w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Anti-inflammatory
activity
and
acute
toxicity
studies
of
hydroalcoholic
extract
of
Herissantia
tiubae
Ana
L.A.
Lima
a,
Adriano
F.
Alves
a,
Aline
L.
Xavier
a,
Talissa
Mozzini-Monteiro
a,
Theresa
R.R.
Oliveira
b,
Fagner
C.
Leite
a,
Wemerson
N.
Matias
a,
Marianna
V.S.C.
Branco
a,
Maria
F.V.
Souza
a,
Marcia
R.
Piuvezam
a,∗aProgramadePós-graduac¸ãoemProdutosNaturaiseSintéticosBioativos,CentrodeCiênciasdaSaúde,UniversidadeFederaldaParaíba,JoãoPessoa,PB,Brazil bDepartamentodeImunologia,InstitutodeCiênciasBiomédicas,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received1October2015 Accepted9November2015 Availableonline19December2015
Keywords:
Malvaceae
Herrisantiatiubae
Acuteinflammation Acutetoxicity
Anti-inflammatoryactivity Mice
a
b
s
t
r
a
c
t
HydroalcoholicextractofaerialpartsofHerissantiatiubae(K.Schum.)Brizicky,Malvaceae,was
eval-uatedinexperimentalmodelsofinflammationandtoxicity.Fortoxicityassays,maleandfemaleSwiss
micewereorallytreatedwithhydroalcoholicextractofH.tiubae(2000mg/kg)andanalyzedby
consump-tionofwaterandfood,bodyweight,mortalityandratesofmajororganweights,aswellasbiochemical
andhematologicalindexes.Foranti-inflammatoryeffect,phlogisticagentssuchascarrageenanoracetic
acidwereusedtoevaluatepawedema,cellmigrationandcytokineproduction.Itwasalsoinvestigated
thehydroalcoholicextractofH.tiubaeinRAW264.7macrophagelineagebynitricoxideandcytokine
productions.SwissmicetreatedwithhydroalcoholicextractofH.tiubaeshowedlowtoxicityand(50or
100mg/kg)wasabletoreducesignificantly(p<0.01,p<0.001)polymorphonuclearcellmigration,TNF-␣
andIL-1productioninthecarrageenan-inducedperitonitis.HoweverthehydroalcoholicextractofH.
tiubae(50,100or200mg/kg)didnotreducecarrageenan-inducedpawedema.Additionally,
hydroal-coholicextractofH.tiubaedidnotpresentcytotoxicityatconcentrationsof6.25,12.5,25or50g/ml
butinducedsignificantlydecreaseofNO,TNF-␣andIL-6productioninmacrophagelineage.Thisstudy
suggeststhathydroalcoholicextractofH.tiubaehasanti-inflammatoryactivitybyinhibitingcell
migra-tionmainlybydecreasingtheinflammatorycytokinelevelsattheinflamedsiteindependentlyofthe
anti-edematogeniceffect.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Malvaceae family hasa wide variety of natural compounds withpharmacologicalpropertiessuchasanti-inflammatory, anal-gesic, anti-rheumatic, among others (Falcão-Silva et al., 2009). HerissantiatiubaeK.Schum.)Brizickyisoneofthespeciesofthis botanicfamilylargely foundin tropicalregions ofSouth Amer-ica,especiallyinnortheasternBrazilanditispopularlyknownas “mela-bode”or“lava-prato”.Theplantisusedinfolkmedicineto treatinfluenzaandfever(Albuquerqueetal.,2007).Phytochemical investigationofH.tiubaedemonstratedthepresenceof polyoxy-genflavonoids,triterpenes,steroid,phenoliccompoundsandtwo glycosylated flavonoids (kaempferol 7-O-␣-l-rhamnopyranoside and 4′,5-dihydroxy-3,6,7,8,3′-pentamethoxyflavone)(Silva etal.,
∗ Correspondingauthor.
E-mail:mrpiuvezam@ltf.ufpb.br(M.R.Piuvezam).
2009). Therefore, the aim of this study was to investigate the anti-inflammatoryactivityofthehydroalcoholicextractoftheaero partsoftheH.tiubae(HtE)anditstoxicityusingdifferent experi-mentalmodels.
