Endothelial Microparticles (EMPs): implications in angiogenesis
2011/2012
Delfim Diogo Ferreira Duarte
Endothelial Microparticles (EMPs): implications in angiogenesis
and angiogenesis-related diseases
Trabalho organizado de acordo com as normas da revista:
Endothelial Microparticles (EMPs): implications in angiogenesis
Delfim Diogo Ferreira Duarte
Mestrado Integrado em Medicina
Área: Bioquímica
Trabalho efetuado sob a Orientação de: Prof. Doutora Raquel Soares
Trabalho organizado de acordo com as normas da revista:
Endothelial Microparticles (EMPs): implications in angiogenesis
and angiogenesis-related diseases
PLoS ONE
Aos meus Pais,
Abstract
Microparticles are submicron membrane vesicles released by activated or dying cells. Endothelial microparticles (EMPs) and platelet microparticles (PMPs) have been studied as biomarkers in several cardiovascular and inflammatory diseases, and as central players in intercellular communication. Asthma is characterized by airway inflammation and increased angiogenesis. Using clinical and laboratorial evaluation, questionnaires, flow cytometry and immunoassays, we studied the role of EMPs, PMPs, inflammatory and angiogenic markers in a group of asthmatic versus non-asthmatic subjects. We also explored the characteristics of EMPs generated upon treatment with the anti-inflammatory and anti-angiogenic agent, isoxanthohumol (IXN), by using electron microscopy, in vitro assays, protein quantification and the developmental retinal angiogenesis model.
PMPs (CD31+/42b+ and CD31/42+/AnV+) were increased among asthmatics. Apoptotic EMPs (CD31+/42b-/AnV+) were augmented in overall asthmatics but the increase was not significant in patients without metabolic syndrome. Soluble intercellular adhesion molecule 1 (sICAM-1) and vascular endothelial growth factor (VEGF) were also significantly higher in the asthma group and might mediate part of microparticles actions in the airways. Endothelial cells treated with IXN, generated EMPs with interesting in vitro anti-angiogenic properties. In the in vivo setting, EMPs did not affect retinal angiogenesis and accumulated in tissue macrophages.
We propose PMPs and apoptotic EMPs as possible asthma biomarkers and participants in asthma pathophysiology. We also present IXN-generated EMPs as effectors that actively interact with endothelial cells and tissue macrophages, leaving them as potential therapeutic agents in inflammatory and angiogenic diseases, such as asthma.
Introduction
Microparticles are small membrane fragments, less than 1.0 um diameter, released from cells by chemical or physical activation such as shear stress, direct lesion or apoptosis. They are characterized by high phospholipidic content and specific surface antigens from the parental cell [1]. Although the shedding process may occur in any cell, studies so far have been mainly dedicated to microparticles from platelet, leukocyte or endothelial origin. Endothelial microparticles (EMPs) have been recognized as possible biomarkers in several cardiovascular and inflammatory conditions, like hypertension, acute coronary syndromes, atherosclerosis, pre-eclampsia, stroke, diabetes, metabolic syndrome and vasculitis [2-5]. EMPs were previously reported as either pro or anti-angiogenic and pro-coagulant [6-9]. Platelet microparticles (PMPs) are the largest fraction of circulating microparticles and were shown to be pro-angiogenic, pro-inflammatory and pro-coagulant [10-13]. PMPs are elevated in diseases such as rheumatoid arthritis, cancer, diabetes and acute coronary syndrome [3,14].
Asthma is a complex disease with a phenotype that is clinically difficult to define. The main physiological feature of asthma is intermittent and reversible airway obstruction, while the dominant pathological feature is airway inflammation sometimes associated with airway structural changes [15]. Taken together, there is evidence both for platelets and endothelium in asthma like inflammation. Increased angiogenesis and vessel remodeling are common features of chronic airway inflammation, while platelets have been associated with airway hiperresponsiveness and bronchial remodeling [16-21]. Nevertheless, the possible role of EMPs or PMPs in asthma has not been explored.
Moreover, microparticles have emerged as exciting biological players due to their possible task as messengers or carriers in cell to cell communication [22]. An interesting feature of microparticles biology is the possibility of altering the sorting of their components by regulating their formation with different stimuli. As an example, this approach has been successful with the generation of pro-angiogenic Sonic Hedgehog-enriched microparticles (Shh+ MPs) [23].
Isoxanthohumol (IXN), a flavonoid of beer and the main xanthohumol metabolite, is an interesting molecule, as it inhibits inflammatory cascades, decreases angiogenesis and modulates adipogenesis [24-26]. We have previously suggested that polyphenols, like IXN, could accumulate in carrying platelets, be transported, released and therefore act
in distant sites [27]. One could hypothesize that IXN accumulates in EMPs and/or alters its composition, and thus changes its effects.
The aim of this study was to characterize EMPs and PMPs in patients with asthma and to investigate the effect and localization of IXN-generated EMPs on in vitro and In vivo vessel formation.
Methods
Subjects and study design
This cross sectional study included a total of 38 participants, 22 subjects with a medical diagnosis of asthma and 16 healthy volunteers. The local Hospital Ethics Committee approved the experimental protocol and all subjects gave written informed consent. Participants were excluded based on the following criteria: less than 18 years, active smoker, cardiovascular disease, active pregnancy or pregnancy in the last 6 months, history of cancer or auto-immune disease. All recruited subjects were classified as with or without metabolic syndrome (MetS), according to the 2009 diagnostic criteria with the exception of elevated fasting glucose, which was not available [28].
Questionnaires, anthropometry and blood pressure
Participants were enquired about their age and current medication. All subjects completed the Asthma Control Questionnaire (ACQ) [29], the Asthma Quality of Life Questionnaire (AQLQ) [30] and the Control of Allergic Rhinitis and Asthma Test (CARAT) [31]. The height and weight were measured at the time of the interview and the body mass index (BMI) calculated as kilogram per meter square. Waist circumference (WC) was determined by measuring the abdominal perimeter around the upper edge of the iliac crest, in the standing position. Systolic and diastolic blood pressures were determined by using a brachial cuff in the dominant arm, in the sitting position.
Lung function and airway inflammation
Spirometric parameters, namely the forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC and maximum midexpiratory flow rate (MMEF 25-75%) were determined using a computerized pneumotachograph spirometer (SensorMedics Vmax 22, SensorMedics, Yorba Linda, USA). The fraction of expired nitric oxide (FeNO) was used to assess airway inflammation and measured using the NIOX system (Aerocrine, Stockholm, Sweden), at a flow rate of 50 ml/s [32].
Blood sampling
Peripheral blood samples were drawn from an antecubital vein using a 21-gauge needle to limit platelet activation, and were processed for flow cytometry in less than 1 hour. The samples were collected in tubes containing citrate acid dextrose (for flow
cytometry analysis), EDTA (for complete blood counts) or no anticoagulants (for biochemical analyses and immunoassays) (BD Vacutainer).
Complete blood counts were obtained using an automated blood counter Sysmex XE-5000 (Emilio de Azevedo Campos, Porto, Portugal). Serum electrolytes, total cholesterol, and high-density lipoprotein cholesterol (HDL-cholesterol) were measured using conventional methods with an Olympus AU5400 automated clinical chemistry analyzer. (Beckman-Coulter, Izasa, Porto, Portugal). serum C-reactive protein (CRP) was assayed using a immuno turbidimetric assay on an Olympus AU5400 automated clinical chemistry analyzer. Low-density lipoprotein cholesterol (LDL-cholesterol) was calculated according to Friedewald`s equation: [LDL-cholesterol = total cholesterol – HDL-cholesterol – (triglycerides/5)].
