w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Original
article
Outcomes
of
allogeneic
hematopoietic
stem
cell
transplantation
for
lymphomas:
a
single-institution
experience
Mira
Romany
Massoud
a,
Paolo
Fabrizio
Caimi
a,b,
Nicole
Ferrari
a,
Pingfu
Fu
b,
Richard
Creger
a,
Robert
Fox
a,
Joanne
Carlson-Barko
a,
Merle
Kolk
a,
Lauren
Brister
a,
Brenda
Wimpfheimer
Cooper
a,b,
Stanton
Gerson
a,b,
Hillard
Michael
Lazarus
a,b,
Marcos
de
Lima
a,b,
Basem
Magdy
William
a,b,∗aUniversityHospitalsCaseMedicalCenter,Cleveland,UnitedStates bCaseWesternReserveUniversity,Cleveland,UnitedStates
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received11May2016 Accepted25July2016
Availableonline18August2016
Keywords:
Transplantation,hematopoietic, allogeneic
Lymphoma,Hodgkin’s Lymphoma,non-Hodgkin
a
b
s
t
r
a
c
t
Introduction:Allogeneichematopoieticstemcelltransplantationofferstheopportunityfor extendedsurvivalinpatientswithHodgkin’sandnon-Hodgkinlymphomaswhorelapsed after,or weredeemedineligible for,autologoustransplantation.Thisstudy reportsthe cumulativeexperienceofasinglecenteroverthepast14yearsaimingtodefinetheimpact ofpatient,disease,andtransplant-relatedcharacteristicsonoutcomes.
Methods:AllpatientswithhistologicallyconfirmeddiagnosisofHodgkin’sornon-Hodgkin lymphomaswhoreceivedallogeneictransplantationfrom2000to2014wereretrospectively studied.
Results:Forty-onepatientswerereviewed:10(24%)hadHodgkin’sand31(76%)had non-Hodgkinlymphomas.Themedianagewas50yearsand23(56%)weremale.Themajorityof patients(68%)hadhadapriorautologoustransplantation.Atthetimeofallogeneic trans-plantation,18(43%)patientswereincompleteandseven(17%)wereinpartialremission. Most(95%)patientsreceivedreduced-intensityconditioning,49%receivedmatchedsibling donorgrafts,24%matched-unrelateddonorgrafts,and27%receiveddoubleumbilicalcord bloodgrafts.The100-daytreatment-relatedmortalityratewas12%.Afteramedianduration offollowupof17.1months,themedianprogression-freeandoverallsurvivalwas40.5and 95.8months,respectively.Onmultivariateanalysis,patientswhohadactivediseaseatthe timeoftransplanthadinferiorsurvival.
Conclusions:Allogeneictransplantationresultsextendsurvivalinselected patientswith relapsed/refractoryHodgkin’sandnon-Hodgkin lymphomas withlowtreatment-related mortality.Patientswhohaveactivediseaseatthetimeofallogeneictransplantationhave pooroutcomes.
©2016Associac¸ ˜aoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense (http://creativecommons.org/licenses/by-nc-nd/4.0/).
∗ Correspondingauthorat:DivisionofHematology,TheOhioStateUniversityComprehensiveCancerCenter,Starling-LovingHallM200F, 320W.10thAve,Columbus,OH43210,USA.