Materialandmethods
Animals
Male and female Swiss mice (n=6/per group, 6–8 weeks, 25–30g)wereusedthroughoutthestudy.Theanimalswere pro-videdfromProf.ThomasGeorgeVivariumof theBiotechnology Center (CBiotec)from Federal University ofParaíba (UFPB), PB, Brazil.Allexperimentalprotocolswereapprovedandperformedin accordancewiththerecommendationsofCommissionofEthicsfor UseofAnimals(CEUA)fromUFPB,whichwasrecordedunder num-ber0508/12.Animalswerekeptinpolypropylenecage,atroom
http://dx.doi.org/10.1016/j.bjp.2015.11.001
temperature(25±2◦C),under12hlight/darkcycle,andfreeaccess tofoodandwater.
Plantmaterialandpreparationofhydroalcoholicextract
AerialpartsofHerissantiatiubae(K.Schum.)Brizicky,Malvaceae, werecollectedinJanuary2010inthecityofJuazeirinho,Paraiba, Brazil.ItwasidentifiedbyDr.MariadeFatimaAgrafromUFPB.A voucherspecimen(n◦2434)isdepositedintheHerbariumLauro PiresXavier– JPBatthesameUniversity.TheaerialpartsofH. tiubae(1kg)weredriedat40◦Cinacirculatingairovenfor96h andgroundtopowder.Driedandpowderedplantmaterialwas sub-mittedtoextractionbymacerationwithethanol–water(70:30)as asolventatroomtemperaturefor72h.Theratioofplant mate-rial:solventwas20:80(w/v)and atthefinal extractionprocess thematerialwasfilteredandconcentratedinrotaevaporator,thus obtainingtheHtE.
ChromatographyoftheHtE
HtEwassuccessivelypartitionedwithhexane,CHCl3,EtOAcand butanol.Kaempferolwasisolatedfromtheethylacetateextract andsubjectedtoaSephadexLH-20gelcolumnelutedwithMeOH. Kaempferolwasquantifiedby meansofHigh Performance Liq-uidChromatography(HPLC)withultravioletdetection.Calibration curvestokaempferolwereconstructedbyusingthestandard addi-tionmethod.Theseparationofkaempferolwasachievedusinga ProminenceChromatographicSystem(Shimadzu®,Tokyo,Japan) equippedwithLC-20ATmultisolventdeliverysystem,degassing systemDGU-20A5, autoinjectorSIL-20A, ovenCTO-20A column anddetectionbyelectronspectroscopyintheultraviolet-visible regionwithdiodearraySPD-M20AUV-VIS.Data werecollected andintegratedthroughsoftwareClassVPV6.14SP1.Themobile phaseconsistedofamixtureofmethanol:water:H3PO4(1:1:0.01, v/v)pHcontrolledat3.1andtheflowrateof1.2ml/mininthe gradi-entmode,wheretheproportionoftheorganicphaseconstitutions by47%for18min,from80%in23minandreturningto47%after 28min.Toperformthechromatographicruns,weusedaC18 col-umn(Phenomenex®)dimensions25cm
×4.6mm×5m,theUV detectorwiththewavelengthof351nm,injectionvolumeof10l temperature50◦C.
TreatmentwithHtE
Forinvivoexperiments,theHtEin2%Tween20(Vetec®)and distilledwater(vehicle)wasorally(p.o.)administeredatdosesof 50,100or 200mg/kg. The untreatedcontrol groupreceivedan equalvolumeofthevehicle.Forinvitroexperiments,theHtEwas dissolvedindimethylsulfoxide(DMSO),thestocksolutionwas ster-ilizedusingadisposablefilterunitof0.22mminporosity(Millipore MillexTM)andusedinthefollowrangeofconcentrations0,6.25, 12.5,25,50,100,200or400g/ml.
Acutetoxicologicaltest
Groupsofmaleandfemale(n=6)Swissmiceweretreatedorally withHtE(2000mg/kg)orvehicle.Theanimalswereobservedfor signsof generaltoxicity in intervalsof 0, 15,30 and 60min,4 and24hlateranddailyfor14 days(Hibbsetal.,1988).During thesetimes,occurrenceof centralnervoussystemchangeswas analyzed:hyperactivity,irritability,aggressiveness,tremors, con-vulsions,catatonia,analgesia,anesthesia,ptosis,decreasedtouch response,ambulation,cleaningcapacity,raise,andautonomic ner-voussystemchanges:diarrhea,constipation,defecation,urination, muscletone,amongothers(Almeidaetal.,1999).Throughoutthe experiment,theconsumptionofwaterandfoodintakeandweight
gainwereobserved. Onday14th,thetreatedanimalsand non-treatedanimalswereeuthanizedbyanesthetic:sodiumthiopental (ThiopentaxR,Cristalia–PharmaceuticalChemicals)andorgans wereremoved:heart,liver,kidneys,spleenandthymusto deter-mineitsindexes.Theweightgainforeachanimalwasdetermined usingtheformula:
%ofweightgain=
animalweightonfirstchallengeanimalweightonlastchallenge
−1×100 Theindexoftheweightorganswascalculatedfollowingthe formulabelow:
Index= organweight(mg)
animalweight(g)
Evaluationofbiochemicalandhematologicalparameters
Onthe14thdayanimalsfastedfor6hwereanesthetizedwith sodiumthiopentalandorbitalsinusbloodwascollectedusinga heparinizedPasteurpipetteandtransferredintotubes(Eppendorf). Thebloodwasanalyzedforhematological(erythrocyteand leuko-cytecounts) and biochemicalparameters (urea, creatinine,uric acid,alaninetransaminase-ALT,aspartatetransaminase-AST, albu-min,totalprotein,triacylglycerides,glucoseandtotalcholesterol).
