Accepted
Article
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as DR TAINA VERAS SANDES-FREITAS (Orcid ID : 0000-0002-4435-0614)
Article type : Original Report
Tuberculosis after kidney transplantation is associated with significantly
impaired allograft function
Silvana Daher Costa1,2, Tainá Veras de Sandes-Freitas1,2, Camilla Neves Jacinto1, Lorena
Vasconcelos Mesquita Martiniano1, Yago Sucupira Amaral¹, Fernando José Villar Nogueira
Paes², Maria Luiza de Mattos Brito Oliveira Sales2, Ronaldo de Matos Esmeraldo2, Elizabeth
de Francesco Daher1
1
Post-Graduation Program in Medical Sciences, Department of Internal Medicine, School of
Medicine, Federal University of Ceará, Fortaleza, Ceará, Brazil
2
Division of Renal Transplantation, Hospital Geral de Fortaleza, Fortaleza, Ceará, Brazil.
Running title: COSTA ET AL.
Correspondence: Silvana Daher Costa
Post-Graduation Program in Medical Sciences,
Department of Internal Medicine,
School of Medicine, Federal University of Ceará,
Fortaleza, Ceará, Brazil.
Accepted
Article
Abstract
Background: This study aimed to evaluate renal function before, during and after the course of tuberculosis (TB) disease in kidney transplant recipients; and assess the risk factors for
non-recovery of baseline renal function.
Methods: We performed a retrospective, single-center cohort study, including all patients with confirmed or presumed TB diagnosis after kidney transplant (n=34, 2.1%). Renal
function was assessed by serum creatinine (Cr) and glomerular filtration rate (GFR) adjusted
for deaths and graft losses.
Results: A significant increase was seen in serum Cr during TB disease and treatment: 1.5 mg/dL at baseline (Crbase), 1.7 mg/dL at diagnosis (P<.001 vs. Crbase), and 2.4 mg/dL during
the peak (P<.001 vs. Crbase). According to AKI-Kidney Disease: Improving Global Outcomes
(KDIGO) classification, 29 (85%) patients had acute kidney injury (AKI): 16 stage 1, 2 stage 2,
and 11 stage 3. Three months after the end of the TB treatment, 5 patients (14.7%) had lost
their graft and 2 others (5.9%) had died. The GFR was lower than the baseline (42.4 mL/min
vs. 51.6 mL/min, P=.007). In the univariate analysis, peak Cr (odds ratio [OR] 1.276, 95%
confidence interval [CI] 0.955-1.705, P=.100), AKI-KDIGO stages 2 or 3 (OR 4.958 95% CI
1.062-23.157, P=.042), severe disease (OR 5.700 95% CI 1.147-28.330, P=.033), and acute
rejection (AR) episodes after TB diagnosis (OR 3.937, 95% CI 0.551-28.116, P=.172) were
associated with non-recovery of baseline renal function. No variable was identified in the
multivariable model.
Conclusion: Post-transplantation TB was associated with a high incidence of AKI and complete recovery of baseline renal function was not achieved after treatment. The severity
of TB disease, AKI, and AR episodes that occurred after TB diagnosis are potential causes for
Accepted
Article
Keywords:
kidney transplantation, renal function, tuberculosis
1 INTRODUCTION
Tuberculosis (TB) is a public health problem and 80% of cases occur in developing
countries.1,2 The incidence in Brazil is 30.9 cases/100 000 inhabitants, with about 70 000
new cases per year and 4.4 thousand deaths.3 TB is the most common neglected tropical
disease occurring after kidney transplantation.4 It occurs by either activation of quiescent
foci of Mycobacterium tuberculosis, transmission via the allograft, or new acquisition from
exposure to another person with the infection. The incidence of TB in transplant recipients
is 20-50 times higher than in the general population, ranging from 0.5% to 1% in North
America to 15% in India and Pakistan.4 Studies in Brazilian transplant centers have reported
incidences of 1.17% to 5%.5-8
Previous studies have shown that TB in kidney transplant recipients (KTRs)
predominantly occurs as extrapulmonary or disseminated forms and is associated with high
mortality.4 In addition, some studies have reported high rates of death-censored graft loss
in the months following the TB diagnosis. Acute rejection (AR) and chronic rejection
secondary to immunosuppressive minimization or withdrawal because of disease severity
are the main reported causes of graft loss. 5,9,10
Little is known about the impact of TB disease and its treatment on renal allograft
function. To the best of our knowledge, no study has systematically evaluated renal function
Accepted
Article
The aim of this single-center retrospective study was to evaluate the incidence of
acute kidney injury (AKI) during TB disease in a cohort of KTRs living in an endemic region, to
assess renal function 3 months after the treatment and evaluate the risk factors for
non-recovery of baseline renal function.
