Article
Printed in Brazil - ©2016 Sociedade Brasileira de Química0103 - 5053 $6.00+0.00
*e-mail: dqmednei@furg.br
Photocatalytic Degradation for Treating Multipesticide Residues Using [Ru(bipy)
3]
Cl
2-Doped TiO
2/SiO
2Based on Surface Response Methodology
Bruno de S. Guimarães,a Arthur A. Bernardes,b Gabriela M. Salcedo,a
Sergiane S. Caldas,a Marianna B. Jorge,c Adalto Bianchini,c Silvana I. Wolkeb and
Ednei G. Primel*,a
aLaboratório de Análises de Compostos Orgânicos e Metais, Escola de Química e Alimentos,
Universidade Federal do Rio Grande, Av. Itália, km 8, s/n, 96203-900 Rio Grande-RS, Brazil
bInstituto de Química, Universidade Federal do Rio Grande do Sul, Av. Bento Gonçalves, 9500,
91501-970 Porto Alegre-RS, Brazil
cInstituto de Ciências Biológicas, Universidade Federal do Rio Grande, Av. Itália, km 8, s/n,
96203-900 Rio Grande-RS, Brazil
This study aimed to develop and optimize an efficient photocatalytic process employing ruthenium [Ru(bipy)3]
2+ doped TiO
2-SiO2 for degrading synthetic wastewater containing six classes of pesticides (bentazone, carbofuran, clomazone, diuron, tebuconazole and pyraclostrobin). To optimize the degradation conditions for the six pesticides and assess the effect of two variables (reaction time and adsorption equilibrium time) on the heterogeneous photocatalytic process, a 22 experimental design with a central composite rotatable design (CCRD) was used. The CCRD was suitable for optimizing the heterogeneous photocatalysis, and the generated surface responses indicated that the best removal conditions were 15 min adsorption equilibrium time and 110 min reaction time. Under these conditions, a pesticide removal between 71.00 and 99.98% was obtained. Furthermore, the system yielded an excellent degree of synthetic wastewater mineralization, with 97.60% total organic carbon (TOC) removal after 110 min.
Keywords: heterogeneous photocatalytic, multipesticide degradation, ruthenium, surface
response methodology
Introduction
Water pollution, contaminations and shortages are problems generating concern with respect to ground and fresh water quality.1,2 Many activities, such as
economic growth; worldwide technological, industrial and agricultural development; and population growth,3 result
in the contamination, pollution and deterioration of water resources. In this context, pesticides, pharmaceuticals, personal care products (PPCPs), polycyclic aromatic hydrocarbons (PAHs), textile dyes, and other substances can threaten the quality of water resources, because they are generally toxic and non-biodegradable.4-6
Recent studies have reported the risk of water contamination by pesticides in Southern Brazil and detected pesticides in ground, surface and drinking waters.7-9
In this context, finding a method to reduce the impact of these pollutants is of great relevance. Among the methods reported to remove organic pollutants from water, the advanced oxidation processes (AOPs) are the most investigated.10
AOPs can be considered appropriate alternatives for treating waters with pesticides. AOPs are based on generating a hydroxyl radical (HO.) as a powerful oxidant (2.8 V standard redox potential vs. hydrogen electrode). These processes can oxidize and mineralize a wide variety of organic compounds by generating water, carbon dioxide and inorganic ions or forming more biodegradable products.10,11
Heterogeneous photocatalysis stands out among the AOPs because it is an easy-to-handle and efficient process for degrading and mineralizing many toxic and recalcitrant pollutants. Moreover, TiO2 is the semiconductor most used
it is cheap, non-toxic, widely available and stable in water under environmental conditions.12,13
To improve the photocatalytic efficiency, TiO2 has been
supported on inorganic supports such as silica, zeolites, and aluminas. Of these supports, silica provides several advantages due to its thermal stability, low cost, and high surface area, and depending on the dispersion, depositing TiO2 onto SiO2 improves the TiO2 irradiation and leads to
better catalytic performance. Approaches such as metal doping and sensitization with [Ru(bipy)3]2+, for example,
have been used to further enhance the photocatalytic activity.14
Although much research on the oxidation of pollutants using heterogeneous photocatalysis has been recently conducted, few studies have reported the simultaneous degradation of several pesticides.15-20 For this reason, a
[Ru(bipy)3]2+ doped TiO2/SiO2 material was chosen for
multivariate pesticide degradation.
