www.bjorl.org
Brazilian
Journal
of
OTORHINOLARYNGOLOGY
ORIGINAL
ARTICLE
Lymphangiogenesis
and
angiogenesis
in
oral
cavity
and
lower
lip
squamous
cell
carcinoma
夽
Mojgan
Alaeddini,
Shahroo
Etemad-Moghadam
∗DentalResearchCenter,DentistryResearchInstitute,TehranUniversityofMedicalSciences,Tehran,Iran
Received18April2015;accepted6June2015 Availableonline5November2015
KEYWORDS
Pathological neovascularization; Lymphangiogenesis; Lip;
Mouth; Squamouscell carcinoma
Abstract
Introduction:Tumorsofthelipandoralcavitydifferinvariousaspects;thereforeaclarification ofthedistinctionsamongthesesitesmayhelptobetterunderstandthebiologicbehaviorof neoplasmsoccurringintheselocations.
Objective: Consideringthatangiogenesisandlymphangiogenesisaretwomajorelementsthat caninfluencevariousaspectsoftumorbiology,weaimedtocomparethesefactorsbetween squamouscellcarcinomaofthelowerlipandoralcavity.
Methods:A totalof84 primarysquamouscellcarcinomasincluding45oraland39lowerlip tumors wereselectedandimmunohistochemicallystained withmonoclonalantibody against D2-40 andCD105.Meanmicrovesseldensitywasassessedintumoraltissue, whilelymphatic vesseldensitywascalculatedinbothneoplastictissueandinvasionfront.Datawerestatistically analyzedusingt-testandp-valuesof<0.05wereconsideredsignificant.
Results:Wefoundameanmicrovesseldensity±standarddeviationof31.94±18.9inoralcavity and27.54±20.8inlowerlipsquamouscellcarcinomas,withnosignificantdifference(p=0.32). Meanlymphaticvesseldensity±standarddeviationwas13.05±8.2and16.57±10.79inoforal cavityandlowerlipneoplastictissue,respectively.Thecorrespondingvalueswere9.94±5.59 and12.50±7.8intheinvasivefront.Significantdifferenceswerenotobservedineitherofthe lymphaticvesseldensityvariablesbetweenthetwosites.
Conclusion: Accordingtoourresults,itseemsthatthesearchforadditionalfactorsotherthan thoserelated tothevasculatureshouldcontinue,tohelpclarify thedifferencesinbiologic behaviorbetweenlowerlipandoralcavitysquamouscellcarcinomas.
© 2015 Associac¸˜ao Brasileira de Otorrinolaringologia e Cirurgia C´ervico-Facial. Published by Elsevier Editora Ltda. This is an open access article under the CC BY license (http:// creativecommons.org/licenses/by/4.0/).
夽 Pleasecitethisarticleas:AlaeddiniM,Etemad-MoghadamS.Lymphangiogenesisandangiogenesisinoralcavityandlowerlipsquamous
cellcarcinoma.BrazJOtorhinolaryngol.2016;82:385---90.
∗Correspondingauthor.
E-mail:shahrooetemad@yahoo.com(S.Etemad-Moghadam). http://dx.doi.org/10.1016/j.bjorl.2015.06.008
PALAVRAS-CHAVE
Neovascularizac¸ão patológica; Linfangiogênese; Lábio;
Boca;
Carcinomadecélulas escamosas
Linfangiogêneseeangiogêneseemcarcinomasdecélulasescamosasdelábioinferior edacavidadeoral
Resumo
Introduc¸ão:Ostumoresdelábioedacavidadeoraldiferememváriosaspectos;portanto,o conhecimentodasdiferenc¸asentreelespodeajudarnamelhorcompreensãodocomportamento biológicodasneoplasiasqueocorremnesseslocais.
