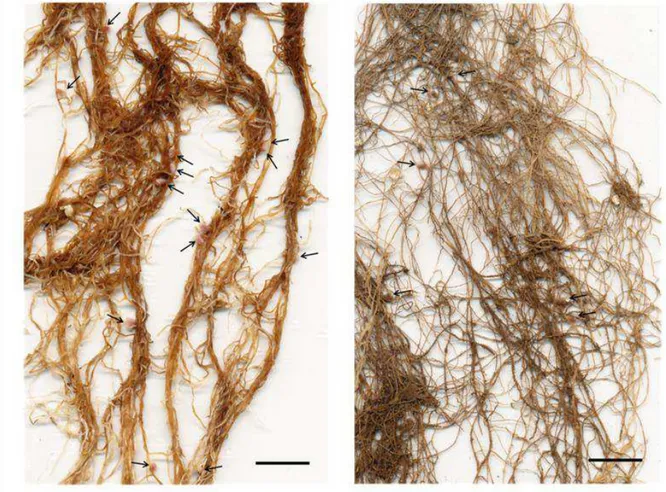
iii Work performed at:
Laboratório de Biotecnologia de Células Vegetais Instituto de Tecnologia Química e Biológica
Universidade Nova de Lisboa
Av. da República, Quinta de Marquês
2780-157 Oeiras
Portugal
PhD Supervisors:
Professor Pedro Fevereiro
Head of laboratory, Laboratório de Biotecnologia de Células Vegetais, Instituto de
Tecnologia Química e Biológica, Universidade Nova de Lisboa.
Assistant Professor, Departamento de Biologia Vegetal, Faculdade de Ciências
da Universidade de Lisboa.
Dr. Dulce Santos
Auxiliary Researcher, Unidade de Veterinária e Zootecnia, Instituto de
Investigação Científica e Tropical.
Professor Tamas Dalmay
Director of Research, School of Biological Sciences, University of East Anglia,
Norwich, UK.
Professor of RNA Biology, School of Biological Sciences, University of East
v Table of contents
Acknowledgments... xi
List of abbreviations... xvii
General Abstract... xix
Resumo geral(in portuguese)... xxi
Chapter 1... 1
Introduction Chapter 2... 47
Identification and characterization of water deficit-responsive microRNAs and their targets in Medicago truncatula Chapter 3... 91
miR408 is involved in seed formation but does not affect photosynthetic activity in water deficit conditions Chapter 4... 127
Reducing miR1507 availability affects Medicago truncatula recovery from water deficit Chapter 5... 155
ix
Acknowledgments
This work could not have been done without the help and support of several people, who have contributed in so many ways for this moment to arrive. I would like to thank:
- Professor Pedro Fevereiro, my supervisor, for giving me the opportunity to do my PhD work at his laboratory and for all the help and support throughout the years.
- Dulce Santos, for teaching me how to be a scientist and how to think and ask the proper questions. Also, thank you for all the long discussions and support throughout the years.
- Tamas Dalmay, for accepting our invitation to be my co-supervisor and for always finding the time to help me and discuss my questions.
- Dr. György Szyttia, Dr. Runchun Jing and Dr. Sara López-Gomollón at the University of East Anglia for teaching me several techniques used in this work and for always being available, either in person or via e-mail, to discuss the work and answer my questions.
- Professor Anabela Bernardes da Silva, for her patience to put up with my endless questions regarding plant physiology and for all the fantastic suggestions. Also, for trusting me and my hands to use the precious equipment from the Faculty of Sciences (University of Lisbon) in the photosynthesis analysis.
x
- Cátia Nunes, for all the help and precious advices regarding plant physiology and for teaching me how to deal with "assim mais ou menos" issues. I could not have done it without you, really. Also, thank you for the discussions about the future of everything. Hope it all turns out amazing for you.
- Mara Alves, my partner in crime (all of them!), for pretty much everything. Thank you for keeping my sanity, for calling my attention every time you had to and for always being there for me. This is also your thesis and I am pretty sure by now you know more about plant small RNAs than most people. Also, thank you for the precious help with the statistical analysis. I could wish you all the luck in the world for your PhD, but I won't because I know you'll do beyond great!
- Matilde Cordeiro, for introducing me to the work in the laboratory when I first arrived at the Plant Cell Biotechnology lab back in 2006 and for being much more than a lab colleague. You're always a breath of fresh air and I wish I could be with you more often. You known it takes one to know one and nobody gets my PhD dramas like you do! You better finish that thesis quickly, so we can be unemployed together!
- Zé Ricardo, who entered my life as just another new kid in the lab and suddenly became a huge part of it. Thank you for all the moments we have spent together and I am sorry for not being available during the last months. I promise I'll make it up to you once I am free again.
- Susana Leitão for the friendship and support throughout the years, both in and outside the laboratory. Thank you for making everyday work much more pleasant. Wish you all the best in the future, though I know you’ll do great!
xi - Leonor Tomaz, for all the help with the in vitro culture techniques and for keeping the laboratory in proper conditions, making it much easier to work. Also, thank you for the non-working time we have spent in and outside the laboratory.
- André Alcântara, for bugging me every single time with the most amazing and exciting questions and for being the best grammar nazi we could have ever had in the lab.
- Nuno Almeida, for the time spent in the laboratory discussing bacteria transformation and DNA cloning. Somehow I will never forget the way you looked at me when I dropped a whole box of yellow tips in the floor during my first days in the lab. I was so young and so scared (just had to mention this, sorry). Hope by now you have finished the famous paper and that one day I'll get to meet your son-in-law... it won't take long, I'm sure!
- Victor Carocha, for all the help during my last months of bench work and for the amazing time we have spent together in the radioactive room. - Helena Garcês and Sofia Duque for the help with the Medicago truncatula
transformation protocol.
- Rita Morgado for keeping my in vitro cultures alive while I have been absent from the lab.
- Jorge Paiva for reviewing this thesis and for all the suggestions on how to improve it.
- To all the other present and former members of the Plant Cell Biotechnology laboratory who have helped me improve as a researcher throughout the years.
xii
laboratory, especially the "minis" at ITQB's bar (yes, if I am known throughout all your theses for drinking beer, I'll return the favour).
- Dr. Nelson Saibo, also from the GPlants laboratory, for providing the Gateway vectors used in this work.
- Ana Milhinhos, from the Forest Biotech laboratory (ITQB), for being the best PhD Program partner I could have had and for all the science-related (and others not so much!) discussions at ITQB's bar. Also, a huge acknowledgment for teaching me how to do PCR site-directed mutagenesis, which made my life so much easier.
- To everyone else I met during the classes at ITQB's PhD Program for being such nice people and for making that period and every day at ITQB much more pleasant.
- Ana Paula Regalado, for allowing us to have random moments of fun at the institute. We already miss the Christmas chocolates and the amazing decorative napkins.
- Jorge, Maria João, Puga, Nuno and Pedro, for being the most amazingly random and weird group of people. Thank you for the fun times and the brilliant non-sense discussions that, in a strange way, have always helped me keeping my sanity.
- To all my other friends who have helped me throughout the years and with whom I know I can always count on, even though we don't see each other that often.
