REVISTA
PAULISTA
DE
PEDIATRIA
www.rpped.com.br
ORIGINAL
ARTICLE
Relation
between
safe
use
of
medicines
and
Clinical
Pharmacy
Services
at
Pediatric
Intensive
Care
Units
Lucas
Miyake
Okumura
a,∗,
Daniella
Matsubara
da
Silva
b,
Larissa
Comarella
baHospitaldeClínicasdePortoAlegre,PortoAlegre,RS,Brazil
bHospitalInfantilWaldemarMonastier,CampoLargo,PR,Brazil
Received15January2016;accepted17March2016
Availableonline16April2016
KEYWORDS
Criticalcare; Drug-relatedside effectsandadverse reactions;
IntensiveCareUnits, Pediatric;
PharmacyService, Hospital;
Patientsafety; Medicationerrors
Abstract
Objective: ClinicalPharmacyServices(CPS)areconsideredstandardofcareandisendorsed bytheJointCommissionInternational,theAmericanAcademyofPediatrics,andtheAmerican CollegeofClinicalPharmacy.InBrazil,singleexperienceshavebeendiscreetlyarisingandthe importanceoftheseservicestochildrenandadolescentscarehasledtointerestingresults, butcertainlyareunderreported.Thisshortreportaimstodiscusstheeffectofimplementing abedsideCPSataBrazilianPediatricIntensiveCareUnit(PICU).
Methods: Thisisacross-sectionalstudyconductedina12bedPICUcommunityhospital,from CampoLargo/Brazil.Subjectswith<18yearsoldadmittedtoPICUwereincludedfordescriptive analysisifreceivedaCPSintervention.
Results: Of 53 patients accompanied, we detected 141 preventable drug-related problems (DRPs) whichwere solvedwithin clinicians(89%acceptance ofallinterventions). The most commoninterventionsperformedtoimprovedrugtherapyincluded:preventingincompatible intravenous solutions(21%) andacomposite ofinadequatedoses(17%duetolow, highand non-optimizeddoses).AmongthetoptenmedicationsassociatedwithDRPs,fivewere antimi-crobials.ByanalyzingthecorrelationbetweenDRPsandPICUlengthofstay,wefoundthat74% ofallvariationsonlengthofstaywereassociatedwiththenumberofDRPs.
Conclusions: AdversedrugreactionsduetoavoidableDRPscanbepreventedbyCPSina mul-tifacetedcollaborationwithotherhealthcareprofessionals,whoshouldattempttouseactive andevidence-basedstrategiestoreducemorbidityrelatedtomedications.
©2016SociedadedePediatriadeS˜aoPaulo.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBYlicense(http://creativecommons.org/licenses/by/4.0/).
∗Correspondingauthor.
E-mail:lucasokumura@yahoo.com.br(L.M.Okumura).
http://dx.doi.org/10.1016/j.rppede.2016.04.001
PALAVRAS-CHAVE
Cuidadosintensivos; Efeitoscolaterais relacionadosa medicamentose reac¸õesadversas; UnidadesdeCuidado IntensivoPediátrico; Servic¸odeFarmácia, Hospital;
Seguranc¸ado paciente;
Errosdemedicac¸ão
Relac¸ãoentreousosegurodemedicamentoseServic¸osdeFarmáciaClínicaem UnidadesdeCuidadosIntensivosPediátricos
Resumo
Objetivo: Servic¸osdeFarmáciaClínica (SFC)sãoconsiderados umpadrão deatendimentoà saúdeesãoendossadospela JointCommissionInternational,aAmericanAcademy of Pedi-atrics,eaAmericanCollegeofClinicalPharmacy.NoBrasil,experiênciasisoladasvêmsurgindo discretamenteeaimportânciadessesservic¸osparaocuidadodecrianc¸aseadolescentestêm levadoaresultadosinteressantes,masquecertamentesãosub-relatados.Esteartigoóriotem comoobjetivodiscutiroefeitodaimplantac¸ãodeumSFCàbeiradoleitoemumaUnidadede CuidadosIntensivosPediátricos(UCIP)brasileira.