Immunoassay
Luminex-based immunoassays were performed using Milliplex Map (Millipore, Billerica, MA), according to the manufacturer’s instructions. The selected analytes were adiponectin, sICAM-1, IL-10, TNFα and VEGF. Briefly, the appropriate cytokine standards, controls and plasma samples were diluted in assay buffer and added to pre-wet filter plates. Fluorescent beads containing the respective antibodies were added and incubated on a plate shaker overnight, at 4ºC. The wells were washed, and the samples were incubated with detection antibodies, for 30 minutes at room temperature and with streptavidin-phycoerythrin, under the same conditions. After washing, beads were resuspended with sheath fluid and analyzed on a Luminex 200 system (Luminex corp., Austin, TX). Mean fluorescent intensity data (MFI) were analyzed using the Luminex 100 Integrated System version 2.3 (Luminex Corporation, USA). All measurements were performed in duplicate.
Microparticles isolation and characterization
Blood samples in acid citrate dextrose tubes were centrifuged at 200 g, for 10 minutes, at 4ºC, without acceleration or brake. The upper part of the plasma, platelet rich plasma (PRP), was collected, without disturbing the white buffy coat layer on top of the cell compartment, and centrifuged at 1500g, 10 minutes, at 4ºC. The supernatant was collected, resulting in supernatant – platelet poor plasma (PPP). Supernatant-PPP was stained for flow cytometry analysis, with a BD FACS Calibur flow cytometer, with the BD CellQuest software. 100 µl of PPP were incubated with 8 µl of CD31-FITC, 8 µl of CD42b-PE-Cy5.5 and 4 µl of Annexin V-APC fluorochrome-labeled antibodies (BD
Biosciences, San Jose, CA) for 20 minutes, at 4ºC, in the dark. Negative controls for CD31 e CD42b were also used: anti-mouse IgG1, k (unknown specificity) FITC and anti-mouse IgG1, k PE-Cy5. Filtered Isoton (BD FACS Flow) was added and samples were transferred to TruCount tubes (Becton Dickinson) to allow quantification of particles by flow cytometry analysis. Values were reported as counts per microliter and percentage.
MPs were defined according to their size (less than 1 µm) and according to their phenotype. CD31+/CD42b+ events were classified as platelet microparticles (PMPs), CD31+/CD42b- events as endothelial microparticles (EMPs) and annexin V+ events as apoptotic microparticles (platelet or endothelial) (Figure S1).
Cell culture
Human umbilical vein endothelial cells (HUVEC) were purchased from ScienceCell Research Labs (San Diego, USA), used between passages 3 and 7, and cultured in 0.2% gelatin-coated flasks Aldrich, Portugal), using M199 medium (Sigma-Aldrich, Portugal) supplemented with 20% fetal bovine serum (FBS) (Invitrogen Life Technologies, Scotland, UK), 1% penicillin/streptomycin (Invitrogen Life Technologies, Scotland, UK), 0.01% heparin (Sigma-Aldrich, Portugal) and 20 µg/mL endothelial cell growth supplement (Sigma-Aldrich, Portugal), and maintained at 37º C in a humidified 5% carbon dioxide atmosphere.
EMPs generation
EMPs were generated as described before [9] with modifications. At passage 6, upon confluency, HUVEC were serum-starved, at 37°C in 2% FBS supplemented M199 medium for 2h, and stimulated with (1) 10 ng/ml human recombinant tumor necrosis factor alpha (TNFα) (PeproTech, Rocky Hill, USA) for 24h, or (2) 10 ng/ml human recombinant granulocyte-macrophage colony-stimulating factor (GM-CSF) (ImmunoTools, Germany) for 24h, or (3) 10 µM IXN (Alexis, Switzerland) for 1h followed by co-incubation with TNFα for 24h. A (4) untreated group (control) was also considered. Next, media was collected and centrifuged at 1,200 g for 5 minutes at 10 ºC to remove cell debris. The cell-free supernatant was then centrifuged at either 100,000 g for 90 minutes or 20,000 g for 20 minutes at 10 ºC to obtain a pellet enriched in EMPs. The pellet was then re-suspended in 400 µl of sterile phosphate buffer saline (PBS) and either used immediately or stored at -80ºC.
Electron Microscopy
EMPs obtained from centrifugation at 100,000 g were fixed in 2.5% gluteraldehyde in 0.01M PBS overnight, at room temperature (RT). The pellet was subsequently washed in 0.1M cacodylate buffer, postfixed in 2% osmium tetroxide in the same buffer for 30 minutes, dehydrated with progressive ethanol and embedded in Epon 812 (Taab, Aldermaston, Berkshire, U.K.). Ultra-thin sections were obtained, stained with lead citrate and uranyl acetate and visualized and photographed in a Jeol JEM-100CX II (Jeol, Tokyo) electron microscopy at 80 kV. Microparticles were defined as rounded, small (<1.0 µm) particles with double membrane.
Cell viability
HUVEC were allowed to grow until 70-90% confluence and incubated with 15-120 µl (with completion for the highest volume of 120 µl) of microparticles from different origins or vehicle (cell medium) for 24 h. After the incubation period, cells were washed twice with PBS and their viability was assessed using Cell Titer 96 Aqueous ONE Solution Reagent (MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] colorimetric assay (Promega, Madison, USA), according to the instructions provided by the manufacturer. Optical density was measured at 492 nm. All samples were assayed at one experiment in triplicate, and the mean value was calculated. The results are given as mean ±SEM and are expressed as percentage of control, which was considered to be 100%.
In vitro wound-healing assay
HUVEC (1,0 x 104 cells/mL) were established in 24-well plates coated with 0.2% gelatin and allowed to form confluent monolayers. The monolayers were scratched using a sterile 200 µl pipette tip to form a denude area and cells were washed. Equal 60 µl volumes of either EMPs from different origins or PBS (vehicle) were added in 3% FBS-supplemented medium. Cell migration was sequentially visualized on an inverted microscope (Nikon TMS, Japan) and photographed (Nikon D40x, Japan) for 12 hours. To quantify migration ImageJ 1.43m (NIH, USA) was used and the “healed area” was calculated (%Healed = [(Original wound area – Time point wound area)/Original wound area] x 100) for 6h and 12h time points. The experiments were done in triplicate.
EMPs uptake by HUVEC
HUVEC (1,0 x 104 cells/mL) were seeded in glass coverslips in 24-well plates. EMPs were tracked by labeling with the lipophilic dye Dil (Vybrant Dil, Invitrogen, CA, USA). After reaching sub-confluence, cells were incubated with 30 µl volumes of labeled
EMPs from different origins or vehicle (sterile PBS) for 18 hours. Then, the cells were permeabilized in 4% BSA, 0.1% Triton X-100, 0.01M PBS for 1 hour, incubated with FITC-conjugated Griffonia simplicifolia (Bandeiraea) isolectin B4 (1:50, Sigma-Aldrich, MO, USA) for 1 hour at RT, washed 3 x 5 minutes in 0.01% Tween-20, 0.01 M PBS, labeled with DAPI (Roche Diagnostics, Basel, Switzerland) for 5 minutes and washed again for 3 x 5 minutes. The coverslips were mounted using ProlongGold antifade mounting media (Invitrogen, CA, USA). Finally, samples were imaged on a Zeiss Imager Z1 microscope in ApoTome mode for optical sectioning, with AxionVision 40 v4.8.2.0 software (Carl Zeiss MicroImaging, Germany) and 10 selected 20x fields of view (FOV) per well were captured per well and analyzed using ImageJ 1.43m (NIH, USA). The parameter evaluated was the ratio (number of cells co-localizing with EMPs)/(total number of cells). The experiments were done in triplicate (i.e. 30 FOV per treatment).
Protein extraction and preparation
Proteins were isolated and extracted either from HUVEC or EMPs, using the TRIzol® isolation reagent (Invitrogen, CA, USA), supplemented with protease inhibitors (Complete miniprotease inhibitor cocktail tablets, Roche Diagnostics, Switzerland) and phosphatase inhibitors (phosphatase inhibitor cocktail 1 and 2; P5726 and P2850 Sigma-Aldrich, USA), dissolved in 200 µL SDS 1% and kept at -80ºC until use.
