Ciências Veterinárias
Assessment of the Biodegradable Membrane Potential on
Bone Regeneration in a Rat Critical Mandibular Defect
(D issert ação Pro visó r ia)
Tiago José Silva M oura
Orientador: Carlos Alberto Antunes Viegas
Co-orientador: João Filipe Martins Freire Requicha
UNIVERSIDADE DE TRÁS-OS-MONTE S E ALTO DOURO
Ciências Veterinárias
Assessment of the Biodegradable Membrane Potential on
Bone Regeneration in a Rat Critical Mandibular Defect
(D issert ação Pro visó r ia)
Tiago José Silva M oura
Orientador: Carlos Alberto Antunes Viegas
Co-orientador: João Filipe Martins Freire Requicha
UNIVERSIDADE DE TRÁS-OS-MONTE S E ALTO DOURO
This project was developed as an original scientific dissertation and presented at School of Agricultural and Veterinary Sciences – Veterinary Sciences Department of University of Trás-os-Montes e Alto Douro, so that its author can obtain the Master degree in Veterinary Sciences, in accordance with the chapter III of the Decreto-Lei n. º 74/2006 from March, 24.
Abstract
Periodontal disease is an inflammatory pathology prevalent in dogs and humans that can result in tooth loss as well in systemic health implications. As the current treatments are sometimes ineffective, Tissue Engineering strategies have paved for new therapies.
The purpose of this project was to develop a new scaffold with osteoconductive properties for use in bone and periodontal regeneration. We proposed a double layer scaffold comprising a starch poly-ɛ-caprolactone (SPCL) membrane obtained by solvent casting, which sustains the guided tissue regeneration principle, and a SPCL wet-spun fiber mesh functionalized with osteoconductive silanol groups. The potential of this biomaterial as vehicle of stem cells and as an osteoconductive matrix was previously assessed by culturing it with canine adipose stem cells.
In this study, the performance of the SPCL-Si double layer scaffold was assessed in the mandibular rodent model, and compared with empty defects (negative control) and collagen commercial membranes (positive control).
After 8 weeks of implantation, micro-computed tomography and histomorphometrical analysis (samples processed by the Donath technique), revealed that the SPCL-Si double layer scaffold conduced higher new bone formation, compared to empty defects and collagen.
This bioactive double layer scaffold was shown to have large potential for use in alveolar bone and periodontal regeneration.
Resumo
A doença periodontal é um processo inflamatório prevalente tanto em canídeos como em humanos e que pode resultar em esfoliação dentária bem como em implicações de saúde sistémicas. Como os tratamentos atuais são por vezes ineficazes, a Engenharia de Tecidos pode fornecer estratégias para o desenvolvimento de novas terapias.
O objetivo deste projeto consistiu no desenvolvimento uma nova matriz com propriedades osteocondutoras para a regeneração óssea e periodontal. Propusemos uma matriz de dupla camada que compreende uma membrana de amido poli-ɛ-caprolactona (SPCL) obtido por evaporação de solvente, a qual sustenta o princípio da regeneração tecidular guiada, e uma malha de fibra de SPCL funcionalizada com grupos silanol osteocondutores. O potencial deste biomaterial para servir como um veículo de células estaminais e como matriz osteocondutora foi previamente avaliado por cultura com células estaminais adiposas caninas.
Neste estudo, o desempenho da matriz de dupla camada SPCL-Si foi avaliado no modelo mandibular de rato sendo comparado com defeitos vazios (controlo negativo) e com membranas comerciais de colagénio (controlo positivo).
Após 8 semanas de implantação, através de microtomografia computadorizada e análise histomorfométrica (amostras processadas pela técnica de Donath), foi concluído que a matriz de dupla camada SPCL-Si conduziu a formação de osso novo em maior quantidade, quando comparado com os defeitos vazios e com o colagénio.
Esta matriz bioativa de dupla camada mostrou ter um grande potencial para ser usado na regeneração do osso alveolar e do periodonto.
Palavras-chave: Periodonto; Osso; Regeneração; Biomateriais; Engenharia de tecidos; Modelo roedor.
Index
1. INTRODUCTION ... 1
1.1. Tissue Engineering principles ... 1
1.2. Current elements in bone healing ... 1
1.3. Bone Tissue Engineering importance and future directions ... 2
1.4. Guided Tissue Regeneration in bone regeneration ... 3
1.5. Bone regeneration biological principles ... 4
1.6. Biomaterials for bone tissue engineering ... 6
1.6.1. Polymers and bone organic matrix similarity ... 9
1.6.1.1. Starch Poly-ɛ-Caprolactone (SPCL) ... 10
1.6.2. Ceramics and bone mineral matrix resemblance ... 10
1.6.2.1. Silanol groups ... 11
1.7. Scaffold technical considerations ... 12
1.8. Biocompatibility as the paramount criterion ... 12
1.8.1. In vitro assessment of cytocompatibility ... 13
1.8.2. In vivo assessment of biocompatibility ... 14
1.8.3. In vitro and in vivo SPCL background ... 15
1.9. Animal models for biocompatibility assessment... 15
1.10. Bone ingrowth models ... 16
1.10.1. Mandibular model ... 16 1.11. Methods of assessment ... 17 1.11.1. Imaging ... 17 1.11.1.1. Microradiography ... 17 1.11.1.2. Micro-computed tomography ... 18 1.11.2. Histology... 18 1.11.2.1. Histomorphometry ... 18 1.11.2.2. Donath technique ... 19
1.11.2.3. Specific bone marker analysis ... 20
2. OBJECTIVES ... 21
3. MATERIALS AND METHODS ... 22
3.1. Materials... 22
3.2. Animals ... 23
3.4. Postoperative care ... 24
3.5. Micro-computed tomography analysis ... 25
3.6. Histomorphometric analysis... 25
3.7. Statistical analysis... 26
4. RESULTS... 27
4.1. Micro-computed tomography findings ... 27
4.2. Bone histomorphometric analysis ... 28
4.3. Semi-quantitative evaluation of the biocompatibility... 29
4.4. Histomorphometric analysis... 29
5. DISCUSSION ... 35
6. CONCLUSION ... 41
Figures Index
Figure 1 – Schematic representation of GBR principals ... 4 Figure 2 – Bone grafting procedure in a bone defect. ... 6 Figure 3 – SPCL double-layer scaffold.. ... 11 Figure 4 – Schematic representation of the rat (Rattus norvegicus) mandible (left lateral view). ... 17 Figure 5 – Mandibular model surgical procedure. ... 24 Figure 6 – Representative micro-computed tomography images of the f. masseterica showing the induced bone CSD after 8 weeks of implantation... 27 Figure 7 – Representative slides of the results obtained with blank, collagen and SPCL-Si double layer scaffold (Lévai Laczkó stain) ... 28 Figure 8 – Quantification of the new bone formed in the defect. ... 30 Figure 9 – Percentage of new formed bone in blank, collagen and SPCL-Si groups in the defined ROI after 8 weeks of implantation. ... 31
Tables Index
Table 1 – Biomaterials tested in scaffolds manufacturing aimed at use in bone and periodontal
tissue engineering ... 7
Table 2 – Overview of critical size defect dimensions and implantation time points used in several reported studies using the rat mandibular model ... 16
Table 3 – Semi-quantitative classification, adapted from the annex E of the ISO norm 10993-6:2007 ... 29
Table 4 – Descriptive statistics that quantitatively describes the main features of the collected data from blank, collagen and SPCL-Si groups ... 31
Table 5 – Shapiro-Wilk test ... 32
Table 6 – Levene’s test ... 32
Table 7 – One-way ANOVA test ... 33
Table 8 – Welch and Brown-Forsythe tests. a – Asymptotically F distributed. ... 33
Abbreviations and Acronyms
® Registered Trademark
2D Two dimensional
3D Three dimensional
ANOVA Analysis of Variance
BCP Biphasic Calcium-Phosphate
BMPs Bone Morphogenetic Proteins
BMSCs Bone Marrow Stem Cells
BTE Bone Tissue Engineering
CaP Calcium-Phosphate
CD Cluster of Differentiation
CSD Critical Size Defect
Df Degrees of Freedom
DNA Deoxyribonucleic Acid
d-PTFE High-Density Polytetrafluoroethylene
ECM Extracellular Matrix
EEC European Economic Community
e-PTFE Expanded Polytetrafluoroethylene
et al. Et alii
GBR Guided Bone Regeneration
GTR Guided Tissue Regeneration
HA Hydroxyapatite
hASCs Human Adipose Stem Cells