- To my brother André for performing the statistical analysis of the photosynthesis data. Obrigadinha também por me chateares o juízo e essas coisas todas.
xiii o meu irmão tenhamos tido as condições ideais para chegar onde chegámos. Ninguém de fora consegue perceber o quanto de vocês está nesta tese.
- "Ao meu primo Ricardo pela inspiração, apoio moral, etc, etc" (palavras do próprio).
- A toda a família Dionísio (sogros, tios e avós emprestados, Ana, Mafalda e Bernardo) por me tratarem sempre como mais uma filha/sobrinha/neta e por tão bem terem ensinado o rapaz a participar nas lides domésticas. - Ao Duarte por todos os dias me ajudar a ser uma pessoa melhor e por todas as horas de descanso que tenho tido desde o início do último ano. - To everyone I may have forgotten to mention. It is the thesis' fault that I cannot remember everything, I am sorry.
xv List of abbreviations
µg - microgram
µmol - micro mole
ABA - abscisic acid
Amax - net photosynthesis rate under saturating light ANOVA - analysis of variance
ATP - Adenosine-5'-triphosphate
CaMV - cauliflower mosaic virus
cDNA - complementary DNA
cpm - counts per minute
CT - control
DNA - deoxyribonucleic acid
dsRNA - double-stranded RNA
DW - dry weight
ECM - embryo conversion medium
EDC - 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide
EIM - embryo inducing medium
EPM - embryo proliferation medium
FW - fresh weight
GHME - guanidine hydrochloride-MES-EDTA
GI - gene of interest
gs - stomatal conductance GSP - gene-specific primer
HindIII - Haemophilus influenzae III
LNA - locked nucleic acid
m - meter
M - molar
MA - main axis
xvi
miRNA - microRNA miRNA* - microRNA star
mM - millimolar
mol - mole
MOPS - 3-(N-morpholino) propanesulfonic acid
mRNA - messenger RNA
MS - Murashige and Skoog
MWS - moderate water stress
ng -nanogram
nt - nucleotide
PCR - polymerase chain reaction
phasiRNA - phased small interference RNA
qPCR - quantitative polymerase chain reaction
RACE - rapid amplification of cDNA ends
REC - recovery
RNA - ribonucleic acid
RT - reverse transcription
RWC - relative water content
s - second
SDS - sodium dodecyl sulfate
siRNA - small interference RNA
snRNA - small nuclear RNA
sRNA - small RNA
SSC - saline-sodium citrate
ssDNA - single-stranded DNA
SWS - severe water deficit
tasiRNA - trans-acting small interference RNA
TBE - Tris-Borate EDTA
TF - transcription factor
xvii General abstract
The decrease in water availability is one of the main factors responsible for crop losses worldwide. In order to improve their tolerance to water deprivation, plants have developed a variety of morphological, biochemical and molecular adaptations that involve the modulation of gene expression. Over the last decade, small RNAs, namely microRNAs (miRNAs), have been shown to participate in these mechanisms in several plant species. However, little is known about their role in legumes, a plant family of high economical and ecological value.
Therefore, the main goal of this study was to acquire more knowledge on the miRNA-mediated regulation of gene expression in response to water deficit in the model legume Medicago truncatula. For this purpose, the investigation was divided in three tasks: (1) identification of miRNAs differentially expressed under water deficit conditions, (2) prediction and expression analysis of their target transcripts and (3) functional characterization of some selected miRNAs.
Several conserved and non-conserved miRNAs differentially expressed in plants subjected to water deprivation were identified. According to the analysis of their targets, the water deficit-responsive miRNAs can be arranged in three functional groups: those related to root architecture (miR166 and miR169); the miRNAs involved in the maintenance of copper homeostasis (miR398a/b and miR408); and those predicted to target transcripts encoding for proteins involved in plant-pathogen interactions (miR1507 and miR2089).
Taking the previous analysis into consideration, a conserved (miR408) and a non-conserved (miR1507) miRNA were selected for functional characterization, in order to understand more about their role in the responses to water deficit. For this purpose, the technique of target mimicry
xviii
in legumes. The photosynthetic activity of the transformed plants under water deficit conditions and after re-watering was determined, as a way to assess their tolerance to this condition.
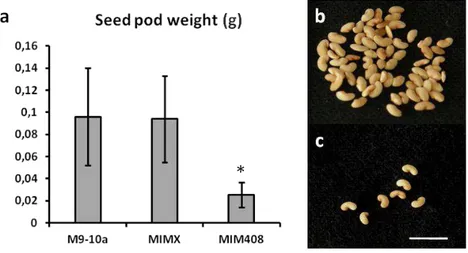
The conserved miR408, whose function in plants is still poorly understood, was selected due to its high up-regulation in water-deprived plants. The obtained results show that this miRNA is not involved in the regulation of photosynthesis under water deficit. However, further evidence supports the hypothesis that miR408 is up-regulated upon water deprivation in order to increase the levels of free copper inside the plants, which could compensate for a reduced nutrient absorption in the roots caused by water deficit. Additionally, the results strongly suggest that miR408 is involved in seed development, as miR408-impaired plants exhibit a severely reduced seed set when compared to the wild-type.
miR1507 was selected because it was one of the non-conserved water deficit-responsive miRNAs with higher expression. According to the analysis performed, this miRNA does not seem to be involved in the regulation of plant development. However, it was shown that miR1507-impaired plants exhibit a lower net photosynthesis rate upon re-watering, implying that miR1507 could be related to the ability of the plants to recover from water deficit.
xix Resumo geral (in portuguese)
A decrescente disponibilidade de água é um dos principais factores responsáveis pelas perdas de produção agrícola a nível mundial. De forma a aumentar a sua tolerância à escassez de água, as plantas desenvolveram uma série de adaptações morfológicas, bioquímicas e moleculares, que envolvem a modulação da expressão génica. Durante a última década, tem sido demonstrada em várias espécies vegetais a participação de pequenos RNAs, nomeadamente de microRNAs (miRNAs), nestes mecanismos. No entanto, pouco se sabe sobre a sua função em leguminosas, uma família de plantas com elevada importância económica e ecológica.
Assim, o principal objectivo deste estudo era adquirir mais conhecimentos sobre a regulação da expressão de genes mediada por miRNAs na resposta ao défice hídrico da leguminosa modelo Medicago truncatula. Para tal, o trabalho foi dividido em três tarefas: (1) identificação de miRNAs diferencialmente expressos em condições de défice hídrico, (2) previsão e análise dos seus transcriptos alvo e (3) caracterização funcional de alguns miRNAs seleccionados.
Vários miRNAs conservados e não conservados foram identificados como sendo diferencialmente expressos em plantas privadas de água. De acordo com a análise dos respectivos alvos, estes miRNAs podem ser organizados em três grupos funcionais: os relacionados com a arquitectura da raiz (miR166 e miR169); os miRNAs envolvidos na manutenção da homeostasia de cobre (miR398a/b e miR408); e os que têm como alvos previstos transcriptos de proteínas envolvidas em interacções planta-patogénio (miR1507 e o miR2089).
xx
de forma a desenvolver plantas transgénicas com reduzida disponibilidade de cada um dos miRNAs escolhidos. Esta foi a primeira vez que esta técnica foi aplicada em leguminosas. A actividade fotossintética das plantas transformadas foi determinada em condições de défice hídrico e após recuperação por retoma da irrigação, como forma de avaliar a sua tolerância à escassez de água.