2 MATERIALS AND METHODS
Retrospective cohort study including patients who received a kidney allograft between
January 1994 and November 2014 in a single center in Fortaleza, Ceará, located in the
Northeast of Brazil, and had TB disease after transplant. The study was approved by the
local Ethics Committee.
Patients who received multiorgan transplants, those who lost to follow-up within the
first 6 months after transplantation, and patients with insufficient clinical data were
excluded. All patients treated for TB for at least 6 months or until death were included. In
the study period, TB diagnosis was based on positive acid-fast bacilli (AFB) smear, high
adenosine deaminase levels, histological findings, and positive culture for M. tuberculosis.
Empirically treated patients were also included when clinical presentation was suggestive,
extensive clinical investigation excluded other diagnoses, and clinical improvement was
observed after treatment. These cases were defined as “presumed TB.”
According to local practice, patients with prior TB at any time before transplantation,
patients with tuberculin skin test (TST) ≥ 5mm, or patients that had contact with active TB
received treatment for latent TB infection (LTBI), with isoniazid 300 mg/day for 6 months.11
To improve adherence, patient education and instructions were provided at every visit and
Accepted
Article
Disseminated TB was defined as the involvement of bloodstream, bone marrow,
liver, or two or more noncontiguous sites, or miliary TB.12 Severe TB disease was defined as
severe sepsis and/or intensive care unit admission.
Patients were treated according to the Brazilian guidelines: until 2009, the standard
treatment consisted of rifampicin, isoniazid, and pyrazinamide (RIP) for 2 months, followed
by rifampicin and isoniazid (RI) for at least 4 additional months, depending on the site and
immunosuppressive status. From 2009 on, ethambutol (E) was added to the first 2 months
of treatment.13,14
Renal function was assessed by serum creatinine (Cr) and estimated by glomerular
filtration rate (GRF)15 using four-variable MDRD formula before the diagnosis (baseline,
Crbase, GFRbase), at TB diagnosis, and before treatment (Crdiag, GFRdiag), during the TB
treatment (the highest Cr, Crmax, GFRmin), and 3 months after the end of the treatment (Crpost,
GFRpost). Baseline Cr was defined as the mean of the two last measurements prior to TB
diagnosis. Patients with AKI were classified according to the Kidney Disease: Improving
Global Outcomes (KDIGO) staging criteria using the highest Cr obtained since TB diagnosis
up to the end of treatment, as follows: 16
Stage 1: increase in Cr ≥ 0.3mg/dL; or increase in Cr of 1.5-1.9 times the baseline; or urinary
volume <0.5 mL/kg/hour for 6-12 hours.
Stage 2: increase in Cr of 2-2.9 times the baseline; or urinary volume <0.5 mL/kg/hour for
≥12 hours.
Stage 3: increase in Cr to ≥ 4.0 mg/dL or start of dialysis; or urinary volume <0.3 mL/Kg/hour
≥24 hours or anuria >12 hours.