Carrageenan-inducedmicepawedema
GroupsofSwissmice(n=6)weretreated(p.o.)withvehicle, indomethacin(10mg/kg-Roche®)orHtE(50,100or200mg/kg) 1hbeforeadministrationofcarrageenanat2.5%(Sigma-Aldrich®) injectedsubcutaneouslyintotheplantarregionofthelefthindpaw andphosphatebuffersaline(PBS)inrighthindpaw.Negative con-trolgroupreceived20lPBSinjectionsinbothpaws.Pawdiameter wasmeasuredwithadigitalmicrometerat1,2,3,4,6and24h afterstimulation.Resultswereexpressedasdifferencebetween thediameterofleftandrightpaws(DeVasconcelosetal.,2011).
Carrageenaninducedperitonitis
Mice(n=6)wereorallytreated withHtE(50or 100mg/kg), indomethacin 10mg/kg or vehicle 1hbefore carrageenan (1%) intraperitoneal injection. The basal groupreceivedsaline. After 4htheanimalswereeuthanizedbyxylazineandketamine over-doseandtheperitonealcavitywashed with2ml ofsterilecold PBS,followedby aone-minmassageand collectionofthefluid (Guerra et al., 2011;Pinheiro et al.,2013).Exudates were cen-trifuged(10min,266g,4◦C)andthepelletofcellsresuspended in1mlofPBS(4◦C),dilutedinTurksolutionintheratioof1:40 andtotalcellswerecountedinatNeubauercameraunderoptical microscope(NikonE200,Melville,NY–EUA).Differentialcell mea-surementwasmadeincytocentrifuge–254×g,15min(Cytospin –BioResearch,Washington–USA),slidestainedinFastPanoptic (RenyLab)andcountedunderopticalmicroscope(100×objective). For eachslide aminimum of100cells werecountedinoptical microscopeunder1000magnification(Sousaetal.,2010).
Cytotoxicassay
Minutes
50000
B
40000
30000
20000
10000
0
0 10 20
Kaempferol
30
mA
U
A
40000
30000
20000
10000
0
0 10 20
Kaempferol
30 40
0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0
0.5 1.0 2.0 3.0 4.0 5.0 4.5 3.5 2.5 1.5 0.0 15.25 15.50 15.75 mn0.0
1.0 2.0 3.0 4.0 5.0 mAU 0.7
0.6 0.5 0.4 0.3 0.2 0.1 0.0
7 8 6 5 4 3 2 1 0
15.25 15.50 15.75 16.00 mn 15.25 15.50 15.75 16.00 mn
17.25 17.50 17.75 18.00 mn 0.0
2.5 5.0 7.5 mAU
Minutes
mA
U
Fig.1.Chromatographicprofileat370nmof(A)samplesolutionofkaempferolstandardUSPandyourDADspectrumand(B)sampleofthehydroalcoholicextractofthe aeropartsoftheHerissantiatiubae(HtE)andyourDADspectrum.
Table1
EffectofhydroalcoholicextractoftheaeropartsoftheHerissantiatiubae(HtE)ontoxicalparametersofmaleandfemaleSwissmice.
