www.jped.com.br
ORIGINAL
ARTICLE
Metabolic
syndrome
in
children
and
adolescents
with
phenylketonuria
夽
,
夽夽
Viviane
C.
Kanufre
a,b,c,∗,
Rosângelis
D.L.
Soares
a,b,c,
Michelle
Rosa
A.
Alves
a,c,d,
Marcos
J.B.
Aguiar
a,b,c,
Ana
Lúcia
P.
Starling
a,b,c,
Rocksane
C.
Norton
a,b,caUniversidadeFederaldeMinasGerais(UFMG),BeloHorizonte,MG,Brazil
bHospitaldasClínicas,UniversidadeFederaldeMinasGerais(UFMG),BeloHorizonte,MG,Brazil
cNúcleodeAc¸õesePesquisaemApoioDiagnóstico(NUPAD),UniversidadeFederaldeMinasGerais(UFMG),BeloHorizonte,
MG,Brazil
dPontifíciaUniversidadeCatólicadeMinasGerais,BeloHorizonte,MG,Brazil
Received13January2014;accepted5June2014 Availableonline25October2014
KEYWORDS Phenylketonuria; Metabolicsyndrome; Childrenand adolescents; Overweight; Diet
Abstract
Objective: Thisstudyaimedtoidentifymarkersofmetabolicsyndrome(MS)inpatientswith phenylketonuria(PKU).
Methods: Thiswasacross-sectionalstudyconsistingof58PKUpatients(agesof4-15years): 29patientswithexcessweight,and29withnormalweight.Thebiochemicalvariablesassessed werephenylalanine(phe),totalcholesterol,HDL-c,triglycerides,glucose,andbasalinsulin. ThepatientshadHomeostasisModelAssessment(HOMA)andwaistcircumferenceassessed.
Results: Nointer-group difference was found for phe. Overweightpatients hadhigher lev-els oftriglycerides,basal insulin, andHOMA, but lowerconcentrations ofHDL-cholesterol, whencomparedtotheeutrophicpatients.Totalcholesterol/HDL-cwassignificantlyhigherin theoverweightgroup.ApositivecorrelationbetweenbasalinsulinlevelandHOMAwithwaist circumferencewasfoundonlyintheoverweightgroup.
Conclusion: Theresults ofthisstudy suggestthatpatients withPKUand excessweightare potentiallyvulnerabletothedevelopmentofmetabolicsyndrome.Therefore,itisnecessary toconductclinicalandlaboratorymonitoring,aimingtopreventmetabolicchanges,aswellas excessiveweightgainanditsconsequences,particularlycardiovascularrisk.
©2014SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
夽 Pleasecitethisarticleas:KanufreVC,SoaresRD,AlvesMR,AguiarMJ,StarlingAL,NortonRC. Metabolicsyndromeinchildrenand
adolescentswithphenylketonuria.JPediatr(RioJ).2015;91:98---103.
夽夽StudyconductedatNúcleodeAc¸õesePesquisaemApoioDiagnóstico(NUPAD),HospitaldasClínicas,UniversidadeFederaldeMinas
Gerais(UFMG),BeloHorizonte,MG,Brazil.
∗Correspondingauthor.
E-mail:vikanufre@gmail.com.br(V.C.Kanufre).
http://dx.doi.org/10.1016/j.jped.2014.06.006
PALAVRAS-CHAVE Fenilcetonúria Síndromemetabólica; Crianc¸ase
adolescentes; Excessodepeso; Dieta
Síndromemetabólicaemcrianc¸aseadolescentescomfenilcetonúria
Resumo
Objetivo: DeterminarmarcadoresbioquímicosdasíndromemetabólicaempacientescomPKU.
Métodos: Foramavaliados dois grupos de pacientescom PKU, 4a 15 anosde idade, com excessode peso(29)e eutróficos(29). Asvariáveis bioquímicas avaliadasforam a fenilala-nina(phe),colesterol total,HDL-c,triglicérides,glicoseeinsulinabasal.Foideterminadoo HOMAemensuradaacircunferênciadacintura.