Therefore, this method can be considered an appropriate alternative for treating water containing pesticides. With this in mind, the TiO2/SiO2 and [Ru(bipy)3]2+doping system was
investigated based on a previous study showing this system to be the most active for the photodegradation of diuron.14
The heterogeneous photocatalysis mechanism using a TiO2 semiconductor as the photocatalyst begins by
generating hole/electron pairs (h+/e-) in the semiconductor
valence bands (h+
(VB)) and conduction bands (e-(CB))
according to equation 1. This process occurs when the TiO2 is irradiated by ultra-violet (UV) light with energy
equal to or higher than the corresponding band gap energy (for example, > 3.21 eV for TiO2 P25).15-21
TiO2 + hv TiO2(h+(VB)+ e-(CB)) (1)
The valence bands (h+
(VB)) may be scavenged via the
oxidation of some species, such as H2O, HO-, or organic
compounds, while the conduction bands (e
-(CB)) reduce O2 to
form hydroxyl radicals (HO.) and superoxide radical anions (O2.-), which can oxidize and mineralize a wide variety of
organic compounds.15-21
Therefore, this study aims to develop and optimize an efficient photocatalytic process using ruthenium [Ru(bipy)3]2+ doped TiO2-SiO2, a new catalyst, to degrade
synthetic wastewater containing six different pesticides (bentazone, carbofuran, clomazone, diuron, tebuconazole and pyraclostrobin). A 22 experimental design with a
central composite rotatable design (CCRD) was used to optimize the degradation conditions for these six pesticides and evaluate the effect of two variables (reaction time and adsorption equilibrium time) on the heterogeneous photocatalytic process.
Experimental
Reagents and chemicals
Tetraethylorthosilicate (TEOS), titanium(IV) isopropoxide, cetyltrimethylammonium chloride 25 wt.%, bentazone, carbofuran, clomazone, diuron, tebuconazole, pyraclostrobin, carbofuran-3-hidroxy and 3,4-dichloroaniline (3,4-DCA) analytical standards (purity > 99%) were purchased from Sigma-Aldrich (São Paulo, Brazil). Chromatography grade methanol and acetonitrile were supplied by Mallinckrodt (Phillipsburg, NJ, USA). Analytical grade phosphoric (85%) and formic (98%) acids were purchased from Merck (Darmstadt, Germany). Ultrapure water was
produced using a Direct-Q UV3® system (Millipore,
Bedford, MA, USA).
Preparation of [Ru(bipy)3]Cl2 doped TiO2/SiO2
The [Ru(bipy)3]Cl2·6H2O complex was prepared
according to the literature procedure.22
A solution of cetyltrimethylammonium chloride 25 wt.% (1.2 mL, 3.5 mmol) and 5.0 mol L-1 of urea
(10 mL, 50 mmol) was added to a 2.0 mol L-1 HCl solution
(30 mL). The mixture was stirred vigorously before adding TEOS (3.2 mL, 14.07 mmol). After 10 minutes, [Ru(bipy)3]Cl2·6H2O (14.20 mg; 0.018 mmol) and titanium
isopropoxide (2.1 mL, 7.03 mmol) were added and stirred at 750 rpm and 25 ºC until a solid formed. After the reaction, the obtained solid was extracted using a mixture of hexane and ethanol (1:1) via a Soxhlet extraction to remove the surfactant and complex. Excess complex was removed by washing with distilled water. The solid was dried in an oven at 50 ºC for six hours to yield 900 mg of an orange solid powder.