Parameter(unit) Male(control) Male(HtE) Female(control) Female(HtE)
Feedintake(g) 41.88±2.98 38.13±1.15 37.08±2.73 42.79±1.36 Waterconsumption(ml) 67.50±3.89 59.29±3.05 40.00±4.42 52.29±2.56**
Initialweight(g) 28.60±1.26 25.50±1.29 30.05±0.98 28.17±1.01
Finalweight(g) 38.05±3.22 39.98±1.27 33.14±2.31 33.15±1.07
Weightgain(%) 41.06±6.15 50.19±5.03 14.43±3.46 17.79±1.48
Indexheart(mg/g) 3.93±0.08 4.01±0.17 4.14±0.18 4.08±0.16
Indexliver(mg/g) 61.27±3.71 56.89±1.11 56.53±2.15 56.25±1.78
Indexkidneys(mg/g) 12.66±1.22 11.86±0.99 11.09±0.24 10.70±0.32
Indexspleen(mg/g) 5.59±0.21 6.45±0.53 7.56±0.59 6.97±0.62
Indexthymus(mg/g) 2.94±0.34 2.84±0.24 3.33±0.23 3.89±0.27
Dataareexpressedasmean±S.E.M.andweresubjectedtoanalysisofvarianceofStudent’st-test.
**p<0.01whencomparedtofemalecontrolgroup.Thedataarerepresentativeoftwoexperimentswithn=6(pergroup).
25,50,100,200or400g/ml)at37◦Cin5%CO2 for24h.After 24h,100lof5mg/mlMTTsolution(Sigma-Aldrich®)wasadded toeachwell,followedbyincubationfor4h.Themediumwas aspi-rated,andtheformazancrystalsweredissolvedin100lofDMSO for15min.Theopticaldensityofeachwellwasmeasuredat570nm inamicroplatereader(Bio-Radmodel550, Japan).Treatedcells werecomparedtonon-treatedcells.
NOproduction
TheproductionofNOwasdeterminedbymeasuringthe accu-mulatedlevelofnitriteonRAW264.7macrophagesupernatants. Afterpre-incubationofcells(2×105cells/well)at37◦Cin5%CO2 for4h,theplantextract(0,6.25,12.5,25or50g/ml)wasadded andco-stimulatedor notwithlipopolysaccharide-LPS (1g/ml) plusINF-␥(10ng/ml).Thecellswerefurtherincubatedfor24h. AmountsofnitriteweremeasuredusingGriessreagent. Briefly, 50lof cell culture medium was mixed with100l of Griess reagent.Subsequently,themixturewasincubatedatroom tem-peraturefor10minandtheabsorbanceat540nmwasmeasured inamicroplatereader(Bio-Radmodel550,Japan).Thequantityof nitritewasdeterminedfromasodiumnitritestandardcurve(Green etal.,1982).
Cytokineassays
IL-1,TNF-␣and IL-6from cellsupernatantsand peritoneal lavagesweremeasuredbyELISA,usingtherecommended proto-colfromthe antibodies’suppliers.Antibodypairs and standard recombinant cytokines for ELISA assay were purchased from eBiosciences.
Statisticalanalysis
Data were analyzedby Student’s t-test, ANOVAfollowed by Tukeypost-test usingsoftwareGraphPad Prism(GraphPad,San Diego, CA). Valueswere expressedas mean±standard errorof mean (S.E.M.), and results were considered significant when p<0.05.
Resultsanddiscussion
Table2
EffectofhydroalcoholicextractoftheaeropartsoftheHerissantiatiubae(HtE)onbiochemicalandhematologicalparametersofmaleandfemaleSwissmice.
Parameters(unit) Male(control) Male(HtE) Females(control) Females(HtE)
Biochemical
Glucose(mg/dl) 123.00±18.61 87.17±7.99 149.00±6.37 150.50±16.76 Urea(mg/dl) 59.18±4.423 50.88±6.22 54.10±3.78 44.42±1.75 Creatinine(mg/dl) 0.46±0.01 0.50±0.07 0.45±0.08 0.37±0.01 TotalCholesterol(mg/dl) 88.02±4.86 98.48±1.06 83.20±3.05 64.84±16.47 Triacylglycerides(mg/dl) 82.00±10.45 97.4±10.25 64.50±5.58 55.00±16.02 Uricacid(mg/dl) 3.43±0.54 2.45±0.65 2.08±0.19 2.00±0.27 AST(U/l) 175.40±19.56 139.0±14.52 184.90±14.16 195.00±7.82 ALT(U/l) 79.66±2.44 115.1±30.80 103.3±12.25 84.12±8.53 Totalproteins(g/dl) 5.04±0.30 4.99±0.18 4.99±0.24 5.22±0.21 Albumin(g/dl) 2.53±0.10 2.31±0.19 2.79±0.08 2.52±0.18
Hematological
Redblood(106/mm3) 9.28±0.78 8.90±0.43 8.15±0.32 8.77±0.14
Hemaglobin(g/dl) 13.77±0.85 13.98±0.23 12.82±0.45 13.67±0.24 Hematrocit(%) 43.30±2.82 44.54±1.48 39.56±1.72 43.38±0.49 VCM(fm3) 47.00
±0.96 48.80±2.31 48.40±0.40 49.80±0.58 HCM(pg) 14.98±0.43 15.96±0.38 15.78±0.14 15.58±0.15 CHCM(g/dl) 31.85±0.35 31.72±0.35 32.30±0.53 31.22±0.19 Leukocytes(103/mm3) 3.73±0.32 3.76±0.38 4.15±0.93 4.28±0.69
Neutrophil(%) 35.67±6.87 21.60±6.95 18.40±4.69 30.00±1.78 Lymphocytes(%) 58.67±6.08 73.00±6.77 74.60±4.93 65.33±1.33 Monocytes(%) 5.66±1.80 4.20±0.73 6.80±1.39 7.16±1.56
Dataareexpressedasmean±S.E.M.andweresubjectedtoanalysisofvarianceofStudent’st-test.Thedataarerepresentativeoftwoexperimentswithn=6(pergroup). Band,basophilandeosinophilcellswerenotfoundinthedifferentialcount.
