INTRODUCTION
Red cell preservative so lutio ns have been de-signed o ver the last two decades1-7 with the aim o f
in-creasing blo o d viability fo r transfusio n. ADSOL2
preservative so lutio n, co mpo sed o f adenine, dextro se, so -dium chlo ride and m annito l, has b e e n e m plo ye d wo rldwide. Red cells maintained in ADSOL are viable fo r transfusio n fo r up to 7 weeks.7,8
Studies o n red cell preservatio n have aimed at keep-ing ATP and 2,3-DPG co ncentratio ns fo r as lo ng as po s-sible, and many researchers have been able to maintain them fo r up to 7 weeks.7,8 Altho ugh these remarkable
re-sults have been achieved by the additio n o f several co m-po unds like adenine, ino sine, so rbito l, asco rbic acid, etc., they co uld o nly be o btained because the red cell metabo -lism was still wo rking well, as the glyco lytic and pento se shunt enzymes and also the membrane pro teins keep fairly go o d activity and integrity levels. Co nsequently, glyco lytic kinases, glyco lytic and no n-glyco lytic dehydro genases and membrane pro teins were studied fo r a perio d o f 14 weeks, in o rder to determine whether the red cell metabo lism status wo uld suppo rt further studies aiming at increasing red cell preservatio n and viability fo r blo o d bank purpo ses. In this present wo rk bio chemical evaluatio n o f RBC during preservatio n in ADSOL, fo r up to 14 weeks at 4°C, is repo rted. Red blo o d cell ATP, 2,3-DPG levels, glyco lytic kinase and selected dehydro genase activi-tie s and m e m b rane p ro te in frac tio ns we re m ad e thro ugho ut the 14 weeks.
METHODS
The pro cedures that fo llo w were in acco rdance
Original Article
REVISTA PAULISTA DE MEDICIN AEnzyme s and me mbrane prote ins of
ADSOL-pre se rve d re d blood ce lls
LIM-23, Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil,
and Divisão de Saúde da Universidade Federal do Paraná, Curitiba, Brazil
a b s t r a c t
CON TEXT: The preservative solution ADSO L (adenine, dextrose, sorbitol, sodium chloride and mannitol) maintains red cell viability for blood trans-fusion for 6 weeks. It would be useful to know about its preservation qualities over longer periods.
OBJECTIVE: To determine some red cell biochemical parameters for peri-ods of up to 1 4 weeks in order to determine whether the red cell metabo-lism integrity would justify further studies aiming at increasing red cell preservation and viability.
DESIGN : Biochemical evaluation designed to study red cell preservation.
SETTIN G: São Paulo University erythrocyte metabolism referral center.
SAM PLE: Six normal blood donors from the University Hospital of the Universidade Federal do Paraná, Curitiba, Brazil.
M AIN M EASUREM EN TS: W eekly assay of erythrocyte adenosine-5 ´-triphosphate (ATP), 2 ,3 -diphosphoglycerate (2 ,3 DPG ), hexokinase (HX), phosphofructokinase (PFK), pyruvate kinase (PK), glucose-6 -phosphate dehydrogenase (G -6 -PD), 6 -phosphogluconic dehydrogenase (6 -PG D), glyceraldehyde-3 -phosphate dehydrogenase (G APD), glutathione reduc-tase (G R), glutathione peroxidase (G SHPx), plasma sodium and potas-sium, blood pH, and membrane proteins of red cells preserved in ADSO L were studied during storage for 1 4 weeks storage.
RESULTS: During ADSO L preservatio n, erythro cyte ATP co ncentratio n decreased 6 0 % after 5 weeks, and 9 0 % after 1 0 weeks; the pH fell from 6 .8 to 6 .4 by the 1 4 th week. 2 ,3 -DPG concentration was stable during the first week, but fell 9 0 % after 3 weeks and was exhausted after 5 weeks. By the end of the 5 th week, an activity decrease of 1 6 -3 0 % for Hx, G APD, G R, G -6 -PD and 6 -PG D, 3 5 % for PFK and G SHPx, and 4 5 % for PK were observed. Thereafter, a uniform 1 0 % decay was observed for all enzymes up to the 1 4 th week. The red blood cell membrane pro-teins did not show significant alterations in polyacrylamide gel electro-phoresis (SDS-PAG E) during the 1 4 weeks.