O miRNA conservado, miR408, cuja função em plantas é ainda pouco conhecida, foi seleccionado devido ao elevado aumento de expressão em plantas privadas de água. Os resultados obtidos demonstram que este miRNA não está envolvido na regulação da fotossíntese em défice hídrico. No entanto, são apresentados dados que sugerem que a expressão do miR408 é mais elevada em condições de falta de água de forma a aumentar a quantidade de cobre livre nas plantas, o que poderá compensar a reduzida absorção de nutrientes, provocada pela escassez de água. Adicionalmente, os resultados sugerem o envolvimento deste miRNA na formação de sementes, uma vez que as plantas com menos miR408 disponível apresentam um número total de sementes muito reduzido quando comparado com o das plantas wild-type.
O miR1507 foi seleccionado por, de entre os miRNAs não conservados induzidos por défice hídrico, ser um dos que apresentou maiores níveis de expressão. De acordo com a análise efectuada, este miRNA não parece participar na regulação do desenvolvimento das plantas. No entanto, demonstrou-se que as plantas com reduzida disponibilidade de miR1507 apresentam uma taxa fotossintética mais baixa após a retoma da irrigação, sugerindo que o miR1507 pode estar envolvido nos processos de recuperação de uma situação de défice hídrico.
Chapter 1.
Introduction
Part of the present chapter was published on the following book chapter:
Trindade I, Santos D, Dalmay T, Fevereiro P (2011) Facing the environment: small RNAs and the regulation of gene expression under abiotic stress in plants.
3 Chapter 1. Table of Contents
The Fabaceae family... 5 Impact of legumes in agriculture, nutrition and economy... 5
Medicago truncatula as a model legume... 6 Impact of water deficit - current status and future perspectives... 7 Plant responses to water deficit... 8 Morphological responses to water deficit... 8 Photosynthetic responses to water deficit... 9 Water deficit-induced oxidative stress... 10 Impact of water deficit on legume metabolism... 12 Regulation of gene expression under water deficit conditions... 13 Epigenetic and transcriptional regulation of gene expression... 14 Regulation of gene expression after transcription... 15 Plant small RNAs... 16 MicroRNAs... 18 Small interference RNAs... 20 Roles of small RNAs in plant development... 21 Small RNAs and the regulation of gene expression in response
4
5 The Fabaceae family
Legumes are a group of species belonging to the Fabaceae family, which is the third largest flowering family of plants, following Orchidaceae and Asteraceae (Doyle and Luckow, 2003), and the second most important to humanity, only behind the Graminiae (Graham and Vance, 2003). Some of the most important species from this group include soybean (Glycine max), common bean (Phaseolus vulgaris), pea (Pisum sativum), chickpea (Cicer arietinum), broad bean (Vicia faba), cowpea (Vigna unguiculata), chickpea (Cicer arietinum) and lentils (Lens esculenta).
Impact of legumes in agriculture, nutrition and economy
A large majority of legumes are able to establish symbiosis with soil bacteria, leading to the formation of root nodules, where atmospheric nitrogen (N2) fixation takes place (reviewed by Oldroyd and Downie, 2008).
Due to this ability, they are often used as forage and in agriculture, as a strategy to improve soil fertility and nutrient availability (Graham and Vance, 2003). Additionally, natural N2 fixation enables a reduction in the application
of nitrogen-enriched fertilizers in agriculture, which can be beneficial to the surrounding environment, as recently observed in a study regarding the introduction of legumes in crop rotation systems (Nemeceka et al., 2008).
6
Besides all the benefits in agriculture and nutrition, legumes have also been used in a variety of industries to produce flour, bread, snacks, milk, oil and several non-edible products like biodegradable plastics, gums and biodiesel (reviewed by Graham and Vance, 2003), illustrating their potential impact in economy.
Medicago truncatula as a model legume
Over the last two decades, since it was proposed as a model system for the Fabaceae family, Medicago truncatula, commonly known as barrel medic, has emerged as an important tool in the study of legume molecular biology (Barker et al., 1990; Cook, 1999).
M. truncatula is an autogamic plant that has a relatively small genome (around 470 Mbp; Young et al., 2003) and a short lifecycle (Cook, 1999). It has an extensive sinteny with other legumes of higher economical value, meaning most of the knowledge gathered from the analysis of this plant can be transferred to those species (Choi et al., 2004).
7 Impact of water deficit - current status and future perspectives
Plants are constantly challenged by conditions of low water availability that can be caused by several environmental stresses like drought, salinity, cold and elevated temperatures (Taiz and Zeiger, 2002). Water deficit, defined as the water content of a cell or tissue that is below the one registered at a fully hydrated state, has been widely reported to induce severe constrains to plant development (Taiz and Zeiger, 2002), and is generally achieved when water losses exceed water uptake (Lawlor and Tezara, 2009).
In Europe, particularly in the southern countries, water deficit has become a real concern during the last two decades. In 2003, the European gross primary productivity suffered a reduction of 30% due to high temperatures and low precipitation (Ciais et al., 2005). Moreover, during the 1900's, water exploitation indexes above 20%, which is the threshold for a country or region to be considered water stressed, were registered in Spain, Italy, Cyprus and Malta, according to the Eurostat (BIO Intelligence Service, 2012).
Over the past years, several reports have raised awareness for the fact that extreme weather conditions are predicted to become more frequent in a near future, which is likely to have a strong impact in crop production. For instance, it is estimated that by 2030, global water demand solely for agriculture may have increased by more than 30% as a consequence of foreseen climate changes (Foresight, 2011). This is expected to affect the economy of several countries as well, as crop yields are predicted to register significant declines between 2000 and 2050 (Nelson et al., 2010). Consequently, in an optimistic scenario, maize, rice and wheat prices are predicted to increase during this period by around 87.3%, 31.2% and 43.5%, respectively (Nelson et al., 2010).
8
to reduce productivity losses in sub-optimal environments.
Plant responses to water deficit
Plants have developed a variety of morphological and biochemical strategies to improve their performance when water is less available. These can be generally divided in desiccation postponement, desiccation tolerance, when plants have the ability to function at low water contents, and drought escape, when they complete their life cycle before the beginning of the dry season (Taiz and Zeiger, 2002).
Morphological responses to water deficit
Water enters plants in the roots and is lost mainly in the leaves by evapotranspiration through the cuticle and the stomata (Taiz and Zeiger, 2002). When water availability is compromised, stomata closure is induced, as a way to minimize water losses (Taiz and Zeiger, 2002). Interestingly, this process has been widely reported to be regulated by chemicals that are translocated from the roots to the shoots, including the phytohormone abscisic acid (ABA; reviewed by Davies et al., 2002). Simultaneously, particularly under severe stress, an increase in cuticle thickness, due to an abnormal wax deposition, is frequently observed as a way to additionally minimize leaf permeability (Taiz and Zeiger, 2002).
Water-deprived plants are usually more susceptible to light-induced oxidative stress, which can cause several damages in the cellular metabolism (Chaves et al., 2003). Therefore, some have developed different strategies to minimize the exposure to light when water availability is compromised. Among these, higher leaf hair density, which increases light reflection, and changes in leaf orientation (or curling in grasses) are frequently observed (Comstock and Mahall, 1985; Taiz and Zeiger, 2002).
