Assessment of survival of patients with metastatic clear cell
renal cell carcinoma after radical cytoreductive nephrectomy
versus no surgery: a SEER analysis
_______________________________________________
Wen-Jun Xiao
1,2, Yao Zhu
1,2, Bo Dai
1,2, Hai-Liang Zhang
1,2, Ding-Wei Ye
1,21 Department of Urology, Fudan University Shanghai Cancer Centre, Shanghai, People’s Republic of
China; 2 Department of Oncology, Shanghai Medical College, Fudan University, Shanghai, People’s
Republic of China
ABSTRACT
ARTICLE
INFO
______________________________________________________________ ______________________
Purposes: To examine the factors related to the choice of cytoreductive nephrectomy (CN) for patients with metastatic clear cell renal cell carcinoma (mCCRCC), and com-pare the population-based survival rates of patients treated with or without surgery in the modern targeted therapy era.
Materials and Methods: From 2006 to 2009, patients with mCCRCC were identified from SEER database. The factors that affected patients to be submitted to CN were examined and propensity scores for each patient were calculated. Then patients were matched based upon propensity scores. Univariable and multivariable cox regression models were used to compare survival rates of patients treated with or without surgery. Finally, sensitivity analysis for the cox model on a hazard ratio scale was performed.
Results: Age, race, tumor size, T stage and N stage were associated with nephrectomy univariablely. After the match based upon propensity scores, the 1-, 2-, and 3-year cancer-specific survival rate estimates were 45.1%, 27.9%, and 21.7% for the no-sur-gery group vs 70.6%, 52.2%, and 41.7% for the surno-sur-gery group, respectively (hazard ratio 0.42, 95%CI: 0.35-0.52, log-rank P<0.001). In multivariable Cox proportional hazard regression model, race, T stage, N stage and median household income were significantly associated with survival. Sensitivity analysis on a hazard ratio scale indi-cated that the hazard ratio might be above 1.00 only when the unknown factor had an opposite effect on survival which was 3-fold than CN.
Conclusion: The results of our study showed that CN significantly improves the survi-val of patients with metastatic CCRCC even in the targeted therapy era.
Key words:
Carcinoma, Renal Cell; Nephrectomy; SEER Program; Propensity Score; Molecular Targeted Therapy
Int Braz J Urol. 2015; 41: 288-95
_____________________
Submitted for publication: November 02, 2013
_____________________
Accepted after revision: June 06, 2014
INTRODUCTION
Renal cell carcinoma (RCC) is a common malignancy, and represents more than 3% of adult solid malignant tumors (1). Most RCCs are diagnosed in early-stage disease, but approxi-mately 25% of RCC patients have systemic me-tastases at initial diagnosis (2, 3). Two
tyrosine kinase inhibitors (VEGFR-TKIs) in renal carcinoma. The unprecedented antitumor activi-ty and relatively favorable toxiciactivi-ty profile of the modern targeted therapies resulted in a shift of the standard systemic therapy for mRCC (9-11). So, in the era of VEGFR-TKIs, it is uncertain the appropriate role of CN. The present study will explore factors associated with CN and evaluate its role in modern day practice within a large North American population-based dataset.
MATERIALSN AND METHODS
Data for the current analysis were deri-ved from the Surveillance, Epidemiology, and End Results (SEER) registry. The SEER has col-lected clinical and pathological data in 19 spe-cified geographic areas of the United States (US) since 1973, including the Atlanta, Detroit, San Francisco-Oakland, Seattle-Puget Sound metro-politan areas and the states of Connecticut, Ha-waii, Iowa, New Mexico, and Utah. These data are highly representative of the demographic makeup of the United States, especially in terms of geography, socioeconomic status, race/ethni-city, and age (12).