hPDL Human Periodontal Ligament
IBM SPSS® International Business Machines Statistical Package for Social Science®
IP Intraperitoneal
ISO International Standards Organization
Micro-CT Micro-Computed Tomography
MSCs Mesenchymal Stem Cells
n Sample size
PCL Polycaprolactone
PDL Periodontal Ligament
PGLA Polyglatic Acid
PHB Poly(Hydroxybutyrate)
PLA Polylactic Acid
PLGA Poly Glycolic Acid
PLLA Poly(L-Lactic Acid)
PPF Poly(Propylene Fumarates)
PRP Platelet Rich Plasma
RM Regenerative Medicine
ROI Region of Interest
RT-PCR Reverse Transcriptase Polymerase Chain Reaction
RUNX2 Runt-Related Transcription Factor 2
SAFE® Scientific Animal Food & Engineering®
SC Subcutaneous
SID Semel in die
Si-OH Silanol Group
SPCL Starch-Poly(ɛ-Caprolactone)
SPCL-Si Starch-Poly(ɛ-Caprolactone) functionalize with silanol groups
TCP Tricalcium-Phosphate
TE Tissue Engineering
UV Ultra-Violet
VEGF Vascular Endothelial Growth Factor
WSFM Wet-spun Fiber Mesh
T o my P a r en t s a n d B r o t h e r J o s é, Ma r i a a n d P ed r o
Acknowledgments
This Master dissertation resulted from an interdisciplinary cooperation , with numerous contributions from different professionals and institutions, which I can not fail to highlight. For this reason, I wish to express my sincere thanks to:
University of Trás-os-Montes e Alto Douro, in the person of its Magnificent Rector, Professor Dr. Carlos Alberto Sequeira, I express my appreciation for the support given to the realization of this Master dissertation.
Professor Carlos Viegas, my supervisor, for the scientific expertise, availability and generosity, always revealed throughout this year of work. I am also sincerely grateful for the professional criticisms, corrections and suggestions made during guidance. This project would not be possible without his work ability and competence.
João Requicha, my co-supervisor, for the enthusiasm putted in the project since the beginning, for all the dedication and friendship demonstrated throughout the project. I also could not forget to underline his scientific advices, ideas and suggestions to this dissertation.
3B’s Research Group, in the name of Rui Reis, Manuela Gomes and Isabel Leonor, for all the technical support in the construct and manufacturing of the double layer scaffold and aid in the micro-computed tomography assessment of samples.
Histomorphometric Laboratory of the Surgery Unit of the Faculty of Veterinary of Lugo (University of Santiago de Compostela), in the name of Fernando Muñoz, Mónica Muñoz and María, for the support in the histomorphometric and semi-quantitative analysis.
Completed a particularly important step in my life, I could not forget to express the deepest gratitude to all of those who supported me during this long walk:
All my family for having always believed in me and all the support over the years.
Manuel e Ídília, my third grandparents, as an example of love and affection.
All Gonçalvez family, particularly my cousins Carlos and Vera Moura for the amazing unit and example.
Frank Haise and his beautiful family for having taught me how much it weighs a brick.
Turma 3, where I grew in all these years, and all my year colleagues.
1. INTRODUCTION
1.1. Tissue Engineering principles
Tissue Engineering (TE) is an emerging entity in medical research that develops new technological applications aimed at repair, replace and regenerate biological functions that have been compromised by injury or disease (Daar et al., 2007). Achieving this goal requires an interdisciplinary approach between knowledge and skills from a variety of disciplines that include Stem Cell Biology, Cell, Tissue and Organ Transplantation, Material Science, Genetics and Cell and Molecular Biology (Berthiaume et al., 2011). New approaches are being proposed to satisfy all the required elements for in vivo repair, by stimulate and support tissue intrinsic capability to regenerate and heal (Salgado et al., 2004a; Gurtner et al., 2007). Regeneration implies the restore and maintenance of the lost morphological and physiological state. A multicelular body unfolds from a single cell through complex processes, with cells and their environment interactions affecting this course. Reproduce, stimulate or otherwise head this process, is the shared goal between TE diverse contributing disciplines (Daar et al., 2007). Providing three central elements – matrix, cells and signalling molecules, is necessary for most TE procedures, to reproduce the sequence of overlapping events that occur during tissue formation and growth (Ikada, 2006). The ultimate goal is then to translate the developed approaches to the medical practice, so they can be used at a meaningful scale, with cost viability and in a wide spectrum of applications (Williams et al., 2005).
1.2. Current elements in bone healing
Bone has an intrinsic potential to regenerate itself but, over a critical size defect (CSD) this ability is not verified (Baroli, 2009). A bone CSD is the smallest size wound, in a specific bone and animal specie, which will not heal spontaneously during the animal’s lifetime (Huh et al., 2005). In such defects, the migration of the fibroblasts is faster than osteoblasts and their regenerative activity, which leads to the deposition of a fibrous connective tissue in the bone defect with consequent function lost (Liu et al., 2010). CSDs stem from a variety of pathologic processes as congenital malformations, cancer, trauma and infections, and bone regeneration only occurs if an osteogenic substitute material fills the bone flaw. In an induced CSD it is possible to exclude the spontaneous bone osteogenesis, providing a practical standard for testing bone substitute materials or methods (Ma et al., 2009; Liu et al., 2010).
Today, standard clinical approaches to enhance bone regeneration in non-union or CSDs are centred in different bone grafting methods, as autologous and allogeneic grafts. These medical practices exhibit relatively satisfactory results but they also exhibit limitations to their use (Dimitriou et al., 2011). Autologous bone remains the gold standard bone grafting material but, its inconvenients are related with the limited amount of available bone, and morbidity of the donor site. Furthermore, allogeneic bone substitutes have reduced osteoinductive properties, and are associated to immunogenic and rejection reactions, and transmission of infectious agents (Petite et al., 2000; Dimitriou et al., 2011).
1.3. Bone Tissue Engineering importance and future directions
In humans, gingivitis affects 50-90 % of adults worldwide, depending on its precise definition (Albandar et al., 2002). Chronic periodontitis affects about 30 % of the adult population (Nares, 2003). As recently stated by the World Health Organization, the advanced disease with deep periodontal pockets affects 10-15 % of adults, leading to enormous spending efforts by health care providers. Periodontal diseases are an important health problem in Human Medicine, due to its enormous prevalence and life threatening implications on systemic health (Petersen et al., 2005). A positive correlation has been previously reported between periodontal diseases and preterm birth, low birth weight and other adverse pregnancy outcomes, diabetes, cardiovascular diseases and stroke (Petersen et al., 2005; Persson et al., 2008).
With the ongoing understanding of tissue regeneration at the cellular and molecular level, TE has the potential to develop novel treatments as alternatives or adjuncts to the actual therapies (Porter
et al., 2009; Dimitriou et al., 2011).
Bone Tissue Engineering (BTE) includes a broad range of settings and approaches added to apply the TE concept to Orthopaedics, seeking to repair, augment, replace and regenerate the bone tissue. By providing engineered synthetic bone substitutes with mimicking properties, BTE can overcome the bone grafting limitations and potentially treat medical conditions currently untreatable (Porter et al., 2009; Dimitriou et al., 2011).