Western blot
20 µg of protein were subjected to 8% or 12% SDS-PAGE with a 5% stacking gel. After electrophoresis, proteins were blotted into a Hybond nitrocellulose membrane (Amersham, Arlington, USA), using a mini-transblot electrophoretic transfer cell (Amersham Biosciences, USA). Immunodetection for VEGFR2 (Cell Signaling, MA, USA) and β-actin (Santa Cruz Biotechnology), was accomplished with enhanced chemiluminescence (ECL kit, Amersham Biosciences, USA).
The relative intensity of each protein blotting analysis was measured using a computerized software program (Biorad, CA, USA) and normalized with β-actin bands to compare the expression of proteins in the different treatment groups.
Animals
All research animals were obtained, maintained and used in experiments conducted according to accepted standards of human animal care (European Community guidelines (86/609/EEC) and Portuguese Act (129/92) for the use of experimental animals). A veterinarian accompanied every step involving animal care and
manipulation. The developmental retinal angiogenesis model was done using C57BL/6 mice pups.
Intravitreal injection
At post-natal day (P) 7, mice pups were anesthetized with inhaled isoflurane and the eyelids softly opened with forceps to perform intravitreal injection, by inserting a 33-gauge needle, equipped in a Hamilton syringe, in the vitreous. The needle entered the eye in a 45º angle, close to the limbus, to avoid damage of the lens or touching the retina. The injection lasted 20-30 seconds and hydroxypropyl methylcellulose (Methocel) was placed immediately to avoid significant leakage and to lubrify the eye. Dil-labeled EMPs generated from 20.000 g centrifugation in 0.5 µl vehicle (sterile PBS) or control (0.5 µl vehicle) were injected in contralateral eyes of the same animal, to avoid individual variability. Mice were kept with their mothers for 5 days until P12, at which time point they were euthanized with pentobarbital 6 mg/kg body weight and their eyes enucleated and fixed in 4% p-formaldehyde overnight (Figure S2).
Retinal Immunoflourescence and analysis
For whole mount staining, retinas were dissected out, washed 3 x 5 minutes in PBS, permeabilized in 4% BSA, 0.1% Triton X-100, 0.01M PBS overnight at 4ºC, incubated with FITC-conjugated GSB isolectin B4 (1:50, Sigma-Aldrich, MO, USA) for 1 hour at RT for endothelial labeling and washed with 0.01% Tween-20 in 0.01 M PBS for 3 x 5 minutes and 5 x 10 minutes. In some cases cell nuclei were labeled with DAPI (Roche Diagnostics, Basel, Switzerland). Retinas were flattened with radial relaxing incisions and mounted using ProlongGold antifade mounting media (Invitrogen, CA, USA). Finally, samples were imaged on a Zeiss Imager Z1 microscope in ApoTome mode for optical sectioning, with AxionVision 40 v4.8.2.0 software (Carl Zeiss MicroImaging, Germany) and selected fields were captured, merged using Adobe Photoshop CS3 and analyzed using ImageJ 1.43m (NIH, USA) and AngioTool (CCR, NCI, USA) softwares [33]. The parameters analyzed were the vasculature length per retinal radius (ratio between two parallel radial distances measured from the center of the retina to the tip of the vascular network and to the edge of the retinal leaf), the vessel density (four 20x FOV per retina, and three retinas per treatment were selected) and junctions density (the same FOV used for vessel density measurements were selected and branchpoints quantified).
Statistical analysis
For the human studies, descriptive statistics of all variables were expressed as medians and interquartile ranges. The nonparametric test Mann-Whitney U test was used to compare variables between two groups. Correlations between variables were determined using the Spearman rank test. Microparticles variables had positively skewed distributions and were log transformed before the correlation study, to improve normality. Statistical difference was considered if p<0.05. Analyses were performed using the statistical package PASW Statistics, 18.0 version (SPSS Inc; Chicago, IL). Experimental results are expressed as mean ± SEM and/or as percentage of control, which was considered to be 100%. Statistical significance of difference between various groups was evaluated by analysis of variance (ANOVA) followed by the Bonferroni post-test. For comparison between two groups, Student’s t-test was used. Statistical difference with a confidence interval of 95% was considered if p< 0.05 (GraphPad Prism, version 5.03).
Results
Baseline characteristics
The baseline characteristics are shown in Table 1. Asthmatic participants were significantly older and had higher BMI, waist circumference and systolic blood pressure when compared to healthy volunteers. Every asthmatic participant was medicated with at least one inhaled corticosteroid.
Asthma control, lung function and airway inflammation
As listed in Table 1, asthmatic subjects had higher AQLQ and ACQ scores and lower CARAT scores and had, as expected, an obstructive pattern of spirometry. Airway inflammation, expressed as FeNO, was significantly increased among asthmatics.
Complete blood counts and lipid profile
No differences in blood counts, total cholesterol and cholesterol lipoproteins values were found between asthmatics and non-asthmatics (Table 1)
Serum biomarkers
Inflammatory (CPR, IL10 and TNFα) and adipose tissue (adiponectin) biomarkers were not significantly altered between groups (Table 2). Interestingly, the endothelium-born factors closely associated with inflammation and angiogenesis, VEGF (p=0.021) and sICAM-1 (p=0.01), were markedly increased in the asthma group (Table 2).
Cell microparticles
Microparticles were identified based on the surface expression of PECAM-1 or CD31, platelet glycoprotein Ib or CD42b and phosphatidylserine binding to annexin V. PMPs express both CD31 and CD42b (CD31+/42b+) and EMPs only express CD31 (CD31+/42b-). As predictable, PMPs (CD31+/42b+) and apoptotic PMPs (CD31+/42b+/AnV+) represent nearly overlapping populations and had similar values (Table 2). Hence, only one of these populations (apoptotic PMPs) will be considered for future comparisons to avoid confusion. EMPs (CD31+/42b-), on the other hand, had a clearly distinct and smaller subgroup of apoptotic EMPs (CD31+/42b-/AnV+), reflecting
a unique mechanism that governs the translocation of phosphatidylserine to the outer membrane of microparticles (Table 2).
In the asthma group the increased number of total EMPs (CD31+/42b-) was not significant (p=0.336), but there was a significant elevation of apoptotic EMPs (CD31+/42b-/AnV+) when compared to non-asthmatics (p=0.021) (Table 2) (Figure 1, A and B). PMPs were also significantly increased among asthmatic subjects (p=0.033) (Table 2) (Figure 2, A).
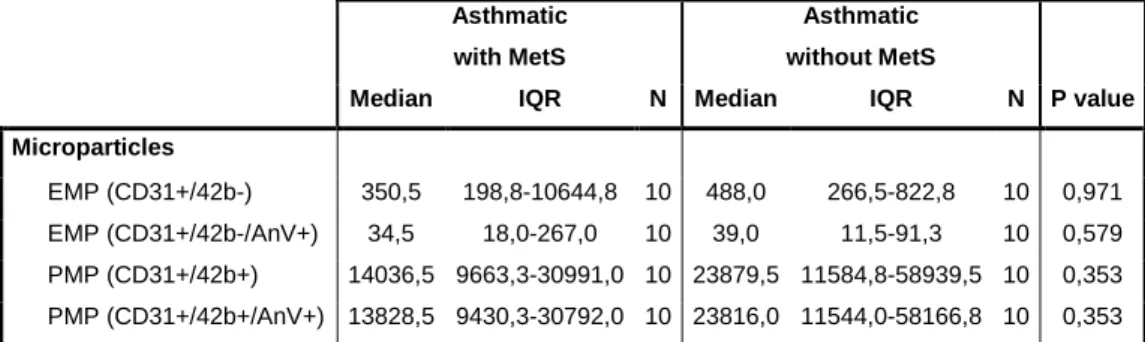
Because the association between elevated EMPs and MetS has been previously reported [4] we excluded subjects with MetS from both groups and performed the same comparisons (Table 3). Despite the higher counts in asthmatics, total (p=0.807) and apoptotic EMPs (p=0.103) were not significantly different between groups (Table 3) (Figure 1, C and D). Nevertheless, PMPs remained significantly elevated among asthmatics without MetS (p=0.031) (Table 3) (Figure 2, B).