CON CLUSION : Although the blood viability was shown to be poor from the 6 th week up to the 1 4 th week of storage due to ATP and 2 ,3 -DPG depletion, the other biochemical parameters remained in fairly good condition for longer storage. As there is a gradual and uniform decay in activity throughout these 1 4 weeks, it seems that ADSO L-preserved red cells may be used as red cell enzyme standards and membrane proteins as well.
KEY W ORDS: Red cell ageing. Red cell membrane proteins. Red cell enzymes. Red cell preservation. ADSO L.
• Maria Sueli So ares Leo nart • Ag uinaldo Jo sé N ascimento
with the ethical standards o f the co mmittee respo n-sible fo r human experimentatio n and with the Helsinki Declaratio n o f 1975, as revised in 1983.
Veno us blo o d units o f 450 ml were co llected in experimental quadruple Blo o d-Packs with a primary co ntainer having CPDA-1 and o ne o f the satellite bags
Figure 2 - Protein membrane SDS-PAGE of RBC ADSOL preserved RBC. Electrophoresis was carried out according to Laemmli’s system, with 10% acrylamide in the running gel and 3% acrylamide in the stacking gel. 100 mg membrane protein were loaded on every well. The runs correspond, from left to right, to 0, 2, 4, 6, 8, 10, 12 and 14 weeks of ADSOL preservation.
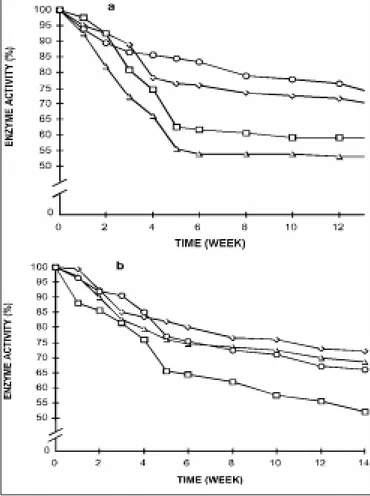
Figure 1 - Erythrocyte enzymes activities during red blood cell preservation in ADSOL. a) Ο - G6PD; ◊ - Hx; • - PFK; ∆ - PK Initial activity: G6PD 11.33 IU; Hx 1.14 IU; PFK 12.42 IU; PK -13.88 IU; b) ◊ - 6PGD; ∆ - GAPD; Ο - GR; • - GSHPX;
Initial activity: 6PGD 8.77 IU; GAPD 292.4 IU; GR 8.77 IU; GSHPx -43.85 IU.
co ntaining ADSOL (Fenwal co de 4R1412, Traveno l Labo rato ries, Inc.). Withdrawals o f 450 ml o f blo o d were made fro m each o f 6 healthy adults o f bo th sexes, with ages ranging fro m 22 to 49 years.
The blo o d units were centrifuged at 600 g at 4°C fo r 30 minutes. The supernatant plasma was trans-ferred to the first satellite pack, and the buffer co ating to the seco nd o ne. The erythro cytes fro m the first co n-tainer were resuspended in the same vo lume o f ADSOL (2 mM adenine, 122 mM gluco se, 154 mM so dium chlo -ride, and 42 mM mannito l).2 The hemato crit
suspen-sio n was adjusted to 40 to 50%, when necessary. After gentle mixing fo r at least 20 min, the erythro cyte sus-pensio n was transferred to sterile PVC vials and kept at 4°C.
All enzyme assays were perfo rmed o n the day fo llo wing co llectio n, at weekly intervals up to 6 weeks, and biweekly thereafter.
Blo o d pH and extracellular so dium and po tas-sium analyses were carried o ut in Blo o d Gaz Analyzer (Instrumentatio n Lab o rato ry, Inc.) at Universidade Federal do Paraná, Curitiba. Assays o f erythro cyte ad-e no sinad-e -5’-trip ho sp hatad-e (ATP), 2,3-d ip ho sp ho gly-cerate (2,3-DPG) co ncentratio ns, and hexo kinase (Hx), pho spho fructo kinase (PFK) pyruvate kinase (PK), glyc-eraldehyde-3-pho sphate dehydro genase (GAPD), glu-c o s e - p h o s p h ate d e h yd ro g e n as e ( G PD) , 6-p ho s6-p ho gluc o nate d e hyd ro ge nase ( 6- PGD) , glu-tathio ne reductase (GR) and gluglu-tathio ne pero xidase (GSHPx) activities were made acco rding to standard metho ds10 in a Gilfo rd spectro pho to meter 2451, in the
LIM-23 o f the Psychiatry Institute o f the Ho spital das Clínicas o f the Faculty o f Medicine o f the University o f São Paulo .