There are three main research lines within BTE: 1) scaffold/matrix based therapies, 2) cell based therapies, and 3) signalling molecules based therapies (Salgado et al., 2004b; Ikada, 2006). An ideal single model is not currently available, and the most promising outcomes result from a combination of the aforementioned research strategies (Porter et al., 2009).
A scaffold is a three-dimensional architectural environment, which serves as a temporary support, onto cells can grow, differentiate and synthesise the extracellular matrix (ECM)
(Farhadi et al., 2006; Stamatialis et al., 2008). A scaffold based therapy consists on the surgical positioning of a structural implant at the injury site (Salgado et al., 2004b). The protocols differ as scaffolds culture-expanded with cells, scaffolds loaded with growth factors or simply the use of acellular scaffolds. This last approach has some benefits, once acellular scaffolds are easier to sterilize, have a wide shelf-life and the lowest potential for infection or immunogenicity. Nonetheless, an acellular scaffold protocol relies on the endogenous ability to recruit osteoprogenitor cells, and design it in order to mimic native bone tissue, is critical to this strategy success (Salgado et al., 2004b; Porter et al., 2009).
A promising therapeutic protocol is the implant of an occlusive acellular scaffold at the damage site immediately after injury, to enhance and guide the tissue regenerative properties during the healing process, a strategy called Guided Tissue Regeneration (GTR) (Retzepi et al., 2010).
1.4. Guided Tissue Regeneration in bone regeneration
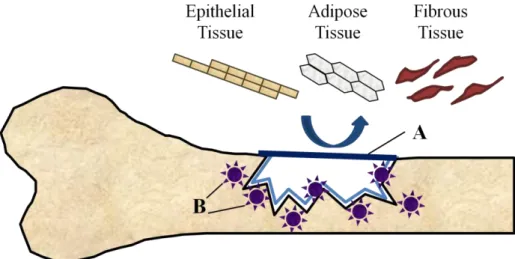
GTR is a research branch within TE that explore the possibility that tissue regeneration could be achieved, when cells with the capacity to regenerate the particular type of lost tissue are allowed to invade the defect, grow and multiply during the healing course. A barrier membrane (figure 1 – A) must mechanically prevent epithelial, adipose and fibrous tissues to engage or grow into the wound. Withal, it must allows, or ideally support, the recruitment of undifferentiated progenitor cells (figure 1 – B) from the remaining healthy tissue, to re-establish the original anatomical and physiological tissue properties (Retzepi et al., 2010). The concept barrier membrane presupposes a two-dimensional material layer implanted at the injury site to create a spatial and temporal void, essential to the repopulation by the tissue regenerative cells (Chen et al., 2010). It serves as a selective barrier between two phases, and remains impermeable to specific undesirable cell populations (Stamatialis et al., 2008). Guided Bone Regeneration (GBR) has its first experimental trials in the final 80’s, and resulted from the application of GTR fundamentals to bone regeneration research (Retzepi et al., 2010).
Figure 1 – Schematic representation of GBR principals. The occlusive membrane must face the bone surface and
support a mechanical exclusion of undesirable soft tissues from growing into the osseous defect. At the same time it must allow to the osteogenic cell population, derived from the parent bone, to engage the osseous wound space to express osteogenic activity (Retzepi et al., 2010). A – Barrier membrane; B – Undifferentiated osteoprogenitor cells.
1.5. Bone regeneration biological principles
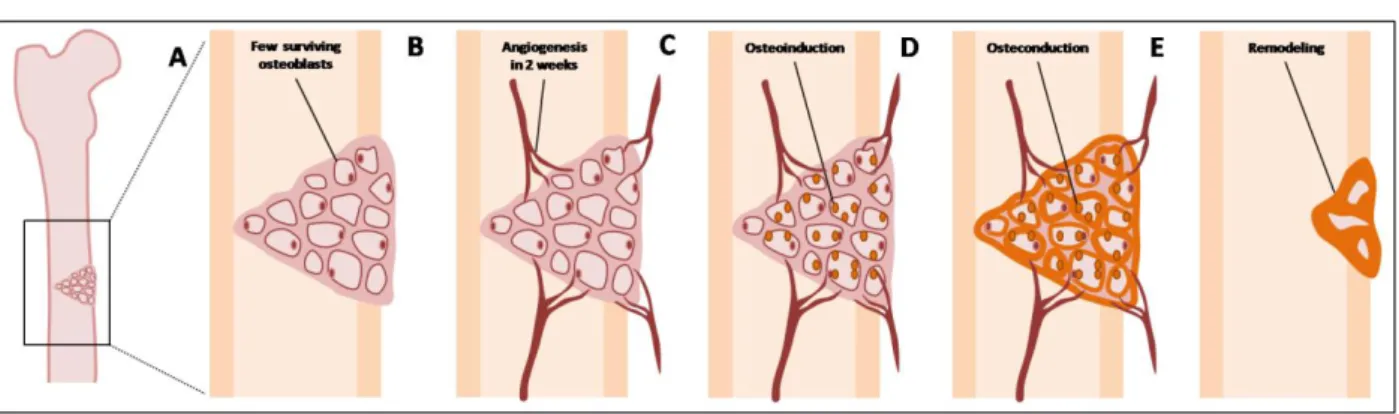
Bone differentiated cells, like osteocytes and osteoclasts, play a minor role in the creation of new bone tissue during the healing course. Undifferentiated cells are essential in this process, because they can be recruited and induced to differentiate into osteoprogenitor cells. Bone has a heterogeneous undifferentiated cellular pool that includes populations like mesenchymal stem cells (MSCs), bone marrow stromal cells (BMSCs) and periosteal cells (Albrektsson et al., 2001; Kneser et al., 2006; Marsell et al., 2011). These populations are attracted from the remaining bone by osteoinductive agents, are induced to become preosteoblasts and ultimately osteoblasts, which are responsible for the production of bone ECM. This process constitutes osteoinduction, that describes the interaction between inductive agents and pre-existing local precursor cells that initiates a cascade of cellular events that towards cells into an osteoblastic phenotype (figure 2 – C) (Salgado et al., 2004a; Muschler et al., 2010; Marsell et al., 2011). Osteoinduction is a normal process in bone healing and starts immediately after injury, being very active during the first week after damage (Albrektsson et al., 2001). In GBR protocols the membrane material and construct must create a microenvironment that promotes osteoinduction, by favouring the migration, homing and differentiation of the osteogenic precursor cells (Salgado et al., 2004b). Bone induction can be attained by two approaches: cell-mediated or growth factor-mediated. In the first protocol, the membrane is previously seeded with undifferentiated or predifferentiated stem cells obtained from different adult tissues. In the second approach osteoinduction is
stimulated by using soluble or membrane-loaded growth-factors (Lind et al., 2001). However, it seems that the local injury is sufficient to recruit previously undifferentiated cells, and evaluate a certain material concerning the osteoinductive properties, could be a merely academic exercise (Albrektsson et al., 2001).
The membrane must be able to preserve a defined tissue volume to restrain the invasion of adjacent soft tissues, and to promote the attachment, migration, proliferation, and survival of the recruited and differentiated osteogenic cells – osteoconduction (figure 2 – D) (Muschler et al., 2010). Bone formation, that started by a primary osteoinduction, is helped to extend due to a favourable structural environment created by the conductive membrane. Osteoconduction is influenced by the type of material used, being possible to categorize it in respect to its osteoconductivity (Albrektsson et al., 2001; Lind et al., 2001).
The osteoinducted and osteoconducted mature osteoblasts starts to form bone matrix, that subsequently mineralizes to mature bone tissue and fills the void or defect – osteogenesis (figure 2 – D) (Muschler et al., 2010). After an ideal time frame, the membrane should be replaced by the newly formed bone, undergoing subsequent integration and remodelling – osseointegration (figure 2 – E). This is the final phenomenon and relies on the extent of the above processes (Albrektsson et al., 2001; Muschler et al., 2010).
