as stem mechanical safety decreases with increasing height/diameter ratio (van Gelder et al.,
2006). Chronic wind stress in fact not only shapes tree architecture (including tropical trees), but also the size structure of tree assemblages across tropical and temperate regions (Webb et al., 1999). It appears that those Serra Grande fragments buffered against wind loading/disturbance (via increased surrounding forest cover) are more able to retain large trees established prior to fragmentation; but also provide suitable conditions for the recruitment of new ones as growing, young trees are less exposed to winds. This rationale is highly consistent with and reinforces the notion that the more closely the matrix resembles the structure of forest fragments the less pronounced are the edge effects (Mesquita et al., 1999; Harper et al., 2005).
In synthesis, this paper outlines some noticeable shifts in the size structure of tree assemblages in response to habitat fragmentation, including the floristic impoverishment of the large-tree stand in edge-affected habitats. Despite the relative lack of knowledge about the mechanisms that make edges and small fragments hostile to large tree species, our findings reinforce the notion that edge-dominated, hyper-fragmented landscapes tend to retain degenerated/altered tree assemblages (sensuSantos et al., 2008). Furthermore, this impoverishment is likely to trigger devastating impacts, via extinction cascades, across other func-tional/taxonomic groups, which require resources exclusively provided by large trees. In the Atlantic forest of northeast Brazil, for instance, the illuminated crowns of large trees offer an irreplace-able habitat for at least two-dozen species of bromeliads (nearly a quarter of the bromeliad flora in this region), which exclusively inhabit the sun-exposed crowns positioned in the forest emergent layer (Siqueira-Filho and Tabarelli, 2006). By retaining only edge-affected habitats, severely fragmented landscapes face a severe threat of biodiversity loss, which clearly impairs their eligibility as future conservation targets and degrade their potential value in terms of ecosystem services and economic opportunities (sensu
Zarin, 2004). This clearly weakens the justification to avoid further deforestation in already severely fragmented Atlantic forest landscapes; one of the most endangered global hotspots of biodiversity (Myers et al., 2000). We hope that our findings encourage wider-scale studies addressing the whole set of proximate mechanisms leading the impoverishment of the large-tree stand, particularly in edge-affected habitats. This is a crucial step towards the definition of land-use regulations and conservation guidelines designed to avoid a persistent degradation of hyper-fragmented landscapes.
Acknowledgments
We are enormously grateful to Alexandre Grillo for making his tree database available for the authors; Luis Antoˆnio Bezerra and Table 2
Indicator tree species (sensuDufreˆne and Legendre, 1997) recorded in 20 forest interior plots in the Coimbra Forest, with their respective families, regeneration strategies and indicator values (IV) at Usina Serra Grande, Brazil
Indicator species Family R. strategya IV Randomized IV p
Virola gardneri Myristicaceae S-tolerant 87.7 29.16.1 0.001
Manilkara salzmanii Sapotaceae S-tolerant 45.9 195.3 0.001
Balizia pedicellaris Fabaceae LL pioneer 38.8 17.25.0 0.006
Sloanea obtusifolia Elaeocarpaceae S-tolerant 37.3 154.5 0.001
Diplotropis purpurea Fabaceae S-tolerant 36.3 20.76.2 0.02
Ficus guaranitica Moraceae LL pioneer 34.2 18.34.9 0.011
Sloanea guianensis Elaeocarpaceae S-tolerant 33.4 144.8 0.004
Couepia rufa Chrysobalanaceae S-tolerant 32 20.65.1 0.045
Pterocarpus violaceus Fabaceae LL pioneer 30.6 16.24.7 0.009
Aspidosperma spruceanum Apocynaceae LL pioneer 30 11.54.3 0.001
Aspidosperma discolor Apocynaceae LL pioneer 25 103.9 0.007
Pradozia lactescens Sapotaceae S-tolerant 25 103.9 0.011
Jose´ Clodoaldo Bakker for authorizing our fieldwork at the Usina Serra Grande landholding; CNPq (research grant to M. Tabarelli), Fundac¸a˜o O Botica´rio de Protec¸a˜o a` Natureza; and Conservac¸a˜o Internacional for essential financial support. Two anonymous referees offered constructive criticisms on the manuscript.