Non-recovery of baseline renal function was defined as graft loss or Crpost at least 25%
Accepted
Article
2.1 Statistical analysis
Continuous variables are expressed as means and standard deviations, or medians and
range, when standard deviations were higher than means. Discrete variables are expressed
as frequencies and percentages. The Shapiro-Wilks test was used to verify the normality of
quantitative variables. The last observation carried forward analysis was used for missing
GFR values, attributing 10 mL/min for patients who lost the graft and the last available GFR
for those who died or lost to follow up. Friedman’s test was used to compare renal function
over time. Logistic regression was used for uni- and multivariable analysis. Variables with
P-value <.20 in the univariate analysis were included in the multivariable model. The
statistically significant difference was assumed when P-value was <.05. Statistical analyses
were performed using SPSS 20.0 for Windows (SPSS Inc, Chicago, IL, USA).
3 RESULTS
3.1 Population and demographics
Of 1604 patients submitted to kidney transplantation during the study period, 34 (2.1%)
were diagnosed with post-transplant TB. Patients were predominantly young (41.0±12.7
years old), males (70.6%), non-Caucasian (76.5%), and were submitted to deceased-donor
transplantations (58.8%). Induction therapy with depleting or non-depleting antibodies was
used in 61.8%. The TB diagnosis was attained 25.5 (1-168) months after the transplantation
and 30 patients (88.2%) were receiving calcineurin inhibitor-based regimens. Fifteen (44.1%)
patients had AR episodes 10.5 (1-196) months before the TB diagnosis (Table 1).
Of the 34 patients, 1 received LTBI therapy because of TB disease that occurred 14
years prior to kidney transplantation; 21 patients had no clinical indication for isoniazid; 10
Accepted
Article
treatment because of TST ≥ 5mm, but accidentally they did not receive it (Figure 1). Of the
10 patients who developed TB disease despite LTBI treatment, in 3 the disease occurred
early after transplantation (2, 3, and 4 months), still during isoniazid therapy. Two of these 3
had the disseminated forms, including in the urinary tract. In the remaining 7 patients, TB
disease occurred after isoniazid therapy, late after transplantation (mean of 38 months,
ranging from 8 to 90 months). In a single patient, close contact with a relative who had
active pulmonary TB was documented and a new exposure was considered likely. There
were no reports of noncompliance to LTBI treatment in the medical records.
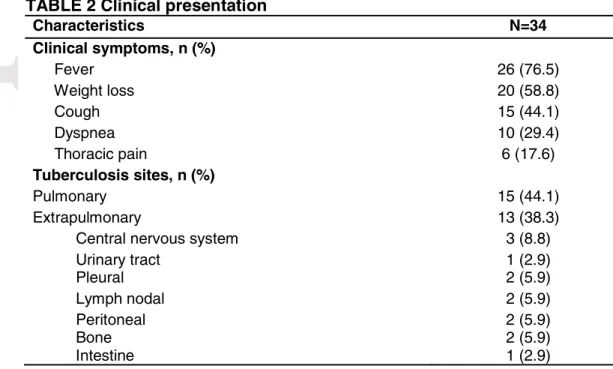
3.2 Clinical presentation, diagnosis, and treatment
The main symptoms were fever (76.5%), weight loss (58.8%), and cough (44.1%). Fifteen
patients (44.1%) had isolated pulmonary involvement; 13 (38.2%) had one extrapulmonary
site; 1 (2.9%) had pleuropulmonary TB; 4 (11.8%) had disseminated TB; and 1 patient who
had prolonged fever of unknown origin were empirically treated for TB.
Thirty-one patients (91.2%) had a confirmed TB diagnosis. In 16 (47.0%), AFB smear
microscopy using Ziehl-Neelsen stain was positive. In 5 patients (14.7%), high adenosine
deaminase levels in biological fluids were observed. In 10 patients (29.4%), tuberculous
granuloma on histological examination of biopsy samples was found, and positive culture
for M. tuberculosis was obtained in 4 patients. Of note, in most patients, M. tuberculosis
culture was not found in the medical records and laboratory results. Three patients were
treated based on high clinical suspicion, but had no diagnostic confirmation (presumed TB):
two presented with fever and pulmonary infiltrates. They had negative bronchoalveolar
lavage smears and cultures and no alternative diagnosis on available serological and
Accepted
Article
improvement in pulmonary infiltrates. One patient had fever of unknown origin and weight
loss. Extensive clinical investigation was performed and no cause was found. After
treatment, there was clinical improvement and fever resolution. For detailed information
about clinical presentation and diagnosis, see Table 2.