Objetivo:Considerandoqueaangiogêneseealinfangiogênesesãodoiselementosimportantes quepodeminfluenciardiversosaspectosdabiologiadostumores,objetivamoscompararesses fatoresentreocarcinomadecélulasescamosas(CCE)delábioinferioredacavidadeoral. Método: Nototal,foramselecionados84 CCEsprimários (45tumoresdacavidadeoral e39 tumores delábio).Essestumores foramcoradosporprocessoimuno-histoquímicocom anti-corpomonoclonalanti-D2-40eCD105.Avaliamosadensidademédiademicrovasos(DMV)no tecidotumoral,enquantoqueadensidadevascularlinfática(DVL)foicalculadatantonotecido neoplásicocomonofrontdeinvasão.Osdadosforamestatisticamenteanalisadoscomousodo testetevaloresdep<0,05foramconsideradossignificantes.
Resultados: ChegamosaumamédiaparaDMV±DPde31,94±18,9paraCCEsnacavidadeorale de27,54±20,8nolábioinferior,semdiferenc¸asignificante(p=0,32).AsmédiasparaDVL±DP foramde13,05±8,2 e16,57±10,79 notecidoneoplásico dacavidadeorale lábioinferior, respectivamente.Osvalorescorrespondentesforam9,94±5,59e12,50±7,8nofrontinvasivo. Nãoforamobservadasdiferenc¸assignificantesnasduasvariáveisDVLentreosdoislocais. Conclusão:Deacordocomosnossosresultados,apesquisaporfatoresadicionais,além daque-les relacionadosà vasculatura,deve ter continuidade,para auxiliarno esclarecimentodas diferenc¸asdocomportamentobiológicoentreCCEsnolábioinferiorenacavidadeoral. © 2015 Associac¸˜ao Brasileira de Otorrinolaringologia e Cirurgia C´ervico-Facial. Publicado por Elsevier Editora Ltda. Este ´e um artigo Open Access sob uma licenc¸a CC BY (http:// creativecommons.org/licenses/by/4.0/).
Introduction
Squamouscell carcinomas(SCCs)originatefromepithelial cellsofvariousorgansandtheirbiologicbehaviordepends ondifferentfactors,oneofwhichistheanatomiclocation ofthetumor.1 Agoodexampleofthisfactisthe consider-ableetiologicandprognosticdifferencesbetweenSCCsof thelipandoralcavity,withlipneoplasmsdemonstratinga lowertendencytowardregionallymph-nodemetastasisand ahighersurvivalrateofapproximately90%.2,3
Many factors are involved in the etiopathogenesis of SCC.ContrarytoSCCoftheoralcavitywheretobaccouse is the most well-known etiologic factor, chronic exposure to sunlight has been suggested as an important element in SCC of the lower lip, which is known to receive more ultravioletradiationthantheupperlip.2,4,5Recentstudies have shown that the expression of some markers related totumormicroenvironmentandneoplasticcellsoflipSCCs are different from those of the oral cavity.2,3 Therefore, it seems that the differences between these sites are not limited to etiology and prognosis, but may also be relatedtomolecular factors associated withtheir stroma andcellularstructures.2,3Consequently,anumberof inves-tigatorsbelievethat SCCofthe lipshouldberegarded as aseparate entityandbe evaluatedassuch. Onthe other hand, some cellular-molecular studies on these locations havenotshownany biologicaldifferencein theevaluated markers.6,7
Angiogenesis is an important and fundamental process inthe progressionandmetastasis of malignancies. Before
1960,researchersbelievedthatnutritionandbloodsupply ofneoplastictissuesweresimplyprovidedthroughdilation ofbloodvesselsavailableinthetumor.Subsequentstudies revealedthatangiogenesis,theformationofnewblood ves-sels,isvitaltothegrowthandpropagationofmalignancies.8 Developmentofanetworkofnewbloodvesselsinthetumor is essential to provide nutrients and oxygen and remove waste products.Fortheinitiationofangiogenesis,various moleculesarereleasedfrommalignantcells,whichsend sig-nalstothesurroundinghosttissues.Thismayresultinthe activationofcertaingenes,followedbyproteinproduction, leadingtotheinductionofangiogenesis.9,10
Lymphangiogenesis is the formation of new lymphatic vesselsfrompre-existingvasculatureandsimilarto angio-genesis has several induction mechanisms.11 The growth of lymphatic vessels occurs in a variety of normal and pathologicprocesseslikewoundhealing,inflammation,and progressionofmalignancies.12,13
compare angiogenesis and lymphangiogenesis between lowerlipandoralcavitySCCusingCD105andD2-40markers.