Because VEGFR-TKIs were mainly used for clear cell renal cell cancer (13), its diagnos-tic codes (“International Classification of Disea-se for Oncology”, 3rd edition, histology coding 8310) were used as inclusion criteria. The pre-sence of histology coding 8310 and AJCC sta-ge M1 (derived AJCC 6th edition) resulted in the
identification of 2305 patients with metastatic clear cell renal cell cancer (mCCRCC) from 2006 to 2009. Patients who underwent a radical sur-gical removal of kidney, were included in the surgery group. Only patients who did not un-dergo surgery were included in the “no surgery group”. The duration of survival after nephrec-tomy or from the date of mRCC diagnosis was determined according to the SEER survival time definition. We selected 2006 as the initial year because 2005 was the time of initial regulatory approval for VEGFR-TKI’s use in renal carcino-ma. Follow-up was defined as the time between date of diagnosis and date of death or November 2011 whichever came first.
Statistical analyses
Baseline statistics were analyzed with me-ans for continuous variables (age, tumor size) and proportions for categorical variables (race/ethnicity, stage). The baseline differences of two groups were compared using chi-squared for categorical varia-bles. T-test was used for comparison of age, while Wilcoxon test was used for comparison of tumor size. Cox proportional hazard regression model was used to calculate the survival rate.
As patients were not randomly selected for nephrectomy, a propensity score was calculated re-flecting the probability of a person undergoing ne-phrectomy given a set of known covariates. A mul-tivariable regression model, including these factors univariably associated with nephrectomy, was used to obtain the propensity of nephrectomy for each patient. Then the patients without surgery were ma-tched by the patients with surgery based upon the propensity score. This would adjust the bias due to imbalance of certain prognostic variables associated with nephrectomy to a certain extent. Then a cox survival model for the matched patients, including all variables associated with survival, was made to evaluate the beneficial effect of nephrectomy. Sensi-tivity analysis for this model on a hazard ratio scale was also performed (14). Analyses were conducted using the R statistical package (the R foundation for Statistical Computing, version 2.15.2).
RESULTS
Among 1505 assessable patients, 1038 (68.97%) were men, and the average age was 62.1 years. The no-surgery patients (n=460) were sig-nificantly older (65.77 vs 60.51 years, P<0.001), had almost the same proportion of women (31.1% vs 31.0%, P>0.5), and had smaller tumors (median size, 7.5 vs 9.0 cm, P<0.001) than the surgery
pa-tients (n=1045). Race, T stage and N stage were also associated with nephrectomy (Table-1). At 1, 2 and 3 years of follow-up, the cancer-specific survival rate was 44.9%, 28.3%, and 20.6% for the no-surgery group vs 72.7%, 54.1% and 47.2% for the surgery group, respectively (hazard ratio 0.39, 95%CI: 0.33-0.46, log-rank P<0.001).
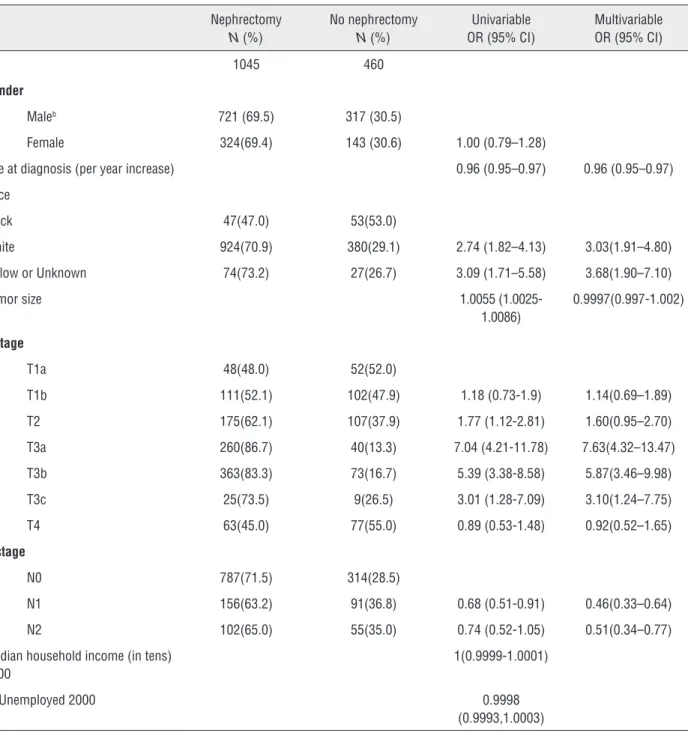
Table 1 - Uni- and multivariable regression models to estimate factors associated with nephrectomy.
