Tecnologia Química e Biológica, Universidade Nova de Lisboa, Oeiras, Portugal.
Acknowledgments
This thesis is the result of a long scientific and personal journey, full of good and some “not so good” moments. Now that this journey is finishing, there are many people to whom I would like to address my sincere gratitude for their contribution to the conclusion of this “task”. First of all I would like to thank my supervisors Cristina Silva Pereira and Andrew Hursthouse. Thank you, Cristina, for your scientific guidance, for your commitment and constant enthusiasm and optimism. Thank you for some wise advices I will always remember. Andrew, I’m really grateful for your constant encouragement and for being always available each time I needed.
I would also like to thank all present and former members of Applied and Environmental Mycology lab. Your valuable support and help during so many occasions were crucial. Cátia, Sandra and Joana, thank you for the important contribution to the work developed on fungal biodegradation of PCP. Thank you for your constant help and support, particularly in the “not so good” moments. Cristina Leitão, thank you so much for your indispensable help with the HPLC and UPLC, for your competence and for your kindness and pacience with me. Thank you Isabel for your help and time devoted to this work. We shared some of the most exciting moments, at the beginning of our works. Tiago, Diego and Celso thank you for the careful reading of parts of the thesis. Thank you also, Diego, for preparing the nice microscopic photos of M. plumbeus, in particular the one chosen for the cover of the thesis. Thank you so much Paula for your help at the end of the writing of the thesis. Thank you, Helga, Marija, Adélia, Rui and Marina, for your constant help and support.
early times. I would also like to thank Carmo Barreto, for the crucial help with the “fungi stuff”.
I would also like to thank Oscar Núñez, Prof. Teresa Galceran and Héctor Gallart-Ayala, for the MS analysis of the PCP degradation metabolites. Thank you for receiving us warmly in the beautiful city of Barcelona. Thank you, Oscar, for being available to answer all my doubts.
I would like to thank Jenny Renaut and Sebastien Planchon for protein MS analysis. Thank you, Jenny, for answering all my questions.
I would also like to thank Pedro Lamosa for NMR analysis of the PCP degradation metabolites, Joana Ferreira for the help with GC and Abel Oliva for allowing me to enter his lab at any hour to use the spectrophotometer for, at least, a couple of years.
To my friends, I want to thank for keeping me as “emotionally healthy” as possible. Vanessa, thank you for your indispensable support in several moments and for being a true friend! Catarina, thank you for your wise advices and for the feeling that no matter what, you’ll be there, if I need! Thank you João for your important support and help at the early times of this journey. Thank you Gonçalo for your “assistance” and for all the times you offered to help. Thank you Mariana and Nuno for the good times we spent together in the last years and for your friendship. Thank you to all my friends that are still waiting for me, despite my long “absence”.
“Once we accept our limits, we go beyond them”
Summary
Pentachlorophenol (PCP) is a synthetic compound introduced as a wood preservative and used for decades in agricultural and industrial applications. Today, the production and use of PCP is restricted due to its toxicity and environmental impact. However, extensive use in the past and its environmental persistence has resulted in substantial contamination of PCP worldwide. The detection of PCP in the human population and in remote environments, such as the Arctic, is still being reported.
Bioremediation technology exploits the catabolic capability of microorganisms to treat pollutants. To ensure robust application of this technology, the detailed biodegradation mechanisms of the target pollutants need to be fully investigated.
Filamentous fungi are ubiquitous in nature and are known decomposers of organic molecules, including a large range of pollutants. Basidiomycota strains are the most widely investigated in the bioremediation of organic pollutants, including PCP, due to their ability to secrete ligninolytic enzymes which efficiently breakdown these compounds.