E-mailaddress:basem.william@ousmc.edu(B.M.William). http://dx.doi.org/10.1016/j.bjhh.2016.07.003
Introduction
Hodgkin’s and non-Hodgkin lymphoma (HL and NHL) are a heterogeneous group of hematologic malignancies with variedaggressivenessandmanytherapeuticoptions.An esti-mated66,360newcasesofNHLwerediagnosedintheUnited Statesin2011.B-cellnon-Hodgkin(B-NHL)lymphomas com-priseapproximately85%ofthesecases.Transplantation,both autologousandallogeneic,hasaroleinthemanagementof B-celllymphoma, withmore than 5000hematopoietic cell transplantations(HCTs)being performedannuallyinNorth America forthis indication. Diffuse largeB-cell lymphoma (DLBCL)isthemostcommontypeoflymphomaseenin devel-opedcountries, accounting for30% of all newlydiagnosed NHL.Itisanaggressivelymphoma,and whentreatedwith anthracyclineandrituximab-basedchemotherapy,onlyhalf ofthepatientsarecuredwithupfronttherapy.1
High-dosetherapyfollowedbyautologousstemcell trans-plantation(autoSCT)hasbeenthestandardcareforpatients with relapsed B-NHL. The efficacy of autoSCT as salvage for such patients, in the post-rituximab era, was recently questioned by the Collaborative Trial in Relapsed Aggres-siveLymphoma(CORAL)whichdemonstratedadismal23% progression-freesurvival(PFS)attwoyears.2Historically, allo-geneic transplantation (alloSCT) was considered an option afterfailureofautoSCTbutsomecentershavetransplanted higher risk B-NHL cases at first relapse or second com-plete remission. No prospective comparative studies have beencompletedinthissetting.1TheeffectivenessofalloSCT in B-NHL has been attributed to a graft-versus-leukemia (GVL) effect because of the elimination of tumor cells by alloimmune effector lymphocytes. Durable responses were demonstratedafteralloSCTinfollicularlymphomas(FL) how-evera higher transplant-relatedmortality (TRM) related to myeloablativeconditioningregimenslimitedthewidespread useofalloSCTforFL.1Earlierstudies,includingalargeanalysis fromtheCenter forInternationalBloodand Marrow Trans-plantResearch(CIBMTR),3 demonstrated a differentialGVL effect among B-NHL patients with low/intermediate grade histologies, such as FL and mantle cell lymphoma (MCL), being more sensitive to GVL compared to their aggressive counterparts(DLBCLandBurkitt’slymphoma).Theadventof reduced-intensityconditioning (RIC) regimens hasrenewed interest in alloSCT,which reduces TRM whilemaintaining aGVL effect and thereforeallowsthe treatment ofelderly patientsandpatientswithcomorbidities.4Amorerecent anal-ysisfromtheCIBMTRhasshownthatdiseasestatuswasthe maindeterminingfactorforoutcomesafteralloSCT regard-lessoftheintensityofconditioningregimeninDLBCL.5Given the limited efficacy of autoSCT in the post-rituximab era and the decreased TRM with RIC, it islikely that the use ofalloSCTinB-NHLwillexpand.Furtherunderstanding of the factors likely to predict a more robust GVL response, andpotentiallybetterclinicaloutcomes,willbeveryuseful inselecting patients with B-NHLwho are likely to benefit from alloSCT.T-cell NHL accountsfor only30% ofall NHL andrepresentsahighlyheterogeneousgroupwheretherole ofalloSCTremainsundefined.6AlloSCTisusuallyofferedto patientswith HLas asalvage therapy followingrelapse or
progressionafterautoSCT.FailureofautoSCTmaybesalvaged byalloSCTwithextendedsurvivalinhighlyselectedpatient populations.7
Thisstudyreportsthecumulativeexperienceofonesingle center aimingtodefinethe impactofpatient, disease,and transplant-relatedcharacteristicsonoutcomes.