ofinformationaboutpre-clinicaltoxicityofthisplant,preliminary toxicologicalevaluationwascarriedout.HtE(2000mg/kg)acute oraltreatmentdidnotinducesignsofgeneraltoxicity(Table1). However,itwasobservedasignificantincrease(p<0.01)inwater consumptioninthefemaletreatedgroupascomparedtothefemale controlgroup.Thisresultmaybeassociatedwithsusceptibility anddifferentialsensitivitytodrugsbetweengenders(Anderson, 2008). In addition, no significant changes in biochemical and
hematologicalparameterswereobservedinthetreatedanimals ascomparedwiththecontrolanimals,asshowninTable2.
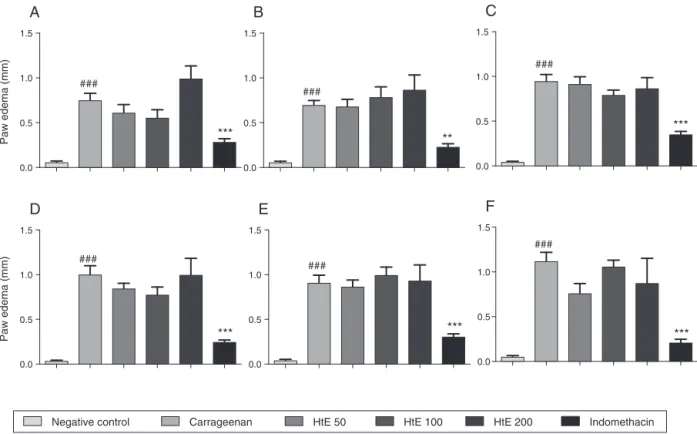
Wealsoevaluatetheanti-inflammatoryeffectofHtE consid-eringitsuseinfolkmedicinetotreatdiseaseswithinflammatory characteristics(Albuquerqueetal.,2007).Thepawedemainduced bycarrageenan is widelyused todetermineanti-edematogenic activityofcompounds. Inthis model,carrageenan promotesan immediatephase(rangefrom0through6h)thatinvolvesseveral
0.0 0.5 1.0 1.5
###
***
A
Paw edema (mm)
0.0 0.5 1.0 1.5
###
**
B
0.0 0.5 1.0 1.5
###
***
C
0.0 0.5 1.0 1.5
###
***
D
Paw edema (mm)
0.0 0.5 1.0 1.5
###
***
E
0.0 0.5 1.0 1.5
###
***
F
Negative control Carrageenan HtE 50 HtE 100 HtE 200 Indomethacin
0 5 10 15
###
** ***
***
A
Negative control
HtE 50
Indomethacin HtE
100 Carrageenan 1%
Total cells/ml x 10
6
0 2 4 6
###
** ***
***
B
Negative control
HtE 50
Indomethacin HtE
100 Carrageenan 1%
PMN cells/ml x 10
6
0 1 2 3 4
C
Negative control
HtE 50
Indomethacin HtE
100 Carrageenan 1%
MN cells/ml x 10
6
Fig.3.EffectofhydroalcoholicextractoftheaeropartsoftheHerissantiatiubae(HtE)inthecarrageenan-inducedperitonitismodel.Determinationofthetotalcellularity (A),polymorphonuclearcells(PMN)(B)andmononuclearcells(MN)(C).Thedatarepresentthemean±S.E.M.oftotalanddifferentialcellcounts.###p<0.001vsnegative group,**p<0.01and***p<0.001vspositivecontrolgroup(carrageenan)afteranalysisbyANOVAonewayfollowedbyTukeypost-test.Thedataarerepresentativeoftwo experimentwithn=6(pergroup).