Based on the identification by LC-HRMS (Q-TOF) of several PCP-derived metabolites produced by M. plumbeus during PCP degradation, a transformation pathway was proposed (Chapter III). The metabolites identified include tetra- and tri-chlorohydroquinones (TeCHQ and TCHQ, respectively) and phase II-conjugated metabolites, resulting from the conjugation of PCP, TeCHQ and TCHQ with sulfate, glucose or ribose. The presence of sulfate-glucose conjugates was reported for the first time in fungi. Phase II conjugation is a well-known detoxification strategy employed by diverse organisms to increase the solubility of xenobiotics and facilitate their excretion. The conversion of PCP into TeCHQ does not seem to involve the activity of either extracellular ligninolytic enzymes or intracellular cyt P-450 monooxigenases, as
previously suggested for the Basidiomycota
Phanerochaete chrysosporium and Mucor ramosissimus, respectively. The proposed transformation pathway involves several reactions of phase II conjugation and both oxidative and reductive dechlorination, thought to occur intracellularly.
needs further investigation. PCP degrading enzymes were not detected in the fungal secretome preliminary identified. This also supports the hypothesis of the intracellular degradation pathway proposed in Chapter III.
Sumário
O pentaclorofenol (PCP), um composto sintético que actua como conservante de madeira, foi usado durante décadas em aplicações agrícolas e industriais. Hoje em dia, a sua produção e utilização encontram-se restringidas devido à sua toxicidade e impacto ambiental. No entanto, resultado da sua utilização extensiva no passado e da sua persistência no ambiente, os níveis de contaminação de PCP são elevados em todo o mundo. A deteção de PCP na população humana e em localizações remotas, tais como o Ártico, continua a ser relatada.
A tecnologia de bioremediação explora a capacidade catabólica dos microorganismos para tratar poluentes. Para garantir uma aplicação robusta desta tecnologia, os mecanismos de biodegradação dos poluentes-alvo têm de ser investigados detalhadamente.
degradação de PCP, independentemente das condições de cultura. A via de transformação usada por esta espécie era até aqui desconhecida, justificando a sua seleção para um estudo mais aprofundado.
Com base na identificação por LC-HRMS (Q-TOF) de vários metabolitos do PCP produzidos pelo M. plumbeus, foi proposto uma via de transformação de PCP (Capítulo III). Os metabolitos identificados incluem tetra e tri-clorohidroquinonas (TeCHQ e TCHQ, respectivamente) e metabolitos de conjugação (fase II), resultantes da conjugação de PCP, TeCHQ e TCHQ com sulfato, glucose ou ribose. A presença de conjugados sulfato-glucose foi aqui relatada pela primeira vez em fungos. As reações de conjugação (fase II) constituem estratégias de destoxificação bem conhecidas, utilizadas por diversos organismos para aumentar a solubilidade de xenobióticos e facilitar a sua excreção. A conversão de PCP em TeCHQ não parece envolver a atividade de enzimas extracelulares ou de monooxigenases do sistema cyt P-450, como anteriormente tinha sido sugerido para o Basidiomycota Phanerochaete chrysosporium e para o Mucor ramosissimus, respectivamente. A via de transformação proposta envolve várias reações de conjugação (fase II) e desclorinação oxidativa e redutiva que parecem ocorrer no interior das células.
identificada após exposição ao PCP é uma álcool desidrogenase dependente de NAD e Zn, que foi anteriormente associada à destoxificação de xenobióticos. Apesar do envolvimento deste enzima nos passos iniciais de transformação do PCP ser pouco provável, é possível que participe noutros passos desta via, aspecto que necessita ser melhor investigado. Não foram detectados enzimas que degradem PCP no secretoma fúngico preliminarmente identificado. Este resultado vai ao encontro da hipótese de uma via de degradação intracelular, como a proposta no Capítulo III.
