DOI:
10.2298/SOS0701009Z
UDK 662.785:661.183.8:549.521.4
Effect of Polymorphism of Al
2O
3on Sintering and Grain Growth
of Magnesia Aluminate Spinel
Z. Zhihui
1,2*
), L. Nan
1, R. Guozhi
1,21
Hubei Province Key Lab of Refractories and Ceramics,Wuhan University of Sci. &
Tech., Wuhan, Hubei, P.R.China, 430081.
2
College of Materials Science and Engineering, Shandong University of Science and
Technology, 579 Qianwangang Road, Huangdao District, Qingdao, Shandong
Province, China, 266510.
Abstract:
The effect of polymorphism of Al2O3 on sintering and grain growth of magnesia
aluminate spinel was studied. γ- Al2O3 and α- Al2O3 were mixed with MgO according to the
stoichiometric MA ratio, respectively, and pressed into billets with a 20mm diameter and
15mm height, and then were sintered at temperature from 1250oC to 1400oC in air
atmosphere. Bulk density, apparent porosity and grain size were measured. The results
indicated that the grain size of MA with γ-Al2O3 is larger than the grain size of MA with α-
Al2O3. This is because the activation energy of grain growth of magnesia aluminate spinel
prepared by γ-Al2O3 is lower than that by α-Al2O3, the former is 159KJ/mol and the latter is
217KJ/mol.
Keywords:
Alumina polymorphism; Magnesium aluminate spinel; Grain growth.Introduction
Magnesium aluminate spinel (MA) is widely used in refractories because of its high melting point (2135оC), good mechanical strength and excellent chemical resistance et al [1-3]. Especially in recent years, as the hazardous character of chrome bearing materials is exposed, MA becomes more important [4]. At present, people usually use Alpha aluminum (α-Al2O3) as a raw material in the formation of MA because it is a readily available industrial
material. However the production temperature of α- Al2O3 is high, usually up to 1400oC in
industry [5], which results in a decrease of its activity and an increase in energy consumption. At the same time, the process of α- Al2O3 transformation to MA is accompanied by a large
volume expansion, because of its higher density, making it difficult to obtain a dense sintered body [6].
In order to avoid these disadvantages, some researches used various additives to enhance the MA formation and to densify the material at lower temperatures. Ritwik Sarkar et al [7-8] studied the effect of various oxides additives on the densification of reaction sintered
_____________________________
*)
and presynthesised stoichiometric MA. They found TiO2 showed the greatest beneficial effect
and that Cr2O3 showed some benefit on densification of all the presynthesised and reaction
sintered MA. However, V2O5 and B2O3 showed detrimental effects on the densification
behavior of presynthesised and reaction sintered MA. Kostic et al reported [9] that the fluorine ion from AlF3 or CaF2 increased the solid-state reaction synthesis of magnesium
aluminate spinel by increasing the cation vacancy. Although these additives can improve the densification of MA to a certain extent, they introduce impurities in the MA. Other researches improved the densification of MA using high-energy ball milling of MgO, Al2O3, or their
mixture [10-11]. However, one of the drawbacks attributed to this technique is the lack of reproducibility and the difficulty in comparing results obtained by different authors using different mills [12]. On the other hand, high energy ball mills create a high energy consumption.
Gamma aluminum (γ- Al2O3) has a lower density than α- Al2O3, and when γ- Al2O3 is
used as a raw material to synthesize MA, the volume expansion is lower in the formation of MA. Also, the temperature at which boehmite transfers to γ- Al2O3 is rather low, resulting in
γ- Al2O3 powder with higher activity and a low energy consumption [5]. Furthermore, γ-
Al2O3 has the same crystal structure as MA. They are a cubic system. But α- Al2O3 is a
rhombohedral system. In our former research, we found that γ- Al2O3 is beneficial for
synthesizing MA [13]. In this paper, the effect of polymorphism of Al2O3 on the grain growth
of MA was studied.
2. Experimental procedures
The starting materials used in this study are γ- Al2O3, α- Al2O3 and an analytical
reagent grade of MgO. γ- Al2O3 was produced from boehmite heated at 700oC for 4h, and α-
Al2O3 was produced from gibbsite heated at 1400 oC for 4h. Composition, particle size and
surface area of the two kinds of Al2O3 and MgO used in this study are given in tab. I and their
X-ray patterns shown in Fig.1.