Nephrectomy
N (%)
No nephrectomy
N (%)
Univariable OR (95% CI)
Multivariable OR (95% CI)
N 1045 460
Gender
Maleb 721 (69.5) 317 (30.5)
Female 324(69.4) 143 (30.6) 1.00 (0.79–1.28)
Age at diagnosis (per year increase) 0.96 (0.95–0.97) 0.96 (0.95–0.97)
Race
Black 47(47.0) 53(53.0)
White 924(70.9) 380(29.1) 2.74 (1.82–4.13) 3.03(1.91–4.80)
Yellow or Unknown 74(73.2) 27(26.7) 3.09 (1.71–5.58) 3.68(1.90–7.10)
Tumor size 1.0055
(1.0025-1.0086)
0.9997(0.997-1.002)
T stage
T1a 48(48.0) 52(52.0)
T1b 111(52.1) 102(47.9) 1.18 (0.73-1.9) 1.14(0.69–1.89)
T2 175(62.1) 107(37.9) 1.77 (1.12-2.81) 1.60(0.95–2.70)
T3a 260(86.7) 40(13.3) 7.04 (4.21-11.78) 7.63(4.32–13.47)
T3b 363(83.3) 73(16.7) 5.39 (3.38-8.58) 5.87(3.46–9.98)
T3c 25(73.5) 9(26.5) 3.01 (1.28-7.09) 3.10(1.24–7.75)
T4 63(45.0) 77(55.0) 0.89 (0.53-1.48) 0.92(0.52–1.65)
N stage
N0 787(71.5) 314(28.5)
N1 156(63.2) 91(36.8) 0.68 (0.51-0.91) 0.46(0.33–0.64)
N2 102(65.0) 55(35.0) 0.74 (0.52-1.05) 0.51(0.34–0.77)
Median household income (in tens) 2000
1(0.9999-1.0001)
% Unemployed 2000 0.9998
(0.9993,1.0003)
All factors univariably associated with nephrectomy were included in the multivariable logistic regression model which is presented in Ta-ble-1. Race, T stage, N stage and age at diagnosis remained significantly associated with nephrec-tomy. This multivariable model was also used to estimate the propensity score for each patient (the probability of undergoing a nephrectomy).
After the match process based upon pro-pensity scores, 442 no-surgery patients were ma-tched with 442 surgery patients. In this mama-tched population (n=884), the 1-, 2-, and 3-year esti-mate was 45.1%, 27.9%, and 21.7% for the no--surgery group vs 70.6%, 52.2%, and 41.7% for the surgery group, respectively (hazard ratio 0.42, 95%CI: 0.35-0.52, log-rank P<0.001, Figure-1).
DISCUSSION
As we know, the use of CN in the cytoki-ne therapy era was supported by two randomi-zed trials conducted by the Southwest Oncology Group and by the European Organization for Re-search and Treatment of Cancer (4-6). In the era of molecular targeted therapy, there has been some retrospective data about the combined use of CN with systemic targeted therapy (15-17). But their results were controversial and the number of pa-tients was small. The present population-based study may be one of the large sample investiga-tions to explore the prognostic factors associated with CN and evaluate its role in modern molecular targeted therapy era.