Methods
ThetransplantdatabaseoftheUniversityHospitalsCase Med-icalCenter(UHCMC) wassearchedtoidentifypatientswith HL and NHL who received alloSCT from 2000to 2014. All patientsincludedintheanalysishad acentrally-confirmed histologicdiagnosis ofHLorNHL.Thestudywasapproved bytheUHCMCinstitutionalreviewboard.Allpatientshada comprehensiveevaluationbeforealloSCTtoensurethatthey had adequate cardiac,pulmonary, renal,and hepatic func-tions per institution protocol. All patients included in the analysisreceivedeithermobilizedperipheralbloodstemcells (PBSC)ordoubleumbilicalcordblood(dUCB)grafts.Allofthe transplantations wereperformedonaninpatientbasisand patientsreceivedcareinprivateroomswithlaminarairflow. PBSCgraftswereeitherfromamatchedsiblingdonor(MRD) orhumanleukocyteantigen(HLA)7/8or8/8matched(toHLA A, B,C,DR, andDQ loci)unrelateddonor(MUD).UCBasa graftsourcewasonlyconsideredintheabsenceofanadult HLA-matched related or unrelated donor. For dUCB grafts, aminimumof1.5×107totalnucleatedcells/kgofrecipient bodyweightcelldosewasrequired.AlsodUCBunitshadto bematchedforatleastthreeHLAloci(A,B,DRB1)between eachother andtotherecipient.Mosttransplantsemployed RICwithfludarabineandcyclophosphamide(FluCy)withor without rabbit antithymocyteglobulin(ATG). Mostpatients whoreceiveddUCBgraftswerealsoconditionedwith200cGy oftotalbodyirradiation(TBI).Allpatientsreceivedgraftvs. hostdisease(GVHD)prophylaxiswithacalcenurininhibitor (cyclosporineAortacrolimus)withorwithoutreduced-dose methotrexate(5–15mg/m2)onDays+1,+3,+6,+11after trans-plantormycophenolatemofetil(for30daysaftertransplant). Most patients received growth factor support, supportive transfusions,andprophylacticantimicrobialagentsper insti-tutional protocol. Calcenurin inhibitors (CI) were tapered from 120 days after transplant with most patients being tapered off CIs by six months in the absence of active GVHD.
Definitions
Table1–Patientcharacteristics.
Patientcharacteristic
Age–Median(Range),years 50(45–55)
Malegender–n(%) 23(56)
Histology–n(%)
Hodgkin’slymphoma 10(24) Non-Hodgkinlymphoma 31(76) DiffuselargeB-celllymphoma 9(22) Follicularlymphoma 9(22) Mantlecelllymphoma 6(15)
T-celllymphoma 2(4)
Others 5(12)
DiseasestatusatalloSCT–n(%)
Completeremission 18(43)
Partialremission 7(17)
Stabledisease 8(19)
Progressive(active)disease 8(19)
AnnArborStagingatpresentation–n(%)
Earlystage(I/II) 6(15) Advancedstage(III/IV) 35(85) Extranodaldiseaseatpresentation–n(%) 6(15) PriorautoSCT–n(%) 24(58) IntervalfromdiagnosistoalloSCT≥1year 39(95) HCT-CI–median(range) 0(0–3)
alloSCT: allogeneic stem cell transplantation; autoSCT: autol-ogous stem cell transplantation; HCT-CI: hematopoietic cell transplantation-specificcomorbidityindex.
chemotherapy)orhad ahigh-riskFLwithanHLA-identical sibling donoravailable. Acute GVHD was graded based on theInternational BoneMarrow TransplantRegistry (IBMTR) SeverityIndex8;gradesC/Dwerecategorizedas“severeacute
Table2–Allogeneictransplantcharacteristics.
Transplantcharacteristic
Donortype–n(%)
Siblingdonor 20(49)
Matchedunrelateddonor 10(24) Umbilicalcordblood 11(27)
Conditioningregimen
Fludarabine-cyclophosphamide(±Rituximab) 22(53) Fludarabine-cyclophosphamide-TBI 12(30)
Others 7(17)
Conditioningregimenintensity
Reduced-intensity 39(95)
Myeloablative 2(5)
Anti-thymocyteglobulin–n(%) 27(77) CD34+celldose(×106)–median(95%
confidenceinterval)
6.2(5.26–6.67)
GVHDprophylaxis–n(%)
Cyclosporine±methotrexateorMMF 37(90) Tacrolimus/methotrexate 4(10) SevereacuteGVHD–n(%) 10(24) Moderate/severechronicGVHD–n(%) 8(20)
±:withorwithout;TBI:totalbodyirradiation;GVHD:graftvs.host disease;MMF:mycophenolatemofetil.