Resultados: As concentrac¸ões de phe, de colesterol total e de glicose foram equi-valentes entre os grupos. Os pacientes com excesso de peso apresentaram maiores concentrac¸õesdetriglicérides,deinsulinabasal,maioresvaloresdadeterminac¸ãodoHOMA, menores concentrac¸ões deHDLcolesterol evalores mais elevadosdarelac¸ão docolesterol total/HDL-c.Houvecorrelac¸ãopositivaentreadosagemdeinsulinabasaledoHOMAcoma circunferênciadacinturanospacientesdogrupocomexcessodepeso.
Conclusões: Osresultados desteestudo sugeremquepacientescomPKUeexcessodepeso sãopotencialmente vulneráveis ao desenvolvimentodasíndrome metabólica.Há,portanto, necessidadedeacompanhamentoclínico-laboratorialqueprevinaasalterac¸õesmetabólicas,o ganhoexcessivodepesoeassuasconsequências,emespecialoriscocardiovascular.
©2014SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
Phenylketonuria (PKU), an inborn error of amino acid metabolism characterized by the loss or reductionin the activityof hydroxylasephenylalanine (phe)enzyme,leads toelevatedbloodlevelsofpheanditsmetabolites, result-ingin neurologicaldamage thatculminates in irreversible mental retardation.1,2 Disease control is accomplishedby
prescribingadietfreeofanimalproteinandwithrestricted vegetableproteinconsumption.Duetothediet characteris-tics,someresearchershaveassociatedPKUtoatendencyof excessiveweightgainandmetabolicsyndrome(MS).3---6
Con-versely,excessweightandmetabolicalterationsassociated with it have been associated to increased cardiovascular risk,whichhaspromptedresearchersworldwidetoconsider theimportanceofearlyidentificationanddamage preven-tioninat-riskpopulations.7---11
Duetotheparticularitiesoftheirdiet,PKUpatientscan beconsidereda vulnerablegrouptometabolic abnormali-tiesandexcessweight.Proteinrestrictionfavors---andeven stimulates --- the consumption of carbohydrate-rich foods (especially simple carbohydrate) and lipids, in particular, increasingtheriskofweightgain.
MSwouldbepresentinthispopulationduetoboth the diet and the disease itself. The detection of parameters that identify the presence of MS may prevent the emer-gence of other diseases in these patients, for instance, diabetesandcardiovasculardisease.TheInternational Dia-betesFederation(IDF)considersthemeasurementofwaist circumference(WC),associatedwiththemeasurementsof HDLcholesterol(HDL-c),triglycerides(TG),andglucoseto beparametersfortheidentificationofMS.Thelatterwould bedefinedbyWCmeasurements>90thpercentile, associ-atedwithatleasttwoofthefollowingfindings:highlevels ofTG,reducedHDL-c, andincreaseinblood pressureand fastingglucose.11
This study sought todetermine some markers ofMS in patientswithPKUtreated attheSpecialGenetics Depart-mentofHospitaldasClinícas,UniversidadeFederaldeMinas Gerais(SEG-HC-UFMG),toidentifyrisksandtopromote bet-terclinical and laboratory control of the diseaseand the adoptionofspecialprotocolsforpreventingcardiovascular damage.
Methods
Astudyofcaseseriesinvolving58childrenandadolescents withPKU,aged4to15yearswasconducted.Datacollection wasperformedbetweenOctoberof2008andNovemberof 2009.Patientswereselected,scheduled,andsubmittedto clinicalandlaboratoryassessment.
The study was approved by the Ethics Committee of theUniversidadeFederaldeMinasGerais(COEP-UFMG).An informedconsentwassigned by aparent,legal guardian, and/orPKUpatientolderthan6yearsold,afterdue expla-nations.
The groups were defined according to the body mass index (BMI) calculated according to the formula: BMI=weight (kg)/height2 (m). The value obtained was assessed using the growth curves of the World Health Organization (WHO) for children aged 0-5 years (2006) and 5-19 years (2007), considering as cutoffs for over-weight and obesity BMI > 85th percentile and > 97th percentile,respectively.Thegroupswereconstitutedas fol-lows:29 patients with normalweight and 29 withexcess weight.