Among demographic variables, age and race except gender appeared to be independent factors associated with nephrectomy. Black pe-ople appeared less likely to be submitted to CN than White or Asian group, while older people were less likely, either. But these factors may be confounded by performance status, comorbidi-ty, economic conditions and so on. Kader et al. (18) found that the prognosis amongst elderly is comparable to the younger patients if they survive the surgery. So age alone should not be a criterion whether or not to treat patients with cytoreductive surgery (8).
Tumor size and TNM stage were signifi-cantly associated with the probability of nephrec-tomy as well. Local or regional advanced tumors make surgical intervention a high risk and have been shown to be associated with a poor prognosis (19, 20). However, the majority of these patients in the present study were still submitted to CN: more than 70% of patients with T3 disease and more than 60% of patients with positive lymph node. This indicated the importance of surgical removal of primary tumor, tumor thrombus and positive lymph node in the mRCC setting. As T1a were con-cerned, more than half of these patients included in our study did not receive any therapy for renal car-cinoma. However, this did not mean that the local treatment was unimportant. Due to smaller tumors, these patients might choose other local therapy ra-ther than “radical nephrectomy”, including local tu-mor destruction, thermal ablation, electrocautery,
Figure 1 - Surgery group.
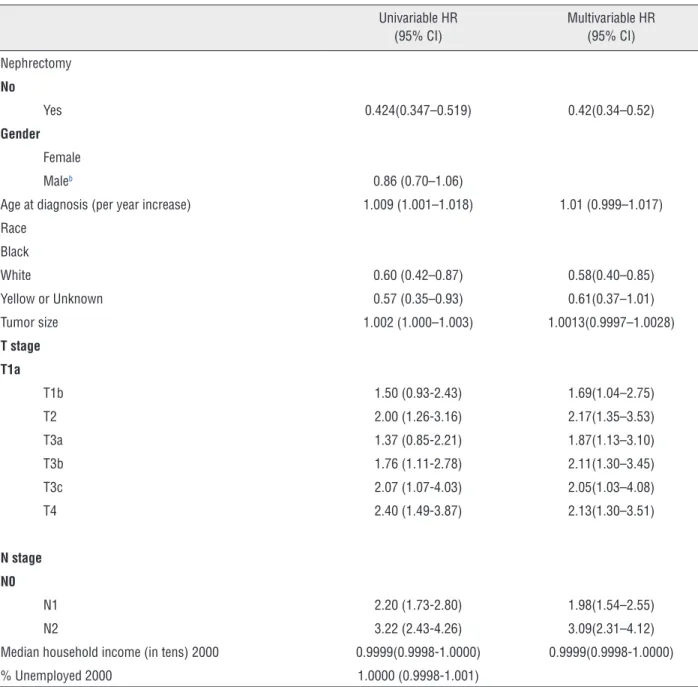
In Table-2 the results of the univariable and mul-tivariable Cox proportional hazard regression analyses are presented. Race, tumor size, T stage, N stage and age at diagnosis were univariable-ly associated with cancer-specific survival. Less median household income associated with better cancer-specific survival, although this was not statistically significant (P=0.056). In multivariable Cox proportional hazard regression model, race, T stage, N stage, and median household income significantly associated with survival.
Table 2 - Cox Proportional Hazards regression analyses including nephrectomy, propensity score and other prognostic variables.
Univariable HR (95% CI)
Multivariable HR (95% CI)
Nephrectomy
No
Yes 0.424(0.347–0.519) 0.42(0.34–0.52)
Gender
Female
Maleb 0.86 (0.70–1.06)
Age at diagnosis (per year increase) 1.009 (1.001–1.018) 1.01 (0.999–1.017)
Race
Black
White 0.60 (0.42–0.87) 0.58(0.40–0.85)
Yellow or Unknown 0.57 (0.35–0.93) 0.61(0.37–1.01)
Tumor size 1.002 (1.000–1.003) 1.0013(0.9997–1.0028)
T stage T1a
T1b 1.50 (0.93-2.43) 1.69(1.04–2.75)
T2 2.00 (1.26-3.16) 2.17(1.35–3.53)
T3a 1.37 (0.85-2.21) 1.87(1.13–3.10)
T3b 1.76 (1.11-2.78) 2.11(1.30–3.45)
T3c 2.07 (1.07-4.03) 2.05(1.03–4.08)
T4 2.40 (1.49-3.87) 2.13(1.30–3.51)
N stage
N0
N1 2.20 (1.73-2.80) 1.98(1.54–2.55)
N2 3.22 (2.43-4.26) 3.09(2.31–4.12)
Median household income (in tens) 2000 0.9999(0.9998-1.0000) 0.9999(0.9998-1.0000)
% Unemployed 2000 1.0000 (0.9998-1.001)
HR = hazard ratio; CI = confidential interval
cryosurgery and so on. These cases were beyond the scope of the present study and have been ex-cluded, which might result in the low proportion of patients receiving CN.