Photoreactor and light source
Photodegradation experiments
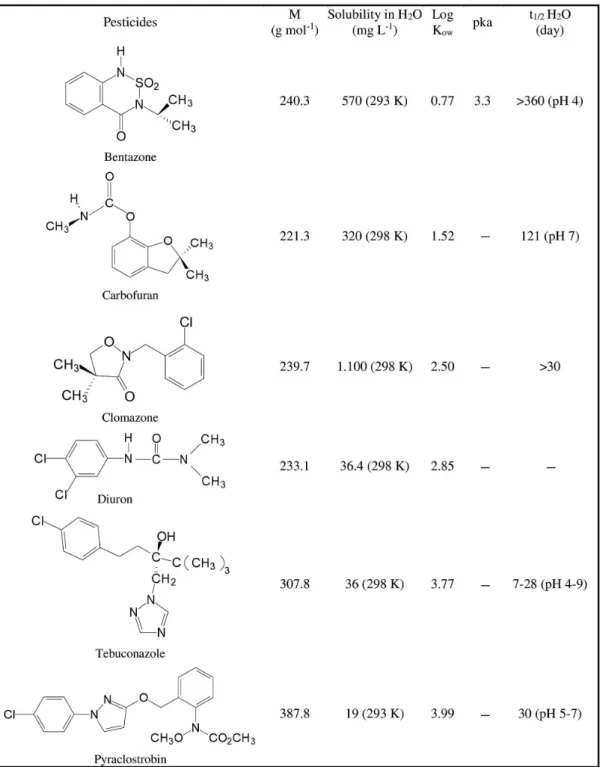
The synthetic wastewater used during the degradation experiments was prepared in the laboratory by adding 10 mg of each pesticide (bentazone, carbofuran, diuron, clomazone, tebuconazole and pyraclostrobin, see Figure 1) to 1 L of distilled water.
For all experiments, 250 mL of the synthetic wastewater (containing 10 mg L-1 of each pesticide) and 20 mg of the
catalyst at a pH of 7 were used. The mass of catalyst and pH conditions were defined according to previous study developed by Bernardes et al.14 Sodium hydroxide or
phosphoric acid solutions were used to adjust the pH. The effects of the reaction time and adsorption equilibrium time on the pesticide degradation were evaluated via an experimental design. The adsorption equilibrium time ranged from 0 to 30 min while the reaction time was evaluated from 10 to 110 min.
Figure 1. Chemical structures of the selected pesticides. M: molecular weight; Log Kow: octanol-water partition coefficient; pka: acid dissociation constant;
Sampling and analysis
The pesticide removal was monitored via high performance liquid chromatography (HPLC) with a diode array detector (LC-DAD). The LC-DAD separation was performed using an HPLC apparatus consisting of a Thermo BDS Hypersil C18 5 µm (250 × 4.6 mm i.d.) column from Thermo Scientific (USA), a Waters 600 pump model with an associated Waters 2996 Photodiode Array Detector (Milford, MA, USA) and Rheodyne 20 µL loop injector connected to the Empower PDA software for data acquisition. The UV spectra were recorded from 210-400 nm. For the LC-DAD analysis, the mobile phase was methanol (A) and ultrapure water pH 4.0 (B) acidified with phosphoric acid 1:1 (v/v). The gradient elution mode was: 0-6 min, 40% A; 6-7 min, 40-75% A; 7-25 min, 75% A; 25-26 min, 75-40% A; and 26-30 min, 40% A. The flow rates were: 0-6 min, 0.8 mL min-1; 6-7 min,
0.8-1.2 mL min-1; 7-25 min, 1.2 mL min-1; 25-26 min,
1.2-0.8 mL min-1 and 26-30 min, 0.8 mL min-1, for a 30 min
total running time.
Liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) was used to confirm the pesticide degradation and evaluate the formation of carbofuran-3-hydroxy and 3,4-DCA, which are byproducts of the pesticides carbofuran and diuron, respectively.9,23 The LC-ESI-MS/MS was performed
using a Waters Alliance 2695 Separations Module (Milford, MA, USA). The mass spectrometry was performed using a Micromass Quattro Micro API (Waters, Milford, MA, USA) with an ESI interface connected to the Masslynx software version 4.1, 2005, for data acquisition. The liquid chromatography was performed on an XTerra analytical column 3.5 µm (50 × 3 mm, i.d.) (Waters, Milford, MA, USA). The LC-ESI-MS/MS determinations were performed as described by Demoliner et al.9
The total organic carbon (TOC) content was measured using a TOC Analyzer (TOC, model TOC-V CPH, Shimadzu, Japan) with a non-purgeable organic carbon (NPOC) analysis.
Aliquots were collected before and after the treatment to characterize the sample and monitor the pesticide removal under different conditions. All aqueous samples were withdrawn and filtered using a Millex® PTFE 0.45 µm
filter, Millipore (Bedford, USA), before the analysis as described by Guimarães et al.24 The samples were mixed
with methanol and stored at −18 °C to stop the reaction. Nevertheless, the samples were mixed with Na2S2O3 for
the TOC content analysis.24
The pesticide removal (%) monitored by LC-DAD was determined using equation 2.