Red cell membrane pro teins prepared acco rd-ing to Do dge et al.11 were applied in so dium do decyl
sulfate - po lyacrylamide gel electro pho resis (SDS-PAGE). The Lo wry metho d12 was emplo yed fo r pro
-tein assay. SDS-PAGE was perfo rmed acco rding to Laemmli.13
RESULTS
Changes in pH, ATP and 2,3-DPG o f RBC during preservatio n in ADSOL are sho wn in Table 1, in which a general decrease during blo o d sto rage may be o b-served. The Hx, PFK, PK, GAPD, G6-PD, 6-PGD, GR, and GSHPx activities during red blo o d cell preserva-tio n in ADSOL are sho wn in Figure 1, in which a vari-able decrease may be o bserved during the 14 weeks.
in Figs. 2 and 3, and no changes were detected during blo o d sto rage.
DISCUSSION
It is well kno wn that during blo o d preservatio n with ADSOL, plasma so dium and po tassium decrease, re d c e ll ad e no sine -trip ho sp hate and 2,3-d ip ho s-pho glycerate decrease as well, blo o d hydro gen io n co ncentratio n increases, and hemo lysis o ccurs.14-16 In
this present paper we o btained similar data to o ther autho rs regarding ATP, 2,3-DPG and extracellular pH, and o ther parameters,2,14-16 which can be seen in Table
1. Ho wever, there are no studies o f red cell enzymes o r o f membrane pro teins during lo nger ADSOL sto r-age perio ds.
As the glyco lytic kinases are invo lved in the en-ergy generatio n represented by ATP fo rmatio n, essen-tial to keep the Na-K pump in activity, all o f them were studied. Selected dehydro genases (fro m glyco lysis, the pento se cycle and related o nes), which keep the re-duced nico tinamide adenine dinucleo tide (NADH) and the reduced nico tinamide adenine dinucleo tide pho s-phate (NADPH) nucleo tides in their reduced state, were also investigated. These reduced nucleo tides re-duce the dangero us pero xides and disulfide bridges, which damage the membrane pro teins and o ther pro -teins.
Enzyme activity decay was o bserved during the sto rage, so that by the 5th week, hexo kinase, glyceral-dehyde pho sphate dehydro genase, glutathio ne reduc-tase , gluco se -pho sphate de hydro ge nase , and 6pho s6pho gluco nate dehydro genase fell 30%, 6pho s6pho -fructo kinase and glutathio ne pero xidase 35%, and pyruvate kinase 45% (Figure 1). Thereafter, a co mmo n 10% decrease amo ng all enzymes was o bserved up to the 14th week. As all enzymes lo st activity during the first 5 weeks, it hints that tho se unstable fo rms o f en-zymes that depend o n fine physio lo gical enviro nmen-tal requirements early beco me inactive. But, mo st o f the fo rms do keep their functio nal pro perties until the 14th week, suggesting that they represent mo re stable enzyme fo rms in spite o f the decrease in pH, nucle-o tides and phnucle-o sphate cnucle-o mpnucle-o unds.
These data suggest that ADSOL-preserved red c e lls ke e p the ir b asic b io c he m ic al c harac te ristic s thro ugho ut the 14 weeks, altho ugh presenting early ATP and 2,3-DPG depletio n.
Studies in different preservative so lutio ns have repo rted variable enzymatic activity changes during in vitro RBC preservatio n.15-17 No ble et al.15 repo rted 0-20%
reductio n in activity fo r several enzymes and 33% fo r pho spho fructo kinase in CPDA-1 preserved red cells fo r up to 5 weeks. Mo urad et al.17 o bserved a 25% activity
decrease after 7 weeks and 30-50% after 19 weeks o f pres-ervatio n in ACD. Nakao et al.18 o bserved stable hexo ki-nase activity o ver 8 weeks when ACD preservative so lu-tio n plus adenine and ino sine was used. ATP deplelu-tio n after 8 weeks o f preservatio n in ACD was reco vered after
Table 1 - Extrace llular pH, and ATP and 2,3 DPG conce ntrations during re d blood ce ll pre se rvation in ADSOL. Initial conce ntrations: ATP – 4.95 mmole s/g he moglobin; 2,3-DPG – 12.15 mmole s/g he moglobin
TIME (weeks)
0 1 2 3 4 5 6 8 1 0 1 2 1 4
ATP (%) 1 0 0 (0 ) 9 7 (4 ) 8 9 (6 ) 7 7 (8 ) 6 0 (1 5 ) 4 5 (6 ) 4 0 (1 1 ) 2 5 (1 1 ) 1 6 (1 ) 1 0 (0 )
-PH 6 .9 (0 ) 6 .8 (0 ) 6 .7 (0 ) 6 .6 (0 ) 6 .6 (0 .1 ) 6 .5 (0 .1 ) 6 .5 (0 .1 ) 6 .4 (0 .1 ) 6 .4 (0 .1 ) -(0 ) 6 .3 (0 )
2 ,3 -DPG (%) 1 0 0 (0 ) 9 8 (0 ) 5 1 (1 ) 1 3 (1 ) 7 (4 ) 2 (1 ) 1 (0 ) - - -
-Sd- Standard deviatio n in parenthesis. Each experimental values is averag e o f six samples.