Methods
Samples
Thisretrospectivestudywasperformedonindividualswith primary SCC who were consecutively visited at the Can-cerInstituteofImamKhomeiniHospitalComplex,affiliated withTehran University of Medical Sciences between 2007 and2012, usingthepatientrecord archiveof thisCenter. Cases whichhad ahistory ofchemotherapy, radiotherapy, oranyothertreatmentpriortosurgerywerenotincludedin thiswork.Excisionalbiopsysampleswithsignificant necro-sisandinadequatetissuewerealsoexcludedfromthestudy. Ageandsexwererecordedforeachsubjectaccordingtothe clinicaldataprovidedintheirmedicalcharts.Formalin-fixed paraffin blocksof alllesions correspondingtothe patient chartsoftheselectedcaseswereretrievedfromthe pathol-ogyarchive tobeusedfor immunohistochemical analysis. ThisprojectwasapprovedbytheethicscommitteeofTehran UniversityofMedicalSciences(coden◦70-10646).
Immunohistochemicalstaining
Formalin-fixed paraffin-embedded tissue sections (3m)
weremountedonpoly-l-lysine-coatedslidesandsubjected todeparaffinization in xylene, followed by rehydrationin graded alcohol and antigen retrieval. ForD2-40, this was doneby immersingthespecimensin citratebuffer (0.1M, pH 6) and heating in a microwave oven for 2 cycles of 15mineach,andforCD105pretreatmentwithproteinaseK wasperformedfor5min.Endogenousperoxidasewasthen blockedbyincubatingthesectionsinasolutionof3% hydro-genperoxideandmethanolforhalfanhour.Afterwashing withTris-bufferedsaline(TBS),thespecimensweretreated witheitherD2-40(D2-40,DakoCytomation)orCD105(SN6h, Dako, Glostrup, Denmark) monoclonal antibodies for 1h in ahumid chamber at1:1000 and1:30 dilutions, respec-tively. TBS was used for rinsing before incubating with EnVisionSystem (DakoCytomation, Glostrup,Denmark)at room temperature for 30min. Antigen---antibody reaction wasvisualizedwithdiaminobenzidine,andcounterstaining wascarriedoutwithMayer’shematoxylin.Positivecontrols consisting of breast carcinoma with known immunoreac-tionsforD2-40andnormallivertissueforCD105alongwith negativecontrols (omission of primaryantibody for nega-tivecontrol)wererunsimultaneouslywiththeexperimental slides.
Stainingevaluation
Microvessel density (MVD) and lymphatic vessel density (LVD) were quantified according tothe method described previously.20 In brief, using a double-headed microscope (OlympusBH2, Tokyo,Japan), fivehotspotswereselected at100×magnificationbytwooralpathologists,followedby microvesselcountingat400×(fieldsize:0.18mm2)and
cal-culatingthemeanmicrovesselcountforeach sample.Any possibledisagreementswereresolvedbyconsensus.
Figure1 Representativesectionoflymphaticvesseldensity usingimmunohistochemistrywithmonoclonalantibodyagainst D2-40(originalmagnification200×).
Statisticalanalysis
Statisticalanalysiswasperformedusingt-test,andp<0.05 wasconsideredsignificant.
Results
Weobtainedatotalof 84casesof SCC,45 ofwhichwere locatedintheoralcavityand39inthelowerlip.Ofthe39 lowerlipSCCsamples,5(13.5%)werefemaleand34(86.5%) weremale,correspondingto31(68.9%)maleand14(31.1%) femalepatientswithSCCoftheoral cavity.Theagerange ofthepatientswithlowerlipandoralcavitySCCwas31---90 (mean:65)and19---64(mean:61)years,respectively.Oral cavitytumorswerelocatedinthetongue(21:46.7%),floor ofthemouth(8:17.8%),buccalmucosa(6:13.3%),gingiva (6:13.3%)andmaxilla(4:8.9%).