0 100 200 300 400
###
***
***
***
A
Negative control
HtE 50
Indomethacin HtE
100 Carrageenan 1%
TNF-α
pg
/m
l
0 200 400 600
##
**
**
B
Negative control
HtE 50
Indomethacin HtE
100 Carrageenan 1%
IL
-1
β
pg
/m
l
0 200 400 600 800 1000
###
C
Negative control
HtE 50
Indomethacin HtE
100 Carrageenan 1%
IL
-6
p
g
/m
l
Control 6.25 12.5
25 50 100 200 400
0 1 2 3 4
***
*** ***
HtE μg/ml
Absorbance (A)
Fig.5.EffectofhydroalcoholicextractoftheaeropartsoftheHerissantiatiubae(HtE) onRAW264.7macrophages.Thedatarepresentsmean±S.E.M.opticaldensities ofcelllysateaccordingtothetreatments.***p<0.001vscontrolafteranalysisby ANOVAonewayfollowedbyTukeypost-test.Thedataarerepresentativeoftwo independentexperiments.
mediators(histamine,cytokinesandNO)and,alatephase(range from6through96h)whereitisobservedtheleukocytemigration (Posadasetal.,2004).HtE(50,100,or200mg/kg)didnotreducethe edemaatbothphasesdemonstratingnoanti-edematogeniceffect (Fig.2A–F).
InordertostudytheHtEeffectoncellmigrationtotheinflamed site, the carrageenan-induced peritonitis was performed. The mechanismofactionbywhichcarrageenaninducesthe inflamma-toryprocessesisasynergismamongseveralmediators(bradykinin, serotonin,prostaglandins,leukotrieneB4)(Pinheiroetal.,2013). Asshown in Fig.3A–C,theHtE (50or 100mg/kg)significantly reduced(p<0.01and p<0.001,respectively)theleukocytesand polymorphonuclearcell(PMN)numbersintotheperitonealbutdid notchangemononuclearcellnumber(MN).Thesefindings sug-gested that HtEpresents anti-inflammatoryeffect byinhibiting cellmigrationtotheinflamedsitewithoutdecreasestheedema process.Similarresultsweredescribed byPaiva andcolleagues (2013)wherePseudobombaxmarginatum,Malvaceae,extractalso inhibitedthemigrationofPMNwithoutaffecttheMNcell migra-tion.
Control Control
INF-γ+ LPS
INF-γ+ LPS
6.25 12.5 25 50 6.25 12.5 25 50
0
2
4
6
8
10
**
***
***
***
###
A
0 2 4 6 8 10
###
INF-γ + LPS (–) HtE μg/ml INF-γ + LPS (+)
HtE μg/ml
B
NO
–2 (
μ
M)
NO
–2 (
μ
M)
Fig.6. EffectofhydroalcoholicextractoftheaeropartsoftheHerissantiatiubae(HtE)onNOproductionbyRAW164.7macrophagesexposed(A)ornot(B)toINF-␥
(10ng/ml)+LPS(1g/ml).Thedatarepresentmean±S.E.M.concentrationsofnitrite.Thedatarepresenttwoindependentexperiments.***p<0.001and**p<0.01vsthe INF-␥+LPSgroup,###p<0.001vscontrolgroupafteranalysisbyANOVAonewayfollowedbyTukeypost-test.Thedataarerepresentativeoftwoexperimentsintriplicate.
0 2000 4000 6000 8000
###
***
***
**
***
A
T
NF
-α
pg
/m
l
0 500 1000 1500
2000 ###
*
***
***
B
IL
-6
p
g
/m
l
HtE (μg/ml) INF-γ + LPS HtE (μg/ml)
INF-γ + LPS
Control
INF-γ+ LPS 6.25
12.5 25 50 Control
INF-g+ LPS
6.25 12.5 25 50
Thecarrageenan-inducedperitonitisalsoinvolvesincreasesof TNF-␣,IL-1andIL-6levelsattheperitonealfluid,whichpresents akeyroleininflammatoryprocesses(Lorametal.,2007).Inthis regard,weanalyzedtheHtEeffectonthecytokinelevelsatthe peritonealexudateinducedbycarrageenaninjection.Fig.4AandC showstheHtE(50or100mg/kg)decreasedsignificantly(p<0.001) thelevelsofTNF-␣withoutreducingIL-6,respectively.However, only HtEat doseof 100mg/kg was ableto reducethe amount (p<0.01)ofIL-1inperitonealfluid(Fig.4B).ThereductionofIL-1
andTNF-␣levelsbyHtEmayberesponsiblefortheinhibitionof leukocytemigration,sincethesepro-inflammatorycytokines pro-moteexpressionofendotheliumadhesionmoleculesinvolvedin permeabilityandleukocytetransendothelialmigration(Schmidt etal.,2013).