Months after transplant
Probability of progression-free survival
120 96
72 48
24 0
0 20 40 60 80 100
A
B
n=41
Months after transplant
Probability of overall survival
120 96
72 48
24 0
0 20 40 60 80 100
n=41
Figure1–Progression-free(A)andoverallsurvival(OS)after allogeneictransplantation(with95%confidenceintervals).
GVHD”forthepurposeofthisanalysis.ChronicGVHDwas gradedaccordingtotheNationalInstituteofHealth(NIH)2015 consensuscriteria.9TomaintainconsistencyinGVHDgrading, many cases were graded retrospectively. The hematopoi-eticcell transplantation-specificcomorbidityindex(HCT-CI) was calculated pre-transplant as described previously.10 TRM was defined as the cumulative incidence of death within 100 days afteralloSCT without evidence of disease progression.
Statistical
analysis
Results
Patientcharacteristics
Forty-onealloSCTswereperformedbetween2000and2014: 10(24%)forHLand 31(76%)forNHL.Themedianagewas 50years(range:16–69years)and23(56%)patientsweremale. Themajorityofpatients(58%)hadundergoneapriorautoSCT. AtthetimeofalloSCT,18(43%)patientswereinCRandseven (17%)wereinPR.ThemedianHCT-CIwas0(range:0–3).Patient characteristicsaresummarizedinTable1.
Transplantprocedure
TransplantcharacteristicsareshowninTable2.Thesources for hematopoietic stem cells were HLA-matched sibling donors(49%),doubleumbilicalcordblood(27%)and matched-unrelateddonors(24%).Mostpatients(95%)receivedreduced intensityconditioningpriortoalloSCTwiththecommonest regimen utilized being fludarabine and cyclophosphamide
(83%). The median CD34+ cell dose was 6.2×106/kg. All patients,butone,engraftedneutrophils.Mostpatients(90%) received cyclosporine A-basedregimens forGVHD prophy-laxis.TheincidenceofsevereaGVHD(GradeC/D)was24%and moderate/severecGVHDwas20%.
Survival
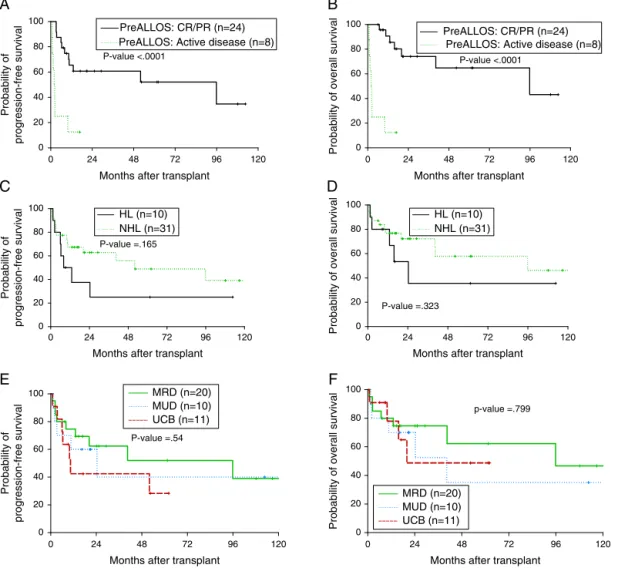
Medianfollow-upwas17.1months(range:0.8–130.7months). MedianOSwas95.8monthsandtheone-yearOSwas77.3% (Figure1A).MedianPFSwas40.5monthsand theone-year PFSwas62.8%(Figure1B).Fivepatientsdiedwithin100daysof alloSCTgivingaTRMrateof12%.Bymultivariateanalysis,only remissionstatuspriortoalloSCTwassignificantforinferior outcomes(Figure2AandB).Thehazardofdeathwasreduced by 95% in patients who had achieved complete or partial remission(CR/PR)comparedtothosewhohadactivedisease priortoalloSCT(p-value=0.001).AgeatalloSCT,gender, sub-typeoflymphoma(Hodgkin’svs.non-Hodgkin),HCT-CIand graftsourcewerenotassociatedwithoutcomesinthe Cox-multivariatemodel(Figure2C–F).