The employed GBR protocol must be able to provide objective and quantifiable parameters in relation to osteoinduction, osteoconduction, osteogenesis and osseointegration. Specificities as specie anatomy, physiology and histology make some models better suited for investigation of certain domains. It is also important that detects and predicts clinically relevant differences between methods and materials (Muschler et al., 2010). The material and design determinates the functionality of the construct to a high extent. Although the final requirements are dependent on the specific purpose of the membrane, several general characteristics need to be considered for all designs (Stamatialis et al., 2008).
Figure 2 – Bone grafting procedure in a bone defect. A – Graft application – Hematoma formation with the release
of cytokines and growth factors. B – Graft vascularization – inflammation, migration and proliferation of the MSCs and formation of fibrovascular conjunctive tissue around the graft. C – Osteoinduction phase – revascularization by the infiltration of blood vessels in the bone tissue graft. D – Osteoconduction and osteogenesis phases – substitution, in variable length, by osteoclastic resorption of the grafted bone trabeculae with the simultaneous formation of non lamellar bone tissue by osteoblasts with origin in the receptor tissue. E – Reshuffle phase – conversion in lamellar bone influenced by mechanical forces acting over the receptor local of the graft. Adapted from Stevenson (1999) and Bauer et al. (2000). (Stevenson, 1999; Bauer et al., 2000).
1.6. Biomaterials for bone tissue engineering
When a certain substance, other than drug, or combination of substances in a therapeutic or diagnostic system are in contact with biological fluids, it is defined as a biomaterial (Stamatialis
et al., 2008). Biomaterials can be used for any period of time, as a whole or as a part of a system,
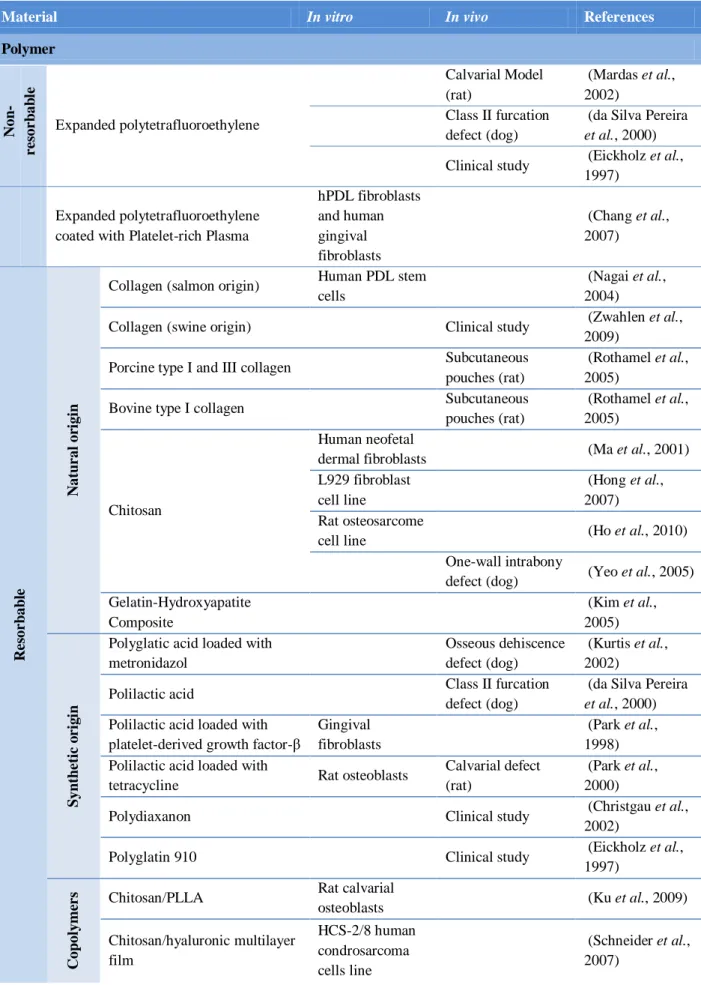
and treats, augments, or replaces any tissue, organ, or function (An et al., 1999). They must show to be biocompatible, biodegradable, non-immunogenic, non-toxic and not trigger any other adverse biological response that could affect the regenerative process (Salgado et al., 2004a; Stamatialis et al., 2008) The appropriate material selection to BTE scaffolds is of major importance, since the material properties will determinate the scaffold ability to respond to specific and required purposes (table 1) (Salgado et al., 2004a; Puppi et al., 2010).
Table 1 – Biomaterials tested in scaffolds manufacturing aimed at use in bone and periodontal tissue engineering.
Material In vitro In vivo References
Polymer N on -r e sor b ab le Expanded polytetrafluoroethylene Calvarial Model (rat) (Mardas et al., 2002) Class II furcation defect (dog)
(da Silva Pereira
et al., 2000)
Clinical study (Eickholz et al., 1997)
Expanded polytetrafluoroethylene coated with Platelet-rich Plasma
hPDL fibroblasts and human gingival fibroblasts (Chang et al., 2007) R e sor b ab le N atu r al or igi n
Collagen (salmon origin) Human PDL stem cells
(Nagai et al., 2004)
Collagen (swine origin) Clinical study (Zwahlen et al., 2009)
Porcine type I and III collagen Subcutaneous pouches (rat)
(Rothamel et al., 2005)
Bovine type I collagen Subcutaneous pouches (rat)
(Rothamel et al., 2005)
Chitosan
Human neofetal
dermal fibroblasts (Ma et al., 2001) L929 fibroblast
cell line
(Hong et al., 2007) Rat osteosarcome
cell line (Ho et al., 2010) One-wall intrabony
defect (dog) (Yeo et al., 2005) Gelatin-Hydroxyapatite Composite (Kim et al., 2005) S yn th e ti c o r ig in
Polyglatic acid loaded with metronidazol
Osseous dehiscence defect (dog)
(Kurtis et al., 2002) Polilactic acid Class II furcation
defect (dog)
(da Silva Pereira
et al., 2000)
Polilactic acid loaded with platelet-derived growth factor-β
Gingival fibroblasts
(Park et al., 1998) Polilactic acid loaded with
tetracycline Rat osteoblasts
Calvarial defect (rat)
(Park et al., 2000)
Polydiaxanon Clinical study (Christgau et al., 2002)
Polyglatin 910 Clinical study (Eickholz et al., 1997) C op ol yme r
s Chitosan/PLLA Rat calvarial
osteoblasts (Ku et al., 2009) Chitosan/hyaluronic multilayer film HCS-2/8 human condrosarcoma cells line (Schneider et al., 2007)
The first BTE protocols used non-resorbable biomaterials, which contributed to important discoveries regarding bone formation and regeneration. They were also responsible for the establishment of basic BTE protocol requirements as surgical procedure, healing time and post-operative care (Hammerle et al., 2003; Strietzel et al., 2006). These biomaterials retain their shape and structure, do not grow with the patient or change the shape in response to load, and need a second surgical procedure for removal. Increasing effectiveness and knowledge in resorbable biomaterials made this category lose importance in clinical practice, and their use is now limited to specific indications (Hammerle et al., 2003; Gentile et al., 2011).