Métodos: Esseéumestudotransversal,realizadoemumaUCIPdehospitaldacomunidadecom 12leitos,emCampoLargo,Brasil.Foramincluídosindivíduoscom<18anosinternadosemUCIP paraanálisedescritiva,quandoreceberamumaintervenc¸ãodoSFC.
Resultados: De 53 pacientesacompanhados, foram detectados 141Problemas Relacionados aMedicamentos(PRM)evitáveisqueforamresolvidos emconjunto comosmédicos(89%de aceitac¸ãode todasas intervenc¸ões).Asintervenc¸õesmais comunspara melhoraraterapia medicamentosaforam:prevenc¸ãodesoluc¸õesintravenosasincompatíveis(21%)edoses inade-quadas(17%devidoadosesbaixa,altaenãootimizadas).Entreosdezprincipaismedicamentos associadosàPRM,cincoeramantimicrobianos.Aoanalisaracorrelac¸ãoentreoPRMetempo depermanêncianaUCIP,verificamosque74%detodasasvariac¸õesnotempodepermanência eramassociadascomonúmerodePRM.
Conclusões: Reac¸õesadversasamedicamentosdevidoaPRMevitáveispodemserprevenidas porSFCemumacolaborac¸ãomultifacetadacomoutrosprofissionaisdesaúde.Taisproblemas podemser evitadospormeiodeestratégias ativasebaseadasem evidênciaspara reduzira morbidaderelacionadaamedicamentos.
©2016SociedadedePediatriadeS˜aoPaulo.PublicadoporElsevierEditoraLtda.Este ´eum artigoOpenAccesssobumalicenc¸aCCBY(http://creativecommons.org/licenses/by/4.0/).
Introduction
The increasing number of medications being approved to adultswithpotentialuseonPediatrics,1theneed totreat clinicallychallenging diseases, andthe ethical issues sur-roundingpediatricsresearchput childrenand adolescents atmorerisksassociatedtomedicationadverseevents.2,3To illustratethisscenario,anested-cohortstudyconductedby Bellisandcolleagues2 demonstratedthatunapproved pre-scriptions were associated with an augmented hazard of havinganadverseevent(hazardratio1.30,95%CI1.20---1.30, p<0.001).
Todetectmedicationadversereactionsandtoavoid pre-ventable drug-related problems (DRPs), many accredited hospitals4---7 have been putting effortsto implement Clin-ical Pharmacy Services (CPS). Since the last decade, the multifacetedcollaborationbetween Pediatricians, Critical CarePhysicians and Clinical Pharmacists wasendorsed by theAmericanAcademyofPediatrics,5 AmericanCollegeof ClinicalPharmacyandmanystudiesinthefield.5---9
Despitethewell-stablishedimportance5---9ofCPSto chil-drenandadolescents,in thelastyears,Brazilhasstarted theimplementationofsingleexperiencesaroundthe coun-try,especiallyforPICUpatients,whichhasledtointeresting butunderreportedresults.
This study is endorsed by the evolving role of CPS in Brazil, which has been due to the recent approval of a legislation about clinical activities developed by
pharmacists10; and the increasing interest of Latin Amer-ican health institutions to get accredited.11 Noteworthy, AccreditationOrganizations,suchastheJointCommission International, advocates that strategies to prevent medi-cationerrors,likewisepharmacists-drivenclinicalservices, should be implemented to reduce the number of drug-relatedundesiredevents.12
Theaimofthisshortreportistodescribethe implemen-tationandresultsofaCPSdirectedtoPICUinpatientsina Braziliansetting.
Method
This study complies with Helsinki’s Declaration and was approvedbytheLocalEthicsCommittee.
responsibletoprovideCPS toinpatients (PICUand30bed generalpediatricwards).