Months after transplant
Probability of progression-free survival 120 96 72 48 24 0 0 20 40 60 80 100
A
B
C
D
E
F
PreALLOS: CR/PR (n=24) PreALLOS: Active disease (n=8) P-value <.0001
Months after transplant
Probability of overall survival
120 96 72 48 24 0 0 20 40 60 80 100
PreALLOS: CR/PR (n=24) PreALLOS: Active disease (n=8)
P-value <.0001
Months after transplant
Probability of progression-free survival 120 96 72 48 24 0 0 20 40 60 80 100 HL (n=10) NHL (n=31) P-value =.165
Months after transplant
Probability of overall survival
120 96 72 48 24 0 0 20 40 60 80 100 P-value =.323
Months after transplant
Probability of progression-free survival 120 96 72 48 24 0 0 20 40 60 80
100 MRD (n=20)
MUD (n=10) UCB (n=11)
P-value =.54
Months after transplant
Probability of overall survival
120 96 72 48 24 0 0 20 40 60 80 100 p-value =.799 HL (n=10) NHL (n=31) MRD (n=20) MUD (n=10) UCB (n=11)
Figure2–Impactofdiseaseandtransplant-relatedfactorsonprogression-free(PFS)andoverallsurvival(OS)after
allogeneictransplant;remissionstatuspriortotransplant(A,B),lymphomasubtype;Hodgkin’svs.non-Hodgkinlymphoma
Discussion
This study confirms that long-term survival is achievable after alloSCT in patients with lymphomas. As with most retrospective studies inpatients submitted toalloSCT, the heterogeneityinrelationtodisease entities, remission sta-tus,transplantcenterchoiceofconditioningregimens,graft source,method of GVHDprophylaxis, and supportive care practices make any direct comparisons challenging. With mostpatientsinthe current cohortreceiving RIC,the out-comesofalloSCTwerefavorablewith77%and44%ofpatients aliveatoneandtenyearsaftertransplantation.Thisisdespite thefactthatlessthanhalfofthepatientswereinCRatthe timeoftransplantation.Theresultsherecomparefavorably withpatientsreceivingRICalloSCTasreportedinrecent reg-istrystudiesfromtheCIBMTRwithone-yearsurvivalratesof 41%,56%,and77%inpatientswhohadDLBCL, HL,andFL, respectively.5,13,14The100-dayTRMrateofthisstudywas12% whichcomparesfavorablywithsomereports(25%,15%and 13%).5,13,14
Theresultsinthisstudy suggest similaroutcomes with dUCBgraftscomparedtofullymatchedadultdonors.Limited data have been published on the role ofdUCB alloSCT in patientswithlymphoma.AstudyfromEurocord-Netcordof 104adultpatientswithlymphoidmalignancieswhoreceived UCBalloSCTreportedone-yearPFSandOSof40%and48%, respectively although only 25% of patients received dUCB units and48% received low-dose TBI.Theuse oflow-dose TBIinthatreportwasassociatedwithlowerriskof engraft-ment failure.15 A more recent study of 27 patients who receiveddUCBalloSCTforrelapsed/refractoryHLattwo cen-tersreporteda26%TRMat100dayswithtwo-yearPFSand OSof41%and56%,respectively.16 Inthisstudy,5/11dUCB transplants(45%)hadrelapsed/refractoryHL.ThePFSandOS forthe11transplantswere42%and48%,respectivelyattwo years,whichiscomparable.Inthissameseries,thetwo-year PFSandOS fortheentirelymphomacohort were 52%and 62%and40%and35%forMRDandMUDgrafts,respectively, suggestingcomparableoutcomesforadultdonors(Figure2E andF).