Immunohistochemicalevaluation ofLVD wasperformed separatelyintheneoplastictissueandtumorinvasivefront (Fig.1),whileMVDwasassessedgenerallyintheSCCtissues (Fig.2).
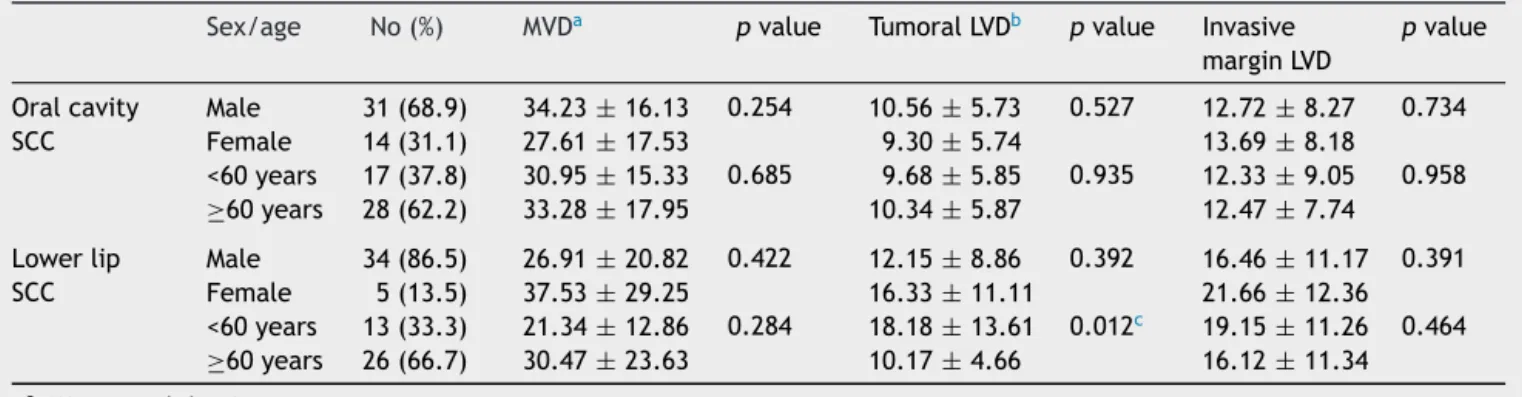
Table1 Comparisonofmicrovessel-andlymphaticvessel-densityinoralcavityandlowerlipSCC.
Sex/age No(%) MVDa pvalue TumoralLVDb pvalue Invasive marginLVD
pvalue
Oralcavity SCC
Male 31(68.9) 34.23±16.13 0.254 10.56±5.73 0.527 12.72±8.27 0.734 Female 14(31.1) 27.61±17.53 9.30±5.74 13.69±8.18
<60years 17(37.8) 30.95±15.33 0.685 9.68±5.85 0.935 12.33±9.05 0.958 ≥60years 28(62.2) 33.28±17.95 10.34±5.87 12.47±7.74
Lowerlip SCC
Male 34(86.5) 26.91±20.82 0.422 12.15±8.86 0.392 16.46±11.17 0.391 Female 5(13.5) 37.53±29.25 16.33±11.11 21.66±12.36 <60years 13(33.3) 21.34±12.86 0.284 18.18±13.61 0.012c 19.15±11.26 0.464 ≥60years 26(66.7) 30.47±23.63 10.17±4.66 16.12±11.34
aMicrovesseldensity. b Lymphaticvesseldensity. c Significant(p<0.05).
Mean MVD±SD was31.94±18.9in the oral cavityand 27.54±20.8inthelowerlip.Thehighestandlowestmean MVDvalues (92.00 and 5.30, respectively) were observed intheoralcavitySCCgroup.Nosignificantdifferencewas foundbetweenthelowerlipandoralcavity(p=0.32)
Mean LVD±SDin thetumoral tissuewas13.05±8.2in theoralcavityand16.57±10.79inthelowerlipSCCgroup. Tumor invasive margin demonstrated a mean LVD±SD of 9.94±5.59and12.50±7.8intheoralcavityandlowerlip, respectively.Thehighest andthelowestamountsofmean LVDwereobservedinthetumorfrontwhencomparedwith the neoplastic tissue,with the highest value being 48.67 in an oral cavity tumor and the lowest counting 0.67 in thelowerlip.Neithertumoraltissue(p=0.105)norinvasive front(p=0.098)showedsignificantdifferencesbetweenthe lowerlipandoralcavity.