References
Aide, T.M., Grau, H.R., 2004. Ecology, globalization, migration, and Latin American ecosystems. Science 305, 1915–1916.
Barlow, J., Peres, C.A., 2008. Fire-mediated dieback and compositional cascade in an Amazonian forest. Phil. Trans. R. Soc. B 363, 1787–1794.
Barlow, J., Peres, C.A., Lagan, B.O., Haugaasen, T., 2003. Large tree mortality and the decline of forest biomass following Amazonian wildfires. Ecol. Lett. 6, 6–8. Barroso, G.M., Morim, M.P., Peixoto, A.L., Ichaso, C.L.F., 1999. Frutos e Sementes:
Morfologia Aplicada a` Sistema´tica de Dicotiledoˆneas. Universidade Federal de Vic¸osa, Vic¸osa.
Brokaw, N.V.L., 1982. Treefalls: frequency, timing, and consequences. In: Leigh, Jr., E.G., Rand, A.S., Windsor, D.M. (Eds.), The Ecology of a Tropical Forest: Seasonal Rhythms and Long-Term Changes. Smithsonian Institution Press, Washington, DC, USA, pp. 101–108.
Brown, K.A., Gurevitch, J., 2004. Long-term impacts of logging on forest diversity in Madagascar. Proc. Natl. Acad. Sci. U.S.A. 101, 6045–6049.
Castilho, C.V., Magnusson, W.E., Arau´jo, R.N.O., Luiza˜o, R.C.C., Luiza˜o, F.J., Lima, A.P., Higuchi, N., 2006. Variation in aboveground tree live biomass in a central Amazonian forest: Effects of soil and topography. Forest Ecol. Manage. 234, 85–96.
Chambers, J.Q., Higuchi, N., Schimel, J.P., 1998. Ancient trees in Amazonia. Nature 391, 135–136.
Chambers, J.Q., Santos, J., Ribeiro, R.J., Higuchi, N., 2001. Tree damage, allometric relationships, and above-ground net primary production in central Amazon forest. Forest Ecol. Manage. 152, 73–84.
Clark, D.A., Clark, D.B., 1992. Life history diversity of canopy and emergent trees in a neotropical rain forest. Ecol. Monogr. 62, 315–344.
Clark, D.B., Clark, D.A., 1996. Abundance, growth and mortality of very large trees in neotropical lowland rain forest. Forest Ecol. Manage. 80, 235–244.
Clarke, K.R., Gorley, R.N., 2001. PRIMER v5: User Manual/Tutorial. PRIMER-E Ltd, Playmouth.
Condit, R., Pitman, N., Leigh, E.G., Chave, J., Terborgh, J., Foster, R., Nu´n˜ez, P.V., Aguilar, S., Valencia, R., Gorky, V., Muller-Landau, H.C., Losos, E., Hubbell, S., 2002. Beta-diversity in tropical forest trees. Science 295, 666–669.
Curran, L.M., Leighton, M., 2000. Vertebrate responses to spatiotemporal variation in seed production of mast-fruiting Dipterocarpaceae. Ecol. Monogr. 70, 101– 128.
D’Angelo, S.A., Andrade, A.C.S., Laurance, S.G., Laurance, W.F., Mesquita, R.C.G., 2004. Inferred causes of tree mortality in fragmented and intact Amazonian forests. J. Trop. Ecol. 20, 243–246.
Delannoy, C.A., Tossas, A.G., 2002. Breeding biology and nest site characteristics of Puerto Rican Broad-winged Hawks at the Rio Abajo Forest. Caribb. J. Sci. 38, 20–26. Dufreˆne, M., Legendre, P., 1997. Species assemblages and indicator species: the need
for a flexible asymmetrical approach. Ecol. Monogr. 67, 645–666. Ennos, A.R., 1997. Wind as an ecological factor. Trends Ecol. Evol. 12, 108–111. Gascon, C., Williamson, B., Fonseca, G.A.B., 2000. Receding forest edges and
vanish-ing reserves. Science 288, 1356–1358.