The CPS consisted in a systematic service dedicated to: participating in clinical rounds, elaborating institu-tionalprotocols,antiepilepticTherapeuticDrugMonitoring (TDM), reviewingeach ofprescribeddrugdosages, indica-tions, duration of treatments, drug interactions, relative andabsolutecontraindicationsandintravenousdrug incom-patibilities.
We soughttoretrospectivelyanalyzethedemographics (age and sex) and clinical variables (cause of admission, comorbidities,use of vasoactivedrugs, use ofmechanical ventilation,useof artificialnutrition,useof antimicrobial therapyandPICUlengthofstay).Theprevalenceandtypes ofDRPsfoundinsuchvulnerablepopulationattendedbythe CPSduringtheimplementationphase(May,October2012) werealsoreported.
DRPs are defined as all situations that predisposed patients of not having optimized drug therapy, such as: intravenoussolutionsinstabilityandincompatibility,wrong infusiontime,highorlowdosesaccordingtoliterature,need toadjustadoseaccordingtorenalclearanceorTDM(serum concentrations of selecteddrugs), presence of duplicated drugtherapy and wrongpharmaceuticalform.Finally, we assessedthe acceptanceofourservice byquantifying the acceptabilityofCPSinterventionsbyphysiciansandnursing team.
Our conventional sample was calculated based on a 5% alfa, 80% power and r=0.50 as statistically significant correlation for this exploratory analysis, which led to 29 patients.13 Anexploratoryunivariableanalysis(two-tailed, Spearman rho) was performed to assess the association between DRPs and PICU length of stay. All tests were two-sided and p<0.05 was set as null hypothesis rejec-tion.DescriptivestatisticsappliedtoallpatientswithDRPs. The aforementioned covariates were reported as median andinterquartileintervals,anddichotomousvariableswere reportedasabsoluteandrelativenumbers(%)(Table1).
Results
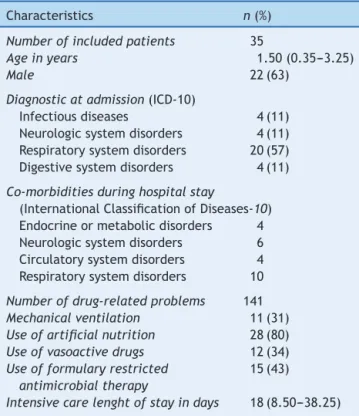
In 5 consecutive months of implementation, 53 patients were accompanied by two part-time clinical pharmacists (5h/daily dedication, except on weekends). 18 patients did not present a DRP, so they were not included in the descriptive analysis. We found 141 DRPs in 35 patients (Tables 1 and 2), who were likely to be male (63%) and were 1.50-years-old in average. Most of them were admitted due to respiratory disorders, such as acute asthma,bronchospasmandbronchiolitis-associated respira-tory insufficiency. One third (31.40%) needed mechanical ventilation during PICU stay, and 34.30% used vasoactive drugstotreathemodynamicinstability.
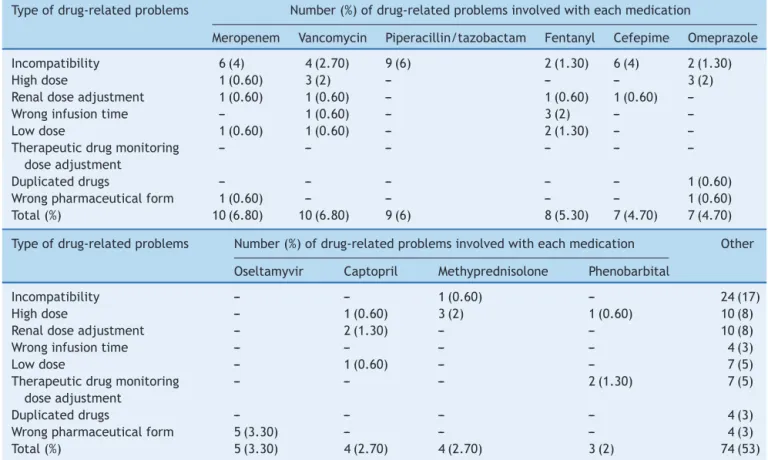
Out of the 141 DRPs detected by CPS, the most common interventions performed to improve drug ther-apy were: preventing incompatible intravenous solutions (21%) and a composite of inadequate doses (17% due to low, high and non-optimized doses) (Fig. 1). Among the top ten medications associated with DRPs, five were antimicrobials: meropenem, vancomycin, piperacillin and tazobactan,cefepimeandoseltamyvir(Table2).