Furthermore, comparison of EMPs and PMPs values between asthmatics with and without MetS revealed no significant differences (Table S1).
Microparticles and serum biomarkers correlations in asthmatic patients
In asthmatics, PMPs values inversely correlated with FeNO (Table S2; Figure 3, A). No other significant correlations with cell microparticles were detected. TNFα positively correlated with C-reactive protein and eosinophils counts (Table S3). There was also a direct correlation between VEGF and eosinophils (Table S3; Figure 3, B). Adiponectin inversely correlated with CARAT score and FEV1 (Figure 3, C), while sICAM-1 directly correlated with AQLQ, ACQ and with C-reactive protein (Table S3).
Microparticles generation
Since IXN is able to prevent inflammation and angiogenesis, two processes associated with asthma, we next examined the effect and localization of EMPs generated upon treatment with this flavonoid. EMPs were generated in cell cultures by incubating HUVEC with PBS (control EMPs), TNFα, IXN+TNFα and GM-CSF. The TNFα and IXN+TNFα treatments induced more cell elongation and confluence comparing to the other groups, which is probably explained by the characteristic TNFα-related cellular activation (Figure S3). Hereafter the different EMPs subsets will be referred as C, TNFα, IXN and GM-CSF, depending on their origin stimulus, to prevent confusion.
Microparticles identification
EMPs were identified in electron microscopy and their morphology revealed (Figure 4). They were round-shaped, smaller than 1µm and had a characteristic bilayered membrane (Figure 4, D and G). The composition of the outer leaflet of EMPs varies and a common identified ligand is phosphatidylserine, a marker of apoptosis. The content of microparticles is heterogeneous, diverges between generation stimuli (Figure 4) and reflects the existence of RNA, membranous content or cytoskeleton proteins that are differentially captured inside the particles and that are more or less electron-dense. The IXN group could not be visualized due to technical limitations.
EMPs affect endothelial cell viability in a dose-dependent manner
It was not possible to count the number of in vitro generated microparticles and hence, it was evaluated the effect of increasing volumes from EMPs suspensions in cell viability to elucidate their toxicity in the following assays. Apart from the Control group we observed a dose-dependent decrease of cell viability, reaching significance in the IXN and GM-CSF groups, at 90 µl and 120 µl (Figure S4). Therefore, the selected volumes for the in vitro studies were 30 and 60 µl of EMPs.
EMPs inhibit endothelial cell migration
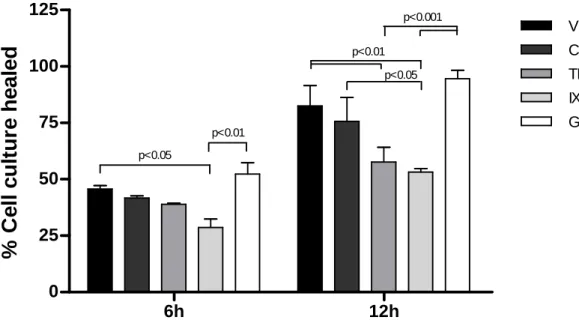
The interaction of EMPs and HUVEC at the site of cell migration was confirmed by immunofluorescence (Figure S5, B). A similar healing profile was observed at the two selected time points, 6 and 12h (Figure 5). Control and GM-CSF groups did not differ significantly from the vehicle treatments and GM-CSF even slightly stimulated the healing process (Figure 5). The strongest inhibition was observed for the IXN group, which impaired significantly the cell migration at 6 and 12h, when compared to the vehicle and GM-CSF groups (Figure 5). TNFα-generated EMPs, only reached significant inhibition at 12h, comparing to the vehicle and GM-CSF (Figure 5).
IXN EMPs have increased uptake by endothelial cells
Dil-labeled EMPs (Dil-EMPs) were incubated with subconfluent HUVEC to assess their interaction. EMPs co-localized with cells and some seemed to accumulate in specific intracellular compartments, with many microparticles collecting at the expected Golgi apparatus site (Figure 6, A, arrowheads). As expected, no Dil-range fluorescence was detected in the vehicle group. Interestingly EMPs originated from IXN+TNFα were
significantly retained by endothelial cells, when compared to EMPs generated from TNFα alone (Figure 6, B)
VEGFR2 is abundant in endothelial cells but not detected in EMPs
Western blot was used to determine if VEGFR2 was present in EMPs and thus playing a role on its effects. VEGFR2 was highly expressed in HUVEC as predicted, but was absent in every EMPs subset (Figure 7).
EMPs accumulate in leukocytes and microglia and do not affect retinal angiogenesis
To investigate the In vivo effects of EMPs, the developmental retinal angiogenesis model was used (Figure S2). It was found an intense accumulation of Dil-EMPs in leukocytes and a particular uptake by microglia modulating the vasculature at pruning and proliferation sites (Figure 8, A-C). The accumulation of EMPs on endothelial cells lining the vessels was scarcer (data not shown). Additionally, we searched for EMPs effect on retinal angiogenesis. No differences in vascular length, vessel density and junctions density were detected (Figure 8, D-F).
Discussion
In the present study, we demonstrated elevated circulating levels of PMPs and apoptotic EMPs in asthma patients. Moreover, we showed that the polyphenol IXN generates VEGFR2-null EMPs with in vitro anti-angiogenic properties and increased uptake by endothelial cells.
The observed increase in PMPs among asthmatics is particularly relevant as it may provide a rationale for underexplored pathophysiologic mechanisms in the airway inflammation. PMPs are pro-inflammatory mediators that induce ICAM-1 upregulation in endothelial cells [12]. Interestingly, we observed a significant increase of sICAM-1 in asthmatics that however, did not correlate with PMPs values. Nevertheless, the measured sICAM-1 represents a systemic evaluation and PMPs may be locally firing endothelial cells. Whether PMPs are generated from local or systemic platelet activation is a question that awaits further investigation. Previously reported migration of platelets in the airways after allergen challenge, suggests a more probable local activation [21]. Furthermore, platelets have been described as essential for leukocyte infiltration in the airways, through the formation of leukocyte-platelet aggregates rich in the surface expression of the adhesion molecule CD11b [17,20]. Again, released PMPs may be carrying platelet-derived factors that stimulate cell trafficking into the airways. A proteomic study of PMPs presents several pro-inflammatory and adhesion candidates, such as CD40L, CD226 or P-selectin, that may be mediating this effect [34] and a similar mechanism involving the induction of IL-8 by IL1α-positive PMPs was revealed in the case of rheumatoid arthritis [14]. The importance of local platelet activation was also highlighted by a study reporting elevated PMPs in atopic dermatitis [35]. Paradoxically, we observed a negative correlation between PMPs and FeNO, an indirect measurement of airway inflammation. On the other hand, it was shown that activated platelets release NO that halts further platelet recruitment [36]. This negative feedback mechanism may apply to this situation, where PMPs release would be prevented by the rising levels of NO in the airways. It is also interesting to observe that microparticles were previously proposed as mediators of disrupted immunological communication in diseases where the hygiene hypothesis is a possibility, which is the case of asthma [37].