Measuresofwaistcircumferencewereanalyzedin accor-dancewiththepercentilesuggestedbyMacCarthyetal.7
triglycerides,glucose,andbasal insulinweredetermined. Lipid and glucose levels were analyzed enzymatically, using the dry chemical technique. Insulin resistance was calculated using the mathematical model of Matthews etal.12Lipidmeasurementswereconductedinaccordance
with the I Guideline of Atherosclerosis in Childhood and Adolescence.13Themeanphe(controlphe)wasdetermined
bythearithmeticmeanofthelast12measurementsandwas consideredinadequatewhenhigherthanthemaximum ref-erencevaluefortheagerange.14Phelevelswereobtained
byultramicrofluorometry,usingtheultramicro-fluorometric test(UMTESTPKU).15
Theresultsobtainedwerestored,tabulatedinan elec-tronicspreadsheet,andanalyzedusingSPSS,release15.0. SampledistributionwasverifiedbytheShapiro-Wilk’stest. Student’st-testwasusedfor variableswithnormal distri-bution,whereasthenonparametricMannWhitneytestwas usedforthosewithnon-normaldistribution.Theassociation analysiswasperformedusingPearson’schi-squaredtestand correlationanalysisusingSpearman’stest.
Results
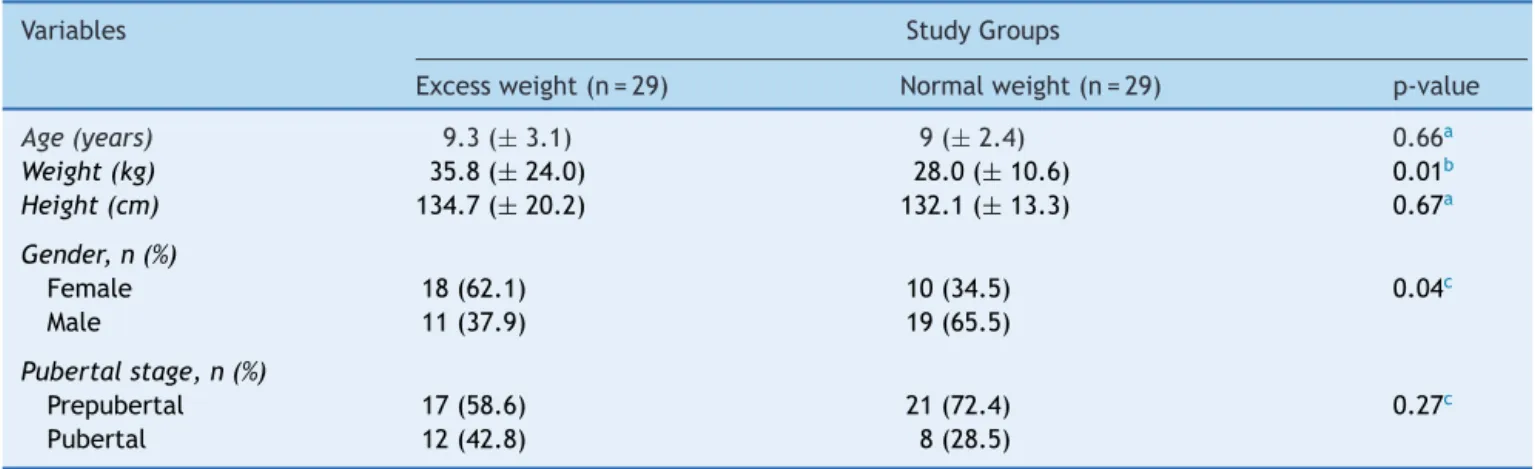
Table1describestheanthropometriccharacteristics, gen-derdistribution,andpubertalstageofeachgroup.
The groups showed similar results in relation to blood phecontrol. There wasnosignificant difference(p=1) in meanphe levelsof the individual: 50% of thetests were adequateinbothgroups.Regardingthephelevelscollected after fasting (p=0.14), 58.6% of tests were adequate in the excess weight group and 41.4% in the normal weight group.
Patients from the excess weight group had higher basal triglyceride and insulin levels, but lower HDL-cholesterol, whencompared to normalweight individuals (Table2).