Historically, aggressive lymphomas were considered to belesssensitive tothe immunologicGVLeffectand hence patients would benefit less from alloSCT.17 Differences in intrinsicantigen-presentingabilitiesofaggressivelymphoma incontrasttoindolentlymphomaswereproposed however itislikelythatthemoreaggressivegrosskineticsof aggres-sive lymphomasoutpace an effective GVL.18 Inthis study, theonlyfactorthatwashighlysignificantininfluencing out-comesafteralloSCTwasremissionstatus.ArecentCIBMTR analysisof533patientswithDLBCLandGrade3FLshowed noimprovementinthePFSorOSwiththeuseof myeloabla-tivecomparedtoRICconditioningregimens.5AnotherCIBMR analysis of 336 patients with NHL (with >50% of patients withFLorMCL)showedthatpre-alloSCTpositronemission tomography (PET) positivity and notdisease histologywas associatedwithincreasedriskofrelapse/progression.19 The resultsofthisstudyconfirmthatdiseasecontrol,ratherthan histology,isthe maindeterminingfactorinoutcomes after alloSCT.
Conclusions
Allogeneictransplantsareassociatedwithextendeddisease controlinpatientswithrelapsedandrefractoryHLandNHL andlowmortalityinselectedpatients.Adequatedisease con-trolpriortoproceedingtoalloSCTisofparamountimportance. dUCBgraftsremainagoodoptionforpatientswhodonothave anavailableadultdonorwiththeoutcomesbeingfavorablein centers withadequateexperience indUCBtransplantation. Thepotentialroleofhaploidenticaltransplantsinthissetting isintriguingandiscurrentlyunderinvestigation.20
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1.AyalaE.HematopoieticcelltransplantationforB-cell lymphoma:anupdate.CancerControl.2012;19(3):175–86. 2.GisselbrechtC,SchmitzN,MounierN,SinghGillD,LinchDC,
TrnenyM,etal.Rituximabmaintenancetherapyafter autologousstem-celltransplantationinpatientswith relapsedCD20(+)diffuselargeB-celllymphoma:finalanalysis ofthecollaborativetrialinrelapsedaggressivelymphoma.J ClinOncol.2012;30(36):4462–9.
3.BiermanPJ,SweetenhamJW,LoberizaFRJr,TaghipourG, LazarusHM,RizzoJD,etal.Syngeneichematopoietic stem-celltransplantationfornon-Hodgkin’slymphoma:a comparisonwithallogeneicandautologoustransplantation– TheLymphomaWorkingCommitteeoftheInternational BoneMarrowTransplantRegistryandtheEuropeanGroupfor BloodandMarrowTransplantation.JClinOncol.
2003;21(20):3744–53.
4.WilliamBM,deLimaM.Advancesinconditioningregimens forolderadultsundergoingallogeneicstemcell
transplantationtotreathematologicmalignancies.Drugs Aging.2013;30(6):373–81.
5.HamadaniM,SaberW,AhnKW,CarrerasJ,CairoMS,Fenske TS,etal.Impactofpretransplantationconditioningregimens onoutcomesofallogeneictransplantationfor
chemotherapy-unresponsivediffuselargeBcelllymphoma andgradeIIIfollicularlymphoma.BiolBloodMarrowTranspl. 2013;19(5):746–53.
6.WilliamB,VoseJ.T-CellLymphomas.In:YounesA,CoiffierB, editors.Lymphoma,vol.43.HumanaPress;2013.p.211–29. 7.SarinaB,CastagnaL,FarinaL,PatriarcaF,BenedettiF,Carella
AM,etal.Allogeneictransplantationimprovestheoverall andprogression-freesurvivalofHodgkinlymphomapatients relapsingafterautologoustransplantation:aretrospective studybasedonthetimeofHLAtypinganddonoravailability. Blood.2010;115(18):3671–7.