9 contributes to a reduction in total leaf area, a phenomenon that is highly sensitive to subtle changes in plant water status and simultaneously leads to a decrease of the evapotranspiration surface, minimizing further water losses (Taiz and Zeiger, 2002). Under prolonged stress situations, some species exhibit an even more dramatic response with the induction of leaf senescence, often followed by abscission, which additionally allows for nutrient re-mobilization (reviewed in Munné-Bosch and Alegre (2004).
Roots, on the other hand, are less sensitive to water deficit and have the ability to keep growing at lower water potentials, when shoot development is already compromised (reviewed in Sharp et al., 2004). This is considered to be a strategy to delay water stress, as it allows root growth into deeper and less dry soil layers, increasing water uptake (Taiz and Zeiger, 2002; Bartels and Sunkar, 2005).
Photosynthetic responses to water deficit
As mentioned above, one of the first lines of defence developed by the plants against low water availability is the regulation of stomatal closure in order to minimize water losses through transpiration (Chaves, 1991; Taiz and Zeiger, 2002). However, this mechanism, associated to a decreased mesophyll conductance, reduces air diffusion inside the leaves, compromising CO2 availability (Chaves et al., 2009). Additionally, water
deficit induces metabolic limitations that are not only related with the lower carbon assimilation, but also with changes in the water status of the cells (Lawlor and Tezara, 2009). Even though there has been some controversy related to the relative contribution of each of these factors, together they all lead to a decrease in the photosynthetic activity in plants subjected to water deficit. Nevertheless, recent studies show evidences indicating that, at least in a majority of the plants analysed, there is a stronger influence of CO2
10
Bota et al., 2004; Galmés et al., 2007). However, the discrepancies observed in the literature suggest that, like several responses to abiotic stress, the extent at which photosynthesis is affected depends on factors like the species analysed, plant growth conditions and the intensity and duration of stress (Lawlor and Tezara, 2009).
The activity of the photosystems is generally affected only when severe water deficit is reached (Lawlor and Tezara, 2009). However, when CO2
availability is compromised, the levels of NADP+, which is the final acceptor of the electron transport chain (Fig.1), decrease and assimilated photons become excessive in relation to the requirements of the subsequent carbon reactions (Lawlor and Tezara, 2009). Under these conditions, the over-produced energy can be used to reduce O2, leading to the accumulation of
reactive oxygen species (ROS; reviewed in Asada, 1999). These have been proposed to contribute to a reduction of the ATP synthase activity and consequent lower rate of ATP production in water-deprived plants (Chaves et al., 2009).
Under more severe water deficit, the decrease in ATP levels, together with the lower CO2 availability, compromise photosynthesis carbon
reactions. In fact, the regeneration of ribulose-1,5-bisphosphate (RuBP), which is a key intervenient of the Calvin cycle, can be affected under these conditions (Seki et al., 2002; Bota et al., 2004). Additionally, the activity of ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco) has been reported to decrease in some species upon water deficit, indicating it could further limit photosynthesis (Bota et al., 2004; Flexas et al., 2006).
Water deficit-induced oxidative stress
11 (e.g. xanthophyll cycle) and photorespiration (Demmig-Adams and Adams, 1996; Noctor et al., 2002). The energy that is not dissipated will ultimately be used to reduce O2, leading to the formation of ROS (Smirnoff, 1993;
Asada, 1999). Additionally, photorespiration, which has been shown to increase under low CO2 conditions, also contributes to the production of
ROS in the peroxisomes (Noctor et al., 2002). All these reactions induce perturbations in the equilibrium between production and scavenging of ROS, like hydrogen peroxide (H2O2), singlet oxygen (1O2) and the radicals
super-oxide (O2-) and hydroxyl (OH-), leading to their abnormal
accumulation (reviewed in Apel and Hirt, 2004; Gill and Tuteja, 2010).
12
In plants, ROS have been shown to be involved in gene expression regulation, through the activation of signalling cascades (Yuasa et al., 2001) and transcription of stress-inducible genes (Desikan et al., 2001). More recently, they were also found to take part in protection against pathogen infection, through the regulation of programmed cell death (Gechev and Hille, 2005; Danon et al., 2006). However, when present in high concentrations, ROS can react with DNA, proteins and lipids, affecting cellular functions (reviewed in Gill and Tuteja, 2010).
Plants have developed several mechanisms to minimize the effects of ROS accumulation. Superoxide dismutases (SODs), which catalyse the conversion of O2- to H2O2 and O2, and catalases (CATs), which convert
H2O2 into H2O and O2, play an important part in defence against oxidative
stress (reviewed in Gill and Tuteja, 2010). Plants also accumulate pools of non-enzymatic antioxidants that, through the action of several enzymes, can be conjugated with ROS, facilitating their elimination. Among these are ascorbate, glutathione (GSH), proline, α-tocopherols, carotenoids and flavonoids (reviewed in Gill and Tuteja, 2010).
Interestingly, when present in non-toxic concentrations, ROS can also act as signalling molecules in plants. Although there is still some controversy around this subject (Kohler et al., 2003), H2O2 has been
reported to act as a secondary messenger in ABA-induced stomata closure (Pei et al., 2000; Zhang et al., 2001; Miao et al., 2006), showing ROS can also be involved in protection against water deficit conditions.
Impact of water deficit in the legume metabolism
13 reasonable to expect high variability regarding legume responses to water deficit. Among the most studied, cowpeas, which exhibit a long root system and small leaves, are regarded as the most drought-tolerant, whereas common beans are usually found on the opposite side of the scale (Graham and Vance, 2003).
Besides all the already mentioned plant responses to water deficit, in legumes, root nodule metabolism and therefore N2 fixation, is also affected,
compromising plant productivity under these conditions (reviewed in Serraj et al., 1999). Several factors have been reported to account for this phenomenon: (1) carbon limitation, which leads to a reduction in the activity of sucrose synthase, essential in nodule metabolism; (2) decreased oxygen availability/permeability, which reduces nodule functioning; (3) feedback regulation by N2 compounds; (4) reduced bacterial motility and,
consequently, infection capacity (reviewed in Serraj et al., 1999). As previously discussed for photosynthesis, the contradictory results found in the literature suggest that the influence of each of these factors may depend on the species and stress conditions. However, experiments with pea using a split-root strategy, in which half of the root system is well-watered and other half is subjected to water deficit, suggest that this decrease in nodulation is regulated by local rather than systemic signals (Marino et al., 2007), which could include locally accumulated ROS (Marino et al., 2006).
Regulation of gene expression under water deficit conditions
14
Epigenetic and transcriptional regulation of gene expression
Abiotic stress in general, including water deprivation, induces epigenetic changes in plant genomes, such as histone post-translational modifications and DNA methylation that affect transcription (reviewed by Chinnusamy and Zhu, 2009). Due to their stability and heritability, these mechanisms have been associated to long-term memory of stress, which could potentially play an important role in plant adaptation (reviewed by Boyko and Kovalchuk, 2011).