Tab. I Compositions (mass %), particle size (μm) and surface area (m2/g) of raw materials
used to make MA
Type Al2O3 SiO2 Fe2O3 TiO2 K2O Na2O IL MgO
Particle size
Surface area
γ- Al2O3 98.00 0.35 0.09 0.02 0.04 0.21 - - 4.61 55.98
a
-Al2O3
97.86 0.88 0.07 0.02 0.08 0.45 - - 5.06 43.36
MgO 0.01 0.14 0.08 0.07 0.09 0.08 - 99.30 5.00 46.03
Note: IL is ignition loss. The operation temperature is 1000-10500C for 1h.
From Fig.1(A) and Fig.1(B), we find that γ- Al2O3 and α- Al2O3 are the dominate
crystal phases, which means at 700oC and 1400 oC , boehmite and gibbsite have already converted to γ- Al2O3 and α- Al2O3, respectively.
γ- Al2O3, α- Al2O3 and MgO were mixed in a stoichiometric ratio of MA by
ball-milling for 5h, using highly pure Al2O3 balls as a milling medium. The mixtures were pressed
apparent porosity of the sintered specimens was measured by the Archimedes method using water as the liquid media. XRD crystalline phase analysis was conducted using Ni filtered Cu Kαat a scanning speed of 2°min-1 and a temperature of 16 oC.
Fig. 1 X-ray patterns of (A) boehmite sintered at 700 oC for 4 h, and (B) Al(OH)3 sintered at
1400 oC for 4h
The MA grain size was measured by the half-peak breadth of the MA {3 1 1} peak using the Warren-Scherrer foumula [14]:
D=
κλ
/(
β
⋅
cos
θ
)
(1) In this formula: D is the grain size (nm);β
is the half-peak breadth (rad);θ
is the diffraction angle(°);λ
is the X-ray wave length, 0.154178nm; andκ
=
1
.3. Results and discussion
3.1 Effect of the sintering temperature and soaking time on sample densification
The bulk density and apparent porosity as a function of sintering temperature and soaking time are shown in Fig.2 and Fig.3 respectively. It is noted that the effect of temperature and soaking time on sample densification is related to the kind of Al2O3 used in
making a sample.
30 32 34 36 38 40 42 44 46 48
0 1 2 3 4
A Soaki ng Ti me ( h)
Ap
pa
re
nt
P
or
os
it
y
(%
)
1250℃ 1300℃ 1350℃ 1400℃
45 47 49 51 53 55 57
0 1 2 3 4
B Soaki ng Ti me ( h)
App
ar
ent
P
or
os
it
y
(%
)
1250℃ 1300℃ 1350℃ 1400℃
Fig. 2 Apparent porosity of samples as a function of sintering and soaking time: (A) γ- Al2O3,
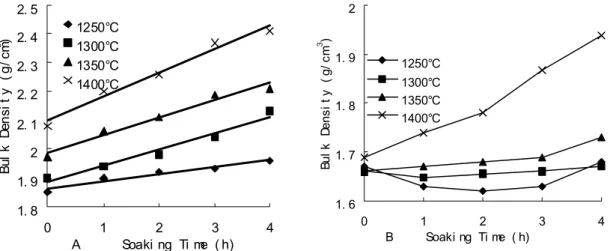
The apparent porosity of samples with γ- Al2O3 decreased and bulk density increased
with increasing sintering temperature and soaking time. And at every sintering temperature, the relationships between apparent porosity, bulk density and soaking time were linear. At same time, the slope of these straight lines increased with increasing sintering temperature.
1. 8 1. 9 2 2. 1 2. 2 2. 3 2. 4 2. 5
0 1 2 3 4
A Soaki ng Ti me ( h)
Bu
lk
D
en
si
ty
(
g/
cm
3 )
1250℃ 1300℃ 1350℃ 1400℃
1. 6 1. 7 1. 8 1. 9 2
0 1 2 3 4
B Soaki ng Ti me ( h)
Bu
lk
D
en
si
ty
(
g/
cm
3 )
1250℃ 1300℃ 1350℃ 1400℃
Fig. 3 Bulk density of samples as a function of sintering and soaking time: (A) γ- Al2O3,
(B) α- Al2O3
As shown in Fig.4 (A), when γ- Al2O3 was used, residual Al2O3 and MgO is less and
the MA formation reaction is close to completion by 1250 oC. In the case of γ- Al2O3, the
effect of the expansion caused by MA formation is small. When α- Al2O3 was used as raw
material, the bulk density and apparent porosity of the samples heated at 1250, 1300 and 1350
oC changes very little with soaking time prolonging. In Fig.2 and Fig.3, however for α- Al 2O3
samples heated at 1400 oC, with increased soaking time, the bulk density increases and the apparent porosity decreases rapidly. As shown in Fig.4 (B), samples heated at 1250, 1300 and 1350 oC for 0 h, contained unreacted α- Al2O3 and MgO.