(2)
where C0 is the initial pesticide concentration and Ct is the
pesticide concentration after the treatment.
The TOC removal (degree of pesticide mineralization) was determined using a TOC Analyzer and the %TOC removal was calculated from equation 3:
(3)
where TOC0 is the initial total organic carbon concentration
and TOCt is the total organic carbon concentration after
the treatment.
Experimental design
This study used a central composite rotatable design (CCRD) with a five level fractional factorial design to construct a second order response surface model (RSM). This CCRD contains three types of points, cube points from the factorial design, axial points and center points, which can be used to calculate the experimental error.1,25
STATISTICA version 7.0 (StatSoft, Inc., 2004) was used for the data processing. An analysis of variance (ANOVA) was performed and the values were significant when
p < 0.05.
The reaction time (Rt) and adsorption equilibrium time (Ae) (independent variables) were optimized using the RSM. The CCRD was adopted to evaluate the combined effects of the two independent variables on one response (% pesticide removal) using eleven experimental sets. The ranges and levels of the two selected experimental parameters are shown in Table 1.
Results and Discussion
TiO2/SiO2 and [Ru(bipy)3]Cl2 doped TiO2-SiO2 materials
were prepared and characterized in a previous study14 for
the photodegradation of the pesticide Diuron. These materials performed well during this photodegradation and their activities were approximately four times that of the commercial catalyst P-25.14 Therefore, this material was
chosen for multipesticide photodegradation.
Optimization of heterogeneous photocatalysis by CCRD 22
removing pesticides because it promoted good pesticide removal in all experiments (removed over 70% of the pesticides diuron, clomazone, tecobunazole and pyraclostrobin under all of the studied conditions); however, for the pesticide carbofuran, the removal ranged between 40 and 96%, while bentazone ranged from 7 to 66%.
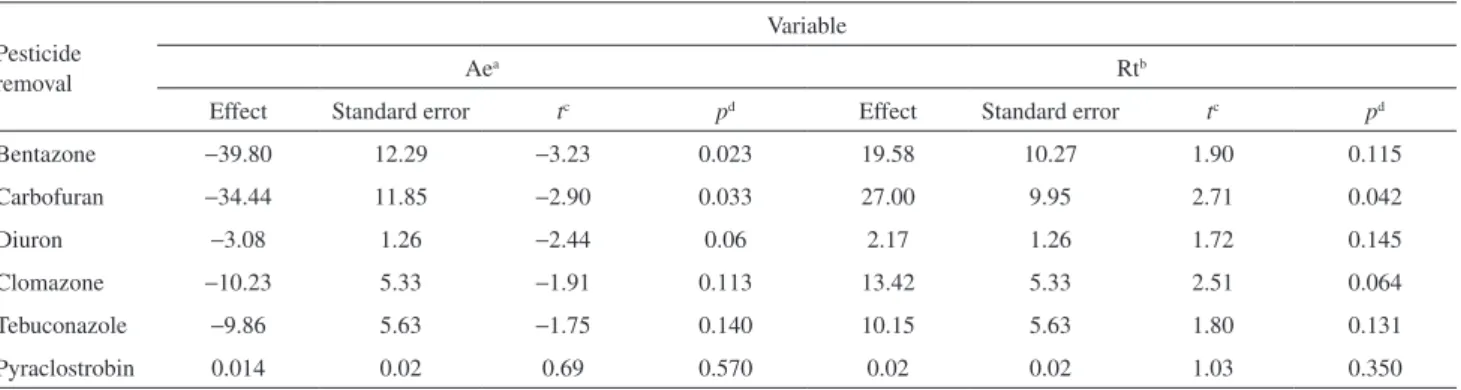
Table 2 shows that only the pesticide removal of bentazone and carbofuran was affected when the independent variables changed from the low (−1) level to the high (+1) level. The removal of the other pesticides occurred regardless of the reaction time and adsorption equilibrium time. In other words, any reaction time and adsorption equilibrium time exhibited high pesticide removal values.
As shown in Table 2, the bentazone removal was only affected by the adsorption equilibrium time, while
both variables affected the carbofuran removal. For the bentazone removal, changing from a low to a high adsorption equilibrium time reduced the removal to 39.80%. For carbofuran removal, the variable adsorption equilibrium time reduced the removal to 34.44%, while changing the reaction time increased the removal by 27.00%.
A second-order model describing the bentazone and carbofuran removal efficiency as a function of the adsorption equilibrium time and reaction time was established using equations 4 and 5.