Table of Contents
Acknowledgments ... iii
Summary ...ix
Sumário ... xiii
Abbreviations ... xxxiii
Chapter I Introduction 1.1. Biological and ecological features of filamentous fungi ... 3
1.1.1. Mucor spp. ... 8
1.2. Fungi as bioremediation agents ... 12
1.2.1. Role of fungal enzymatic systems in biodegradation of pollutants ... 15
1.2.1.1. Extracellular enzymes ... 16
1.2.1.2. Cell-bound enzymes ... 18
1.3. Pentachlorophenol ... 21
1.3.1. Environmental contamination of PCP ... 24
1.3.2. Bioaccumulation of PCP and human exposure ... 28
1.3.3. Toxicity of PCP ... 30
1.4. Fungal degradation of PCP ... 33
1.4.1. Degradation of PCP by Mucorales ... 37
1.5. Cork colonizing fungi... 39
1.6. Aims of the thesis ... 41
Chapter II Screening pentachlorophenol degradation ability by environmental fungal strains belonging to the phyla Ascomycota and Zygomycota
Abstract ... 67
Abbreviations ... 68
Keywords ... 68
Introduction ... 68
Materials and Methods ... 70
Biological ... 70
Fungal cultures ... 71
PCP degradation experiments ... 71
PCP quantification by HPLC ... 72
Glucose quantification ... 73
Detection of PCP degradation intermediates ... 73
Computational interpretation of data ... 74
Results ... 74
Fungal growth behaviour in the presence of PCP ... 74
PCP abiotic degradation ... 75
PCP biotic degradation in cometabolic conditions (GMM) ... 75
Cluster analysis of PCP biotic degradation in co-metabolic conditions (GMM) ... 78
PCP biotic degradation by fungi in metabolic conditions (MM) ... 79
Analysis of PCP metabolic intermediates ... 80
Discussion ... 81
Conclusion... 85
Acknowledgments ... 85
Chapter III Degradation pathway of pentachlorophenol by Mucor plumbeus involves phase II conjugation and oxidation-reduction reactions
Abstract ... 95
Abbreviations ... 96
Keywords ... 96
Introduction ... 96
Materials and Methods ... 98
Reagents ... 98
Fungal strain and cultivation conditions ... 98
PCP biotransformation ... 99
In vivo inhibition of cytochrome P-450 ... 101
Enzymatic assays ... 101
PCP and PCP-derived metabolites analysis ... 103
Liquid chromatography ... 103
Liquid chromatography-high resolution mass spectrometry (LC-HRMS)(Q-TOF) ... 103
Results ... 104
Time course of PCP degradation by M. plumbeus ... 104
Chromarographic analysis of M. plumbeus extracts... 105
Identification of PCP-derived metabolites by LC-HRMS (Q-TOF) ... 108
In vivo inhibition of cytochrome P-450 during PCP degradation ... 112
Enzymatic assays ... 112
Discussion ... 113
Conclusion... 119
Acknowledgments ... 120
Chapter IV The response of Mucor plumbeus to pentachlorophenol: A toxicoproteomics study
Abstract ... 131
Abreviations ... 132
Keywords ... 132
Introduction ... 133
Materials and Methods ... 134
Chemicals ... 134
Fungal strain and cultivation conditions ... 135
Analysis of PCP and PCP derived metabolites ... 136
Proteome analysis ... 137
Protein extraction ... 137
2D gel electrophoresis ... 138
Image and statistical analysis ... 139
Protein identification ... 139
Protein functional classification ... 140
Results and Discussion ... 141
Proteins involved in PCP degradation pathway ... 150
Proteins involved in stress response and oxidoreductase activity ... 151
Proteins involved in energy metabolism ... 153
Proteins involved in cell wall architecture and cytoskeleton ... 155
Mitochondrial proteins ... 156
Extracellular proteome ... 157
Conclusion... 161
Acknowledgments ... 162
Chapter V Final discussion and future perspectives
Abbreviations
aaRS aminoacyl-tRNA synthetase 1-ABT 1-aminobenzotriazole
ABTS 2,2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) ADH alcohol dehydrogenase
ANOVA analysis of variance ATP adenosine triphosphate BSA bovine serum albumin CDA chitin deacetylase
CHAPS 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
CHQ chlorohydroquinone
Cyt P-450 cytochrome P-450
DCBQ 2,6-dichloro-1,4-benzoquinone DCHQ dichlorohydroquinone
2DE bidimensional gel electrophoresis DMSO dimethyl sulfoxide
DNA deoxyribonucleic acid DTT dithiothreitol
EPA Environmental Protection Agency EROD ethoxyresorufin O-deethylase EST expressed sequence tag
EU European Union
GAPDH glyceraldeyde 3-phosphate dehydrogenase GCR glutathione conjugate reductase