Gira˜o, L.C., Lopes, A.V., Tabarelli, M., Bruna, E.M., 2007. Changes in tree reproductive traits reduce functional diversity in a fragmented Atlantic forest landscape. PLoS One 9, e908.
Gorresen, P.M., Willig, M.R., 2004. Landscape responses of bats to habitat fragmen-tation in Atlantic forest of Paraguay. J. Mammal. 85, 688–697.
Gribel, R., Gibbs, P.E., Queiro´z, A.L., 1999. Flowering phenology and pollination biology ofCeiba pentandra(Bombacaceae) in Central Amazonia. J. Trop. Ecol. 15, 247–263.
Grillo, A.S., Oliveira, M.A., Tabarelli, M., 2006. A´rvores. In: Poˆrto, C., Almeida-Cortez, J.S., Tabarelli, M. (Eds.), Diversidade Biolo´gica e Conservac¸a˜o da Floresta Atlaˆn-tica ao Norte do Rio Sa˜o Francisco. Se´rie Biodiversidade no. 14. Ministe´rio do Meio Ambiente, Brası´lia, pp. 191–216.
Harper, K.A., MacDonald, S.E., Burton, P.J., Chen, J., Brosofske, K.D., Saunders, S.C., Euskirchen, E., Roberts, D., Jaiteh, M.S., Per-Anders, E., 2005. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 19, 768–782.
Hubbell, S.P., 2001. The unified theory of biodiversity and biogeography. Mono-graphs in Population Biology, vol. 32. Princeton University Press, Princeton. IBGE, 1985. Atlas Nacional do Brasil: Regia˜o Nordeste. IBGE, Rio de Janeiro. Jones, M.M., Tuomisto, H., Clark, D.B., Olivas, P., 2006. Effects of mesoscale
envir-onmental heterogeneity and dispersal limitation of floristic variation in rain forest ferns. J. Ecol. 94, 181–195.
Laurance, W.F., 2001. Fragmentation and plant communities: synthesis and impli-cations for landscape management. In: Bierregaard, Jr., R.O., Gascon, C., Love-joy, T.E., Mesquita, R.C.G. (Eds.), Lessons from Amazonia: the Ecology and Conservation of a Fragmented Forest. Yale University Press, New Haven, pp. 158–168.
Laurance, W.F., Delamonica, P., Laurance, S.G., Vasconcelos, H.L., Lovejoy, T.E., 2000. Rainforest fragmentation kills big trees. Nature 404, 836–1836.
Laurance, W.F., Lovejoy, L.E., Vasconcelos, H.L., Bruna, E.M., Didham, R.K., Stouffer, P.C., Gascon, C., Bierregaard Jr., R.O., Laurance, S.G., Sampaio, E., 2002. Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv. Biol. 16, 605–618.
Laurance, W.F., Nascimento, H.E.M., Laurance, S.G., Andrade, A., Ribeiro, J.E.L.S., Giraldo, G.P., Lovejoy, T.E., Condit, R., Chave, J., Harms, K.E., D‘Aˆ ngelo, S., 2006. Rapid decay of tree-community composition in Amazonian forest fragments. Proc. Natl. Acad. Sci. U.S.A. 103, 19010–19014.
Laurance, W.F., Nascimento, H.E.M., Laurance, S.G., Andrade, A., Ewers, R.M., Harms, K.E., Luiza˜o, R.C.C., Ribeiro, J.E., 2007. Habitat fragmentation, variable edge effects, and the landscape divergence hypothesis. PloS One 10, e1017. Lorenzi, H., 1998. A´rvores Brasileiras: Manual de Identificac¸a˜o e Cultivo de Plantas
Arbo´reas Nativas do Brasil, vols.1 and 2. Editora Plantarum, Nova Odessa. Lowman, M.D., Nadkarni, N.M., 1995. Forest Canopies. Academic Press, New York. McCune, B., Mefford, M.J., 1999. PC-ORD: Multivariate Analysis of Ecological Data,
Version 4.36. MjM Software, Gleneden Beach, OR, U.S.A.