Table1 Patients’characteristics.
Characteristics n(%)
Numberofincludedpatients 35
Ageinyears 1.50(0.35---3.25)
Male 22(63)
Diagnosticatadmission(ICD-10)
Infectiousdiseases 4(11) Neurologicsystemdisorders 4(11) Respiratorysystemdisorders 20(57) Digestivesystemdisorders 4(11)
Co-morbiditiesduringhospitalstay
(InternationalClassificationofDiseases-10) Endocrineormetabolicdisorders 4 Neurologicsystemdisorders 6 Circulatorysystemdisorders 4 Respiratorysystemdisorders 10
Numberofdrug-relatedproblems 141
Mechanicalventilation 11(31)
Useofartificialnutrition 28(80)
Useofvasoactivedrugs 12(34)
Useofformularyrestricted antimicrobialtherapy
15(43)
Intensivecarelenghtofstayindays 18(8.50---38.25)
Unlessotherwisestated,allvariablesareexpressedasabsolute
and/or relative (%) values. Artificial nutrition includes
par-enteralandenteralnutrition.
All continuousvariablesweredescribedas medianand
inter-quartilerange.
ICD-10,InternationalClassificationofDiseasesEditionn.10.
ByanalyzingtheSpearman-rhocorrelationbetweenDRPs andPICUlengthofstay,wefoundthat74%ofallvariations onPICU lengthof staywere associatedwiththedetected DRPs.
Discussion
Inoursample,theimplementationofaCPSdirectedatPICU inpatientshasshownthevalueofsuchservicesondetecting andsolvingDRPs,whichwereatmostpreventablesituations thatcouldleadtounnecessarymorbidity.
Through an average 33 days of PICU stay (95%CI 20.22---46.38),wefoundthateachpatientcouldbeexposed to as much as 2.6 DRPs, and interventions toward solv-ing them were highly accepted by medical and nursing team (89% acceptability rate). Such acceptance of inter-ventions by PICU team was consistently high, as already demonstratedbefore.8,11,12Themessagebehindthese find-ingsstandsfor agoodCPSimplementationprocess,which had asdeterminants of success: the institutional support andcommunicationbetweenhospital’spharmacymanager, clinicaldirector,PICUnursesandinfectiousdiseaseteam.
Table2 Commondrug-relatedproblemsinpediatricintensivecare.
Typeofdrug-relatedproblems Number(%)ofdrug-relatedproblemsinvolvedwitheachmedication
Meropenem Vancomycin Piperacillin/tazobactam Fentanyl Cefepime Omeprazole
Incompatibility 6(4) 4(2.70) 9(6) 2(1.30) 6(4) 2(1.30)
Highdose 1(0.60) 3(2) --- --- --- 3(2)
Renaldoseadjustment 1(0.60) 1(0.60) --- 1(0.60) 1(0.60) ---Wronginfusiontime --- 1(0.60) --- 3(2) ---
---Lowdose 1(0.60) 1(0.60) --- 2(1.30) ---
---Therapeuticdrugmonitoring doseadjustment
--- --- --- --- ---
---Duplicateddrugs --- --- --- --- --- 1(0.60)
Wrongpharmaceuticalform 1(0.60) --- --- --- --- 1(0.60) Total(%) 10(6.80) 10(6.80) 9(6) 8(5.30) 7(4.70) 7(4.70)
Typeofdrug-relatedproblems Number(%)ofdrug-relatedproblemsinvolvedwitheachmedication Other
Oseltamyvir Captopril Methyprednisolone Phenobarbital
Incompatibility --- --- 1(0.60) --- 24(17)
Highdose --- 1(0.60) 3(2) 1(0.60) 10(8)
Renaldoseadjustment --- 2(1.30) --- --- 10(8)
Wronginfusiontime --- --- --- --- 4(3)
Lowdose --- 1(0.60) --- --- 7(5)
Therapeuticdrugmonitoring doseadjustment
--- --- --- 2(1.30) 7(5)
Duplicateddrugs --- --- --- --- 4(3)
Wrongpharmaceuticalform 5(3.30) --- --- --- 4(3)
Total(%) 5(3.30) 4(2.70) 4(2.70) 3(2) 74(53)
Selecteddrugsaccountsfor67(47%)from141drug-relatedproblems(DRP)foundbypharmacists.Stability,compatibilityanddosewere
commonproblemsidentifiedbyclinicalpharmacists.‘‘Others’’columnreferstodrugsthatwerelesscommon.Onlydrugswithmore
than4DRPswerereported.