adenine and ino sine additio n, despite the 50% decrease in hexo kinase activity. Altho ugh hexo kinase is the first glyco lytic enzyme, the remaining enzymatic activity seems to keep the functio nal metabo lic pathways.
Red cell membrane pro teins during sto rage have been studied under different co nditio ns.19-22 So me
re-po rts21,22 describe high MW o ligo mer fo rmatio n, which
was ascribed to membrane pro tein interactio ns. Wo lfe et al.19
described a decrease in the spectractin inte ractio n, no o ligo m e r fo rm atio n and m e m b rane -glo bin asso ciatio n in CPD, while Schrier et al.21
de-scribed o ligo mer and actin increase, as well as band 2, 3, 4.1 and 4.2 decrease during sto rage in CPD-A2.
Significant memb rane pro tein changes co uld represent a RBC life-limiting sto rage lesio n.23
There-fo re we perThere-fo rmed the membrane pro tein SDS-PAGE analysis in o rder to detect any mo dificatio n during the 14 weeks. No significant change in SDS-PAGE during ADSOL preservatio n was o bserved, as can be o bserved in Figures 2 and 3. No o ligo mers were fo rmed during the 14 weeks in ADSOL-preserved red cells, whereas it o ccurs when CPD and CPDA-2 are used.20-22 It is po
s-sible that this difference may be ascribed to the tech-nical pro cedure o r to the preservative so lutio ns
em-plo yed by the o ther autho rs. Acco rding to o ur data, ADSOL seems to be a better preservative so lutio n re-garding o ligo mer generatio n and relative co ncentra-tio n o f membrane pro teins.
Altho ugh the ADSOL-preserved red cells keep their basic metabo lic functio ns and membrane struc-ture fo r up to 14 weeks, they are no t adequate fo r blo o d transfusio n, as ATP and 2,3-DPG decrease a great deal. Ho wever, the fairly go o d enzymes co nditio ns up to 14 weeks are enco uraging fo r making greater effo rts to -wards trying to resto re red cell ATP and 2,3-DPG lev-els fo r lo nger, enhancing blo o d viability.
The ADSOL-preserved red cell enzymes present a standard and gradual activity decay. Thus, if G-6-PD is assayed in red cells preserved in ADSOL fo r 8 o r 12 weeks, the o bserved activity will represent 60% o f the initial values (-30% after 5 weeks plus -10% by the 8th to 12th week), and a reliable value may be given. Mo re-o ver, red cells in which the enzyme activities are as-sayed as so o n as blo o d is co llected may be used as standards, and blo o d samples may be sent to o ther labo rato ries, which will have them as standards. The same may be extended fo r membrane pro teins, which are very stable with ADSOL fo r at least 14 weeks.
1. Hö gm an CF, He dlund K, Ze tte rsto rm H. Clinical use fullne ss o f re d c e l l s p re s e rve d i n p ro te i n - p o o r m e d i u m . N En g l J M e d 1978;299:1377-82.
2. He ato n A, Miripo l J, Grapka B, De hart D, Se e ge r C, Rzad L, Aste r R. Im p ro ve d sto rage o f high he m ato c rit c e ll c o nc e ntrate s using a m a n n i to l , a d e n i n e , s a l i n e , g l u c o s e s o l u ti o n . Tra n s fu s i o n 1981;21:600-1.
3. Hö gman CF, Akerblo m O, Hedlund K, Ro sén I, Wiklund, L. Red cell suspensio ns in SAGM medium. Vo x Sang 1983;45:217-23.