A resorbable biomaterial degrades and disappears from the implanted site, while ideally, is substituted by the newly formed tissue. The scaffold can be reabsorbed by two processes: i) trough hydrolysis or enzymatic digestion, the biomaterial main chain decreases in molecular weight and eventually disappears; ii) trough chemical process that cross-linked the biomaterial that form an insoluble hydrogel, which is then degraded, generating water-soluble fragments that are washed way from the implanted site (Tabata, 2009). The degradation rate depends on several factors as age of the animal, implantation site, scaffold size or volume, nature and structure of the biomaterial, molecular weight, material phase (crystalline or amorphous) and presence of additives or impurities. This category enables a single surgical procedure, reduce patient discomfort and expenses. Its drawbacks are related with the degradation time and the effect of its reactions and products on bone formation (Gentile et al., 2011). It is plausible that the scaffold Ceramic
β-Tricalcium-Phosphate Human adipose-derived stem cells
(Marino et al., 2010)
Composite
Collagen /Carbonate apatite Subcutaneous implantation (rat) (Matsumoto et al., 2002) Collagen/PLGA/Hydroxyapatite MC3T3-E1 osteoblasts cell line (Liao et al., 2005)
Polycaprolactone/Nano-apatite Rat bone marrow stem cells (Yang et al., 2009) Poly (L-lactide-co-glycolide-co-caprolactone/β-Tricalcium-Phosphate Two-wall intrabony defect (dog) (Kikuchi et al., 2004) Poly(ethyleneglycol terephthalate)/Poly(butylenes terephthalate)/Hydroxyapatite Subgingival implantation (goat) (Jansen et al., 1995)
PLA/Hydroxyapatite nanoparticles Human PDL cells (Mei et al., 2007) Collagen/PLA/Nano-hydroxyapatite Acellular scaffold (Pan et al., 2006)
functions could not be fully guaranteed during all the implantation time, and the degradation process may interfere with the wound healing and bone regeneration process. Therefore, the biodegradability is a feature that should be taken into account and evaluated during the assessment of resorbable biomaterials in BTE protocols (Hammerle et al., 2003; Tabata, 2009; Gentile et al., 2011).
1.6.1. Polymers and bone organic matrix similarity
The bone organic matrix is mainly composed of type I collagen (90 %) and over two hundred different non collagenous proteins (10 %) (Baroli, 2009). These last ones exert important roles such as modulation of tissue repair, calcification, and binding of growth factors. They influence cell adhesion, migration, proliferation, differentiation and apoptosis (Porter et al., 2009; Muschler et al., 2010). As the organic matrix has a central role in the regulation of bone repair, the use of polymers, to mimic these component properties, can be an interesting BTE procedure. Polymers can be either natural or synthetic, and consists of a large macromolecule composed of many repeating units called monomers (Dee et al., 2002).
Natural polymers are obtained from animal or vegetal natural sources and due to the biological matrix similarity, have several protocol, economical and environmental advantages. That includes good biocompatibility and biodegradability with small toxicity and low manufacturing and disposal costs (Salgado et al., 2004a; Puppi et al., 2010). Its disadvantages are associated to inadequate physical properties being rapidly degraded, together with the possible loss of biological properties that compromises their single use in these protocols. They are also associated to immunorejection, hypersensitivity reactions and disease transmission that make proper screening and purification essential procedures in its manufacturing. To increase natural polymers range of use, it is necessary to promote new production procedures that physically or chemically modify them, improving their stability and physical properties (Salgado et al., 2004a; Puppi et al., 2010; Gentile et al., 2011).
Synthetic polymers offer the best therapeutic options because they are very diverse and have predictable and reproducible physical, chemical and degradation properties. They are easily processable and can be manipulated to meet specific requirements by adjusting length, side chains and amount of cross-linked. Their drawbacks have to do with the lack of the natural polymers biological features, which are essential to promote desirable cell responses (Puppi et
(Gentile et al., 2011). The synthetic polymers with the greatest potential for medical applications are poly(α-hydroxyacids) – short chain saturated aliphatic polyesters. They are resorbable and can be easily processed to meet various applications (Puppi et al., 2010).
1.6.1.1. Starch Poly-ɛ-Caprolactone (SPCL)
Starch is produced by all green plants where is the most abundant polysaccharide, which gives it as additional advantage, its almost unlimited source and consequently low attainability costs. It is entirely resorbable and presents in the form of semicristalline granules, being possible to process it by diverse techniques into diverse presentations (Salgado et al., 2004a; Puppi et al., 2010). Starch is commonly blended with thermoplastic polymers to form starch-based polymeric systems, which present a wider range of medical applications. In general they resist better to thermo-mechanical degradation, are less brittle and more easily processable. The structure and functional properties of these biomaterials depend on blend components, material processing technique, incorporation nature of additives and reinforced fillers. Various polymers can be combined with starch, such as poly(ɛ-caprolactone) (PCL) to form starch-poly(ɛ-caprolactone) (SPCL), that can be shaped in diverse items as membranes, three-dimensional scaffolds and microparticles. PCL is a polyester that due to its semi-crystalline nature and hydrophobicity, make its degradation remarkably slow, since the close packed macromolecular arrays retard fluid ingress in the bulk (Puppi et al., 2010).
1.6.2. Ceramics and bone mineral matrix resemblance
Bone has an inorganic component that represents up to 65 % of the ECM. It is made primarily of a variety of calcium-phosphate (CaP) minerals, where the most abundant is impure hydroxyapatite (HA) [Ca10(PO4)6(OH)2] (Baroli, 2009). HA crystals are found within and between the length of collagen fibers, in a precise relationship, that is critical to bone resilience and strength (Porter et al., 2009; Skrtic et al., 2011). A variety of CaP based biomaterials have been proposed based primarily on the compositional similarity with the bone inorganic matrix. Ceramics can be defined as inorganic, nonmetallic and crystalline materials that are submitted to high temperatures during its manufacturing. They are considered bioactive biomaterials as when in contact with a tissue, triggers a specific response at the interface (Puppi et al., 2010). The hydroxycarbonate apatite phase on the scaffold is chemically and structurally equivalent to the mineral phase in bone, providing interfacial bonding and attachment (Le Guehennec et al., 2005;
Seeley et al., 2007). Ceramics are osteoinductive and osteoconductive, possessing low or no biodegradability. Its disadvantages are associated with low mechanical stability that prevents its use in large bone defects (Salgado et al., 2004a).
1.6.2.1. Silanol groups
Silanol groups are functional groups that contain in its structure a silicon atom that is bounded directly to one or more hydroxyl groups (Si-OH). They enhance the apatite formation in a biomimetic solution, which is what all the CaP minerals attempt to mimic. Silanol groups are just an indirect way to produce CaP minerals (Kawai et al., 2007). If a scaffold is functionalized with silanol groups, that are released in locu due to dissolution of the material, the supersaturation of the local fluids in respect to apatite increases, enhancing its nucleation (Oyane et al., 1999; Kawai et al., 2007).
The protocol of this project planned the development of a SPCL double layer scaffold made of two different SPCL presentations: a membrane and a fiber mesh (figure 3). The two components have different functions that are combined to enhance bone regeneration. The SPCL fiber mesh was functionalized with silanol groups in an attempt to augment the biomaterial bioactivity and trigger specific responses at the interface.
Figure 3 – SPCL double-layer scaffold. It comprises two distinct components - a membrane and a fiber mesh
functionalized with silanol groups. Each one was developed to support specific functionalities needed to achieve bone regeneration. The membrane layer has an adequate surface topography for the adhesion and proliferation of undifferentiated progenitor cells and the prevention of the migration of undesirable cell populations. The fiber mesh induces bone formation through the effect of osteoconductive silanol groups, which promote the osteogenic differentiation of the stem cells and ultimately, restore bone tissue (Leonor et al., 2011).
1.7. Scaffold technical considerations
After the proper material choice, the next step is to select an adequate manufacturing technique, given that, it will highly determinate the scaffold biological features. Bone is an elaborate geometric tissue both at a macro and microscopy scale, which needs to be taken into account during membrane design and construct (Salgado et al., 2004a; Porter et al., 2009). The selected methodology must not adversely affect the material properties, and produce homogeneous products with minimal variations between them (Salgado et al., 2004a).