Comparison of MVD and LVD between oral cavity and lowerlipSCCsaccordingtoageandsexisshowninTable1. Basedonourresults, lowerlipSCCpatients youngerthan 60yearsofagedemonstratedsignificantlyhigherneoplastic LVDcomparedtothoseolderthan60years(p=0.012).
Discussion
Metastasisof malignantcells tolymphnodesisoneofthe major prognostic factors in many solid tumors like oral SCC.21Angiogenesisandlymphangiogenesisprovidenew ves-selsthroughwhichmalignantcellscanleavethesurrounding areaoftheprimarytumor.13Differentaspectsofthesetwo processeshavebeenevaluatedinSCCoftheoralcavityand lipusingdifferentmarkers.15---18CD105iscurrentlyemployed for the evaluation of newly formed vessels. This protein preferablybindstotheactiveendothelialcellsinvolvedin theprocessofangiogenesis.Forthisreason,CD105ishighly expressedinproliferatingendothelialcells,whileits expres-sionis weakornegativeinnormalvessels. Thepowerand ability of CD105 for quantitative differentiation between active/proliferatingandnormal/quiescentendothelialcells makes it possible to evaluate newly formed tumor blood vesselsmoreaccurately.22,23
Onthecontrary,fewerstudieshavebeen performedon lymphatic vessels because of an absence of appropriate oculartechniques. Inthepast, manyresearchersbelieved thattumors,due tothelack of lymphaticvessels, cannot
induce lymphangiogenesis. In the recent decade, specific antibodies against lymphatic endothelial cells have been identified,leadingtoamodificationofthegeneralviewpoint towardthisprocess.11D2-40isamarkerthatisexpressedon endothelialcellsoflymphaticvesselsandhasbeenusedfor theevaluationofLVDinrecentyears.24Inthepresentstudy weusedD2-40andCD105toevaluateangiogenesisand lym-phangiogenesisinSCCofthelowerlipandoralcavity.Our resultsshowedhigherMVDintheoralcavitycomparedtothe lip,butthedifferencewasnotsignificant.Inagreementwith ourfindings,M˘arg˘aritescuetal.,25usingCD-105,reportedno significant differencebetween these sites; however, they found MVD tobehigher inlip SCCs. In contrast, Oliveira-Neto etal.17 demonstrated significant differences in MVD between SCCs of the lip and oral cavity, withMVD being higherinoraltumors.Chronicsunexposure,asseenin pho-toagedskin,hasbeensuggestedtodecreasethenumberof bloodvesselsintheupperdermis,26whileoralcavitymucosa isknownforitshighvascularityandefficientbloodsupply. ThisfactmayberesponsibleforthehigherMVDofour intra-oralSCCs.Inaddition,consideringthemetastasis-promoting roleofangiogenesis,thehigherMVDoforal cavitytumors in this study is in line withprevious reports, stating that oralcavitytumorsaremorepronetolymph-node metasta-sisanddemonstratealowersurvivalratewhencomparedto liptumors.2,3
metastasis,ofwhichlymphangiogenesisisonlyoneofthem. Evaluationofothereffectivefactorsmaybetterrevealthe biologicdifferencesbetweenlowerlipandoralcavitySCC. Thestatisticallyinsignificantdifferencesinbothfactors between the lip and oral cavity observed in the current investigation are comparable to previous studies, which found lip cancer to be closely related to upper digestive tractmalignancies.5Additionally,itisnoteworthythatinall oftheabovementioned studies,‘‘lipspecimens’’included theupperandlowerlips,whileweexcludedupperlipSCCs fromour study sample. Consequently, our findings reflect MVDandLVDoflowerliptumorsincomparisontooral neo-plasms, and our results