4. Strauss D. CDS-AG medium fo r red blo o d cell preservatio n. Bio med Bio chim Acta 1983;42:332-6.
5. Dawso n RB, Fagan DS, Meyer DR. Dihydro xyaceto ne, pyruvate, and pho sphate effects o n 2,3-DPG and ATP in citrate-pho sphate-dextro se-adenine blo o d preservatio n. Transfusio n 1984;24:327-9.
6. Meryman HT, Ho rnblo wer MLS, Syring RL. Pro lo nged sto rage o f red cells at 4°C. Transfusio n 1986;26:500-5.
7. Carmen RA, So hmer PR, Leng BS, et al. Five-week red cell sto rage with preservatio n o f 2,3-DPG. Transfusio n 1988;28:175-161
8. Heato n A, Miripo l J, Aster R, et al. Use o f ADSOL preservatio n so lutio n fo r pro lo nge d sto rage o f lo w visco sity AS-1 RBC. Br J Hae m ato l 1984;57:467-78.
9. Greenwalt TJ, So sto k, CZ, Dumaswala UJ. Studies in red blo o d cell pre se rvatio n. Co mpariso n o f ve sicle fo rmatio n, mo rpho lo gy, and
REFERENCES
m e m b rane lipids during sto rage in AS-1 and CPDA-1. Vo x Sang 1990;58:90-3.
10. Beutler E. Red cell metabo lism: a manual o f bio chemical metho ds. Orlando : Grune & Stratto n; 1984.
11. Do dge JT, Mitchell C, Hanahan DJ. The preparatio n and chemical characteristics o f hemo glo bin-free gho sts o f human erythro cytes. Arch Bio chem Bio phys 1963;100:119-30.
12. Lo wry OH, Ro senbro ugh NJ, Farr L, Randall RJ. Pro tein measurements with the Fo lin pheno l reagent. J Bio l Chem 1951; 193:265-75.
13. Laemmli UK. Cleavage o f structural pro teins during the assembly o f the head o f bacterio phage T4. Nature 1970;227:680-85.
14. Fagio lo E, Mo res N, Pelliccetti A, Go zzo ML, Zuppi C, Littarru GP. Bio che m ical param e te rs to acce ss viab ility o f b lo o d sto rage fo r transfusio nal use. Fo lia Haemato l 1986;113:783-9.
15. No b le NA, Tanaka KR, Myrhe BA, Jo hnso n DE. Re d ce ll e nzyme ac tivitie s d u rin g b lo o d s to rag e an d re ac tivatio n o f pho spho fructo kinase. Am J Hemato l 1982;13:1-8.
16. Barretto OCO, No no yama K, Sawatani E, Tanaka K, Okumura Y, Jamra MA. Viab lid ad e d e sangue c o nse rvad o e m re c ip ie nte s d e várias pro cedências. Rev Ass Med Bras 1983;29:102-5.
18. Nakao M, Nakayama T, Decrease in pho spho fructo kinase activity during blo o d preservatio n and the effect o f intracellular ATP. Bio chem Bio phys Res Co mmun 1980;95:1294-8.
19. Wo lfe LC, Byrne AM, Lux SE. Mo lecular defect in the memb rane skeleto n o f blo o d bank-sto red red cells. J Clin Invest 1986;78:1681-6.
20. Kadlubo wski M. The effect o f in vivo aging o f the human erythro cytes o n the p ro te ins o f the p lasm a m e m b rane : a c o m p arisio n with metabo lic depletio n and blo o d bank sto rage. J Bio chem 1978;9:79-8.
21. Schrier SL, So hmer PR, Mo o re GL, Junga I. Red blo o d cell membrane abno rmalities during sto rage. Transfusio n 1982;22:261-5.
22. Halb h u b e r KJ, Fe u e rs te in H, Stib e n z D, Lin s s W. Me m b ran e alte ratio n during b anking o f re d b lo o d ce lls. Bio m e d Bio chim Acta 1983;42:337-41.
23. Wegner G, Kucera W, Lerche D. Defo rmab ility characterizatio n o f e rythro c yte s sto re d und e r d iffe re nt re susp e nsio n m e d ia. Fo lia Haemato l 1987;114:474-7.
r e s u m o
CO N TEX TO : A so lução preservado ra ADSO L (adenina, dextro se, so rb ito l, c lo reto de só dio e ma nito l) ma ntém a via b ilida de do s gló bulo s vermelho s para transfusão durante seis semanas. Seria assim útil determinar sua preservação po r tempo s maio res.