As BTE protocol involves the surgical placement of the scaffold, this must be manufactured in order to be clinical manageable, integrate with host tissues and have cell occlusion properties and space making ability. It must provide sufficient mechanical strength and stiffness to substitute initially for wound contraction forces and later for the tissue remodelling. Bone is always under continuous stress so the scaffold should be plan ideally to match those mechanical properties of living bone. It has to have a high porosity and large ratios surface area/volume to allow the flux and exchange of cells, nutrients and gases, the deposition of the ECM and to promote the neovascularization. Pore size and interconnectivity are also essential parameters usually being the pore size within 200 to 900 µm range for most BTE protocols (Salgado et al., 2004a). An adequate interface is also essential to provide anchorage sites and structural guidance to cells and for integration with the surrounding native tissue. The scaffold surface morphology, hydrophilicity and charge are factors critical for in vivo recruitment, attachment and proliferation of osteoprogenitor cells that can differentiate into osteoblastic lineage and express osteogenic activity.
A better knowledge of the technical features and improvements on the biomaterial construction technology is critical for the success of BTE protocols. This understanding will lead to more refined biomaterial manufacturing with improved performance of the scaffold for a given indication (Gentile et al., 2011).
1.8. Biocompatibility as the paramount criterion
Biocompatibility is associated to the interaction between the biomaterial and the living system, and its ability to perform an appropriate host response in a specific application. The term definition is not consensus but it refers to the biological performance of a certain biomaterial, for a given application and its acceptance for such application, if both host and biomaterial responses are optimal (Stamatialis et al., 2008). Biomaterials are distinct from other classes of materials because of the biocompatibility criterion they all must meet (An et al., 1999;
Stamatialis et al., 2008). They vary from inert biomaterials, which exhibit little or no host response, to interactive biomaterials that are designed to trigger specific and beneficial responses such as, cell adhesion and growth (Dee et al., 2002; Stamatialis et al., 2008). The biocompatibility degree is dependent on the material nature and the manufacturing criteria above mentioned. These two features are engineering in order to enhance the interaction of the biomaterial with the host, suppress undesirable responses and achieve practical benefits (Salgado
et al., 2004a). Previous to clinical use, a new biomaterial must be strictly tested both initial in vitro and then in vivo, in order to determinate whether it conforms to the requirements of
biocompatibility (Stamatialis et al., 2008).
1.8.1. In vitro assessment of cytocompatibility
The term biocompatibility is incorrectly used with in vitro tests, as it can only be used in the case of animals or humans (in vivo), with the correct term being cytocompatibility (Pearce et al., 2007).
This is the primary stage for testing and assesses the cytocompatibility, acute toxicity, degradation rate, potential to promote the cell adhesion and proliferation, and the capacity to induce cell differentiation. It also avoids the unnecessary use of animals in posterior in vivo testing by eliminating cytologically inappropriate biomaterials, embracing the principle of animal reduction (Pearce et al., 2007).
The cytocompatibility of the biomaterials is attained by culturing them with undifferentiated or differentiated stem cells, followed by the performance of specific characterization assays. The cell adhesion and proliferation is a parameter for characterize the cytocompatibility of the biomaterial. It can be assessed by microscopic observation, using an optical microscope after staining with vital stains, as methylene blue, or using more sophisticate imaging techniques, as scanning electron microscopy.
Cell proliferation is evaluated by quantifying the double strain DNA obtained in different cell culturing time points. The cell viability could be assessed by different colorimetric techniques, as
MTS
1.8.2. In vivo assessment of biocompatibility
In vitro systems cannot provide a reproducible approximation of a living organism or clinical
setting. Some processes can only be reliably if created and studied in narrow and uniform conditions. In vivo protocols allow a systematic attempt to optimize a multitude of variables, being essential to design and develop quantitive and reproducible animal models. In a living organism all the processes occur simultaneously, across time and place, and a certain reaction can influence all the others. In vivo tests are essential to determine the general biocompatibility of newly developed biomaterials, for which some knowledge of tissue cytocompatibility is necessary for further research and development (An et al., 1999; Gross, 1999).
The biocompatibility assessment purpose is to determinate the security of a certain biomaterial, device, system or method in a biological environment. It is measure and evaluated the magnitude and duration of possible adverse alterations in homeostatic mechanisms, which determinate the host response. From a practical perspective the in vivo assessment of biocompatibility is carried out to determinate if the device performs as intended, and if presents no significant harm to the subject under conditions that simulate the clinical use (An et al., 1999; Dee et al., 2002).
Extensive efforts have been made by government agencies and regulatory entities, as the International Standards Organization (ISO), to provide procedures, protocols, guidelines and standards that may be used in the in vivo assessment of the biocompatibility of medical devices (Muschler et al., 2010). The norm ISO 10993-6:2007 codify and specifies methodology for evaluate the local effects after the implantation of biomaterials intended for use in sanitary products. It facilitates the protocol design and guarantees standardize procedures between studies.
Subcutaneous or intramuscular implantation is the first in vivo step of testing a biomaterial for its biocompatibility. Implants in the forms or rods, plugs, pins or screws are most often implanted subcutaneously and intramuscularly to study general tissue responses to the biomaterial, its degradation rate and to provide additional information relating to the proposed design criteria in the production of a medical device. The rat is suggested as the first choice for soft tissue degradation studies because of the rich background data available (An et al., 1999; Muschler et
al., 2010).
If the experiment does not reveal any significant toxic effects, and if it is gathered a sufficient amount of data regarding the new biomaterial, a second animal model can be used. It allows evaluating biocompatibility and function of the final product and its potential applications. In case of a BTE protocol the osteoconduction, osteoinduction, osteogenesis and osseointegration
can also be assed in this second animal model. Variables of physical size, anatomy and species-specific histology and physiology make some models better suited for investigate these domains. In a resorbable biomaterial the degradation and replacement process by host tissues can also be investigated. If the new biomaterial shows success in pre-clinical studies, its use can be considered in well controlled human trials (An et al., 1999; Muschler et al., 2010).
1.8.3. In vitro and in vivo SPCL background
SPCL scaffolds have been studied and characterized in 3B’s Research Group over the past few years. SPCL revealed in vitro promising characteristics regarding cytocompatibility (data not published). It also evidenced adequate biological characteristics to serve as a matrix for adult stem cells (Gomes et al., 2006; Tuzlakoglu et al., 2009; Leonor et al., 2011), and with a degradation rate compatible to the bone tissue healing rate (Wikesjo et al., 1991; Leonor et al., 2011). Furthermore, SPCL is a versatile material with good processability, essential for obtaining complex 3D structures. The design and processing of these constructs were based on the 3B’s expertise in scaffolding development, in particular on starch based biodegradable polymers (Gomes et al., 2006; Tuzlakoglu et al., 2009; Leonor et al., 2011).
1.9. Animal models for biocompatibility assessment
There are numerous animal models for testing implant materials in vivo, depending on the final purpose. In orthopedic research, rats, rabbits, dogs and goats are among the most used animals as the first subjects in a new biomaterial testing (Muschler et al., 2010; Albuquerque et al., 2012). The animal selection is relatively easy since there are guidelines establish by professional authorities. It should recreate an environment that is close as possible to the clinical setting in which the therapy will be used. Thus, the animal specie, anatomic site and size, local tissue characteristics as well the employed surgical technique must closely match the features clinically intended. An interdisciplinary approach among researchers, surgeons, veterinarians and animal care staff generally results in an optimization of the ethical and welfare aspects for the animal involved, contributing for a successful outcome (An et al., 1999; Dee et al., 2002; Spector et al., 2011).
Small mammals, like mice (Mus musculus) and rat (Rattus norvegicus), are often used to feasibility testing that includes assessment of biocompatibility, toxicity and screening for adverse reactions (Muschler et al., 2010). The use of inbred strains allow to achieve outcomes after
individuals (reducing the number of animals needed to achieve a statistically significant result) (An et al., 1999).