Bentazone removal (%) = 58.05 – 17.54Ae2 (4)
Carbofuran removal (%) = 89.26 – 16.09Ae2 + 13.50Rt (5)
Table 1. The levels and ranges of variables and pesticide removal in heterogeneous photocatalysis process designed via composite rotatable design
Independent variable Symbol Coded variable (levels)
−1.41 −1 0 +1 +1.41
Reaction time / min Rta 10 30 60 90 110
Adsorption equilibrium time / min Aeb 0 10 15 20 30
Run Rt Ae Pesticide removal / %
Bentazone Carbofuran Diuron Clomazone Tebuconazole Pyraclostrobin
1 −1 −1 7.70 49.86 96.87 78.03 81.86 99.90
2 −1 +1 17.31 40.78 94.49 71.75 78.67 100.00
3 +1 −1 46.86 90.32 99.99 96.40 99.45 100.00
4 +1 +1 38.41 88.54 99.92 97.64 99.61 100.00
5 0 −1.41 40.48 82.5 99.69 93.88 98.17 99.98
6 0 +1.41 31.4 43.29 92.69 68.52 72.42 99.95
7 −1.41 0 53.33 82.63 99.88 92.35 98.33 99.99
8 +1.41 0 66.00 96.61 99.97 98.92 99.72 99.98
9 0 0 65.51 92.91 99.95 97.36 99.11 99.99
10 0 0 65.61 92.9 99.82 97.79 99.17 99.97
11 0 0 65.71 92.85 99.73 97.28 99.14 99.98
a Rt: reaction time; bAe: adsorption equilibrium time.
Table 2. Effects estimates for the experimental design for pesticide removal
Pesticide removal
Variable
Aea Rtb
Effect Standard error tc pd Effect Standard error tc pd
Bentazone −39.80 12.29 −3.23 0.023 19.58 10.27 1.90 0.115
Carbofuran −34.44 11.85 −2.90 0.033 27.00 9.95 2.71 0.042
Diuron −3.08 1.26 −2.44 0.06 2.17 1.26 1.72 0.145
Clomazone −10.23 5.33 −1.91 0.113 13.42 5.33 2.51 0.064
Tebuconazole −9.86 5.63 −1.75 0.140 10.15 5.63 1.80 0.131
Pyraclostrobin 0.014 0.02 0.69 0.570 0.02 0.02 1.03 0.350
The ANOVA for bentazone and carbofuran removal is reported in Table 3. The results show the model is predictive because the calculated F test is approximately twice the F value in both cases (F0.95,1,9 = 5.11 and
F0.95,2,8 = 4.45). Moreover, the regression coefficients
indicate both models are suitable, and the data reported in Table 4 shows that the model is predictive because the adjustment error is insignificant. Thus, both models reported as equations 4 and 5 were used to generate the respective response surfaces described in Figures 2 and 3.
The interaction between the independent variables (Ae vs. Rt) was evaluated via the analysis of response surfaces.
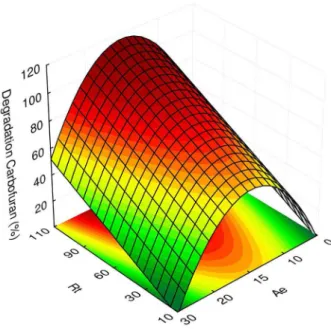
Figure 2 shows that the highest bentazone removal was achieved with a 15 min adsorption equilibrium time, and Figure 3 indicates the highest carbofuran removal was obtained with a 15 min adsorption equilibrium time and 110 min of reaction time.
Therefore, the optimum removal conditions for all of the pesticides in the synthetic wastewater were a 15 min adsorption equilibrium time and 110 min reaction time. Table 3. Analysis of variance by 22 composite rotatable design for pesticides removal
Source of variation Sum of square Degrees of freedom Mean quadratic F test
Bentazone removala
Regression 1900.50 1 1900.50 7.46
Residual 2293.54 9 254.84
Total 4194.04 10 −
Carbofuran removalb
Regression 3057.11 2 1528.55 7.46
Residual 1638.50 8 204.80
Total 4695.61 10 −
Regression coefficient: aR = 0.86, F
0.95,1,9 = 5.11; bR = 0.88, F0.95,2,8 = 4.45.