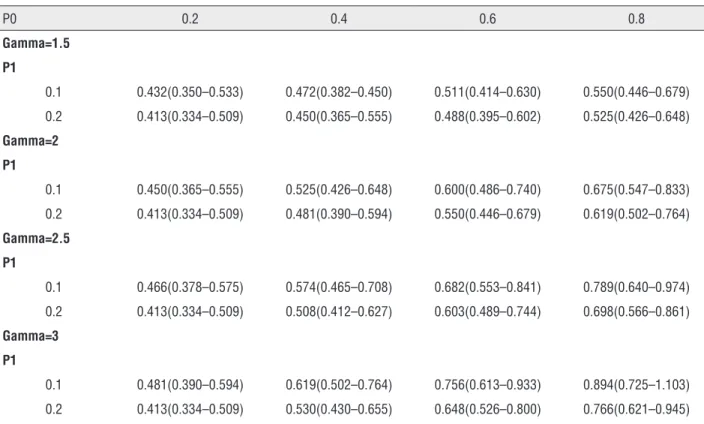
Our results have demonstrated a survival benefit when CN was performed compared with no surgery. The effect of performing CN was re-lated to 2.5-fold increase in cancer-specific
Table 3 - Sensitivity analysis on cytoreductive nephrectomy on a Hazard Ratio scale.
P0 0.2 0.4 0.6 0.8
Gamma=1.5
P1
0.1 0.432(0.350–0.533) 0.472(0.382–0.450) 0.511(0.414–0.630) 0.550(0.446–0.679)
0.2 0.413(0.334–0.509) 0.450(0.365–0.555) 0.488(0.395–0.602) 0.525(0.426–0.648)
Gamma=2
P1
0.1 0.450(0.365–0.555) 0.525(0.426–0.648) 0.600(0.486–0.740) 0.675(0.547–0.833)
0.2 0.413(0.334–0.509) 0.481(0.390–0.594) 0.550(0.446–0.679) 0.619(0.502–0.764)
Gamma=2.5
P1
0.1 0.466(0.378–0.575) 0.574(0.465–0.708) 0.682(0.553–0.841) 0.789(0.640–0.974)
0.2 0.413(0.334–0.509) 0.508(0.412–0.627) 0.603(0.489–0.744) 0.698(0.566–0.861)
Gamma=3
P1
0.1 0.481(0.390–0.594) 0.619(0.502–0.764) 0.756(0.613–0.933) 0.894(0.725–1.103)
0.2 0.413(0.334–0.509) 0.530(0.430–0.655) 0.648(0.526–0.800) 0.766(0.621–0.945)
an observational study, other factors unavaila-ble from SEER database may affect the survival rates of the patients with mRCC, including co-morbidities, performance status, the number of metastatic sites and laboratory variables (eg, he-moglobin, calcium, lactate dehydrogenase) (21, 22). If these unmeasured variables were inclu-ded in the analysis it would possibly change the conclusions. Sensitivity analysis is a way to see how much of a relationship needs to exist with the unmeasured variable before the conclusions change. In present study, the sensitivity analysis demonstrated the hazard ratio might be above 1.00 only when the unknown factor had an op-posite effect on survival which was 3-fold than cytoreductive nephrectomy. So we could say that the survival benefit related to cytoreductive nephrectomy was not due to an unfavorable per-formance status or multiple comorbidities in the patients in the no-surgery group, and the lack of performance status and/or baseline comorbidity data did not spuriously inflate the survival bene-fit of surgery (23).