The importance of the airways vasculature and increased angiogenesis in asthma was also emphasized in our study [16]. In the asthma group, we found augmented levels of the main angiogenic factor, VEGF, and its positive correlation with eosinophils, a major cell type in asthma and a site of VEGF release [38]. In line with previous observations,
we also found that the increased levels of the endothelium-born sICAM-1 correlated with asthma control scores [39]. Additionally, we detected increased levels of apoptotic EMPs, but not total EMPs, in asthmatics, which may address interesting possibilities in asthma pathology. As an example, apoptotic EMPs, from atherosclerotic lesions, were proved to be ICAM-1 deliverers to endothelial cells, which could contribute to monocyte rolling and inflammation local amplification [40]. Notwithstanding, the increased levels of apoptotic EMPs were not significant when we restricted comparisons for subjects without MetS, a condition with known elevated levels of these microparticles [4]. This was probably related to the small sample size used, but once again, underlines the importance of inflammation to endothelial activation. Recently, the association between MetS and asthma symptoms was suggested, while the underlying mechanisms remained unknown [41]. We searched for microparticles alterations between asthmatics with and without MetS, but differences were not significant, rendering PMPs or EMPs unlikely mediators in this relation. Adiponectin inhibits monocyte-endothelial adhesion and decreased serum levels of adiponectin have been associated with both metabolic syndrome and asthma [42-44]. In this study, we found among overall asthmatics, a non significant decline of adiponectin but an inverse correlation with CARAT, an asthma and rhinitis score that punctuates higher for better control. These observations are in accordance with previous findings of increased adiponectin levels in rhinitis [45].
Owing to the probable participation of microparticles and vascular factors in asthma pathological modifications we then asked if the anti-inflammatory and anti-angiogenic molecule, IXN, could generate EMPs (IXN-EMPs) with interesting therapeutic properties in endothelial cells.
We observed diminished endothelial cell viability with higher quantities of IXN-EMPs. Additionally, IXN-EMPs revealed the strongest decrease in cell migration between groups. The overall anti-angiogenic effect of IXN-EMPs is in accordance to previous observations with EMPs generated from TNFα alone [46]. We also confirmed the co-localization of microparticles with HUVEC and identified a significant increase in the uptake of IXN-EMPs, suggesting that IXN might be altering the composition of microparticles during their formation. In the case of EMPs, the exposure of surface proteins related to angiogenesis, such as matrix metalloproteases (MMP), vascular endothelial cadherin (VE-cadherin) and T-cadherin is established [47]. We asked if the main VEGF receptor, VEGFR2, commonly used to identify endothelial progenitor cells (EPCs) [48] and active endothelial cells, could be present and differentially expressed in EMPs. Western blotting revealed its absence in every EMPs group, including
IXN-EMPs, leaving VEGFR2 as a questionable mediator of their anti-angiogenic properties within EMPs. An appealing possibility is that IXN itself incorporates inside carrying EMPs and unloads into target cells, such as endothelial cells. However, this hypothesis, along with the characterization of differences in lipids and protein content of IXN-EMPs, requires more investigation.
We also evaluated EMPs originated from GM-CSF treatment (GM-CSF-EMPs), an underexplored cytokine in microparticles studies, produced by endothelial cells and eosinophils and involved in their survival and proliferation [49-51]. Electron microscopy of GM-CSF-EMPs revealed double membrane vesicles morphologically similar to EMPs from the other groups but with apparently less organized content. Contrary to IXN, GM-CSF induced the formation of EMPs that did not affect cell migration and with unchanged interaction with cultured endothelial cells, comparing to control EMPs. Consequently, GM-CSF-EMPs seem to be much less promising agents in impaired angiogenesis.
To further explore the outcomes and localization of these EMPs, we used the developmental retinal angiogenesis model, which allows clear visualization and quantification of possible anti or pro-angiogenic compounds effects in an In vivo setting [52]. We observed unaltered angiogenesis by EMPs that may be explained by the retinal vascular stabilization achieved at the used time points. The outcome might be different in pathological angiogenesis, where vessels are more immature and leaky, similarly to angiogenesis in chronic airway inflammation [53]. Interestingly, labeled EMPs strongly collected in tissue macrophages or microglia, a cell type involved in vascular pruning and a major participant in vessel fusion [54]. EMPs may act as modulators of macrophage-endothelial interaction, by affecting the expression of adhesion molecules.
In summary, this study highlights a new role for cell microparticles in human diseases by reporting increased levels of PMPs and, to a lesser extent, apoptotic EMPs in asthma patients. Our observations open new perspectives for futures approaches to inflammation and angiogenesis in asthma, by including microparticles as active players. We also suggest that microparticles actions might be shaped by interesting compounds, such as IXN and we present IXN-EMPs as anti-angiogenic microparticles that warrant additional examination to better elucidate their potential therapeutic use.
Acknowledgments
I would like to deeply thank everyone involved in this work, namely Tiago Taveira Gomes, Rita Negrão, Raquel Costa and Raquel Soares, my advisor, from the Department of Biochemistry, Oksana Sokhatska, Carmo Palmares and André Moreira, from the Department of Immunology, Carla Martins, from the Department of Immunoallergology, Hospital São João, and João Tiago Guimarães, from the Department of Clinical Pathology, Hospital São João and Department of Biochemistry, Faculty of Medicine, University of Porto. I thank Delminda Magalhães and the excellent technical assistance of Elisa Nova and Victor Mata, from the Department of Experimental Biology and Department of Electronic Microscopy and Dr. Stirling Carpenter, from the Department of Pathology and Oncology, Faculty of Medicine, University of Porto. I also thank Christiana Katti, Sumathi Sekaran and Russell Foster, from the Nuffield Laboratory of Ophthalmology, University of Oxford, for providing invaluable training in retina dissection. I finally thank Diana Soares, for the fruitful and motivating discussions during the manuscript preparation and Teresa Rocha and Manuel Pinto for the critical review of the manuscript.
Financial Disclosure
This study was supported by a grant from Fundação Professor Ernesto Morais. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests
References
1. Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM (2005) Membrane microparticles: two sides of the coin. Physiology (Bethesda) 20: 22-27.
2. VanWijk MJ, VanBavel E, Sturk A, Nieuwland R (2003) Microparticles in cardiovascular diseases. Cardiovasc Res 59: 277-287.
3. George FD (2008) Microparticles in vascular diseases. Thromb Res 122 Suppl 1: S55-59.
4. Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, et al. (2006) Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol 98: 70-74.
5. Kumpers P, Erdbrugger U, Grossheim M, Meyer GP, Hiss M, et al. (2008) Endothelial microparticles as a diagnostic aid in Churg-Strauss vasculitis-induced cardiomyopathy. Clin Exp Rheumatol 26: S86-89.
6. Lacroix R, Sabatier F, Mialhe A, Basire A, Pannell R, et al. (2007) Activation of plasminogen into plasmin at the surface of endothelial microparticles: a mechanism that modulates angiogenic properties of endothelial progenitor cells
in vitro. Blood 110: 2432-2439.
7. Chahed S, Leroyer AS, Benzerroug M, Gaucher D, Georgescu A, et al. (2010) Increased vitreous shedding of microparticles in proliferative diabetic retinopathy stimulates endothelial proliferation. Diabetes 59: 694-701.
8. Taraboletti G, D'Ascenzo S, Borsotti P, Giavazzi R, Pavan A, et al. (2002) Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol 160: 673-680. 9. Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, et al. (1999) In vitro
generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 104: 93-102.
10. Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D (2005) Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res 67: 30-38.
11. Mause SF, Ritzel E, Liehn EA, Hristov M, Bidzhekov K, et al. (2010) Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation 122: 495-506.
12. Barry OP, Pratico D, Savani RC, FitzGerald GA (1998) Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest 102: 136-144.
13. Morel O, Morel N, Freyssinet JM, Toti F (2008) Platelet microparticles and vascular cells interactions: a checkpoint between the haemostatic and thrombotic responses. Platelets 19: 9-23.
14. Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, et al. (2010) Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327: 580-583.
15. Maddox L, Schwartz DA (2002) The pathophysiology of asthma. Annu Rev Med 53: 477-498.
16. Detoraki A, Granata F, Staibano S, Rossi FW, Marone G, et al. (2010) Angiogenesis and lymphangiogenesis in bronchial asthma. Allergy 65: 946-958. 17. Coyle AJ, Page CP, Atkinson L, Flanagan R, Metzger WJ (1990) The requirement for platelets in allergen-induced late asthmatic airway obstruction. Eosinophil infiltration and heightened airway responsiveness in allergic rabbits. Am Rev Respir Dis 142: 587-593.