therefore may not be accurately comparedwiththoseinvestigations.Theimportanceofthe exclusiveselectionoflowerlipSCCisreflectedinthefact thattheyhavebeenshowntobebiologicallydistinctfrom upperliptumors.Malignanciesintheselocationsalsodiffer in prevalence, and possibly in etiology: SCC of the lower lip is more common than in the upper lip, and the role of UV light and pipe smoking is more prominent in caus-inglowerlipversus upperlipSCC.19,27 On theotherhand, thegrowthoflowerlipmalignancyisslowerthanits coun-terpart,with abetter prognosis.Forthese reasons,some investigators suggest that SCC of the upper lip should be evaluatedasaseparateentityinordertorendermore reli-ableresults.26---29 Regardingdemographicdata,wefound a significantlyhigherneoplasticLVDinpatientswithlowerlip SCCswhowereyoungerthan60years,ascomparedtothose whowereaged60orolder.Thisisinaccordancewith pre-viousstudiesdemonstratinganaggressivecourseofdisease insomeyoungpatientsandthoseindicating differencesin themolecularprofileofyoungandoldpatientswithSCC.30 ItshouldbementionedthatifwehadaccesstoTNM stag-ing,survivalandmetastasis dataofthepatients,wecould commentontherelationshipofmetastasiswith angiogene-sisandlymphangiogenesisinlowerlipandoral cavitySCC withmorecertainty.
Final
comments
In recent years, studies performed on a number of cel-lular and molecular markers in lip and oral cavity SCCs have revealed some differences, indicating their biologic variations.2,3 On the other hand, the expression of other proteinsshowednodifferencebetweenthesetwogroups.7,8 Basedontheresultsofthecurrentinvestigation, angiogen-esis and lymphangiogenesis do not seem to be helpful in clarifyingthebiologicdifferenceoflowerlipandoral cav-itySCC.Itseemsthatthesearchforadditionalfactorsother thanthoserelatedtothevasculatureshouldcontinuetohelp clarifythedifferencesinbiologicbehaviorbetweenlowerlip andoralcavitySCCs.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.YanW, Wistuba II,Emmert-Buck MR,Erickson HS. Squamous cellcarcinoma---similaritiesanddifferencesamonganatomical sites.AmJCancerRes.2011;1:275---300.
2.BatistaAC,CostaNL,Oton-LeiteAF,Mendonc¸aEF,AlencarRde C,SilvaTA.Distinctiveclinicalandmicroscopicfeaturesof squa-mouscellcarcinomaoforalcavityandlip.OralSurgOralMed OralPatholOralRadiolEndod.2010;109:e74---9.
3.ZancopeE,CostaNL,Junqueira-KipnisAP,ValadaresMC,Silva TA,LelesCR,etal.DifferentialinfiltrationofCD8+andNKcells inlipandoralcavitysquamouscellcarcinoma.JOralPathol Med.2010;39:162---7.
4.SilvermanSJr.Demographicsandoccurrenceoforaland pha-ryngealcancers,theoutcomes,thetrends,thechallenge.JAla DentAssoc.2001;132:7S---11S.
5.deVisscherJG,vanderWaalI.Etiologyofcancerofthelip:a review.IntJOralMaxillofacSurg.1998;27:199---203.
6.CruzMC,PereiraAL,LopesFF,NonakaCF,SilvaRR,FreitasRde A, et al. Immunohistochemicalexpression of E-cadherin and CD44v6insquamouscellcarcinomasofthelowerlipandtongue. BrDentJ.2009;20:64---9.
7.AmaralPereiraAL,LopesFF,daCruzMC,daSilveiraÉ.J.,Pinto LP,deSouzaLB,etal.Roleofintegrinsinthecarcinogenesis ofsquamouscellcarcinomaofthetongueandlowerlip.Appl ImmunohistochemMolMorphol.2013;21:154---8.
8.Folkman J. Tumor angiogenesis: therapeutic implications. N EnglJMed.1971;285:1182---6.
9.Folkman J, Cotran RS. Relation of vascular proliferation to tumorgrowth.IntRevExpPathol.1976;16:207---48.