1.10. Bone ingrowth models
The term bone ingrowth refers to the development of new bone tissue within a scaffold. Bone ingrowth models are developed to address questions involving the feasibility of using various porous biomaterials, desirable scaffold characteristics for bone ingrowth, the effects of interface motion and gaps, the effects of adjuvant therapies during implantation time, and means of enhancing scaffold fixation (An et al., 1999; Spector et al., 2011).
The general principles of bone ingrowth have been identified and it occurs if the scaffold: 1) is made from a biocompatible biomaterial; 2) has an appropriate porosity and integrity; 3) is mechanically stable; 4) is in close contact with the host bone and 5) the implantation site is not infected. All of these characteristics must be taken into account in order to accomplish successful outcomes (An et al., 1999; Dee et al., 2002).
1.10.1. Mandibular model
The mandibular model, along with the calvarial model, was developed to study the amount of bone ingrowth in a certain scaffold. It consists in the surgical creation of a CSD at the fossa
masseterica and posterior filling with a scaffold for a certain implantation time point (table 2).
This anatomical site represents a large bone surface, being useful to test scaffolds with the format of discs (figure 4) (An et al., 1999; Muschler et al., 2010).
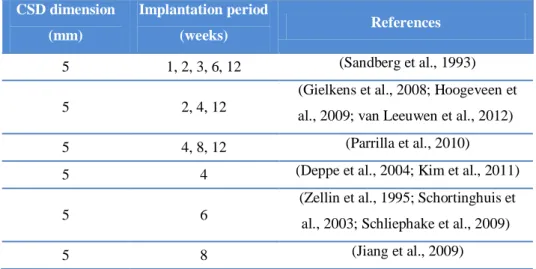
Table 2 – Overview of critical size defect dimensions and implantation time points used in several reported studies
using the rat mandibular model.
CSD dimension (mm) Implantation period (weeks) References 5 1, 2, 3, 6, 12 (Sandberg et al., 1993) 5 2, 4, 12
(Gielkens et al., 2008; Hoogeveen et al., 2009; van Leeuwen et al., 2012) 5 4, 8, 12 (Parrilla et al., 2010) 5 4 (Deppe et al., 2004; Kim et al., 2011)
5 6
(Zellin et al., 1995; Schortinghuis et al., 2003; Schliephake et al., 2009) 5 8 (Jiang et al., 2009)
It is essential that the discs have the same diameter than the CSD so that it can be perfectly adapted to the defect, promoting space occlusion and the migration of the undifferentiated cells from the healthy bone margins. Due to the dimension of the rat mandible this model only allows to test one disc on each side of the mandible (figure 4) (An et al., 1999; Schortinghuis et al., 2003).
Figure 4 – Schematic representation of the rat (Rattus norvegicus) mandible (left lateral view). A – Fossa
masseterica with the created CSD; B – Ramus mandibulae; C – Corpus mandibulae; D – Processus condylar; E – Processus angularis; F – Diastema; G – Incisivus mandibulae; H – Molarem mandibulae.
1.11. Methods of assessment
1.11.1. Imaging
The mineralized matrix thickness and density is strongly correlated with the ability of bone to resist deformation in compression and bending. This feature leads to that in most of the in vivo BTE studies the primary metric assessments are related with volume, distribution and density of the newly formed tissue (Muschler et al., 2010).
1.11.1.1. Microradiography
Microradiography is a radiographic imaging technology commonly employed in the rigorous characterization of the scaffold properties and functions (Schortinghuis et al., 2003; Gielkens et
al., 2008; Hoogeveen et al., 2009). It offers 2D images that can probe and evaluate biological
events at the interface between tissues and the implanted scaffold (Guldberg et al., 2008). Conventional radiographic evaluations are relatively inexpensive and low time consuming,
providing a good overall picture of the defect, and making it a very standardized technology used to assess the bone formation. Its limitations are related with low accuracy and the incapability to reveal the spatial distribution of the formed tissue within the scaffold (Pryor et al., 2006; Muschler et al., 2010).
1.11.1.2. Micro-computed tomography
Micro-computed tomography (Micro-CT) is a high resolution 3D imaging technology that reveals spatial distribution of the newly formed tissue. It provides quantitive volumetric information regarding three-dimensional pattern and distribution of mineralization at the macromolecular level of bone structure, enabling assessment of trabecular number, thickness, connectivity and mineral density (Muschler et al., 2010). It also has the potential to quantify 3D vascular ingrowth that can be tremendously valuable to categorize the potential and viability of certain strategies (Guldberg et al., 2008). Micro-CT was first developed in the early 1980s and is today the most extensively applied imaging modality in BTE (Gielkens et al., 2008). It is able to rapid reconstruction of high resolution 3D images and combined with morphometric analysis algorithms, is currently the gold standard for quantifying 3D changes in the volume and morphology of trabecular and cortical bone (Guldberg et al., 2008; Muschler et al., 2010). It can also be used first to quantify the scaffold architecture, as pore size and interconnectivity, and then connect these parameters with the amount and distribution of mineralized matrix formation. This capability is of major importance in evaluation of a scaffold potential, because bone ingrowth through the membrane is highly dependent to its characteristics. Micro-CT is a non destructive procedure and samples remain available for other tests as histomorphometric evaluation or biomechanical testing (Guldberg et al., 2008).
1.11.2. Histology
1.11.2.1. Histomorphometry
Histomorphometry is the standard secondary metric outcome for bone regeneration evaluation. It characterizes the pattern and distribution of cellularity in the mineralized bone tissue compartment, and the kinetics of bone formation and mineralization. It is also important in portray other key bone tissues as the hematopoietic cells, fat, fibrous tissue and vascularity within the non-mineralized inter-trabecular regions. Histomorphometry can also assess any local inflammatory response or residues from the implanted scaffold (Muschler et al., 2010).
Technical limitations are regarded with the histological section that represents one specific area, which could be not representative of the entire defect. The diversity in histological scorings and stains also make comparison between studies difficult (Schortinghuis et al., 2003). The most common histological stains include haematoxylin-eosin (Srouji et al., 2005; Ebina et al., 2009; Jiang et al., 2009; Kim et al., 2011; Moraes et al., 2011), toluidine blue (Kostopoulos et al., 2001; Donos et al., 2002; Stavropoulos et al., 2003; Moraes et al., 2011), Masson-trichrome (Kim et al., 2011) and Lévai Laczkó stain after processing by the Donath technique (Kostopoulos et al., 2001). Some studies submit the sections to a tetrachrome stain that includes sudan black, toluidine blue, basic fuchsine and light green (Kostopoulos et al., 1994; Donos et
al., 2002).
1.11.2.2. Donath technique
Donath technique is a histological methodology developed by Donath and Breuner in 1982, being routinely used in histological assessment in samples that cannot be processed in paraffin. The specimens normally submitted to this process includes jaw bones with teeth containing fillings, crowns and bridges, thick cortical bones, implants (metals or ceramics), long bones or brittle hypermineralized bone parts. The inclusion of bone in paraffin demands its previous decalcification, which carries a modification of bone morphology. This feature can represent a drawback during bone implants assessment, so that, Donath technique can be an interesting approach to overcome this problem. It consists in a cutting-grinding technique that does not demand the bone sample decalcification. All the process requires very specific equipment and staining methods to obtain thin sections for histological examination.
The specimen suffers a plastic infiltration by glycometacrilate. The infiltration is finalized with vacuum during three consecutive days for make sure that the plastic occupies all the free spaces. The polymerization of the glycometacrilate is by light (photopolymerization) with low intensity and UV light, for the polymerization of the plastic occurs inside the tissue. At the end of this process the sample is included in a block of plastic that is processed by a series of cutting-grinding saws and sandpapers. The final section has about 50 to 200 µm and can be submitted to specific colorations as Lévai and Laczkó stain (Laczkó et al., 1975; Donath, 1995).