18. Gresele P, Dottorini M, Selli ML, Iannacci L, Canino S, et al. (1993) Altered platelet function associated with the bronchial hyperresponsiveness accompanying nocturnal asthma. J Allergy Clin Immunol 91: 894-902.
19. Sullivan PJ, Jafar ZH, Harbinson PL, Restrick LJ, Costello JF, et al. (2000) Platelet dynamics following allergen challenge in allergic asthmatics. Respiration 67: 514-517.
20. Pitchford SC, Yano H, Lever R, Riffo-Vasquez Y, Ciferri S, et al. (2003) Platelets are essential for leukocyte recruitment in allergic inflammation. J Allergy Clin Immunol 112: 109-118.
21. Pitchford SC, Momi S, Baglioni S, Casali L, Giannini S, et al. (2008) Allergen induces the migration of platelets to lung tissue in allergic asthma. Am J Respir Crit Care Med 177: 604-612.
22. Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L (2010) Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int 78: 838-848.
23. Soleti R, Benameur T, Porro C, Panaro MA, Andriantsitohaina R, et al. (2009) Microparticles harboring Sonic Hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis 30: 580-588.
24. Serwe A, Rudolph K, Anke T, Erkel G (2011) Inhibition of TGF-beta signaling, vasculogenic mimicry and proinflammatory gene expression by isoxanthohumol. Invest New Drugs.
25. Negrao R, Costa R, Duarte D, Taveira Gomes T, Mendanha M, et al. (2010) Angiogenesis and inflammation signaling are targets of beer polyphenols on vascular cells. J Cell Biochem 111: 1270-1279.
26. Yang JY, Della-Fera MA, Rayalam S, Baile CA (2007) Effect of xanthohumol and isoxanthohumol on 3T3-L1 cell apoptosis and adipogenesis. Apoptosis 12: 1953-1963.
27. Negrao R, Duarte D, Costa R, Soares R (2011) Could platelet-accumulating polyphenols prevent tumour metastasis? Nat Rev Cancer 11: 685.
28. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, et al. (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120: 1640-1645.
29. Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR (1999) Development and validation of a questionnaire to measure asthma control. Eur Respir J 14: 902-907.
30. Fonseca JA, Delgado L, Costa-Pereira A, Tavares C, Moreira A, et al. (2004) Evaluation of the Asthma Life Quality test for the screening and severity assessment of asthma. Allergy 59: 1198-1204.
31. Fonseca JA, Nogueira-Silva L, Morais-Almeida M, Azevedo L, Sa-Sousa A, et al. (2010) Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy 65: 1042-1048.
32. (1999) Recommendations for standardized procedures for the on-line and off-line measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med 160: 2104-2117.
33. Zudaire E, Gambardella L, Kurcz C, Vermeren S (2011) A computational tool for quantitative analysis of vascular networks. PLoS One 6: e27385.
34. Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, et al. (2011) Deciphering the human platelet sheddome. Blood 117: e15-26.
35. Tamagawa-Mineoka R, Katoh N, Ueda E, Masuda K, Kishimoto S (2009) Platelet-derived microparticles and soluble P-selectin as platelet activation markers in patients with atopic dermatitis. Clin Immunol 131: 495-500.
36. Freedman JE, Loscalzo J, Barnard MR, Alpert C, Keaney JF, et al. (1997) Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Invest 100: 350-356.
37. Martin PI, Sanchez PA, Rewald E (2009) Microparticles and the hygiene hypothesis. Ann N Y Acad Sci 1173: 409-421.
38. Horiuchi T, Weller PF (1997) Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol 17: 70-77.
39. Kobayashi T, Hashimoto S, Imai K, Amemiya E, Yamaguchi M, et al. (1994) Elevation of serum soluble intercellular adhesion molecule-1 (sICAM-1) and sE-selectin levels in bronchial asthma. Clin Exp Immunol 96: 110-115.
40. Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, et al. (2011) Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res 108: 335-343.
41. Lee EJ, In KH, Ha ES, Lee KJ, Hur GY, et al. (2009) Asthma-like symptoms are increased in the metabolic syndrome. J Asthma 46: 339-342.
42. Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, et al. (1999) Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100: 2473-2476.
43. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I (2004) Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol 24: 29-33.
44. Sood A, Cui X, Qualls C, Beckett WS, Gross MD, et al. (2008) Association between asthma and serum adiponectin concentration in women. Thorax 63: 877-882. 45. Ciprandi G, Murdaca G, Marseglia G, Colombo BM, Quaglini S, et al. (2008) Serum
adiponectin levels in patients with pollen-induced allergic rhinitis. Int Immunopharmacol 8: 945-949.
46. Mezentsev A, Merks RM, O'Riordan E, Chen J, Mendelev N, et al. (2005) Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am J Physiol Heart Circ Physiol 289: H1106-1114.
47. Dignat-George F, Boulanger CM (2011) The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 31: 27-33.
48. Werner N, Nickenig G (2007) Endothelial progenitor cells in health and atherosclerotic disease. Ann Med 39: 82-90.
49. Esnault S, Malter JS (2002) GM-CSF regulation in eosinophils. Arch Immunol Ther Exp (Warsz) 50: 121-130.
50. Quesenberry PJ, Gimbrone MA, Jr. (1980) Vascular endothelium as a regulator of granulopoiesis: production of colony-stimulating activity by cultured human endothelial cells. Blood 56: 1060-1067.
51. Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, et al. (1989) Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature 337: 471-473.
52. Dorrell MI, Friedlander M (2006) Mechanisms of endothelial cell guidance and vascular patterning in the developing mouse retina. Prog Retin Eye Res 25: 277-295.
53. Dahlqvist K, Umemoto EY, Brokaw JJ, Dupuis M, McDonald DM (1999) Tissue macrophages associated with angiogenesis in chronic airway inflammation in rats. Am J Respir Cell Mol Biol 20: 237-247.
54. Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, et al. (2010) Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116: 829-840.
Figure Legends
Figure 1. Apoptotic EMPs are increased in asthmatic patients.
A and B, Flow cytometry analysis revealed no significant differences in total EMPs (CD31+/42b-) between asthmatics and non-asthmatics (A, p=0.336) and a significant increase of apoptotic EMPs (CD31+/42b-/AnV+) among asthmatic patients (B, p=0.021). C and D, Total EMPs differences remained non-significant (C, p=0.807) and apoptotic EMPs alterations lost significance (D, p=0.103) when the analysis was restricted to asthmatics and non-asthmatics without metabolic syndrome (MetS).
Figure 2. PMPs are increased in asthmatic patients.
A, PMPs values are significantly increased in asthmatic patients (p=0.033). B, PMPs increase remains significant in asthmatics vs. non-asthmatics without metabolic syndrome (MetS) (p=0.031).
Figure 3. Significant correlations between PMPs, VEGF and adiponectin vs. FeNO, eosinophils and CARAT score respectively, among asthmatics.
A, PMPs values significantly decrease with increasing exhaled NO (FeNO). B, VEGF positively correlates with eosinophils counts. C, Adiponectin decreases with increasing CARAT score.
Figure 4. Representative electron micrographs of endothelial microparticles obtained from the media of HUVEC treated with different stimuli.
Microparticles (*) have variable sizes under 1 µm, are round-shaped, carry heterogeneous content with different electrodensities, representing the differential sorting upon blebbing, and have a membrane bilayer (arrows) with two leaflets (arrowheads, higher magnifications). Note that some microparticles are ruptured, probably due to the technical procedure. Microparticles were released upon PBS (A, B), 10ng/ml TNFα (C, D) and 10 ng/ml GM-CSF (E, F and G) treatments. Microparticles released after 10 nM IXN + 10ng/ml TNFα treatment could not be visualized and are
not represented. Magnifications: x40 000 (A, C, E and F), x53 000 (B), x100 000 (D), x200 000 (G).