●-MgAl2O3, -MgO, ■-α- Al2O3, ▲-γ- Al2O3
Fig. 4 XRD patterns for specimens sintered at different temperatures for 0h: (A) γ-Al2O3
(B) α- Al2O3
soaking time causing the porosity to decrease and bulk density to increase rapidly. From Fig. 2 and Fig. 3, we also found that samples with γ- Al2O3 had higher bulk density and lower
apparent porosity than samples made with α- Al2O3. This result may originate from two
causes; one was the volume change from the formation of MA using different Al2O3 sources.
The other may be the starting crystal structure of γ- Al2O3, which is similar to MA.
3.2 Effect of the sintering temperature and soaking time on the grain growth of
MA
Fig. 5 gives the relationship between the grain size of MA and the soaking time for the different types of Al2O3 when heated at different temperature. It is found that no matter
what kind of Al2O3 raw material was used, the grain size of MA increased with increasing
sintering temperature and soaking time. When the soaking time was less than 1h, the grain size rapidly increased with increasing soaking time. When the soaking time was greater than 1h, the grain size increased slowly with increasing soaking time. This maybe because when the sintering temperature just arrived at the set temperature, the grain size of the new product was very small, specific surface area was large, and the crystal lattice had many deficiencies [15], causing the grain size of MA to rapidly increase with increasing soaking time for the first hour. With the soaking time increasing, the grain size of the MA became large, the specific surface area became smaller, and the crystal lattice was prone to integrity, so the grain growth of the MA decreased. From fig. 5 we also found that the grain size of MA in the samples withγ- Al2O3 was larger than that of MA from α- Al2O3. This can be explained by the
following analysis of activation energy of grain growth for MA.
50 70 90 110 130 150
0 1 2 3 4
A Soaki ng Ti me( h)
Gr
ai
n
Si
ze
of
S
pi
ne
l(
nm
)
1250℃ 1300℃ 1350℃ 1400℃
50 60 70 80 90 100 110 120
0 1 2 3 4
B Soaki ng Ti me( h)
Gr
ai
n
Si
ze
o
f
Sp
in
el
(n
m)
1250℃ 1300℃ 1350℃ 1400℃
Fig. 5 The effect of sintering temperature and time on the grain size of spinel: (A) γ- Al2O3, (B)
α- Al2O3
According to Brook's kinetic model of grain growth [16], grain growth can be showed as follows:
(2)
Kt
G
G
tn−
0n=
Here G and G0 are the grain size of hour t = t and t = 0 of time t respectively, and n and k are constants. The value of n is between 1 and 3 [17]. When grain growth is non-normal, n = 1; when grain growth is normal, n = 2; when grain growth is limited by the impurity or porosity around the grain boundary, n = 3.
In the formula (3):
A is a proportional constant that is related to the transition of ions. And according to Wagner's mechanism [18], in this experiment, the transition ions are mainly Al3+ and Mg2+ ions. Q is the apparent activation energy of grain growth, R is the gas constant, and T is the Kelvin temperature.
When we analyze the grain growth of a certain material by formula (2), we must define the value of n according to experimental conditions. In our experiment, we take n=3 because of impurities and high porosity of the samples. Thus the formula (2) can be expressed as follow:
(4)
Kt
G
G
t3−
03=
And, taking formula (3) into formula (4), can get the following formula (5):
(5)
t
RT
Q
A
G
G
t3−
03=
exp(
−
/
)
After both sides of formula (5) were divided by t and then logarithms were taken, we obtained formula (6):
A
RT
Q
t
G
G
tln
ln
3 0 3+
−
=
−
(6)This means
t
G
G
t3−
03ln
vs. 103/T is linear, as shown in Fig.6, which shows a plot ofthese two parameters.