Melo, F.P.L., Dirzo, R., Tabarelli, M., 2006. Biased seed rain in forest edges: evidence from the Brazilian Atlantic forest. Biol. Conserv. 132, 50–60.
Melo, F.P.L., Lemire, D., Tabarelli, M., 2007. Extirpation of large-seeded seedlings from the edge of a large Brazilian Atlantic forest fragment. E´coscience 14, 124–129. Mesquita, R.C.G., Delamoˆnica, P., Laurance, W.F., 1999. Effects of surrounding
vegetation on edge-related tree mortality in Amazonian forest fragments. Biol. Conserv. 91, 129–134.
Michalski, F., Nishi, I., Peres, C.A., 2007. Disturbance-mediated drift in tree func-tional groups in Amazonian forest fragments. Biotropica 39, 691–701. Myers, N., Mittermeier, R.A., Mittermeier, C.G., Fonseca, G.A.B., Kent, J., 2000.
Biodiversity hotspots for conservation priorities. Nature 403, 845–853. Nascimento, H.E.M., Laurance, W.F., 2004. Biomass dynamics in Amazonian forest
fragments. Ecol. Appl. 14 (Suppl.), 127–138.
Nascimento, H.E.M., Laurance, W.F., Condit, R., Laurance, S.G., D’Angelo, S., Andrade, A.C., 2005. Demographic and life-history correlates for Amazonian trees. J. Veg. Sci. 16, 625–634.
Nepstad, D.C., Tohver, I.M., Ray, D., Moutinho, P., Cardinot, G., 2007. Mortality of large trees and lianas following experimental drought in Amazon forest. Ecology 88, 2259–2269.
Nepstad, D.C., Verı´ssimo, A., Alencar, A., Nobre, C., Lima, E., Lefebvre, P., Schlesinger, P., Potter, C., Moutinho, P., Mendoza, E., Cochrane, M., Brooks, V., 1999. Large-scale impoverishment of Amazon forests by logging and fire. Nature 398, 505–508. Newbery, D.M., Chuyong, G.B., Zimmermann, L., 2006. Mast fruiting of large
ectomycorrhizal African rain forest trees: importance of dry season intensity, and the resource-limitation hypothesis. New Phytol. 170, 561–579. Oliveira, A.A.D., Mori, S.A., 1999. A central Amazonian terra firme forest. I. High tree
species richness on poor soils. Biodivers. Conserv. 8, 1219–1244.
Oliveira, M.A., Grillo, A.S., Tabarelli, M., 2004. Forest edge in the Brazilian Atlantic forest: drastic changes in tree species assemblages. Oryx 38, 389–394. Peres, C.A., 2000. Identifying keystone species plant resources in tropical forests:
the case of gums fromParkiapods. J. Trop. Ecol. 16, 287–317.
Poonswad, P., 1995. Nest-site characteristics of 4 sympatric species of hornbills in Khao-Yai National Park, Thailand. J. Trop. Ecol. 137, 183–191.
Poˆrto, C., Almeida-Cortez, J.S., Tabarelli, M., 2006. Diversidade Biolo´gica e Conser-vac¸a˜o da Floresta Atlaˆntica ao Norte do rio Sa˜o Francisco. Se´rie Biodiversidade no. 14. Ministe´rio do Meio Ambiente, Brası´lia.
Primack, R.B., Corlett, R., 2005. Tropical Rain Forests: An Ecological and Biogeo-graphical Comparison. Blackwell Publishing, New York.
Putz, F.E., 1983. Treefall pits and mounds, buried seeds, and the importance of soil disturbance to pioneer trees on Barro Colorado Island. Panama Ecol. 64, 1069– 1074.
Read, J., Stokes, A., 2006. Plant biomechanics in an ecological context. Am. J. Bot. 93, 1546–1565.