Figure 5. EMPs inhibit in vitro cell migration.
At 6h time point, migration of HUVEC treated with IXN-generated EMPs was markedly decreased (p<0.05 vs. vehicle). At 12h time point, the inhibition of cell migration after IXN-generated EMPs treatment is exacerbated (p<0.01 vs. vehicle) and TNFα -generated EMPs also decrease in vitro culture healing significantly (p<0.01 vs. vehicle). EMPs generated with no stimulus (Control) and GM-CSF-generated EMPs do not affect cell migration, when compared to vehicle treatments.
Figure 6. Endothelial cell uptake of IXN-generated EMPs is increased.
A, Representative captions of HUVEC (isolectin B4, green and DAPI, blue) treated with vehicle (PBS) or EMPs (Dil, red) generated with different stimuli (Control, TNFα, IXN and GM-CSF). EMPs (arrowheads) interact with and accumulate in endothelial cells. Scale bars, 25 µm. B, Quantitative analysis of EMPs co-localizing with HUVEC shows increased uptake of IXN-generated EMPs by endothelial cells (p<0.001 vs. TNF alone).
Figure 7. VEGFR2 is not expressed in EMPs.
Protein quantification in EMPs and in corresponding parental HUVEC was performed with western blot analysis. The angiogenic membrane receptor, VEGFR2 expression was not detected in any of the EMPs fractions and the cytoskeleton marker, β-actin, was dimly expressed. As expected, VEGFR2 was highly expressed in the HUVEC used to generate EMPs.
Figure 8. EMPs accumulate in tissue macrophages and do not affect developmental retinal angiogenesis.
Dil-labeled EMPs (A and B, red; C, green) were injected in the eyes of mice pups and after 5 days their localization and effects in the retinal vasculature (isolectin B4; A and
B, green; C, red) was examined. Tissue macrophages or microglia, responsible for vascular remodeling, incorporate labeled-EMPs (A, higher magnification B and C). Scale bars, 25 µm (A and C) and 15 µm (B). Quantitative analysis of vascular length (D), vessel density (E) and branch-point or junctions density (F) revealed no differences between groups.
Table 1. Characteristics of asthmatic and non-asthmatic subjects
. Asthmatic Non-Asthmatic
Median IQR N Median IQR N P value Baseline characteristics Age (y) 50,5 32,0-58,3 22 29,0 26,0-31,0 15 0,001 Gender (female/male) 15/7 22 12/4 15 BMI (kg/m2) 26,1 23,5-32,6 22 21,7 19,2-25,1 15 0,001 Waist circumference (cm) 95,5 84,5-107,5 22 81,0 78,0-90,0 16 0,001 SBP (mmHg) 133,0 123,3-147,3 22 117,0 112,0-128,0 15 0,006 DBP (mmHg) 66,5 60,3-78,0 22 70,0 64,0-75,0 15 NS
Asthma control scores
AQLQ 14,0 10,8-24 22 0,0 0-0 15 <0,001 ACQ 6,0 1-9,8 22 0,0 0-0 15 <0,001 CARAT 18,0 12,5-23 21 30,0 29-30 15 <0,001 Lung function and airway
inflammation FVC (L) 3,3 2,6-4,3 20 3,9 3,5-5,4 15 0,036 FEV1 (L) 2,4 1,6-3,2 20 3,4 2,9-4,6 15 0,002 FEV1/FVC ratio (%) 70,6 61,0-80,5 20 85,0 79,2-86,6 15 0,001 MMEF 75/25 (L/s) 1,6 0,8-3,1 20 3,8 2,9-4,2 15 <0,001 FeNO (ppb) 41,0 9,2-48,0 15 11,0 5,0-25,0 15 0,016 Blood counts and
cholesterol Eosinophils (103/ µl) 0,15 0,11-0,24 20 0,13 0,10-0,16 11 NS Platelets (103/µl) 251,5 207,3-280,5 20 217,0 186,0-309,0 15 NS Cholesterol (mg/dl) 196,0 179,0-237,0 20 184,5 169,3-210,3 16 NS HDL (mg/dl) 51,0 43,5-57,0 20 52,5 45,8-62,8 16 NS VLDL (mg/dl) 25,6 17,7-49,7 20 22,1 18,7-34,2 16 NS LDL (mg/dl) 121,8 91,4-148,5 20 107,5 89,1-127,1 16 NS Values are expressed as medians and interquartile ranges (IQR); p values were obtained using the Mann-Whitney U test. Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AQLQ, Asthma Quality of Life Questionnaire; ACQ, Asthma Control Questionnaire; CARAT, Control of Allergic Rhinitis and Asthma Test; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; MMEF, maximum midexpiratory flow rate; FeNO, fraction of expired nitric oxide; HDL, high density lipoprotein; VLDL, very low density lipoprotein; LDL, low density lipoprotein.
Table 2. Serum biomarkers and cell microparticles in asthmatic and non-asthmatic subjects
Asthmatic Non-Asthmatic
Median IQR N Median IQR N P value Inflammatory, endothelial and
adipose tissue biomarkers
IL10 (pg/ml) 5,4 2,6-11,3 20 3,9 2,0-6,6 16 NS TNFα (pg/ml) 4,5 3,0-5,6 20 3,4 2,6-4,6 16 NS VEGF (pg/ml) 65,4 38,9-107,3 20 35,3 13,6-53,2 16 0,021 Adiponectin (µg/ml) 16,5 11,6-27,6 15 21,1 14,5-30,4 12 NS sICAM-1 (ng/ml) 104,1 80,1-122,0 20 71,5 54,8-103,2 16 0,01 C-reactive protein (mg/l) 2,9 1,3-7,5 20 1,4 0,7-3,7 16 NS Microparticles (counts/µl) EMP (CD31+/42b-) 404,5 217,8-1030,5 20 209,5 114,3-1534,5 16 0,336 EMP (CD31+/42b-/AnV+) 36,5 12,75-135,50 20 11,0 6,0-35,5 16 0,021 PMP (CD31+/42b+) 17540,0 11170,8-45158,8 20 6107,0 2374,0-19753,0 16 0,033 PMP (CD31+/42b+/AnV+) 17388,0 10542,5-45030,8 20 5654,5 2358,8-19637,3 16 0,033 Values are expressed as medians and interquartile ranges (IQR); p values were obtained using the Mann-Whitney U test. Abbreviations: IL10, interleukin 10; TNFα, tumor necrosis factor alpha; VEGF, vascular endothelial growth factor; sICAM-1, soluble intercellular adhesion molecule 1; EMP, endothelial microparticles; PMP, platelet microparticles.
Table 3. Cell microparticles in asthmatic and non-asthmatic subjects, without metabolic syndrome Asthmatic without MetS Non-Asthmatic without MetS
Median IQR N Median IQR N P value
Microparticles (counts/µl)
EMP (CD31+/42b-) 488,0 266,5-822,8 10 222,0 124,0-1656,0 15 0,807 EMP (CD31+/42b-/AnV+) 39,0 11,5-91,3 10 12,0 6,0-37,0 15 0,103 PMP (CD31+/42b+) 23879,5 11584,8-58939,5 10 7244,0 3250,0-20259,0 15 0,031 PMP (CD31+/42b+/AnV+) 23816,0 11544,0- 58166,8 10 7191,0 3237,0-20164,0 15 0,031 Values are expressed as medians and interquartile ranges (IQR); p values were obtained using the Mann-Whitney U test. Abbreviations: MetS, metabolic syndrome; EMP, endothelial microparticles; PMP, platelet microparticles.