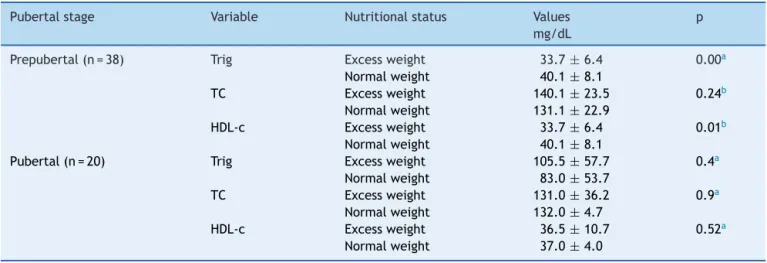
The patients’ lipid profile, when analyzed according to pubertal stage, showed higher triglyceride and lower HDL-c levels in the group of prepubertal patients with excessweight.Nostatisticallysignificant differenceswere
observed between thetwo groups of patients at pubertal stage(Table3).
Thegroupwithexcessweighthadsignificantlyhigher val-ues oftotal cholesterol/HDL-cratio,blood levelsof basal insulin,andHOMAdetermination(Table4).
Therewasa positive correlation between basal insulin level(p=0.00)andHOMA(p=0.08)withWC,butonlyinthe groupwithexcessweight.
Discussion
Excess weightis acurrent publichealth problem in many partsofthe world.InBrazil,despite preventionprograms undertaken by health managers and professionals, preva-lencerateshaveincreasedinallagegroups.16
The concern for obesity in PKU patients resulted from theroutineobservationofclinicalcaretopatientsin SEG-HC/UFMG,confirmedbyassessmentsperformedin2007and 2009.
Cross-sectional investigations identified increasing prevalence rates of excess weight and obesity, going from 16.8% and 8.8% in 2007 to 19.8% and 9.6% in 2009, respectively.
The occurrence of excess weight in a population with PKU, whose diet is periodically monitored by nutrition-ists,hasoneof itspossible justificationsin therestrictive andmonotonouscharacterofthediet,especiallyforolder children and adolescents, who have autonomy in dietary choices.
Dietary transgressions cause variations in the labora-tory control of the diseasestarting at thisage group and metabolic abnormalities that, in the medium term, can translate into excessive weight gain and increased car-diovascular risk. Several studies confirmed these control difficultiesinpatientsthatacquireddietaryautonomy.17---20
Inthisstudy,thegroupofoverweightpatientswasexpected tohavehigherconcentrationsofbloodphe---duetodietary transgressions---asreportedbyMcBurnieetal.,3whichcould
explaintheexcessweightinthesepatients.However,itwas verifiedthatmost meanblood phetests wereelevated in
Table1 Characterizationoftwogroupsofphenylketonuriapatientsaged4to15yearsthatparticipatedinthestudy.
Variables StudyGroups
Excessweight(n=29) Normalweight(n=29) p-value
Age(years) 9.3(±3.1) 9(±2.4) 0.66a
Weight(kg) 35.8(±24.0) 28.0(±10.6) 0.01b
Height(cm) 134.7(±20.2) 132.1(±13.3) 0.67a
Gender,n(%)
Female 18(62.1) 10(34.5) 0.04c
Male 11(37.9) 19(65.5)
Pubertalstage,n(%)
Prepubertal 17(58.6) 21(72.4) 0.27c
Pubertal 12(42.8) 8(28.5)
aStudent’st-test(mean
±SD).
b Mann-Whitney’stest[median(
±Q3-Q1)].
Table2 BloodlevelsoftotalcholesterolandHDL-c,triglycerides,bloodglucose,andbasalinsulininphenylketonuriapatients withexcessweightandnormalweight.
Variables Studygroups
Excessweight(n=29) Normalweight(n=29) p-value
Cholesterol(mg/dL)
Total 136.2(±22.2) 130.7(±20.2) 0.32a
HDL-c 34.0(±7.5) 37.0(±8.0) 0.00b
Triglycerides(mg/dL) 109.0(±43.0) 74.0(±40.5) 0.00b
Glycemia(mg/dL) 73.8(±6.4) 76.3(±6.3) 0.13a
Basalinsulin(uUI/mL) 8.4(±8.95) 3.8(±5.15) 0.02b
a Student’st-test(means
±SD).
b Mann-Whitney’stest[median(
±Q3-Q1)].