Table 4. Predicted optimum condition for removing bentazone and carbofuran by the photocatalytic degradation
Variable Response functions
Aea / min Rtb / min Bentazone removal / % Carbofuran removal / %
Actual Predicted Adj. err. Actual Predicted Adj. err.
15 110 66.05 58.05 12.11 96.61 108.35 12.15
aAe: adsorption equilibrium time; bRt: reaction time. Adj. err.: adjustment error.
Figure 2. Response surface for bentazone removal as a function of
Considerations for the adsorption equilibrium time
The fact that both pesticides had some resistance to degradation by the photocatalytic system makes necessary the use of a high Rt. Notably, high Ae values decrease the degradation, which also occurred for low Ae values. This decrease may occur due to the initial excitation of these compounds, which can adsorb to the catalyst surface and inject these excited electrons into the solid semiconductor.26
Moreover, the difference between the energy levels of the semiconductor and the redox potential of the adsorbed substrate drive the electron transfer process at the surface. Therefore, the photocatalytic efficiency of the system depends on various factors, such as the electron-hole recombination, that compete with the separation of the generated charges.
Removal efficiency and mineralization using optimized conditions
Applying the optimum removal conditions (pH 7.0, 20.0 mg catalyst, 15 min Ae and 110 min Rt) to the synthetic wastewater containing 10 mg L-1 of each pesticide yielded
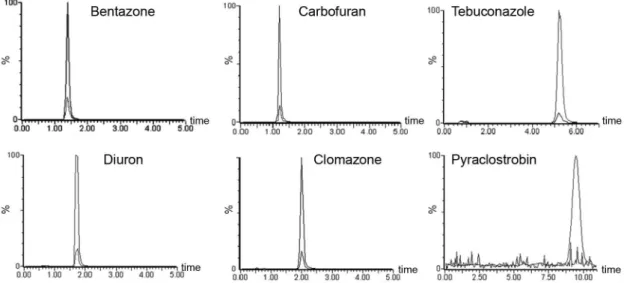
the highest observed removal efficiencies. Figure 4 shows the chromatographic profile of the synthetic wastewater before and after the heterogeneous photocatalysis treatment.
Figure 5 shows the removal values for each pesticide by the heterogeneous photocatalysis under the optimal conditions. These values ranged between 71.00 and 100.00%, which proves this system is suitable for and extremely efficient at removing these pesticides. Moreover, the system being developed yielded an excellent degree of synthetic wastewater mineralization, with 97.60% of
the TOC removed after 110 min. The TOC value reduced from 105.00 to 2.50 mg L-1 of carbon, which complies with
Brazilian laws regarding effluent disposal.27 Furthermore,
carbofuran and diuron byproducts were also removed during the synthetic wastewater treatment. These compounds were detected in lower concentrations (0.36 µg L-1 of 3,4-DCA
and 92 µg L-1 of carbofuran-3-hidroxy) after the treatment.
Conclusions
The developed system proved to be efficient in the removal of the six pesticides. Moreover, the central composite rotatable design was suitable for optimizing the heterogeneous photocatalysis. The generated surface responses indicated the best removal conditions were 15 min Ae and 110 min Rt. Under these conditions, Figure 4. Chromatograms of synthetic wastewater before and after treatment by heterogeneous photocatalysis. Experimental conditions: pH 7.0, 20.0 mg catalyst, 15 min adsorption equilibrium time and 110 min reaction time.
pesticide removals between 71.00 and 99.98% were achieved. Furthermore, the system yielded an excellent degree of synthetic wastewater mineralization with 97.60% TOC removal after 110 min.
Acknowledgments
The authors acknowledge the financial support and fellowships granted by the Brazilian agencies CAPES and FURG. Part of this study was supported by a grant from the Brazilian agencies FAPERGS/CNPq (process number 010/0022-0), CNPq/CAPES (process number 552318/2011-6), CNPq (process number 477083/2011-00), FAPERGS (process number 810-25.51/13-3), and FAPERGS (process number 831-25.51/13-0). E. G. Primel received a productivity research fellowship from the Brazilian agency CNPq (DT 310517/2012-5).
References
1. Sakkas, V. A.; Islam, M. A.; Stalikas, C.; Albanis, T. A.;
J. Hazard. Mater.2010, 175, 33.
2. Sahoo, C.; Gupta, A. K.; J. Hazard. Mater.2012, 215, 302. 3. Oturan, M. A.; Oturan, N.; Edelahi, M. C; Podvorica, F. I.; El
Kacemi, K.; Chem. Eng. J. 2011, 171, 127.