For all patients, TB treatment was a combination of three or four antituberculous
drugs for 12.2 ± 6.9 months (range 3-36 months). Eleven patients (32.4%) had previously
received standard treatment without ethambutol (RIP); 22 (64.7%) were treated with the
current standard treatment with this drug17; 1 patient (2.9%) could not receive
pyrazinamide because of intolerance and was treated with rifampicin, isoniazid, and
ethambutol. One patient was diagnosed with multidrug resistant (MDR) TB 30 months after
transplantation. Nine months after the standard treatment for urinary TB (RIP) without
clinical improvement, MDR was confirmed and an additional period of 12 months of
treatment with streptomycin, ethambutol, and ofloxacin was employed. The patient did not
meet the criteria for LTBI treatment.
Clinical management after TB diagnosis, including immunosuppressive drug
monitoring, was performed at the discretion of the attending physician.
3.3 Renal function and outcomes
The mean serum Cr at baseline (Crbase) was 1.5 mg/dL (0.9-3.5 mg/dL), rising to 1.7mg/dL
(1.0-6.5 mg/dL) at diagnosis (Crdiag) (P<.001 vs Crbase). The mean highest serum Cr during TB
treatment (Crmax) was 2.4 mg/dL (1.2-11mg/dL) (P <.001 vs Crbase). Three months after the
end of the TB treatment, 5 patients (14.7%) had lost their grafts (1 AR, 1 immune interstitial
fibrosis and tubular atrophy (IF/TA), 1 nonimmune IF/TA, 2 sepsis); and other 2 (5.9%) had
Accepted
Article
functioning grafts, Crpost was 1.5 mg/dL (0.9-3.9 mg/dL) (P=.700 vs Crbase) (Figure 2).
Considering the last observation carried forward adjustment, the estimated GFR
corresponding to these periods (GFRbase, GFRdiag, GFRmin, GFRpost) were 51.6 mL/min, 43.4
mL/min, 29.4 mL/min, and 42.4 mL/min, respectively (P=.029 GFRdiag vs GFRbase,P<.001
GFRmin vs GFRbase, and P=.032 GFRpost vs GFRbase) (Figure 3).
Twenty-nine patients (85.3%) showed AKI criteria at any time from TB diagnosis to
the end of the treatment: 16 were KDIGO stage 1, 2 were stage 2, and 11 were stage 3.
AKI was observed at TB diagnosis in 9 patients and the causes were as follows:
prerenal (n=1), urinary obstruction (n=1), sepsis (n=2), and amphotericin (n=1). The etiology
was unclear in 4 patients. The remaining 20 patients developed AKI after TB diagnosis and
treatment start (mean of 91 days, ranging from 15 to 613). Reported causes were prerenal
(n=2), sepsis (n=5), amphotericin (n=1), and tacrolimus nephrotoxicity secondary to
clarithromycin association (n=1). The etiology was unclear in 9 patients. Of note, all 10
patients who developed severity criteria (severe sepsis and/or intensive care unit
admission) had AKI, 6 of them KDIGO 2/3.
Three patients had AR episodes during TB treatment. The first patient showed AR
Banff grade IIA 27 months after transplantation and 2 months after the onset of TB
treatment. There was prolonged low exposure to tacrolimus. Despite treatment with
anti-thymocyte globulin, the patient lost the graft. The second patient had a Banff IA rejection 4
months after transplantation and 2 months after the onset of TB treatment. Tacrolimus was
withdrawn after TB diagnosis as a minimization strategy. The patient was successfully
treated with methylprednisolone pulse therapy. The third patient had a borderline infiltrate
32 months after transplantation, in month 10 of TB treatment. Although low exposure to
Accepted
Article
dysfunction. There was no unequivocal evidence of noncompliance. He received
methylprednisolone and Cr levels decreased.