8.RowlingsPA,PrzepiorkaD,KleinJP,GaleRP,PasswegJR, Henslee-DowneyPJ,etal.IBMTRSeverityIndexforgrading acutegraft-versus-hostdisease:retrospectivecomparison withGlucksberggrade.BrJHaematol.1997;97(4):855–64. 9.FilipovichAH,WeisdorfD,PavleticS,SocieG,WingardJR,Lee
SJ,etal.NationalInstitutesofHealthconsensusdevelopment projectoncriteriaforclinicaltrialsinchronic
graft-versus-hostdisease:I.Diagnosisandstagingworking groupreport.BiolBloodMarrowTranspl.2005;11(12):945–56. 10.SorrorML,MarisMB,StorbR,BaronF,SandmaierBM,
(HCT)-specificcomorbidityindex:anewtoolforrisk assessmentbeforeallogeneicHCT.Blood.2005;106(8): 2912–9.
11.KaplanEL,MeierP.Nonparametricestimationfrom incompleteobservations.JAmStatAssoc.
1958;53(282):457–81.
12.CoxDR.Regressionmodelsandlife-tables.JRStatSocSeries BStatMethodol.1972;34(2):187–220.
13.DevettenMP,HariPN,CarrerasJ,LoganBR,vanBesienK, BredesonCN,etal.Unrelateddonorreduced-intensity allogeneichematopoieticstemcelltransplantationfor relapsedandrefractoryHodgkinlymphoma.BiolBlood MarrowTranspl.2009;15(1):109–17.
14.KlyuchnikovE,BacherU,KrögerNM,HariPN,AhnKW, CarrerasJ,etal.Reduced-intensityallograftingasfirst transplantationapproachinrelapsed/refractorygradesone andtwofollicularlymphomaprovidesimprovedoutcomesin long-termsurvivors.BiolBloodMarrowTranspl.
2015;21(12):2091–9.
15.RodriguesCA,SanzG,BrunsteinCG,SanzJ,WagnerJE, RenaudM,etal.Analysisofriskfactorsforoutcomesafter unrelatedcordbloodtransplantationinadultswithlymphoid malignancies:astudybytheEurocord-Netcordand
lymphomaworkingpartyoftheEuropeangroupforblood andmarrowtransplantation.JClinOncol.2009;27(2):256–63. 16.ThompsonPA,PereraT,MarinD,OranB,PopatU,Qazilbash M,etal.Doubleumbilicalcordbloodtransplantiseffective therapyforrelapsedorrefractoryHodgkinlymphoma.Leuk Lymphoma.2016;57(7):1607–15.
17.ButcherBW,CollinsRHJr.Thegraft-versus-lymphomaeffect: clinicalreviewandfutureopportunities.BoneMarrow Transpl.2005;36(1):1–17.
18.KlyuchnikovE,BacherU,KrollT,SheaTC,LazarusHM, BredesonC,etal.Allogeneichematopoieticcell
transplantationfordiffuselargeBcelllymphoma:who,when andhow?BoneMarrowTranspl.2014;49(1):1–7.
19.BachanovaV,BurnsLJ,AhnKW,LaportGG,AkpekG, Kharfan-DabajaMA,etal.Impactofpretransplantation (18)F-fluorodeoxyglucose-positronemissiontomography statusonoutcomesafterallogeneichematopoieticcell transplantationforNon-Hodgkinlymphoma.BiolBlood MarrowTranspl.2015;21(9):1605–11.
20.KanateAS,MussettiA,Kharfan-DabajaMA,AhnKW,DiGilio A,BeitinjanehA,etal.Reduced-intensitytransplantationfor lymphomasusinghaploidenticalrelateddonorsvs