10.CarmelietP,JainRK.Angiogenesisincancerandotherdiseases. Nature.2000;407:249---57.
11.Zhang Z, Helman JI, Li LJ. Lymphangiogenesis, lymphatic endothelialcells and lymphaticmetastasis inheadand neck cancer---areviewofmechanisms.IntJOralSurg.2010;2:5---14. 12.Bruyère F, Noël A. Lymphangiogenesis: in vitro and in vivo
models.FASEBJ.2010;24:8---21.
13.Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573---83.
14.López-GranielCM,TamezdeLeónD,Meneses-GarcíaA, Gómez-Ruiz C, Frias-Mendivil M, Granados-García M, et al. Tumor angiogenesisasaprognosticfactorinoralcavitycarcinomas. JExpClinCancerRes.2001;20:463---8.
15.ArteseL,RubiniC,FerreroG,FioroniM,SantinelliA,PiattelliA. Microvesseldensity(MVD)andvascularendothelialgrowth fac-torexpression(VEGF)inhumanoralsquamouscellcarcinoma. AnticancerRes.2001;21:689---95.
16.Kyzas PA, Stefanou D, Agnantis NJ. Immunohistochemical expression of vascular endothelial growth factor correlates with positive surgical margins and recurrence in T1 and T2 squamouscell carcinoma (SCC)ofthelowerlip. OralOncol. 2004;40:941---7.
17.Oliveira-Neto HH,Gleber-Netto FO,de SousaSF, Franc¸a CM, AguiarMC,SilvaTA,etal.Acomparativestudyofmicrovessel densityinsquamouscellcarcinomaoftheoralcavityandlip. OralSurgOralMedOralPatholOralRadiol.2012;113:391---8. 18.WatanabeS,KatoM,KotaniI,RyokeK,HayashiK.Lymphatic
vesseldensityandvascularendothelialgrowthfactor expres-sion in squamous cell carcinomas of lip and oral cavity: a clinicopathological analysiswithimmunohistochemistry using antibodies to D2-40, VEGF-C and VEGF-D. YonagoActa Med. 2013;56:29---37.
19.LindqvistC,TeppoL.Isupperlipcancer‘‘true’’lipcancer?J CancerResClinOncol.1980;97:187---91.
20.AlaeddiniM,SalahS,DehghanF,EshghyarN,Etemad-Moghadam S. Comparison of angiogenesis in keratocystic odontogenic tumours, dentigerous cysts and ameloblastomas. Oral Dis. 2009;15:422---7.
22.Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene. 2003;22:6557---63.
23.Sharma S, Sharma MC,SarkarC.Morphology ofangiogenesis inhumancancer:aconceptualoverview,histoprognostic per-spective and significanceofneoangiogenesis. Histopathology. 2005;46:481---9.
24.LongattoFilhoA,OliveiraTG,PinheiroC,deCarvalhoMB, Curi-oniOA, MercanteAM,etal.Howuseful istheassessmentof lymphaticvasculardensityinoralcarcinomaprognosis?WorldJ SurgOncol.2007;5:140.
25.M˘arg˘aritescu C, Simionescu C, Mogoant˘a L, Badea P, Pirici D, Stepan A, et al. Endoglin (CD105) and microvessel den-sityinoralsquamouscellcarcinoma.RomJMorpholEmbryol. 2008;49:321---6.
26.SawaneM,KajiyaK.Ultravioletlight-inducedchangesof lym-phatic and blood vasculature in skin and their molecular mechanisms.ExpDermatol.2012;21:22---5.
27.Moore SR, Allister J, Roder D, Pierce AM, Wilson DF. Lip cancer in South Australia, 1977---1996. Pathology. 2001;33: 167---71.
28.Knabel MR, Koranda FC, Panje WR, Grande DJ. Squamous-cell carcinoma of the upper lip. J Dermatol Surg Oncol. 1982;8:487---91.
29.DawnA1,LawrenceN.Significantdifferencesinnonmelanoma skin cancers of the upper and lower lip. Dermatol Surg. 2013;39:1252---7.