(intravenous and oral routes prescribed). Sub-therapeutic doses of phenobarbital were corrected by pharmacists, eitherbyliterature-basedinformationorbyTDM.
Few studies8,14---17 were alreadypublished in PICU sett-ings,butnonecomesfromLatinAmericancountries.Asingle randomizedcontrolledtrial8 assessedthe effectivenessof CPSin reducinginpatient lengthof stay.An observational studyconductedinFrench-speakingcountriesdescribed966 interventionsdone to solve DRPin 270 patients, through 6 months of CPS implementation.14 Other researches had alsoshowedpositive results. Acohort study conductedin United States included 1120 patients andfound that half ofpatientswereexposedtomedicationerrors.Theyfound that 28% of all problems detected were related to dose, and other 18% with wrong route of administration.15 In United Kingdom, antibiotics and inotropes were reported tobethe top drugs associated withmedicationserrors.16 Our study showed similar results by having meropenen, piperacillin and tazobactam, vancomycin, cefepime and oseltamyviraspartthe toptenmedicationsassociated to DRP.
Unfortunately,somestudies16didnotspecifythedetails ofthemedicationerrorsdetected,whichareindispensable for PICU pharmacists. To overcome such lack of descrip-tiveinformation,ourstudyidentifiedthatweightvariation, acute kidney injury and TDM led to dose adjustment
interventions, namely: vancomycin, captopril and pheno-barbital.
Figure1 IncompatibilityproblemsavoidedbyClinical Phar-macyServices.
pharmacistsinthisactivity,whoconsultedeachotherwhen discrepancies/inconsistencieswerefound.
Every ten patients admitted to PICU, six had a DRPs detectedbyCPSandfivereceivedan interventionto opti-mize drugtherapy. PICU setting has a high prevalence of compatibilityandstabilityDRPs(Table2),anddose adjust-mentsshouldbepromptlyassessedespeciallyoninadequate therapeuticdrugserumconcentrations,weightchangesand other risk factors that may change drug distribution and excretion,suchasacutekidneyinjury.Basedonour imple-mentationexperience,CPSmightbeafeasibletechnology thatimproveinfants,childrenandadolescentscare. Pedia-tricians’andstakeholdersshouldattempttopreventDRPsby usingactiveandevidence-basedstrategiestoreduce avoid-ablemorbidity-relatedtomedications.4---6,8,9
Funding
LMOreceivesamonthlyscholarshipfromtheBrazilian Min-istryofEducation. BythetimeofCPSimplementation,he wasasixthyearpharmacystudent.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
We would like to thank Dr. Leonardo Cavadas Soares (FormerClinicalDirector),whoprovidedanoutstanding pre-ceptorship, scientific and clinical support among Clinical PharmacyServicesimplementation.Theservicereportedin thismanuscriptwassupportedbyallhealthcare profession-alsfromWaldemarMonastierChildren’sHospital,especially thosededicatedtocriticallyillchildren.