CONCLUSIONS
Although the data came from the United States and there might be different clinical selec-tion criteria used for cytoreductive nephrectomy candidates in other countries, the results of our study have shown that cytoreductive nephrec-tomy significantly improves the survival of pa-tients with metastatic clear cell renal cell carcino-ma even in the targeted therapy era. According to SEER database, race, T stage, N stage and age at diagnosis were significantly associated with ne-phrectomy, while race, T stage, N stage and me-dian household income were significantly associa-ted with cancer-specific survival. We hope that our result sheds some light on the ongoing clinical trial of this aspect (24).
ACKNOWLEDGEMENTS
This work was supported by National Na-tural Science Foundation of China (grant number 81001131).
REFERENCES
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61: 69-90. Erratum in: CA Cancer J Clin. 2011;61:134.
2. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007; 109: 1763-8.
3. Chow WH, Devesa SS. Contemporary epidemiology of renal cell cancer. Cancer J. 2008; 14: 288-301.
4. Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R; European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group: Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001; 358: 966-70.
5. Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001; 345: 1655-9.
6. Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004; 171: 1071-6.
7. Jeldres C, Baillargeon-Gagne S, Liberman D, Isbarn H, Capitanio U, Shariat SF, et al. A population-based analysis of the rate of cytoreductive nephrectomy for metastatic renal cell carcinoma in the United States. Urology. 2009; 74: 837-41. 8. Aben KK, Heskamp S, Janssen-Heijnen ML, Koldewijn EL, van
Herpen CM, Kiemeney LA, et al. Better survival in patients with metastasised kidney cancer after nephrectomy: a population-based study in the Netherlands. Eur J Cancer. 2011; 47: 2023-32. 9. Halbert RJ, Figlin RA, Atkins MB, Bernal M, Hutson TE, Uzzo
RG, et al. Treatment of patients with metastatic renal cell cancer: a RAND Appropriateness Panel. Cancer. 2006; 107: 2375-83.
10. Margulis V, Wood CG. Pre-surgical targeted molecular therapy in renal cell carcinoma. BJU Int. 2009; 103: 150-3.
11. Margulis V, Wood CG, Jonasch E, Matin SF. Current status of debulking nephrectomy in the era of tyrosine kinase inhibitors. Curr Oncol Rep. 2008; 10: 253-8.
12. Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, et al. SEER Cancer Statistics Review, 1975-2010, National Cancer Institute. Bethesda, MD, available at: http:// seer.cancer.gov/archive/csr/1975_2010/ based on November 2012 SEER data submission, posted to the SEER web site. 2013.
13. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007; 356: 125-34. Erratum in: N Engl J Med. 2007;357:203.
14. Snow G: Sensitivity analysis for Observational studies. Available at: http://cran.r-project.org/web/packages/obsSens/ obsSens.pdf
15. Crispen PL, Blute ML. Role of cytoreductive nephrectomy in the era of targeted therapy for renal cell carcinoma. Curr Urol Rep. 2012; 13: 38-46.
16. Choueiri TK, Xie W, Kollmannsberger C, North S, Knox JJ, Lampard JG, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011; 185: 60-6.
17. You D, Jeong IG, Ahn JH, Lee DH, Lee JL, Hong JH, et al. The value of cytoreductive nephrectomy for metastatic renal cell carcinoma in the era of targeted therapy. J Urol. 2011; 185: 54-9.
18. Kader AK, Tamboli P, Luongo T, Matin SF, Bell K, Jonasch E, et al. Cytoreductive nephrectomy in the elderly patient: the M. D. Anderson Cancer Center experience. J Urol. 2007; 177: 855-60; discussion 860-1.