At the transcriptional level, transcription factors (TFs) have been described as major regulators of gene expression under abiotic stress conditions. Many TFs have been shown to regulate responses to water deficit in plants, which are known to comprise both ABA-dependent and ABA-independent regulatory pathways (reviewed by Yamaguchi-Shinozaki and Shinozaki, 2005). TFs are proteins that act by binding to DNA conserved cis-acting elements and inducing the activation or repression of stress-responsive genes (Nakashima et al., 2009). The ones binding to the dehydration-responsive/C-repeat elements (DRE/CRT; bearing the conserved sequence A/GCCGAC) and ABA-responsive elements (ABRE; with the conserved sequence C/TACGTGG/TC) play a major role in the regulation of the responses to water deficit. Interestingly, the analysis of these regulators has unveiled a high degree of cross-talk between the molecular responses to different environmental stimuli in plants. For instance, DRE-binding TFs (DREBs) were found to be involved in responses to osmotic stress and cold (Yamaguchi-Shinozaki and Shinozaki, 1994; Nakashima et al., 2009).
15 genes, like other TFs, phospholipase C, RNA-binding proteins, sugar transporters, protease inhibitors, Late Embryogenesis Abundant (LEA) proteins and enzymes involved in the synthesis of compatible solutes (Nakashima et al., 2009). Interestingly, TFs have also been associated to the photosynthetic responses to abiotic stress, as they can be involved in the regulation of stomatal and non-stomatal mechanisms under these conditions (reviewed by Saibo et al., 2009).
Regulation of gene expression after transcription
Following transcription, post-transcriptional and post-translational mechanisms of regulation of gene expression have also been shown to play an important role in the responses to abiotic stress conditions (reviewed by Hirayama and Shinozaki, 2010).
16
Arabidopsis (Arabidopsis thaliana) tolerance to this condition (Catala et al., 2007).
Transcript processing can also induce changes in gene expression upon certain environmental stimuli (Chinnusamy et al., 2007; Hirayama and Shinozaki, 2010). For instance, alternative splicing, the process through which a single gene can originate different transcripts, is widely known to regulate gene expression in plants subjected to low and high temperatures (reviewed by Chinnusamy et al., 2007) and has been reported upon water deficit as well (Egawa et al., 2006). This can be due to changes in the levels and distribution of splicing factors or to alteration in the expression and phosphorylation status of proteins involved in this mechanism (reviewed by Barbazuk et al., 2008). Additionally, in 2004, plant small RNAs were also found to be involved in plant responses to environmental stimuli (Jones-Rhoades and Bartel, 2004; Sunkar and Zhu, 2004). Since then, they have emerged as important intervenients in these processes in several species.
Plant small RNAs
Evidence for the existence of RNA-mediated silencing mechanisms in plants first appeared in the late 1990's, when short antisense RNA molecules were isolated from tomato plants where post-transcriptional gene silencing had been detected (Hamilton and Baulcombe, 1999). Since then, the knowledge on sRNAs has broadened and these molecules have been identified as important players in a wide variety of processes in plants. Back in 2002, when the first set of plant microRNAs (miRNAs) was cloned (Reinhart et al., 2002), there were only 218 entries in the public miRNA database (miRBase; Griffiths-Jones, 2004) whereas nowadays more than 21 000 entries can be found (release 19).
17 duplexes by RNase III Dicer-like (DCL) proteins (reviewed by Bartel, 2004). S-adenosyl methionine-dependent methyltransferase Hua Enhancer 1 (HEN1) methylates these molecules at the 3' end, in a sequence-independent manner, protecting them from uridylation and degradation (Park et al., 2002; Li et al., 2005; Yu et al., 2005). There are four loci in Arabidopsis and rice (Oryza sativa) that encode for DCL proteins (Liu et al., 2005). Although some functional redundancy has been observed, each DCL seems to have a specific role in sRNA biosynthesis pathways (Bouche et al., 2006; Henderson et al., 2006).
Following methylation, sRNAs are incorporated into ARGONAUTE (AGO) proteins, in order to act upon targets in a sequence-specific manner (Llave et al., 2002b; Rhoades et al., 2002; Vazquez et al., 2004b). Plants have multiple loci encoding for AGO proteins: 10 are known in Arabidopsis, at least 18 in rice (Mallory and Vaucheret, 2010) and 12 were predicted in
Medicago truncatula (Capitão et al., 2011). AGO4, AGO6 and AGO9 are usually involved in silencing at the transcriptional level, by association with 24-nt sRNAs, whereas AGO1 and AGO7 participate in post-transcriptional gene silencing and are usually loaded with 21 and 22-nt sRNAs (reviewed by Mallory and Vaucheret, 2010). Recently, roles for AGO2 in responses to virus infection, antibacterial immunity and as an AGO1 substitute have also been described (Harvey et al., 2011; Maunoury and Vaucheret, 2011; Zhang et al., 2011). With few exceptions, the main characteristics that influence the loading of AGO proteins in plants are both the size of the sRNAs and the nature of the nucleotide at the 5' end (Mi et al., 2008; Mallory and Vaucheret, 2010).
18
MicroRNAs
MicroRNAs are usually transcribed from intergenic regions (Llave et al., 2002a; Reinhart et al., 2002) by Polymerase II (Pol II) into 5' capped and 3' polyadenylated primary transcripts, in a way similar to protein-coding genes (Xie et al., 2005). These precursors (named pri-miRNAs) have regions of self-complementarity and can therefore fold back into stem-loop structures (Park et al., 2002; Meyers et al., 2008), yielding the dsRNA region, needed to trigger RNA silencing mechanisms. The hairpin precursors, called pre-miRNAs, are released from the pri-miRNAs by DCL1 in the nucleus, which then processes the pre-miRNAs into smaller duplexes bearing the mature miRNA and the complementary strand (miRNA*; Reinhart et al., 2002; Henderson et al., 2006). In plants, both HYPONASTIC LEAVES 1 (HYL1) and SERRATE (SE) proteins seem to interact with DCL1 and are also required for this process (Han et al., 2004; Lobbes et al., 2006).
19
20
proteins could be part of a plant RNA silencing complex. In the same work, Hsp90 was found to be required for the loading of sRNAs into AGO1, presumably by inducing conformational changes in this protein (Iki et al., 2010).
There are also other miRNAs that are processed into 22-nt molecules, which is thought to be related to asymmetry in the hairpin precursor that is cleaved into a 22-nt mature miRNA and a 21-nt miRNA* (Cuperus et al., 2010). Recently, these molecules that are also loaded into AGO1, were found to trigger the production of secondary sRNAs from the 3' product of miRNA-directed cleavage (Chen et al., 2010; Cuperus et al., 2010; Shivaprasad et al., 2012).
Small interference RNAs
A second and less studied group of sRNAs is known to exist in plants. These molecules, generally called siRNAs, are cleaved by DCL proteins from long dsRNA molecules that do not form hairpins (Carrington and Ambros, 2003). These are produced by either the transcription of inverted repeats or by the action of RNA-dependent RNA polymerases (RDRs) that convert single-stranded RNA transcripts into dsRNA (Dalmay et al., 2000). The best studied siRNAs in plants fall into two distinct categories: RDR2-dependent (or heterochromatin associated) siRNAs and trans-acting siRNAs (ta-siRNAs; Ramachandran and Chen, 2008).