Table3 Comparisonbetweenthemeanormedianofserumlevelsoftriglycerides,totalcholesterol,andHDL-cin phenylke-tonuriapatientswithnormalweightandexcessweight,accordingtopubertalstage.
Pubertalstage Variable Nutritionalstatus Values
mg/dL
p
Prepubertal(n=38) Trig Excessweight 33.7±6.4 0.00a
Normalweight 40.1±8.1
TC Excessweight 140.1±23.5 0.24b
Normalweight 131.1±22.9
HDL-c Excessweight 33.7±6.4 0.01b
Normalweight 40.1±8.1
Pubertal(n=20) Trig Excessweight 105.5±57.7 0.4a
Normalweight 83.0±53.7
TC Excessweight 131.0±36.2 0.9a
Normalweight 132.0±4.7
HDL-c Excessweight 36.5±10.7 0.52a
Normalweight 37.0±4.0
Trig,triglycerides;TC,totalcholesterol.
a MannWhitneyTest[median(±Q3-Q1)].
b Student’st-test(means±SD).
both groups and that the excess weight group showed a highernumberofadequatetestsforthevariablefastingphe. Belanger-Quintanetal.5andRochaetal.20suggestedthat
the excessweight ofPKU patients wasrelatedto disease severity. As in the present study most of the participants inboth groups hadthesevere formofthedisease, itwas notpossibletocorrelateexcessweightwithdisease sever-ity.Therefore,itappearsthattheexcessweightdetectedin thesepatientsisnotnecessarilyrelatedtofood transgres-sionordiseaseseverity.
Non-adherence to or inadequacy of nutritional guide-lines for the treatment of PKU can cause abnormalities
resultingfromlack or excessof nutrients,21---24 andexcess
weight(anditsconsequences)is themostfrequent.Thus, asdemonstratedbyFreedmanetal.,25patientswithexcess
weightevaluatedin this study showed a positive correla-tionbetweenmeasuresofWCandlevelsofbasalinsulinand HOMAdeterminations.
The low concentrations of cholesterol observed in this studymaybeexplainedbygeneticfactors,bycholesterol biosynthesisinhibition,andalsobyanexclusivevegetarian diet.10,26---29PKUpatientswithexcessweight,inadditionto
centralobesityexpressedbytheWC,hadhighblood concen-trationsoftriglyceridesandreducedHDL-c,suggestingthe
Table4 Comparisonbetweentotalcholesterol/HDL-c,bloodlevelsofbasalinsulin,andHOMAinphenylketonuriapatients withexcessweightandnormalweight.
Studygroups
Excessweight Normalweight pa
Totalcholesterol/HDL-c 4.00(±0.84) 3.33(±0.81) 0.00
Insulin(uUI/mL) 8.40(±8.95) 3.8(±5.15) 0.01
HOMA 1.64(±1.59) 0.70(±1.10) 0.03
presenceofMS. LowconcentrationsofHDL-candhigh con-centrationsofLDL-c, evenisolated,havebeen considered asgoodpredictorsofcardiovascularrisk.29,30
According toBurrows etal.,8 children and adolescents
haveathree-fold higherriskofdeveloping MSwhenbasal insulinandHOMAare>75thpercentile.Inthisstudy,ahigher percentageofpatientswithhighlevelsofbasalinsulinand HOMAwasidentifiedamongthosewithexcessweightand atpubertal stage(31% vs.14%).Dietarycharacteristics of PKUpatients associatedwiththe physiological changesof pubertymayexplainthesefindings,whichshouldconstitute warningfactors for the diagnosis of MS. The WHO (World HealthOrganization)31 and EGIR (European Group for the
StudyofInsulinResistance)32 considerinsulinresistanceto
beoneofthemarkersofMS.InpatientswithPKUandexcess weight,elevatedHOMAvalueswerealsofound.