19. Kutikov A, Egleston BL, Canter D, Smaldone MC, Wong YN, Uzzo RG. Competing risks of death in patients with localized renal cell carcinoma: a comorbidity based model. J Urol. 2012; 188: 2077-83.
20. Thomas K, David G, Christopher PE, Andrea T: Current and Future Trends in the Treatment of Renal Cancer. Eur urol suppl. 2007; 6: 374-84.
21. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007; 356: 115-24. 22. Mekhail TM, Abou-Jawde RM, Boumerhi G, Malhi S, Wood L,
Elson P, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005; 23: 832-41.
23. Lin DY, Psaty BM, Kronmal RA: Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics. 1998; 54: 948-63.
24. Carmena. Randomized Phase III Trial Evaluating the Importance of Nephrectomy in Patients Presenting With Metastatic Renal Cell Carcinoma Treated With Sunitinib. Available at: https:// clinicaltrials.gov/ct2/show/NCT00930033?term=renal+cance r&recr=Open&intr=nephrectomy&rank=2. accessed in 2009.
_______________________ Correspondence address:
EDITORIAL COMMENT
In this edition of Int Braz J Urol, Doe et al. describe an interesting retrospective analysis of the role of cytoreductive nephrectomy (CN) in patients with metastatic renal cell carcinoma (mRCC) from the Surveillance, Epidemiology, and End Results (SEER) database. This is a widely researched and discussed topic in uro-oncology precisely because we have few (possibly none) high quality studies with level of evidence that can guide practice and help clinical management of such patients in the targeted therapy era.
The concept of cytoreduction is one of the pillars of modern surgical oncology and is applied in various types of neoplasms such as ovarian, colonic, bladder and more recently some groups have evaluated its role in prostate cancer (1, 2). In the mRCC scenario, two randomized phase III trials investigated the role of CN in the treatment of patients with mRCC during the immunotherapy era (3, 4). The combined analysis of such trials demonstrated a median survival of 13.6 months in the CN group versus 7.8 months in the sys-temic therapy-only group. The 31% difference in risk of death was statistically significant (p=0.002) favoring surgery (5). And this was considered the standard approach until the emergence of targe-ted therapy. Since then, the role of CN has been questioned.
Retrospective studies have confirmed the importance of CN in the targeted therapy era. Ho-wever, unlike immunotherapy with interferon or IL-2, it is known that TKIs and mTOR inhibitors might present an objective response on the prima-ry tumor too. That said, some key questions must be answered. Who deserves to undergo CN? What are the criteria used to select candidates for CN? When CN must be offered, prior or after systemic therapy? Is there any place for nephron-sparing surgery? Evidence suggests that patients at low or intermediate risk groups, with good perfor-mance status, low extrarenal tumor burden and no central nervous system metastasis are the ideal
candidates to CN (6). The remaining issues remain unanswered.
The SURTIME trial (EORTC 30073, NCT01099423) and the CARMENA trial (NCT00930033) are currently investigating the utility of CN in the targeted therapy era and its ideal timing. Until preliminary results of such studies are published, the clinical management of mRCC patients will be based on extrapolations from the immunotherapy era; and despite all the limitations, from some interesting retrospective studies such as Doe et al. study.
REFERENCES
1. Al Rawahi T, Lopes AD, Bristow RE, Bryant A, Elattar A, Chattopadhyay S, et al. Surgical cytoreduction for recurrent epithelial ovarian cancer. Cochrane Database Syst Rev. 2013 Feb 28;2:CD008765.
2. Heidenreich A, Pfister D, Porres D. Radical cancer surgery of renal cell and prostate carcinoma with hematogenous metastasis: benefits. Urologe A. 2014;53:823-31.
3. Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655-9.
4. Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R; European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966-70.
5. Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171:1071-6.
6. Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794-9.
Walter Henriques da Costa, MD