After the onset of TB treatment, mean tacrolimus trough levels decreased from
6.2±2.3 ng/mL (before treatment) to 1.9±1.0 ng/mL (first concentration after treatment),
reaching 1.7±0.8 ng/mL (the lowest value). Similar underexposure occurred with
cyclosporine, for which C2 levels were reduced from 619±213 ng/mL to 408±161 ng/mL,
reaching 295±149 ng/mL. Significant variability was seen in the intervals between
immunosuppressive drug monitoring and in the management of doses according to
concentrations. Nine patients had their immunosuppressive regimens minimized or
completely withdrawn.
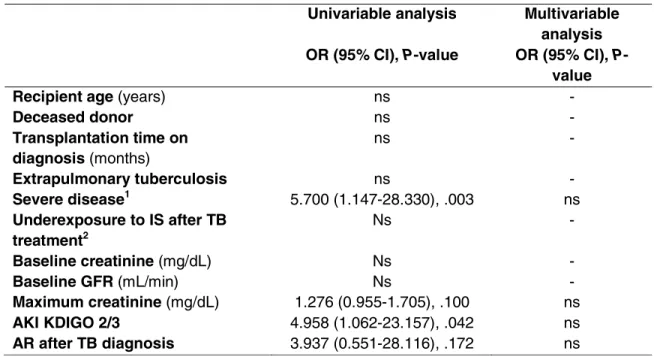
Variables associated with non-recovery of baseline renal function after TB treatment
in the univariate analysis were maximum Cr (OR 1.276, 95% CI 0.955-1.705, P=.100), AKI
KDIGO stages 2 or 3 (OR 4.958 95% CI 1.062-23.157, P=.042), severe disease (OR 5.700 95%
CI 1.147-28.330, P=.033), and AR episode after TB diagnosis (OR 3.937, 95% CI 0.551-28.116,
P=.172). In the multivariable analysis, none of the tested variables was significant (Table 3).
We also compared the 5 patients who lost their grafts with the 29 who persisted
with functioning grafts. As a result, the mean maximum Cr was higher in those who lost the
graft (4.3±3.0 vs. 1.6± 2.3 mg/dL, p=.005). All these 5 patients had AKI KDIGO 2/3 (100% vs.
20.7%, P=.002).
4 DISCUSSION
This study showed that TB disease is associated with a significant incidence of AKI and
permanent allograft impairment. Severe AKI and AR episodes, which are common events
Accepted
Article
In our cohort of KTRs, the demographics and clinical characteristics of TB disease
were quite similar to those described by other transplant centers located in endemic
regions, with late occurrence, high incidence of extrapulmonary and disseminated forms,
difficulty in attaining a definitive diagnosis, and prolonged treatments.2,7,18 Studies
performed in low-incidence countries have demonstrated earlier clinical presentation.19,20
This is probably a result of a higher incidence of reactivation or donor-derived infection than
a new acquisition of TB. The high percentage of patients with a history of previous
treatment for AR indicates a possible state of over immunosuppression. Interestingly,
despite the routine treatment of LTBI for high-risk patients, TB disease was diagnosed as
early as 1 month after transplantation. These cases probably occurred in patients with
latent TB that were not identified by the available methods. Early TB disease after
transplantation in patients with or without isoniazid treatment, may even be due to
unrecognized active disease at the time of transplantation or donor-derived infection.
Compared with Brazilian studies, TB incidence was somewhat lower. 5-8 It is possible that the
routine use of LTBI therapy for at-risk patients might be the reason for this finding.
Our data showed a significant increase in serum Cr during and after TB diagnosis
and, consequently, a high prevalence of AKI, including the severe forms. Graft dysfunction
has been previously described in other TB cohorts, but little has been reported about the
causes of AKI.6,8 In our study, prerenal azotemia, sepsis, AR, and drug nephrotoxicity were
the main identified causes of graft dysfunction. The pharmacokinetics interaction between
the antituberculous drugs, especially rifampicin, and calcineurin inhibitors is an important
cause of the increased incidence of AR during TB treatment. Furthermore, in clinically
severe patients, the staff often minimizes maintenance immunosuppression, which is also a
Accepted
Article
Three months after TB treatment, when adjusting GFR for deaths and graft losses,
patients did not recover the baseline renal function, showing a permanent damage to the
allograft function and structure. Interestingly, the TB disease and AKI severity, and AR
episodes were associated with this outcome. These variables were not significant in the
multivariable analysis, probably owing to the small sample size.