As in the study by Rocha et al.,20 the present results
demonstrate that patients with PKU and excess weight behavesimilarlytoobeseindividualswithoutagenetic dis-ease. However, PKU patients with excess weight may be moresusceptibletoMSduetofactorsinherenttothe dis-ease. Thus, a review of the treatment protocol used for thesepatientsisadvisable,characterizedbythe introduc-tion of simple procedures that can be conducted in any outpatientcare facility.Generalguidelinesshould empha-size the need to maintain the food free of phe, control theover-consumption ofsugar, aswell asthe importance of incorporating physical activity into everyday life, as appropriatetoeachindividual.Clinicalcontrolmustinclude regular measurement of WC and, when necessary, moni-toringofbloodconcentrationsofcholesterol,triglycerides, glucose,andbasalinsulin.
Patients withexcess weightshowed higher blood con-centrationsoftriglyceridesandbasalinsulin,highervalues oftotalcholesterol/HDL-CratioandHOMAandlower HDL-c levels. The results of this study suggest that patients withPKUand excess weightarepotentially vulnerable to thedevelopmentofMS.Itis,therefore,necessary to con-ductclinicalandlaboratorymonitoringtopreventmetabolic changes, as well as excessive weight gain and its con-sequences, particularly cardiovascular risk. Additionally, specificdietaryguidelinesshouldbeemphasizedinclinical practice,especiallyforpatientswithexcessweight,inorder topreventmetabolicinadequacies.
Funding
FAPEMIG(Fundac¸ãodeAmparoàPesquisadoestadodeMinas Gerais).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Acosta PB,Yaniccelli S.The Rossmetabolic formulasystem, nutritionsupportprotocols.4thed.Columbus:Ross
Laborato-ries;2001.Libraryofcongress.
2.ScriverCR,KaufmanS.Hyperphenylalaninemia:phenylalanine hydroxylasedeficiency.In:ScriverCR,SlyWS,ChildsB,Beaudet
AL,ValleD,KinzlerKW,editors.Themetabolicandmolecular basisofinheriteddisease.8thed.NewYork:McGraw-Hill;2001.
p.1667---724.
3.McBurnieMA,KronmalRA,SchuettVE,KochR,AzengCG. Phys-icalgrowthofchildrentreatedforphenylketonuria.AnnHum Biol.1991;18:357---68.
4.ScaglioniS,VerduciE,FioriL,LammardoAM,RossiS,Radaelli G, et al. Body mass index rebound and overweight at 8 yearsofageinhyperphenylalaninaemicchildren.ActaPaediatr. 2004;93:1596---600.
5.Belanger-QuintanaA,Martínez-PardoM.Physicaldevelopment inpatientswithphenylketonuriaondietarytreatment:a retro-spectivestudy.MolGenetMetab.2011;104:480---4.
6.Burrage LC, McConnell J, Haesler R, O’Riordan MA, Sut-ton VR, Kerr DS, et al. High prevalence of overweight and obesity in females with phenylketonuria. Mol Genet Metab. 2012;107:43---8.
7.McCarthyHD,JarrettKU,CrawleyHF.Thedevelopmentofwaist circumferencepercentilesinBritishchildrenaged5.0-16.9y. EurJClinNutr.2001;55:902---7.
8.BurrowsAR,LeivaBL,WeistaubG,CeballosSX,GattasZV,Lera ML,etal.Síndromemetabólicoenni˜nosyadolescentes: aso-ciaciónconsensibilidadinsulínicayconmagnitudydistribución delaobesidad.RevMedChil.2007;135:174---81.
9.Daniels SR, Greer FR. Committee on nutrition Lipid screeningand cardiovascularhealth inchildhood. Pediatrics. 2008;122:198---208.
10.Williams RA, Mamotte CD, Burnett JR. Phenylketonuria: an inbornerrorofphenylalaninemetabolism.ClinBiochemRev. 2008;29:31---40.
11.ZimmetP,AlbertiKG,KaufmanF,TajimaN,SilinkM,Arslanian S, et al. The metabolic syndrome in children and adoles-cents --- an IDF consensus report. Pediatr Diabetes. 2007;8: 299---306.
12.MatthewsDR,HoskerJP,RudenskiAS,NaylorBA,TreacherDF, TurnerRC. Homeostasis modelassessment:insulin resistance andB-cellfunctionfromfastingplasmaglucoseandinsulin con-centrationsinman.Diabetologia.1985;28:412---91.
13.SociedadeBrasileiradeCardiologia.Diretrizdeprevenc¸ãoda aterosclerosenainfânciaenaadolescência.ArqBras,Cardiol. 2005;85:3---36.