The first sub-group comprises siRNAs that are transcribed by RNA polymerase IV (Pol IV) and further processed to dsRNA by RDR2 (Herr et al., 2005). These sRNAs are mostly cleaved into 24-nt duplexes by DCL3 (Gasciolli et al., 2005; Song et al., 2012) and loaded into AGO4 to guide target silencing, usually by promoting DNA or histone methylation (Zilberman et al., 2003; Herr et al., 2005).
21 2004b). The biogenesis of these ta-siRNAs requires miRNA-AGO1-mediated cleavage of target transcripts, called TAS genes (Allen et al., 2005; Allen and Howell, 2010), and seems to be related to the production of 22-nt miRNAs (Chen et al., 2010; Cuperus et al., 2010). The RNA polymerase RDR6, together with SUPRESSOR OF GENE SILENCING 3 (SGS3) protein, processes the cleavage products (Vazquez et al., 2004b; Montgomery et al., 2008b), yielding long dsRNA molecules that are subjected to DCL4-mediated phased cleavage of 21-nt dsRNA duplexes (Gasciolli et al., 2005; Yoshikawa et al., 2005; Song et al., 2012). One of the strains from each of these duplexes is then loaded into an AGO protein, according to the criteria previously mentioned, in order to promote the silencing of target transcripts (Mi et al., 2008). Four TAS genes have been identified in Arabidopsis: TAS1, TAS2 (both targeted by miR173; Montgomery et al., 2008b), TAS3 (cleaved by miR390; Allen et al., 2005) and TAS4 (targeted by miR828; Rajagopalan et al., 2006). Interestingly,
TAS3 seems to undergo a specific pathway that is conserved in several plant species and different from what is observed for other TAS genes. This pathway depends on miR390, a 21-nt long conserved miRNA, and requires AGO7 to be bound to the miRNA in order to cleave TAS3 transcripts (Adenot et al., 2006; Montgomery et al., 2008a).
Roles of small RNAs in plant development
22
Jacobsen et al., 1999; Chen et al., 2002; Vaucheret et al., 2004; Vazquez et al., 2004a). Later, other strategies, like the over-expression of a miRNA or of a non-cleavable version of its target transcripts, have also contributed to increase the knowledge on the role of plant small RNAs (Chen, 2004; Wang et al., 2005). In 2007, Franco-Zorrilla et al. identified in Arabidopsis a mechanism through which miR399 is naturally down-regulated due to its sequestration by the INDUCED BY PHOSPHATE STARVATION1 (IPS1) non-coding RNA. Additionally, the authors found that it was possible to engineer this transcript in order to make it complementary to other miRNAs and reduce their availability in plants (Franco-Zorrilla et al., 2007). Recently, the development of a collection of Arabidopsis mutants based on this technique, known as target mimicry, has highlighted the role of several miRNAs in the regulation of plant development (Todesco et al., 2010).
One of the main pathways through which small RNAs participate in plant development is through the regulation of auxin signalling. For instance, miR160 was found to target the auxin response factors ARF10,ARF16 and
ARF17, members of a large family of TFs that regulate auxin-responsive genes, participating in processes like root growth, leaf and flower development and seed germination (Mallory et al., 2005; Sorin et al., 2005; Wang et al., 2005; Liu et al., 2007). miR167, on the other hand, targets
ARF7 and ARF8 and was shown to be involved in anther and ovule development (Wu et al., 2006). Additionally, ta-siRNAs originated from the miR390-induced cleavage of TAS3 are also involved in auxin regulation by targeting ARF3 and ARF4 transcripts (Allen et al., 2005). These small RNAs have been implicated in processes like lateral root development and leaf polarity (Garcia et al., 2006; Schwab et al., 2009; Marin et al., 2010).
23 The miR165/miR166 group has been related to the establishment of leaf abaxial/adaxial patterning (Emery et al., 2003; Juarez et al., 2004; Kidner and Martienssen, 2004). These miRNAs target transcripts encoding for members of class III HD-ZIP TFs, like ATHB15, PHABULOSA (PHB), PHAVULOTA (PHV) and REVOLUTA (REV), that have also been implicated in vascular development and root growth (McHale and Koning, 2004; Kim et al., 2005). In maize, miR166 was found to accumulate in the abaxial part of leaf primordia and to form a gradient towards the adaxial region (Nogueira et al., 2007). Interestingly, TAS3 transcripts have been shown to accumulate in the adaxial region and seem to be involved in restricting miR166 spatial distribution, thus contributing to the determination of leaf polarity as well (Nogueira et al., 2007).
24
Thus, these miRNAs have been proposed to interact in order to regulate leaf shape and development (reviewed by Schommer et al., 2012).
Additionally, miR172 is known to target APETALA2 (AP2) transcripts and regulate flowering time (Aukerman and Sakai, 2003). Its expression was found to be induced by the SQUAMOSA promoter binding protein-like 9 (SPL9), which, together with SPL3/10 and 15, is a known target of miR156 (Vazquez et al., 2004a; Wu and Poethig, 2006; Schwarz et al., 2008). Interestingly, besides regulating the transition to reproductive phase, miR172 has also been reported to mediate the transition from juvenile to adult phase, while miR156 is known to promote juvenile identity (Wu and Poethig, 2006). These observations led to the proposal of a cooperative model for the regulation of phase transition in plants in which the accumulation of miR156, which is kept at high levels during the juvenile phase, decreases leading to the accumulation of SPL9 and 10 (Wu et al., 2009). This will in turn promote miR172 expression (Wu et al., 2009), down-regulating its target genes, including several flowering repressors (Mathieu et al., 2009). Additionally, SPL3, which accumulates in the adult phase due to lower miR156 levels, regulates flowering time through the direct activation of the genes LEAFY, FRUITFULL, APETALA1 (Yamaguchi et al., 2009) and FLOWERING LOCUS T (Kim et al., 2012).
Interestingly, some of these small RNAs, like miR165/166 and miR172, among others, were reported to be differentially expressed upon different environmental stimuli (Lv et al., 2010; Frazier et al., 2011), suggesting they could also regulate developmental changes under these conditions.
Small RNAs and the regulation of gene expression in response to abiotic stress
25 Reyes, 2010). The fact that plant miRNAs target transcripts in a sequence-specific manner, allowed Jones-Rhoades and Bartel (2004) to computationally predict targets for a conserved group of these regulators. These authors found that the expression of miR395 depends on sulphate availability, and that some other miRNAs can target transcripts that are potentially involved in responses to abiotic stress (Jones-Rhoades and Bartel, 2004). By then, Sunkar and Zhu (2004), analysed two week-old Arabidopsis seedlings, and identified a variety of conserved miRNAs that were differentially expressed upon cold, dehydration, NaCl and ABA treatments. The authors found that, for instance, miR393, miR397b and miR402 were up-regulated in all the implemented conditions. On the other hand, miR389a, which was later found to be related to ta-siRNAs (Allen and Howell, 2010), was down-regulated under all these stresses (Sunkar and Zhu, 2004).
The widespread application of high-throughput sequencing techniques enabled the identification of several stress-responsive small RNAs (reviewed by Trindade et al., 2011), but their role in the context of the responses to environmental stimuli remains to be elucidated. There are however a few exceptions.