Figure 1
A
C
B
Figure 2
Figure 3 A B C r = -0.656 p = 0.011 r = 0.732 p = 0.001 r = -0.723 p = 0.003
Figure 4
A
C
E
G
D
F
B
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
*
C
o
n
tr
o
l
T
N
F
α
G
M
-C
S
F
*
Figure 5 6h 12h 0 25 50 75 100 125 Vehicle C TNFα IXN GM-CSF p<0.05 p<0.01 p<0.05 p<0.01 p<0.001
%
C
e
ll
c
u
lt
u
re
h
e
a
le
d
Figure 6 C TNFα IXN GM-CSF Vehicle B A C TNFα IXN GM-CSF 0 10 20 30 40 50 p<0.001 EMPs origin % H U V E C u p ta k in g E M P s
Figure 7 C TNFα GM-CSF IXN C TNFα GM-CSF IXN EMPs HUVEC VEGFR2 210 KDa β-Actin 43 KDa
Figure 8 Vehicle C TNFα IXN GM-CSF 0 10 20 30 40 V e s s e l d e n s it y ( % ) Vehicle C TNFα IXN GM-CSF 0.0 0.2 0.4 0.6 0.8 1.0 V a s c u la tu re l e n g th p e r re ti n a l ra d iu s Vehicle C TNFα IXN GM-CSF 0.0000 0.0000 0.0001 0.0001 0.0002 J u n c ti o n s d e n s it y A B C D E F
Supporting Figures Legends
Figure S1. Representative flow cytometric plot illustrating the analysis of microparticles.
Example of the analysis of EMPs and apoptotic (annexin V+) EMPs from an asthmatic patient. The gate for microparticles size was achieved by using 0.4 µm and 0.69 µm beads (not represented). The subsequent analysis was performed by gating CD31+ microparticles (in red). In the bottom panel CD31+/CD42b- microparticles (in red) and CD31+/CD42b-/AnV+ microparticles (in yellow) are represented. SSC, side scatter; FSC, forward scatter.
Figure S2. Schematic representation of the developmental angiogenesis model.
The upper panel illustrates the method used to make intravitreal injections of EMPs in mice pups and the following steps used to observe retinal angiogenesis. The lower panels show representative section and confocal images of the three retinal vascular layers of a post-natal day 14 mouse.
Figure S3. HUVEC morphology after 24h treatments with different stimuli in serum-reduced media for generation of microparticles.
Cells treated with PBS (C, control) are sub-confluent and have increased cell death. Treatment with 10 ng/ml TNFα induced cell elongation, resembling a migratory phenotype, with few free spaces. Cells co-incubated with 10 µM IXN and 10 ng/ml TNFα were confluent and had exaggerated elongated shapes. Cells stimulated with 10 ng/ml GM-CSF were similar to the control group, but had sporadic small extensions. All treatments seem to induce less cellular regression with loss of confluence and less cell death, comparing to the control. Scale bar, 100 µm.
Figure S4. Effect of EMPs on HUVEC cell viability.
Endothelial cell viability after incubation for 24h with 15-120 µl EMPs was evaluated by MTS assay. IXN and GM-CSF-generated EMPs significantly increased cell toxicity at higher concentrations (p<0.05 vs. control). Results are means ± SEM and are expressed as percentage of control.
Figure S5. HUVEC migration is affected by EMPs over time.
A, Comparison panels of in vitro endothelial cell migration after incubation with different EMPs populations for 6h and 12h time points. Scale bar, 100 µm. B, Representative image of EMPs (Dil, red) interacting with migrating HUVEC (isolectin B4, green and DAPI, blue) in the wound area.
Supporting Information
Table S1. Cell microparticles in asthmatic subjects, with and without metabolic syndrome
Asthmatic with MetS
Asthmatic without MetS
Median IQR N Median IQR N P value
Microparticles
EMP (CD31+/42b-) 350,5 198,8-10644,8 10 488,0 266,5-822,8 10 0,971 EMP (CD31+/42b-/AnV+) 34,5 18,0-267,0 10 39,0 11,5-91,3 10 0,579 PMP (CD31+/42b+) 14036,5 9663,3-30991,0 10 23879,5 11584,8-58939,5 10 0,353 PMP (CD31+/42b+/AnV+) 13828,5 9430,3-30792,0 10 23816,0 11544,0-58166,8 10 0,353 Values are expressed as medians and interquartile ranges (IQR); p values were obtained using the Mann-Whitney U test. Abbreviations: MetS, metabolic syndrome; EMP, endothelial microparticles; PMP, platelet microparticles.
Table S2. Correlations of cell microparticles with anthropometric and asthma parameters, in asthmatic patients.
BMI (kg/m2
) WC (cm) AQLQ ACQ CARAT FEV1 FEV1/FVC (FEV1%) MMEF 75/25 FeNO Microparticles EMP (CD31+/42b-) R 0,242 0,053 0,043 0,229 -0,093 0,065 0,325 0,158 0,059 P 0,304 0,823 0,857 0,332 0,706 0,798 0,188 0,531 0,840 N 20 20 20 20 19 18 18 18 14 EMP (CD31+/42b-/AnV+) R 0,391 0,226 0,233 0,198 -0,016 -0,281 0,010 -0,228 -0,147 P 0,089 0,338 0,322 0,402 0,947 0,259 0,968 0,362 0,617 N 20 20 20 20 19 18 18 18 14 PMP (CD31+/42b+) R -0,101 -0,132 0,094 -0,176 -0,030 -0,172 0,079 -0,077 -0,674** P 0,673 0,580 0,694 0,457 0,903 0,494 0,754 0,760 0,008 N 20 20 20 20 19 18 18 18 14 PMP (CD31+/42b+/AnV+) R -0,101 -0,132 0,137 -0,135 -0,065 -0,146 0,098 -0,046 -0,656* P 0,673 0,578 0,565 0,571 0,791 0,565 0,699 0,855 0,011 N 20 20 20 20 19 18 18 18 14 Values are Spearman correlation coefficients (R). **, correlation is significant at the 0.01 level (2-tailed); *, correlation is significant at the 0.05 level (2-tailed).
Table S3. Correlations of serum biomarkers with anthropometric and asthma parameters, in asthmatic patients.
BMI (kg/m2
) WC (cm)
AQLQ ACQ CARAT FEV1 FEV1/FVC (FEV1%) MMEF 75/25 FeNO CRP (mg/L) Eosinophils (103 /µL) Biomarkers IL10 (pg/ml) R 0,325 0,339 0,117 0,153 0,032 -0,036 0,020 -0,057 0,029 0,339 0,099 P 0,162 0,143 0,623 0,520 0,897 0,887 0,938 0,823 0,923 0,143 0,695 N 20 20 20 20 19 18 18 18 14 20 18 TNFα (pg/ml) R 0,029 0,270 -0,033 -0,064 -0,110 -0,034 0,069 -0,057 -0,152 0,555* 0,549* P 0,905 0,250 0,889 0,788 0,653 0,893 0,785 0,823 0,604 0,011 0,018 N 20 20 20 20 19 18 18 18 14 20 18 VEGF (pg/ml) R 0,069 0,035 -0,177 -0,183 0,236 0,051 -0,232 -0,067 0,024 0,358 0,732** P 0,772 0,882 0,455 0,440 0,330 0,842 0,354 0,791 0,934 0,121 0,001 N 20 20 20 20 19 18 18 18 14 20 18 Adiponectin (ug/ml) R -0,282 -0,125 0,440 0,315 -0,723** -0,604* 0,143 -0,253 -0,267 0,004 -0,160 P 0,308 0,657 0,100 0,252 0,003 0,022 0,626 0,383 0,455 0,990 0,584 N 15 15 15 15 14 14 14 14 10 15 14 sICAM-1 (ng/ml) R 0,262 0,239 0,476* 0,465* -0,342 -0,119 -0,059 -0,141 -0,203 0,518* 0,021 P 0,265 0,310 0,034 0,039 0,152 0,639 0,817 0,576 0,487 0,019 0,935 N 20 20 20 20 19 18 18 18 14 20 18 Values are Spearman correlation coefficients (R). **, correlation is significant at the 0.01 level (2-tailed); *, correlation is significant at the 0.05 level (2-tailed).