This study has the limitations inherent to a retrospective and single-center study.
These results reflect the clinical presentation of TB disease in patients living in a country
with endemic TB, and may not be extrapolated to low-incidence areas. Although treated as
TB, not all patients had this diagnosis confirmed. Considering the severity of TB in KTRs and
the endemicity in our country, empirical treatment is a relatively common practice in
patients with high clinical suspicion and logistical difficulties for an extensive investigation.
Moreover, in developing countries, even though cultures are more sensitive than
microscopy analysis, diagnosis is primarily based on AFB smear microscopy owing to its
simplicity, lower cost, and fast results. The study period should also be considered. From
1994 to 2014, profound changes have occurred in the transplantation medical care, which
certainly impacted the risk of nephrotoxicity associated with TB treatment. However, to the
best of our knowledge, this is the first study to assess the renal function of patients with TB
after transplantation in a systematic fashion, adding new knowledge about this prevalent
infection. Moreover, the understanding of the risk factors for non-recovery of baseline
allograft function in these patients is an important step in the implementation of preventive
actions.
In conclusion, TB after kidney transplantation was associated with a high incidence
of AKI and, 3 months after the end of the treatment, GFR was significantly inferior to
Accepted
Article
causes for non-recovery of baseline renal function. This study highlights the importance of
the close monitoring of renal function and drug-drug interactions during anti-mycobacterial
therapy, as well as careful management of immunosuppression aiming to prevent excessive
underexposure and consequent rejection episodes. Despite the small sample size, half of
the patients with LTBI who did not receive isoniazid after transplantation developed TB
disease, emphasizing the importance of this treatment.
Acknowledgements
The authors would like to thank Professor Rosa Maria Salani Mota for statistical support.
Author contributions
S.D.C.: Concept/design, data collection, statistics, data analysis and interpretation, drafting
article, critical revision of article; T.V.S.-F: Data analysis and interpretation, drafting article,
critical revision of article; C.N.J.: Data collection, drafting article; L.V.M.M.: Data collection;
Y.S.A.: Data collection, statistics; F.J.V.N.P.: Data analysis and interpretation, drafting article;
M.L.M.B.O.S.: Data analysis and interpretation, drafting article; R.M.E.: Concept/design,
critical revision of article, approval of article; E.F.D.: Concept/design, data analysis and
interpretation, critical revision of article, approval of article
Accepted
Article
REFERENCES
1. Lindoso JA, Lindoso AA. Neglected tropical diseases in Brazil. Rev Inst Med Trop Sao
Paulo. 2009;51:247-253.
2. Canet E, Dantal J, Blancho G, Hourmant M, Coupel S. Tuberculosis following kidney
transplantation: clinical features and outcome. A French multicentre experience in
the last 20 years. Nephrol Dial Transplant. 2011;26:3773-3778.
3. Perspectivas brasileiras para o fim da tuberculose como problema de saúde pública.
Boletim Epidemiológico. Secretaria de Vigilância em Saúde - Ministério da Saúde -
Brasil, volume 47, n 13, 2016. Disponível em:
http://portalarquivos.saude.gov.br/images/pdf/2016/marco/24/2016-009-Tuberculose-001.pdf.
4. Machado CM, Martins TC, Colturato I, et al. Epidemiology of neglected tropical
diseases in transplant recipients. Review of the literature and experience of a
Brazilian HSCT center. Rev Inst Med Trop Sao Paulo. 2009;51:309-324.
5. Marques ID, Azevedo LS, Pierrotti LC, et al. Clinical features and outcomes of
tuberculosis in kidney transplant recipients in Brazil: A report of the last decade. Clin
Transplant. 2013;27:E169-176.