Tobetterunderstandtheanti-inflammatoryeffectoftheextract anditsmechanismsofactionweusedinvitroanti-inflammatory assaybymeasuringtheproductionofNOandcytokinesinRAW 264.7 macrophages. First of all, we demonstrated (Fig. 5) that HtE wasnot toxic for the cells at concentrations rangingfrom 6.25to 50g/ml. As shown in Fig.6, the HtE (6.25–50g/ml) significantly (p<0.01 – p<0.001) decreased NO production by activatedcells(Fig.6A).Inaddition,macrophagesinpresenceof HtEdidnotproduceNO(Fig.6B),demonstratingtheabsenceof endotoxinsorotheragentscapableofinitiatinganinflammatory response.AsshowninFig.7,theHtE(12.5or50g/ml)wasable toinhibitsignificantly(p<0.001–p<0.01)theproductionof
TNF-␣(Fig.7A)andIL-6(Fig.7B).TheHtEat6.25g/mlwasableto reducesignificantly (p<0.001) onlyTNF-␣production (Fig.7A). ThesedatacorroboratewiththeinvivoresultsandwithParkand colleagues(2012)resultswheretheWerckleainsignis,Malvaceae, extractalsoreducedtheinflammatorycytokines(IL-6,IL-1and TNF-␣).
In addition, species in the Malvaceae family are known to producephenolic compoundswhich exhibit antioxidant action, thereforetheycanbeusedtotreatseveraldiseaseswith inflam-matorycharacteristics(Oliveiraetal.,2012)andkaempferolwhich hasbeendescribedtopossesspotentanti-inflammatory proper-ties (Devi et al., 2015; Kadiogluet al., 2015). Therefore, these phyto-constituentspresentinHtEcouldbecontributingto anti-inflammatoryactivitypresentedinthisstudy.
Insummary,weshowedforthefirsttimethatHtEpresented lowtoxicityfollowingoraladministrationatadoseof2000mg/kg andalsopresentedanti-inflammatoryactivity(50and100mg/kg) bymodulatinginflammatorycellsinvivoandinvitro.Thus,these resultscancontributetothemainpopularuseoftheH.tiubae. However,themechanismsofactionofcompoundsofHtEon inflam-matoryprocesseswillbeexploredinfuturestudies.
Authors’contributions
ALAL carried out the study, participated in the toxico-logical and anti-inflammatory assays and wrote the paper; MFVS provided the plant; WNM has performed the chem-ical studies; ALX, TMM and MVSCB provided assistance in the acute toxicity; FCL, AFA, TRRO participated in the anti-inflammatory experiments; MRP supervised the work andcorrectedthemanuscriptforpublication.Alltheauthorshave readthefinalmanuscriptandapprovedthesubmission.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith
thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
This study was financially supported by INCT for Cancer Control, CNPq573806/2008-0,FAPERJE26/170.026/2008, CNPq-Universal14/2012-472853/2012-0andCAPES/Brazil.
References
Albuquerque, U.P., Medeiros, P.M., Almeida, A.L.S., 2007. Medicinal plants of thecaatinga(semi-arid)vegetationofNEBrazil:aquantitativeapproach.J. Ethnopharmacol.114,325–354.
Almeida, R.N., Falcão,A.C.G.M., Diniz, R.S.T., Quintans-Júnior, L.J.,Polari, R.N., Barbosa-Filho, J.M., Agra,M.F., Duarte,J.C., Ferreira, C.D., Antoniolli, A.R., Araújo,C.C.,1999.Metodologiaparaavaliac¸ãodeplantascomatividadeno SistemaNervosoCentralealgunsdadosexperimentais.Rev.Bras.Farm.80, 72–76.
Anderson,G.D.,2008.Genderdifferencesinpharmacologicalresponse.Int.Rev. Neurobiol.83,1–10.
Denizot,F.,Lang,R.,1986.Rapidcolorimetricassayforcellgrowthandsurvival.J. Immunol.Methods89,271–277.