12 12. 2 12. 4 12. 6 12. 8 13 13. 2 13. 4 13. 6 13. 8
0. 6 0. 62 0. 64 0. 66 A T- 1
/ 10- 3
K- 1
ln (G 3 -G 0 3 )/ t 1h 2h 3h 4h 10. 8 11. 3 11. 8 12. 3 12. 8 13. 3
0. 6 0. 62 0. 64 0. 66 B T- 1
/ 10- 3
K- 1
ln (G 3 -G 0 3 )/ t 1h 2h 3h 4h
Fig. 6 Relation between grain size and sintering temperature: (A) γ- Al2O3, (B) α- Al2O3
The average rate of the slope of these straight lines in fig.6 (A) and fig.6 (B) is 19.11 and 26.13 respectively. According to the result of linear regression analysis, the activation energy of grain growth of MA made using γ- Al2O3 is 159KJ/mol, and the activation energy
of grain growth of MA made using α- Al2O3 is 217KJ/mol. From these results, it is obvious
that the activation energy of grain growth for MA with γ- Al2O3 is lower than that of MA with
α- Al2O3. This lower activation maybe due to the similar crystal structure of γ- Al2O3 to MA
structure.
4. Conclusions
1. For the same sintering temperature and soaking time, MA samples made using γ- Al2O3 had
higher bulk density and lower apparent porosity than samples made using α- Al2O3.
2. The grain size of MA in the samples made using γ- Al2O3 is larger than the grain size of
3. At the same time, the activation energy grain growth for MA prepared using γ-Al2O3 is
lower than that of MA prepared using α-Al2O3, the former is 159KJ/mol and the latter is
217KJ/mol. The reason for this difference maybe is that the crystal structure of γ-Al2O3 is
similar to MA.
References
1. J.E. Sheehan, J. Sigalovsty, J.S. Haggerty, J.R. Porter, Ceramica Engineering and Science Proceedings 14 (1993) 660.
2. H.S. Tripathi, B. Mukherjee, S. Das, M.K. Haldar, S.K. Das, A. Ghosh, Ceramics International 29 (2003) 915.
3. G. Baudin, R. Martinez, P. Pena, Journal of the American Ceramic Society 78 (1995) 1857.
4. D.J. Bary, American Ceramic Society Bulletin 64 (1985) 1012.
5. K. Wefers, C. Misra, Oxides and hydroxides of Aluminum, Alcoa Technical Paper No.19, Revised, Aleo Laboratories, 1987.
6. E. Ryshkewitch, Oxide Ceramics, Academic Press, New York, 1960, pp.257-274 7. R. Sarkar, G. Bannerjee, Journal of the European Ceramic Society 20 (2000)
2133.
8. R. Sarkar, S.K. Das, G. Banerjee, Ceramics International 29 (2003) 55.
9. E. Kostic, S. Boskovic, S. Kis, Journal of Materials Science Letter 1 (12) (1982) 507-510
10. L.B. Kong, J. Ma, H. Huang, Materials Letters 56 (2002) 238. 11. Z.H. Zhang, N. Li, Science of Sintering 36 (2004) 73.
12. H. Arik, Materials and Design 25 (2004) 31.
13. Z.H. Zhang, N. Li, Ceramics International 31 (2005) 583.
14. S.Z. Yang, H.P. Zhou, The measurement and experiment for ceramic, Wuhan: Wuhan University of Technology Press, 1990, pp.75-82
15. H.Z. Liu, W.B. H, M.Y. G, R.J. Wu, Journal of Inorganic Materials 17 (2002) 429.
16. L. Wei, G. Lian, L.H. Gui, J.K. A.Guo, Journal of Inorganic Materials 15 (2000) 536.
17. P.W. Lu, Silicate physical chemistry, Nanjing: Southeast University Press, 1991, pp.299
18. C. Wagner, E. Koch, Ibid, B34, (1936) 317.
Са р а: а а Al2O3 а а а а а
а а а а. γ- Al2O3 α- Al2O3 а а MgO а а
МА а а 20
15 а а а а 1250 1400 а
а а. И а а, а а. а а а
а аМА аγ-Al2O3 а аМА аα- Al2O3. а
а а а а а а а а а а а а а γ
-Al2O3 а аα-Al2O3, а 159 КЈ7 а а 217 КЈ/ .