14.WappnerR,ChoS,KronmalRA,SchuettV,SeashoreMR. Man-agement of phenylketonuriafor optimal outcome: a review ofguidelinesfor phenylketonuriamanagementand reportof surveysofparents,patients, andclinicdirectors. Pediatrics. 1999;104:e68.
15.Januario JN, Mourão OG. Manual de organizac¸ão e normas técnicasparatriagemneonatal.BeloHorizonte:Coopmed; Edi-toraMédica;1998.
16.Ministério da Saúde. Instituto Brasileiro de Geografia e
Estatística(IBGE). Pesquisa de Orc¸amentos Familiares
2008-2009. Antropometria e estado nutricional de crianc¸as,
adolescentes e adultos no Brasil. Rio de Janeiro: Instituto
BrasileirodeGeografiaeEstatística;2010.[citedOct102010].
Available from: http://www.ibge.gov.br/home/estatistica/
populacao/condicaodevida/pof/20082009/POFpublicacao.pdf 17.Blau N, Van Sponsen F, Levy HL. Phenylketonuria. Lancet.
2010;3:1417---27.
18.MacDonaldA,Gokmen-OzelH,vanRijnM,BurgardP.Thereality ofdietarycomplianceinthemanagementofphenylketonuria. JInheritMetabDis.2010;33:665---70.
19.WalterJH,WhiteFJ,HallSK,MacDonaldA,RylanceG,Boneh A,etal.Howpracticalarerecommendationsfordietarycontrol inphenylketonuria?Lancet.2002;360:55---7.
21.Demirkol M, Gi˙zewska M, Giovannini M, Walter J. Follow-upofphenylketonuriapatients. Mol Genet Metab.2011;104: S31---9.
22.MacDonald A, Rocha JC, van Rijn M, Feillet F. Nutrition in phenylketonuria.MolGenetMetab.2011;104:S10---8.
23.Trefz F, Maillot F, Motzfeldt K, Schwarz M. Adult phenylke-tonuriaoutcomeandmanagement.MolGenetMetab.2011;104: S26---30.
24.AlvesMR,StarlingAL,KanufreVC,SoaresRD,NortonRdeC, AguiarMJ,etal.Seleniumintakeandnutritionalstatusof chil-drenwithphenylketonuriainMinasGerais,Brazil.JPediatr(Rio J).2012;88:396---400.
25.FreedmanDS,SerdulaMK,SrinivasanSR,BerensonGS.Relation ofcircumferenceandskinfoldthicknessestolipidandinsulin concentrationinchildrenandadolescentstheBogalusaHeart Study.AmJClinNutr.1999;69:308---17.
26.Artuch R, Colomé C, Vilaseca MA, Sierra C, Cambra FJ, Lambruschini N, et al. Plasma phenylalanine is associated withdecreasedserumubiquine-10concentrationsin phenylke-tonuria.JInheritMetabDis.2001;24:359---66.
27.ColoméC,ArtuchR,LambruschiniN,CambraFJ,CampistolJ, VilasecaM.Istherearelationshipbetweenplasma phenylala-nineandcholesterolinphenilketonuricpatientsunderdietary treatment?ClinBiochem.2001;34:373---6.
28.Schulpis KH, Scarpalezou A. Triglicerides, cholesterol, HDL, LDLandVLDLcholesterolinserumofphenylketonuricchildren underdietarycontrol.ClinPediatr(Phila).1989;28:466---9. 29.BarterP,GottoAM,LaRosaJC,MaroniJ,SzarekMG,Kastelein
JP,etal.HDLcholesterol,verylowlevelsofLDLcholesterol, andcardiovascularevents.NEnglJMed.2007;357:1301---10. 30.DenHollanderNC,MulderDJ,GraaffR,ThorpeSR,BaynesJW,
SmitGP,etal.Advancedglycationendproductsandtheabsence ofprematureatherosclerosisinglycogenstoragediseaseIa.J InheritMetabDis.2007;30:916---23.
31.WorldHealthOrganization.Definition,diagnosisand classifica-tionofdiabetesmellitusanditscomplications.ReportofaWHO consultation.Geneve:WHO;1999.