De Vasconcelos, D.I., Leite, J.A., Carneiro, L.T., Piuvezam, M.R., 2011. Anti-inflammatoryandantinociceptiveactivityofouabaininmice.Mediat.Inflamm. 2011,1–11.
Devi,K.P.,Malar,D.S.,Nabavi,S.F.,Sureda,A.,Xiao,J.,Nabavi,S.M.,Daglia,M.,2015. Kaempferolandinflammation:fromchemistrytomedicine.Pharmacol.Res.99, 1–10.
Falcão-Silva,V.S.,Silva,D.A.,Souza,M.F.V.,Siqueira-Junior,J.P.,2009.Modulationof drugresistanceinStaphylococcusaureusbyakaempferolglycosidefrom Heris-santiatiubae(Malvaceae).Phytother.Res.23,1367–1370.
Green,L.C.,Wagner,D.A.,Glogowski,J.,Skipper,P.L.,Wishnok,J.S.,Tannenbaum, S.R.,1982.Analysisofnitrate,nitritean[15N]nitrateinbiologicalfluids.Anal. Biochem.126,131–138.
Guerra, A.S., Malta, D.J., Laranjeira, L.P., Maia, M.B., Colac¸o, N.C., De Lima, M.C., Galdino, S.L., Pitta, I.R., Gonc¸alves-Silva, T., 2011. Anti-inflammatory and antinociceptive activities of indole–imidazolidine derivatives. Int. Immunopharmacol.11,1816–1822.
Hibbs,J.B.,Taintor,R.R.,Vavrin,Z.,Rachlin,E.M.,1988.Nitricoxide:acytotoxic activatedmacrophageeffectormolecule.Biochem.Biophys.Res.Commun.157, 87–94.
Kadioglu,O.,Nass,J.,Saeed,M.E.,Schuler,B.,Efferth,T.,2015.Kaempferolisan anti-inflammatorycompoundwithactivitytowardsNF-Bpathwayproteins. AnticancerRes.35,2645–2650.
Loram,L.C.,Fuller,A.,Fick,L.G.,Cartmell,T.,Poole,S.,Mitchell,D.,2007.Cytokine profilesduringcarrageenan-inducedinflammatoryhyperalgesiainratmuscle andhindpaw.J.Pain8,127–136.
Mosmann,T.,1983. Rapidcolorimetricassayforcellulargrowthand survival: applicationtoproliferationandcytotoxicityassays.J.Immunol.Methods65, 55–63.
Oliveira,A.M.F.,Pinheiro,L.S.,Pereira,C.K.S.,Matias,W.N.,Gomes,R.A.,Chaves, O.S., Souza,M.F.V., Almeida, R.N., Assis,T.S.,2012. Totalphenoliccontent andantioxidantactivityofsomeMalvaceaefamilyspecies.Antioxidants1, 33–43.
Paiva,D.C.,DosSantos,C.A.,Diniz,J.C.,Viana,F.A.,Thomazzi,S.M.,Falcão,D.A.,2013. Anti-inflammatoryandantinociceptiveeffectsofhydroalcoholicextractfrom Pseudobombaxmarginatuminnerbarkfromcaatingapotiguar.J. Ethnopharma-col.149,416–421.
Park,J.W.,Kwon,O.K.,Jang,H.Y.,Jeong,H.,Oh,S.R.,Lee,H.K.,Han,S.B.,Ahn,K.S.,2012. AleafmethanolicextractofWerckleainsignisattenuatesthe lipopolysaccharide-inducedinflammatoryresponsebyblockingtheNF-Bsignalingpathwayin RAW264.7macrophages.Inflammation35,321–331.
Pinheiro,M.M.,Fernandes,S.B., Fingolo,C.E.,Boylan,F.,Fernandes,P.D.,2013. Anti-inflammatoryactivityofethanolextractandfractionsfromCouroupita guianensisAubletleaves.J.Ethnopharmacol.146,324–330.
Schmidt,S.,Moser,M.,Sperandio,M.,2013.Themolecularbasisofleukocyte recruit-mentanditsdeficiencies.Mol.Immunol.55,49–58.
Silva,D.A.,Falcão-Silva,V.S.,Gomes,A.Y.S.,Costa,D.A.,Lemos,V.S.,Agra,M.F., Barz-Filho,R.,Siqueira-Junior,J.P.,Souza,M.F.V.,2009.Triterpenes andphenolic compoundsisolatedfromtheaerialpartsofHerissantiatiubaeandevaluation of5,49-dihydroxy-3,6,7,8,39-pentamethoxyflavoneasamodulatorofbacterial drugresistance.Pharm.Biol.47,128–133.