References
1.Gonc¸alves MG,Heineck I.Frequency of prescriptions of off-labeldrugsanddrugsnotapprovedforpediatricuseinprimary healthcareinasouthernmunicipalityofBrazil.RevPaul Pedi-atr.2016;34:11---7.
2.BellisJR,KirkhamJJ,ThiesenS,ConroyEJ,BrackenLE,Mannix HL,etal.Adversedrugreactionsandoff-labelandunlicensed medicinesinchildren:anestedcase?Controlstudyofinpatients inapediatrichospital.BMCMed.2013;7:238.
3.KorenG,HaslamRH.Pediatricmedicationerrors:predictingand preventingtenfolddisasters.JClinPharmacol.1994;34:1043---5.
4.Bhatt-MehtaV,BuckML,ChungAM,FarringtonEA,Hagemann TM,HoffDS,etal.Recommendationsformeetingthepediatric patient’sneedforaclinicalpharmacist:ajointopinionofthe PediatricsPracticeandResearchNetworkoftheAmerican Col-legeofClinicalPharmacyandthePediatricPharmacyAdvocacy Group.Pharmacotherapy.2012;17:281---91.
5.Rashed AN, Neubert A, Tomlin S, Jackman J, Alhamdan H, AlShaikhA, etal.Epidemiology andpotentialassociatedrisk factors of drug-related problems in hospitalised children in theUnitedKingdom andSaudiArabia.EurJClinPharmacol. 2012;68:1657---66.
6.Tripathi S, Crabtree HM,Fryer KR, GranerKK, ArteagaGM. Impactofclinicalpharmacistonthepediatricintensive care practice: an 11-year tertiary center experience. J Pediatr PharmacolTher.2015;20:290---8.
7.Preventingpediatricmedicationerrors[homepageonthe
Inter-net].Preventingpediatricmedicationerrors. Availablefrom:
http://www.jointcommission.org/assets/1/18/SEA39.pdf [cited23.12.15].
8.ZhangC,Zhang L, HuangL, Luo R, WenJ.Clinical pharma-cistsonmedical careofpediatricinpatients: asingle-center randomizedcontrolledtrial.PLoSONE.2012;7:e30856. 9.SteineckKJ,SkoglundAK,CarlsonMK,GuptaS.Evaluationofa
pharmacist-managedmethadonetaper.PediatrCritCareMed. 2014;15:206---10.
10.Brasil ---Conselho Federalde Farmácia.Resoluc¸ãoN◦ 585de
29 de Agosto de 2013. Ementa: regulamenta as atribuic¸ões clínicas do farmacêutico e dá outras providências. Brasília: CFF; 2013. Available from: http://www.cff.org.br/userfiles/ file/resolucoes/585.pdf
11.Ferracini FT, Almeida SM, Locatelli J, Petriccione S, Haga CS. Implementationandprogressofclinicalpharmacyinthe rationalmedicationuseinalargetertiaryhospital.Einstein. 2011;9:456---60.
12.JointCommissionInternational.JointCommissionInternational accreditationstandardsforhospitals.5thed.OakBrook:Joint CommissionResources;2013.
13.HulleySB,CummingsSR,Browner WS,GradyD,NewmanTB. Designingclinicalresearch:anepidemiologicapproach.4thed. Philadelphia:LippincottWilliams&Wilkins;2013.
15.LarochelleJM,Ghaly M,CreelAM. Clinicalpharmacyfaculty interventionsinaPediatricIntensiveCareUnit:aneight-month review.JPediatrPharmacolTher.2012;3:263---9.
16.RossLM,WallaceJ,PatonJY.Medicationerrorsinapaediatric teachinghospitalintheUK:fiveyearsoperationalexperience. ArchDisChild.2000;6:492---7.
17.Wong IC, GhalebMA, Franklin BD, Barber N. Incidence and natureofdosingerrorsinpaediatricmedications:asystematic review.DrugSaf.2004;9:661---70.