6. Guida JP, Bignotto Rosane D, Urbini-Santos C, Alves-Filho G, Ribeiro Resende M,
Mazzali M. Tuberculosis in renal transplant recipients: A Brazilian center registry.
Transplant Proc. 2009;41:883-884.
7. Romao Junior JE. Tuberculosis in renal transplant recipients: Challenges in
Accepted
Article
8. Meinerz G, da Silva CK, Goldani JC, Garcia VD, Keitel E. Epidemiology of tuberculosis
after kidney transplantation in a developing country. Transpl Infect Dis.
2016;18:176-182.
9. Bodro M, Sabe N, Santin M, et al. Clinical features and outcomes of tuberculosis in
solid organ transplant recipients. Transplant Proc. 2012;44:2686-2689.
10. Liu J, Yan J, Wan Q, Ye Q, Huang Y. The risk factors for tuberculosis in liver or kidney
transplant recipients. BMC Infect Dis. 2014;14:387.
11. de Lemos AS, Vieira MA, Halpern M, et al. Results of implementation of preventive
recommendations for tuberculosis after renal transplantation in an endemic area.
Am J Transplant. 2013;13:3230-3235.
12. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. Am J
Respir Crit Care Med. 2000;161(4 Pt 1):1376-1395.
13. Divisao de Tuberculose CPAVCS. [Changes in tuberculosis treatment]. Rev Saude
Publica. 2010;44:197-199.
14. Conde MB, Melo FA, Marques AM, et al. III Brazilian Thoracic Association Guidelines
on tuberculosis. J Bras Pneumol. 2009;35:1018-1048.
15. Merchan C, Parajuli S, Siegfried J, Scipione MR, Dubrovskaya Y, Rahimian J.
Multidrug-resistant Bacteroides fragilis bacteremia in a US resident: An Emerging
challenge. Case Rep Infect Dis. 2016;2016:3607125.
16. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin
Pract. 2012;120:c179-c184.
17. Castilla J, Moran J, Fernandez-Alonso M, et al. [The 2009 H1N1 flu pandemic in
Accepted
Article
18. Higuita LM, Nieto-Rios JF, Daguer-Gonzalez S, et al. Tuberculosis in renal transplant
patients: The experience of a single center in Medellin-Colombia, 2005-2013. J Bras
Nefrol. 2014;36:512-518.
19. Holty JE, Gould MK, Meinke L, Keeffe EB, Ruoss SJ. Tuberculosis in liver transplant
recipients: A systematic review and meta-analysis of individual patient data. Liver
Transpl. 2009;15:894-906.
20. Lopez de Castilla D, Schluger NW. Tuberculosis following solid organ transplantation.
Transpl Infect Dis. 2010;12:106-112.
Figure legends
FIGURE 1 Distribution of patients with tuberculosis (TB) disease after kidney transplantation according to latent TB screening. TST, tuberculin skin test; LTBI, latent tuberculosis
infection. LTBI therapy was performed using isoniazid 300 mg/day for 6 months
FIGURE 2 Serum creatinine (Cr) before tuberculosis (TB) diagnosis (Crbase), at diagnosis
(Crdiag), the highest creatinine during TB treatment (Crmax) and 3 months after the end of the
treatment (Crpost). P<.001 Crdiag vs. Crbase; P<.001 Crmax vs. Crbase;P=.700 Crpost vs. Crbase
FIGURE 3 Estimated glomerular filtration rate (GFR)15 before tuberculosis (TB) diagnosis (GFRbase), at diagnosis (GFRdiag), the lowest GFR during TB treatment (GFRmin) and 3 months
after the end of the treatment (GFRpost). Last observation carried forward (LOCF) analysis.