MicroRNA regulation upon nutrient deprivation
The most extensively studied miRNA family regarding responses to abiotic stress is probably miR399, whose members are up-regulated by inorganic phosphate (Pi) starvation in several species (Fujii et al., 2005; Bari et al., 2006). These miRNAs were found to target PHOSPHATE2
26
the roots, triggering Pi starvation-inducible genes, including transporters involved in Pi allocation inside the plants (Bari et al., 2006). Interestingly, miR399 was the first plant miRNA to be identified as a phloem-mobile long-distant signal that accumulates in the shoots and targets transcripts in the roots, validating some aspects of the previous model (Pant et al., 2008). Additionally, the action of miR399 was found to be counter-acted by the non-protein encoding transcript IPS1, in order to fine-tune Pi homeostasis (Franco-Zorrilla et al., 2007).
An identical miRNA-mediated regulation mechanism has been reported for members of the sulphate starvation-inducible miR395 family. These miRNAs were shown to target transcripts coding for proteins involved in sulphate assimilation, like the low-affinity SULPHATE TRANSPORTER 2;1 (SULTR2;1) and several ATP sulphurylases (APS; Jones-Rhoades and Bartel, 2004; Allen et al., 2005; Kawashima et al., 2009). Additionally, the SULFUR LIMITATION1 (SLIM1) TF, known to regulate the expression of sulphate-responsive genes (Maruyama-Nakashita et al., 2006), is a major intervenient in this process, as it regulates the expression of miR395 (Kawashima et al., 2009). Recently, this miRNA together with SLIM1 was found to fine-tune the accumulation of APS in order to regulate sulphate flux from the roots to the shoots, as a strategy to improve its assimilation in the leaves under deficiency conditions (Kawashima et al., 2011). In a previous work, the regulation of SULTR2;1 by miR395 had also been associated with sulphate translocation between leaves (Liang et al., 2010). Indeed, sulphate flux was found to increase in Arabidopsis plants over-expressing miR395 (Kawashima et al., 2011), which could explain the previously detected accumulation of sulphate in the leaves of these plants (Liang et al., 2010).
27 2008). Interestingly, besides presenting similar expression profiles under these conditions, they were all found to target transcripts encoding for Cu proteins, like Cu/Zn superoxide dismutases, plantacyanin, cytochrome c
oxidase and several laccases (Yamasaki et al., 2007; Abdel-Ghany and Pilon, 2008). Although there is still further validation missing, this has been proposed to be related to the need to save this micronutrient for more essential proteins, like plastocyanin, which is involved in the photosynthetic electron transport chain (Abdel-Ghany and Pilon, 2008). Interestingly, the expression of copper miRNAs was found to be regulated by the SQUAMOSA promoter binding protein–like7 (SPL7) TF that regulates the expression of several copper-responsive genes and binds to a conserved motif in the miRNA promoter region (Yamasaki et al., 2009).
Generally it seems that miRNAs play an important role in nutrient allocation upon deprivation. Interestingly, like miR399, miR395 and miR398 were also found to accumulate in the phloem sap of Brassica napus upon stress, suggesting they too could participate in systemic responses to abiotic stress (Buhtz et al., 2008).
miR398 as an oxidative stress regulator
Besides being induced by low Cu conditions, the expression of miR398 is also repressed upon oxidative stress (Sunkar et al., 2006; Jagadeeswaran et al., 2009a). Consequently, miR398 is also down-regulated under several adverse conditions, like salt stress, ozone fumigation and even infection with Pseudomonas syringae (Jagadeeswaran et al., 2009a). Additionally to target transcripts encoding for cytochrome c
oxidase (Jones-Rhoades and Bartel, 2004), this miRNA is known to target both cytosolic and plastidic Cu/Zn superoxide dismutases (CSD1 and
CSD2, respectively; Jones-Rhoades and Bartel, 2004; Sunkar et al., 2006), that are responsible for the conversion of O2- to H2O2 (Kliebenstein et al.,
28
inhibition a Cu chaperone for superoxide dismutase (CCSD), that is involved in the generation of functional superoxide dismutases (Beauclair et al., 2010). The reduction in the levels of miR398 upon stress, leads to an increase in CSD transcripts, thus protecting the cells against oxidative damage (Sunkar et al., 2006).
However, the expression of miR398 is not always decreased under abiotic stress conditions. In Arabidopsis its expression decreased after sucrose addition (Dugas and Bartel, 2008) and similar results were registered in common aspen (Populus tremula) subjected to short time treatments with ABA, NaCl and UV-B radiation (Jia et al., 2009). Interestingly, the expression of this miRNA was recently shown to depend on the type of stress imposed and to oscillate with the degree of stress (Frazier et al., 2011), which could explain these discrepancies. However, according to Dugas and Bartel (2008), the induction of this miRNA, and further reduction of CSD transcripts, under these conditions could be caused by a reduction in ROS production as a consequence of some photosynthetic impairment. It could also be that different MIR398 genes are being regulated in different ways, like previously proposed for the precursors of other miRNAs (Reyes and Chua, 2007).
miR169 and the regulation of the responses to osmotic stress
29 translation inhibition of transcripts encoding the NUCLEAR FACTOR Y (NF-Y) TF subunits, reported to regulate the expression of drought-responsive genes (Jones-Rhoades and Bartel, 2004; Li et al., 2008; Zhao et al., 2009). Interestingly, NFYA5, one of those subunits, was shown to be highly expressed in leaves, particularly in guard cells (Li et al., 2008). Additionally, nfya5 knockout mutants, as well as miR169 over-expressing plants, showed higher leaf water losses and increased plant susceptibility to water deprivation (Li et al., 2008). Although further work is still needed in order to fully elucidate its involvement in this mechanism, it is possible that under water deficit, miR169 is down-regulated to promote stomata closure and reduce water losses, while under salt stress it could be induced in order to increase stomatal aperture and, consequently, water uptake in the roots.
Small RNA-mediated regulation of gene expression in legumes
30
2009; Valdes-Lopez et al., 2010), peanut (Arachis hypogaea; Zhao et al., 2010; Chi et al., 2011) and cowpea (Barrera-Figueroa et al., 2011).
However, the functional characterization of these small RNAs has not kept with this pace and little is known about their actual roles in legumes. Prior to these high-throughput sequencing studies, two conserved M. truncatula miRNAs had already been characterized in relation to their role in root growth and development. miR169 was found to target NFYA
transcripts, as also described for other species (Li et al., 2008), and accumulate in root nodules (Combier et al., 2006). The authors found that this regulation led to the accumulation of this TF in the nodule meristematic region, which is crucial for a correct tissue identity and proper nodule development (Combier et al., 2006). Later, miR166 was shown to be involved in the regulation of vascular bundle development by targeting transcripts encoding for HD-ZIP III TFs, like MtCNA1, MtCNA2 and MtHB8, which control lateral root and nodule formation (Boualem et al., 2008).
Nevertheless, during the last 4 years some improvements have been registered towards the functional characterization of legume small RNAs. Recently, the over-expression of soybean miR482, miR1512 and miR1515 led to an increasing number of root nodules, suggesting they could be involved in these processes in legumes (Li et al., 2010). Also, in M. truncatula the Pi-induced miR399 was found to accumulate upon inoculation with arbuscular mycorrhizal fungi, reducing the levels and activity of its target MtPHO2, which was reported to suppress symbiosis (Branscheid et al., 2010).