Ribeiro, J.E.L.S., Hopkins, M.J.G., Vicentini, A., Sothers, C.A., Costa, M.A.S., Brito, J.M., Souza, M.A.D., Martins, L.H.P., Lohmann, L.G., Assunc¸a˜o, P.A.C.L., Pereira, E.C., Silva, C.F., Mesquita, M.R., Proco´pio, L.C., 1999. Flora da Reserva Ducke: Guia de Identificac¸a˜o de uma Floresta de Terra-Firme na Amazoˆnia Central. INPA-DFID, Manaus.
Roosmalen, M.G.M.van, 1985. Fruits of the Guianan Flora. Institute of Systematic Botany, Utrecht.
Santos, B.A., Peres, C.A., Oliveira, M.A., Grillo, A., Alves-Costa, C.P., Tabarelli, M., 2008. Drastic erosion in functional attributes of tree assemblages in Atlantic forest fragments of northeastern Brazil. Biol. Conserv. 141, 249–260.
Silva, J.M.C., Tabarelli, M., 2000. Tree species impoverishment and the future flora of the Atlantic forest of northeast Brazil. Nature 404, 72–73.
Siqueira-Filho, J.A., Tabarelli, M., 2006. Bromeliad species from the Atlantic forest of north-east Brazil: losses of critical populations of endemic species. Oryx 40, 218–224.
Small, A., Martin, T.G., Kitching, R.L., Wong, K.M., 2004. Contribution of tree species to the biodiversity of a 1 ha Old World rainforest in Brunei. Borneo. Biodivers. Conserv. 13, 2067–2088.
Sokal, R.R., Rohlf, F.J., 1995. Biometry. Freeman and Company, New York. Stoner, K.E., Vulinec, K., Wright, S.J., Peres, C.A., 2007. Hunting and plant community
dynamics in tropical forests: a synthesis and future directions. Biotropica 39, 385–392.
Tabarelli, M., Mantovani, W., Peres, C.A., 1999. Effects of habitat fragmentation and plant guild structure in the montane Atlantic forest of southeastern Brazil. Biol. Conserv. 91, 119–127.
Turner, I.M., 2001. The Ecology of Trees in the Tropical Rain Forest. Cambridge University Press, Cambridge.
Turner, I.M., Corlett, R.T., 1996. The conservation value of small, isolated fragments of lowland tropical rain forest. Trends Ecol. Evol. 11, 330–333.
van Gelder, H.A., Poorter, L., Sterck, F.J., 2006. Wood mechanics, allometry, and life-history variation in a tropical rain forest tree community. New Phytol. 171, 367– 378.
Veloso, H.P., Rangel-Filho, A.L.R., Lima, J.C.A., 1991. Classificac¸a˜o da Vegetac¸a˜o Brasileira Adaptado a um Sistema Universal. IBGE, Rio de Janeiro.
Vieira, S., Camargo, P.B., Selhorst, D., Silva, R., Hutyra, L., Chambers, J.Q., Brown, I.F., Higuchi, N., Santos, J., Wofsy, S.C., Trumbore, S.E., Martinelli, L.A., 2004. Forest structure and carbon dynamics in Amazonian tropical rain forests. Oecologia 140, 468–479.
Webb, E.L., Stanfield, B.J., Jensen, M.L., 1999. Effects of topography on rainforest tree community structure and diversity in American Samoa, and implications for frugivore and nectarivore populations. J. Biogeogr. 26, 887–897.
Whitmore, T.C., 1998. An Introduction to Tropical Rain Forests, 2nd edition. Oxford University Press, Oxford.
Wright, S.J., 2005. Tropical forests in a changing environment. Trends Ecol. Evol. 20, 553–560.
Wright, S.J., Muller-Landau, H.C., 2006. The future of tropical forest species. Bio-tropica 38, 287–301.
Zarin, D.J., 2004. Neotropical working forests: concepts and realities. In: Zarin, D.J., Alavalapati, J.R.R., Putz, F.E., Schmink, M. (Eds.), Working Forests in the Neotropics: Conservation Through Sustainable Management? Columbia Uni-versity Press, New York, pp. 1–12.