Accepted
Article
TABLE 1 Demographic characteristics
Characteristic N=34
Recipient age (years)* 41.0 ± 12.7
Gender - male, n (%) 24 (70.6)
Ethnicity, n (%) Caucasian Non-Caucasian
8 (23.5) 26 (76.5)
Etiology of CKD, n (%) Chronic glomerulopathy Unknown Hypertension Diabetes Other 13 (38.2) 10 (29.4) 6 (17.6) 3 (8.8) 2 (5.9)
Time on dialysis before the Tx (months) ** 23 (0-180)
Donor source, deceased, n (%) 20 (58.8)
Induction therapy, n (%) 21 (61.8)
Anti-CD25 5 (14.7)
Depleting antibody 16 (47.1)
Mean time to TB diagnosis after Tx (months)** 25.5 (1-168)
Immunosuppressive regimens at TB diagnosis, n(%)
CsA – MP/AZA ± ST 13 (38.2)
TAC – MP/AZA ± ST 14 (41.2)
TAC – SRL/EVL ± ST 3 (8.8)
SRL/EVL – MP ± ST 4 (11.8)
Rejection episodes before TB, n (%) 15 (44.1)
Time between rejection and TB (months) ** 10.5 (1-196)
*Mean ± SD. **Median (range).
CKD, chronic kidney disease; Tx, transplantation; TB, tuberculosis; CsA, cyclosporine; MPA, mycophenolate; AZA, azathioprine; ST, steroids; TAC, tacrolimus; SRL, sirolimus; EVL, everolimus.
TABLE 2 Clinical presentation
Characteristics N=34 Clinical symptoms, n (%)
Fever 26 (76.5)
Weight loss 20 (58.8)
Cough 15 (44.1)
Dyspnea 10 (29.4)
Thoracic pain 6 (17.6)
Tuberculosis sites, n (%)
Pulmonary 15 (44.1)
Extrapulmonary 13 (38.3)
Central nervous system 3 (8.8)
Urinary tract 1 (2.9)
Pleural 2 (5.9)
Lymph nodal 2 (5.9)
Accepted
Article
Pleuropulmonary 1 (2.9)
Disseminated
Bladder and testicle
Pericardial and lymph nodal Pulmonary and urinary tract Testicular and renal allograft
4 (11.8) 1 (2.9) 1 (2.9) 1 (2.9) 1 (2.9)
Unknown 1 (2.9)
Diagnostic procedures, n (%)
Acid-fast bacilli smear microscopy 16 (47)
Sputum 5 (17.0)
Bronchoalveolar lavage 4 (11.8)
Urine 3 (8.8)
Lymph node 2 (5.9)
Pleural fluid Cerebrospinal fluid
1 (2.9) 1 (2.9) High adenosine deaminase levels
Pleural fluid (>40 U/L) Cerebrospinal fluid (>20 U/L) Ascites (>20 U/L)
5 (14.7) 2 (5.9) 2 (5.9) 1 (2.9) Histopathological analysis Lymph node Renal allograft Pleura Intestine Peritoneum Lung Bone 10 (29.4) 3 (8.8) 1 (2.9) 1 (2.9) 1 (2.9) 1 (2.9) 1 (2.9) 2 (5.9)
Positive culture for Mycobacterium tuberculosis 4 (11.8)
Accepted
Article
TABLE 3 Risk factors for non-recovery of baseline renal function after tuberculosis (TB)
Univariable analysis Multivariable
analysis OR (95% CI), P-value OR (95% CI), P-
value
Recipient age (years) ns -
Deceased donor ns -
Transplantation time on diagnosis (months)
ns -
Extrapulmonary tuberculosis ns -
Severe disease1 5.700 (1.147-28.330), .003 ns
Underexposure to IS after TB treatment2
Ns -
Baseline creatinine (mg/dL) Ns -
Baseline GFR (mL/min) Ns -
Maximum creatinine (mg/dL) 1.276 (0.955-1.705), .100 ns
AKI KDIGO 2/3 4.958 (1.062-23.157), .042 ns
AR after TB diagnosis 3.937 (0.551-28.116), .172 ns
1
Severe sepsis or intensive care unit admission. 2
More than 30% reduction in calcineurin inhibitors concentration, minimization or complete withdrawn of immunosuppression.