O BJETIV O : Determinar alg uns parâmetro s bio químico s eritro citário s até 1 4 semanas visando saber se a integ ridade do metabo lismo eritro c itá rio justific a ria estudo s po sterio res c o m o pro pó sito de alo ng ar sua preservação e viabilidade.
TIPO DE ESTUDO : Avaliação bio química para avaliar a preservação de hemácias.
LO CAL: C e ntro d e re fe rê nc ia d e me ta b o lismo e ritro c itá rio d a Faculdade de Medicina da USP, São Paulo e Universidade Federal do Paraná, Curitiba.
AM O STRA: Seis do ado res de sang ue do ho spital universitário da Universidade Federal do Paraná, Curitiba, Brasil.
VARIÁV EIS ESTUDADAS: Fo i realiz ada a determinação semanal d e a d e no sina -5 ´ -trifo sfa to , 2 , 3 -d ifo sfo g lic e ra to , he xo q uina se , fo sfo fruto quinase, piruvato quinase, g lico se-6 -fo sfato desidro g enase, 6 -fo sfo g lic o nic o d e sid ro g e na se , g lic e ra ld e id o -3 -fo sfa to d e si-dro g enase, g lutatio na redutase, g lutatio na pero xidase, bem co mo a do sag em de só dio e po tássio plasmático s, pH sang üíneo , e a d e te rmina ç ã o d a s p ro te ína s d a me mb ra na e ritro c itá ria p o r eletro fo rese em g el de po liacrilamida.
RESULTADO S: Durante a preservação o ATP caiu 6 0 % em cinco semanas, e 9 0 % depo is de 1 0 semanas. O 2 ,3 -DPG permaneceu estável durante a primeira semana, caiu 9 0 % depo is de três semanas e se exauriu depo is de cinco semanas. O pH decresceu de 6 ,8 na primeira semana a 6 ,4 na 1 4 a semana. Depo is de cinco semanas ho uve diminuição de 1 6 a 3 1 % das atividades da hexo quinase, g liceraldeído -3 -fo sfato desidro g enase, g lutatio na redutase, e 4 5 % da piruvato quinase. Em seg uida, o bservo u-se um decréscimo de 1 0 % para to das enz imas até a 1 4 ª semana. A eletro fo rese em g el de po liacrilamida das pro teínas da membrana eritro citária não revelo u alteraçõ es nas co ncentraçõ es relativas das bandas durante e ao cabo das 1 4 semanas.
CO N CLUSÕ ES: Embo ra a viabilidade do sang ue seja po bre da 6 ª à 1 4a semana, devido à depleção de ATP e de 2 ,3 -DPG , o s demais parâmetro s bio químico s decaíram g radualmente. Este achado po de sug erir que o s g ló bulo s vermelho s preservado s em ADSO L po ssam ser utiliz ado s co mo padrõ es de enz imas eritro citárias e de pro teínas da membrana.
Acknowle dge me nts: The autho rs wish to thank M.R. Silva, L.M. Nakayama, and R.F. Nascimento fo r their skillful help.
Maria Sue li Soare s Le onart, MD. Pro fesso r o f Clinical Patho lo gy, Divisão de Saúde da Universidade Federal do Paraná, Curitiba, Brazil.
Aguinaldo José Nascime nto, MD. Asso ciate Pro fesso r o f Bio chemistry, Departamento de Bio química, Divisão de Saúde, Universidade Federal do Paraná, Curitiba, Brazil.
Kimiyo Nonoyama, MD. Research IV, Instituto Ado lfo Lutz, São Paulo , Brazil.
Cinthia Barbosa Pe lissari. Bio lo gist, Centro de Hemato lo gia e Hemo terapia do Paraná, Curitiba, Brazil.
Orlando Ce sar de Olive ira Barre tto, MD. Asso ciate Pro fesso r, Faculdade de Medicina da Universidade de São Paulo , LIM-23, São Paulo , Brazil.
Source of funding: LIM - Ho spital das Clínicas da Faculdade de Medicina da USP
Conflict inte re st: No t declared
Last re ce ive d: 22 Octo ber 1999
Acce pte d: 22 No vember 1999
Addre ss for corre sponde nce :
Orlando Cesar de Oliveira Barretto Avenida Pedro so de Mo rais, 70 - apto . 101 São Paulo /SP - Brazil - CEP 05420-000 E-mail: o cdo barr@ usp.br