31 enabled the authors to identify miR169*, miR171h, miR5204* and miR5282 as promising regulators of symbiosis in legumes (Devers et al., 2011).
Objectives
This work was developed with the main goal of providing new insights on the roles of small RNA-mediated regulation of gene expression in legumes. Particularly, it was focused on the potential involvement of these regulators in the responses of the model legume Medicago truncatula to water deprivation.
The first part of the work aimed to identify miRNAs that were differentially expressed under water deficit conditions and could potentially be involved in the regulation of plant responses to this stress. In order to achieve this, the expression profile of several conserved and non-conserved miRNAs was analysed in adult plants subjected to different degrees of water deprivation and recovery by re-watering.
Subsequently, in order to understand more about the roles of these regulators in well-watered and water-deprived plants, their targets were predicted in silico, using available softwares, and further validated. Additionally, the expression profile of these transcripts was analysed, to assess if it correlated with the expression of the correspondent miRNA, which could be an extra evidence for miRNA-target regulation.
Finally, taking into consideration the previous results, some miRNAs were selected for a deeper characterization of their roles in legumes. For this purpose, the target mimicry technique was combined with our own
32
References
Abdel-Ghany SE, Pilon M (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. J Biol Chem 283: 15932-15945
Addo-Quaye C, Eshoo TW, Bartel DP, Axtell MJ (2008) Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Curr Biol 18: 758-762
Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouche N, Gasciolli V, Vaucheret H (2006) DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr Biol 16: 927-932
Allen E, Howell MD (2010) miRNAs in the biogenesis of trans-acting siRNAs in higher plants. Semin Cell Dev Biol 21: 798-804
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207-221
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373-399
Araújo S, Duque AS, Santos DM, Fevereiro MP (2004) An efficient transformation method to regenerate a high number of transgenic plants using a new embryogenic line of Medicago truncatula cv. Jemalong. Plant Cell Tiss Org 78: 123-131
Arenas-Huertero C, Perez B, Rabanal F, Blanco-Melo D, De la Rosa C, Estrada-Navarrete G, Sanchez F, Covarrubias AA, Reyes JL (2009) Conserved and novel miRNAs in the legume Phaseolus vulgaris in response to stress. Plant Mol Biol 70: 385-401
Asada K (1999) THE WATER-WATER CYCLE IN CHLOROPLASTS: Scavenging of Active Oxygens and Dissipation of Excess Photons. Annu Rev Plant Physiol Plant Mol Biol 50: 601-639
Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 15: 2730-2741
Aung K, Lin SI, Wu CC, Huang YT, Su CL, Chiou TJ (2006) pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol 141: 1000-1011
Barbazuk WB, Fu Y, McGinnis KM (2008) Genome-wide analyses of alternative splicing in plants: opportunities and challenges. Genome Res 18: 1381-1392
Bari R, Datt Pant B, Stitt M, Scheible WR (2006) PHO2, microRNA399, and PHR1 define a phosphate-signaling pathway in plants. Plant Physiol 141: 988-999
33 Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Mol Biol Rep 8: 40-49
Barrera-Figueroa BE, Gao L, Diop NN, Wu Z, Ehlers JD, Roberts PA, Close TJ, Zhu JK, Liu R (2011) Identification and comparative analysis of drought-associated microRNAs in two cowpea genotypes. BMC Plant Biol 11: 127 Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell
116: 281-297
Bartels D, Sunkar R (2005) Drought and Salt Tolerance in Plants. Crit Rev Plant Sci 24: 23–58
Baumberger N, Baulcombe DC (2005) Arabidopsis ARGONAUTE1 is an RNA Slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci U S A 102: 11928-11933
Beauclair L, Yu A, Bouche N (2010) microRNA-directed cleavage and translational repression of the copper chaperone for superoxide dismutase mRNA in Arabidopsis. Plant J 62: 454-462
Benedito VA, Torres-Jerez I, Murray JD, Andriankaja A, Allen S, Kakar K, Wandrey M, Verdier J, Zuber H, Ott T, Moreau S, Niebel A, Frickey T, Weiller G, He J, Dai X, Zhao PX, Tang Y, Udvardi MK (2008) A gene expression atlas of the model legume Medicago truncatula. Plant J 55: 504-513
BIO Intelligence Service (2012) Water saving potential in agriculture in Europe: findings from the existing studies and application to case studies. In Final report prepared for. European Comission DG ENV
Bohmert K, Camus I, Bellini C, Bouchez D, Caboche M, Benning C (1998) AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J 17: 170-180
Bota J, Medrano H, Flexas J (2004) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol 162: 671-681
Boualem A, Laporte P, Jovanovic M, Laffont C, Plet J, Combier JP, Niebel A, Crespi M, Frugier F (2008) MicroRNA166 controls root and nodule development in Medicago truncatula. Plant J 54: 876-887
Bouche N, Lauressergues D, Gasciolli V, Vaucheret H (2006) An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs. EMBO J 25: 3347-3356
Boyko A, Kovalchuk I (2011) Genome instability and epigenetic modification--heritable responses to environmental stress? Curr Opin Plant Biol 14: 260-266
34
Buhtz A, Springer F, Chappell L, Baulcombe DC, Kehr J (2008) Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J 53: 739-749
Capitão C, Paiva JA, Santos DM, Fevereiro P (2011) In Medicago truncatula, water deficit modulates the transcript accumulation of components of small RNA pathways. BMC Plant Biol 11: 79
Carrington JC, Ambros V (2003) Role of microRNAs in plant and animal development. Science 301: 336-338
Catala R, Ouyang J, Abreu IA, Hu Y, Seo H, Zhang X, Chua NH (2007) The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses. Plant Cell 19: 2952-2966
Chabaud M, de Carvalho-Niebel F, Barker DG (2003) Efficient transformation of Medicago truncatula cv. Jemalong using the hypervirulent Agrobacterium tumefaciens strain AGL1. Plant Cell Rep 22: 46-51
Chaves MM (1991) Effects of Water Deficits on Carbon Assimilation. J Exp Bot 42: 1-16
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103: 551-560
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought — from genes to the whole plant. Funct Plant Biol 30: 239–264 Chen HM, Chen LT, Patel K, Li YH, Baulcombe DC, Wu SH (2010) 22-Nucleotide
RNAs trigger secondary siRNA biogenesis in plants. Proc Natl Acad Sci U S A 107: 15269-15274
Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: 2022-2025
Chen X, Liu J, Cheng Y, Jia D (2002) HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development 129: 1085-1094
Chi X, Yang Q, Chen X, Wang J, Pan L, Chen M, Yang Z, He Y, Liang X, Yu S (2011) Identification and characterization of microRNAs from peanut (Arachis hypogaea L.) by high-throughput sequencing. PLoS One 6: e27530
Chinnusamy V, Zhu J, Zhu JK (2007) Cold stress regulation of gene expression in plants. Trends Plant Sci 12: 444-451
Chinnusamy V, Zhu JK (2009) Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol 12: 133-139
Choi HK, Mun JH, Kim DJ, Zhu H, Baek JM, Mudge J, Roe B, Ellis N, Doyle J, Kiss GB, Young ND, Cook DR (2004) Estimating genome conservation between crop and model legume species. Proc Natl Acad Sci U S A 101: 15289-15294