1.11.2.3. Specific bone marker analysis
The new bone formation can be assessed by analyzing different specific bone markers, namely, collagen type I, RUNX2, bone sialoprotein, osteocalcin and osteopontin, among others. The gene expression of these specific osteoblasts markers can be done by real time PCR. As not all the genes are translated into proteins, it is advised to proceed to the immunohistochemistry, which allows to determinate the expression of the bone proteins in the tissues (Bayliss et al., 2012; Marcos-Campos et al., 2012). In addition, the bone regeneration process can also be monitored by evaluating the presence of bone-specific isoform of alkaline phosphatase serum activities, osteocalcin in the serum and their correlation with the serum minerals as calcium, phosphorus, magnesium and ionized calcium (Dias et al., 2008).
2. OBJECTIVES
Quantify and compare the effect in bone regeneration in a mandibular 5 mm CSD between blank, collagen, and SPCL-Si after 8 weeks of in vivo implantation;
Observe the three-dimensional bone architecture of the mandibles by micro-computed tomography;
Perform a semi-quantitative evaluation of the biocompatibility at the final implantation period according to the ISO norm 10993-6:2007 (Annex E);
The data and conclusions originated from the above procedures regarding SPCL-Si double layer scaffold will also contribute to:
Assess its osteoinduction potential;
Categorize it in terms of its local biological effects;
Consider its potential for use in bone tissue engineering;
Estimate its potentialities to the development of constructs for Veterinary and Human Medicine.
3. MATERIALS AND METHODS
The following experimental protocol includes the use of vertebrates so that, it was performed and conducted accordingly to the international standards on animal welfare defined by the European Communities Council Directive n.º 86/609/EEC of the council November 24, by the portuguese legislation determined at Portaria n.º 1005/92 of October 23, and after approval by the National Ethical Committee for Laboratory Animals.
3.1. Materials
A double-layer membrane based on SPCL, a biodegradable and thermoplastic blend of starch with poly-ɛ-caprolactone (30:70 % w/w), was produced by combining a SPCL patterned solvent casting membrane and a SPCL wet-spun fiber mesh. The membrane and fiber mesh were attached together by dropping chloroform onto the top side of the membrane, allowing it to become slightly soften, and it was applied soft pressure on the fiber mesh to promote the attachment. The combined structures were dried in a hood and cut into discs with 5 mm of diameter.
To obtain the solvent casting membrane, the SPCL (30:70 % w/w) (Novamont®, Italy) was
dissolved in chloroform (Sigma-Aldrich®, Germany) at a concentration of 20 % (w/v). At room
temperature, 3 mL of the polymeric solution was casted onto a 5 cm diameter patterned teflon molds to obtain patterned surface membranes, and onto patterned teflon mold to obtain non-patterned surface membranes as a control. Membranes were dried in a hood and then cut into 5 mm of diameter discs, followed by ethylene oxide sterilization.
In order to prepare the wet-spun fiber meshes (WSFM), the same SPCL solution was loaded into a 5 ml plastic syringe with a metallic needle (21 gauge × 1.5”). The syringe was connected to a
programmable syringe pump (KD Scientific®, World Precision Instruments®, United Kingdom)
to inject the polymer solution at controlled pumping rate of 5 mL per hour to allow the formation of the fiber mesh directly into the coagulation bath. The WSFM structure was created during the processing by the random movement of the coagulation bath: calcium silicate solution where the molar ratio was Si(OC2H5)4 [TEOS, Tetraethoxysilane] / H2O / C2H5OH / HCl / CaCl2 of 1.0 / 4.0 / 4.0 / 0.014 / 0.20 (Sigma-Aldrich®, Germany) (SPCL-Si) (Leonor et al., 2011). SPCL-Si WSFM was dried at 60 ºC for 24 hours.
3.2. Animals
Nine albino, male, 10 weeks old rats (Rattus norvegicus) of the Wistar strain (Charles River
Laboratories®, United Kingdom) were used in this protocol. The mean body weight was of
433,87 ± 21,69 g, ranging from 404,62 g to 476,24 g. The animals were housed in groups of three individuals in standardized plastic cages, maintained with 12 hours day/night cycles, an ambient temperature of 22 ± 2 ºC and constant relative humidity. All the rats were allowed free
access to a pellet rodent diet (A04 rats maintenance diet, SAFE®) and mineral commercial water
ad libitum. They spent two weeks in quarantine, immediately started after arrival and ended
before the surgical procedure, where they were monitored in food and water intake, body weight increase and behavior.
3.3. Mandibular model surgical procedure
The rats were premedicated with dexmedetomidine hydrochloride 0,5 mg/kg IP (Dexdomitor®,
Pfizer®, Finland) and fifteen minutes after, anesthetized with ketamine chloride 75 mg/kg IP
(Imalgene 1000®, Merial®, Portugal). The mandible was pre-operative radiographed to confirm
normal anatomy, and to aid in determining whether adequate bony surface was suitable to encompass a bilateral circular size bone defect with 5 mm of diameter.
The rats were randomly assigned to three experimental groups of three individuals, where the first group received no scaffold (Blank Group – negative control), the second group received the
collagen scaffold (Resodon®, Resorba®, Germany) (Collagen Group – positive control) and the
third group received the SPCL-Si double layer scaffold (SPCL-Si Group – study membrane). In lateral recumbency with the neck extended and the interventional side placed dorsally, the mandibular and hemicervical areas were shaved and aseptically prepared by swabbing with a 10 % dilution of povidone-iodine (figure 5 – A). A mandibular skin incision was made exposing the
musculus masseter (figure 5 – B). After the muscle incision, the borders were pushed away
rostrodorsally and ventrocaudally (figure 5 – C) taking care to prevent damage of the nervus
facialis and the ductus parotideus. The m. masseter was continually dissected and with a
periostotome it was elevated exposing the fossa masseterica in the lateral surface of the ramus
mandibulae (figure 5 – D). Using a 5 mm outer diameter trephine drill (ACE Surgical Supply®, United States of America) mounted in dental technician drill, a bicortical 5 mm circular defect was drilled in both fossa masseterica (figure 5 – E). The surgical field was continuously irrigated with saline solution to reduce thermal damage. After the creation of the circular defect, the
was closed in two layers using a 4-0 resorbable sutures made of glyconate (Monosyn®, B. Braun
Melsungen AG®, Germany) for muscle and 4-0 non-resorbable sutures (Silkan®, B. Braun
Melsungen AG®, Germany) for skin. During this step, care was made to avoid the suture of the
nervus facialis and the ductus parotideus.
The anesthesia was reversed using a single dose of atipamezol chlorhydrate 1 mg/kg IP
(Antisedan®, Pfizer®, Germany). A single dose of butorphanol 0,4 mg/kg IP (Butador®,
Richterpharma AG®, Austria) was administrated for two to four hours postoperative pain relieve.
Figure 5 – Mandibular model surgical procedure. A – Patient positioning and the surgical field after the asepsis; B –
Mandibular skin incision exposing the musculus masseter; C – Incision and dissection of the m. masseter; D – Fossa
masseterica in the lateral surface of the ramus mandibulae; E – F. masseterica containing the created 5 mm bone
CSD; F – Implantation of the scaffold.
3.4. Postoperative care
Antibiotherapy was performed by dissolving in the drinking mineral water enrofloxacin 0,1
mg/mL H2O (Baytril® 5 %, Bayer®, Germany) during six consecutive days. It was also
administrated meloxicam 1,5 mg/kg SC SID (Movalis®, Boehringer Ingelheim®, Germany)
during five consecutive days for pain and inflammation relief.
As the surgical procedure severely affects the mastication, the pellet food was mixed with warm water until it got soft. This mixture was given to animals for two weeks and then the rats were slowly introduced to the pellet food. Other parameters as behavior, water and food intake, weight gain and wound healing were also daily monitored.