4. Cabrera, L. C.; Caldas, S. S.; Rodrigues, S.; Bianchini, A.; Duarte, F. A.; Primel, E. G.; J. Braz. Chem. Soc.2010, 21, 2347. 5. Laurent, F.; Cébron, A.; Schwartz, C.; Leyval, C.; Chemosphere
2012, 86, 659.
6. Capdeville, M. J.; Budzinski, H.; Trends Anal. Chem.2011, 30, 586.
7. Cabrera, L. C.; Costa, F. P.; Primel, E. G.; Quim. Nova2008,
31, 1982.
8. Caldas, S. S.; Demoliner, A.; Costa, F. P.; D’Oca, M. G. M.; Primel, E. G.; J. Braz. Chem. Soc.2010, 21, 642.
9. Demoliner, A.; Caldas, S. S.; Costa, F. P.; Gonçalves, F. F.; Clementin, R. M.; Milani, M. R.; Primel, E. G.; J. Braz. Chem. Soc.2010, 21, 1424.
10. Oller, I.; Malato, S.; Sànches-Pérez, J. A.; Environ. Sci. Technol.
2011, 409, 4141.
11. Guimarães, J. R.; Maniero, M. G.; Araújo, R. N.; J. Environ. Manage.2012, 110, 33.
12. Gupta, A. K.; Pal, A.; Sahoo, C.; Dyes Pigments2006, 69, 224. 13. Vaez, M.; Moghaddam, A. Z.; Mahmoodi, N. M.; Alijani, S.;
Process Saf. Environ. Prot.2012, 90, 56.
14. Bernardes, A. A.; Bulhosa, M. C. S.; Gonçalves, F. F.; D’Oca, M. G. M.; dos Santos, J. H. Z.; Wolke, S. I.; Quim. Nova2011,
34, 1343.
15. Lagunas-Allué, L.; Martínez-Soria, M. T.; Sanz-Asensio, J.; Salvador, A.; Ferronato, C.; Chovelon, J. M.; Appl. Catal. B
2012, 115, 285.
16. Morais, J. L.; Sirtori, C.; Peralta-Zamora, P. G.; Quim. Nova
2006, 29, 20.
17. Cho, M.; Choi, Y.; Chemosphere2007, 70, 97.
18. Yuan, R.; Ramjaun, S. N.; Wang, Z.; Liu, J.; Chem. Eng. J.
2012, 192, 171.
19. Na, S.; Ahn, Y. G.; Cui, M.; Khim, J.; J. Environ. Manage.2012,
101, 104.
20. Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S.; J. Photochem. Photobiol., C 2012,
13, 224.
21. Pozan, G. S.; Kambur, A.; Chemosphere2014, 105, 152. 22. Braddock, J. N.; Meyer, T. J.; J. Am. Chem. Soc.1973, 95, 3158. 23. Lu, L. A.; Ma, Y. S.; Kumar, M.; Lin, J. G.; Chem. Eng. J.2011,
166, 150.
24. Guimarães, B. S.; Kleemann, N.; Caldas, S. S.; Costa, F. P.; Silveira, M. A. K.; Duarte, F. A.; Primel, E. G.; Environ. Sci. Pollut. Res.2014, 21, 584.
25. Ferreira, S. L. C.; Bruns, R. E.; Ferreira, H. S.; Matos, G. D.; David, J. M.; Brandão, G. C.; da Silva, E. G. P.; Portugal, L. A.; dos Reis, P. S.; Souza, A. S.; dos Santos, W. N. L.; Anal. Chim. Acta 2007, 597, 179.
26. Carp, O.; Huisman, C. L.; Reller, A.; J. Hazard. Mater.2004,
32, 33.
27. National Environmental Council, Ministério do Meio Ambiente, Conselho Nacional do Meio Ambiente (CONAMA), Resolution No. 357, 03/17/2005 Dispõe sobre a Classificação dos Corpos de Água e Diretrizes Ambientais para o seu Enquadramento, bem como Estabelece as Condições e Padrões de Lançamento de Efluentes, e Dá Outras Providências. Available at http:// www.mma.gov.br/port/conama/res/res05/res35705.pdf, accessed on April 2016.
Submitted: January 27, 2016 Published online: April 15, 2016