GMM glucose minimal media Gr glucose residual concentration GST glutathione S-transferase
HCB Hexachlorobenzene
HPLC high performance liquid chromatography
HQ hydroquinone
HRMS high resolution mass spectrometry HSP heat shock protein
IARC International Agency for Research on Cancer IEF isoelectric focusing
JGI Joint Genome Institute
Ko/w octanol/water partition coefficient LC liquid chromatography
LDS lignin degrading system LiP lignin peroxidase
MALDI matrix-assisted laser desorption/ionization MIC minimal inhibitory concentration
MIPS Munich Information Center for Protein Sequences
MM minimal media
MnP manganese dependent peroxidase
MS mass spectrometry
NAD nicotinamide adenine dinucleotide NaPCP sodium pentachlorophenate
NATO North Atlantic Treaty Organization
NCBI Nacional Center for Biotechnology Information NK cells natural killer cells
NMR nuclear magnetic resonance
P5CDH 1-pyrroline-5-carboxylate dehydrogenase PAH polycyclic aromatic hydrocarbons
PCA pentachloranisole
PCEB pentachloroethoxybenzene PCP pentachlorophenol
PCPd pentachlorophenol decay PCPL pentachlorophenyl laurate PDB potato dextrose broth PDH proline dehydrogenase PeCB pentachlorobenzene
pKa negative base-10 logarithm of the acid dissociation constant PMSF phenylmethanesulfonyl fluoride
PVPP polyvinylpolypyrrolidone
Q-TOF MS quadrupole time-of-flight mass spectrometry RBBR remazol brilliant blue R
RNA ribonucleic acid
ROS reactive oxygen species SAM S-adenosyl-L-methionine SDS sodium dodecyl sulfate SOD superoxide dismutase
T4 thyroxine
TCHQ trichlorohydroquinone TCA tricarboxylic acid 2,4,6-TCA 2,4,6-trichloroanisole TCP trichlorophenol
TCPG 2,4,6-trichlorophenol-1-O-glucoside TeCA tetrachloroanisole
TeCBQ tetrachloro-1,4-benzoquinone TeCHQ tetrachlorohydroquinone
TEMED N,N,N’,N’-tetramethylethylenediamine TFA trifluoroacetic acid
TIC total ion chromatogram
TOF MS time-of-flight mass spectrometry
Tr retention time
Tris 2-amino-2-hydroxymethyl-propane-1,3-diol tRNA transfer ribonucleic acid
TTR transthyretin
UPLC ultra performance liquid chromatography VA veratryl alcohol
Chapter I
1.1. Biological and ecological features of filamentous fungi
Fungi are ubiquitous in nature, accounting for up to 75% of the soil biomass [1]. They form one of the kingdoms of life [2], which includes moulds, mushrooms, lichens, rusts, smuts and yeasts [3]. To date, the number of fungal species described is close to 100,000, but approximately 1.5 million are estimated to exist [1].
According to the fungal genomics resource MycoCosm [4], over 150 assembled genome sequences are now available. This high number, when compared with other eukaryotes, is partially explained by the small size of the fungal genome, which is typically around 30-40 Mb [3].
conditions over extensive periods of time [15]. These characteristics are the main reasons for the successful fungal colonization of diverse environments.
After Whittaker created the Fungi kingdom in 1969 [17], the later underwent significant changes in classification. Until approximately a decade ago, the fungal kingdom was considered to include four phyla, namely Chytridiomycota, Zygomycota, Ascomycota and Basidiomycota [18]. A new comprehensive phylogenetic classification was proposed in 2007 by Hibbett et al. [19], and is schematically shown in Figure 1.
In the classification of Hibbett et al. [19], the phyla Basidiomycota and Ascomycota were grouped in the subkingdom Dikarya, whereas the fungi belonging to the traditional Zygomycota phylum were distributed amongst the phylum Glomeromycota and four subphyla incertae sedis, namely Kickxellomycotina, Zoopagomycotina, Entomophthoromycotina and Mucoromycotina. The later subphylum includes the Mucorales, the order comprising the genus Mucor. In this thesis, the traditional classification which includes Mucor spp. in the Zygomycota phylum will be used to facilitate discussion.
Fungi can generate a variety of morphologies, including yeast cells (either spherical, ellipsoidal or cylindrical), pseudohyphae or hyphae [22]. Hypha, the basic unit of filamentous fungi [23], are tubular cells that extend only at their apices (polarized growth) and ramify into a mycelium [24]. In Ascomycota and Basidiomycota, the Spitzenkörper or “apical body”, a dense spheroidal cluster of vesicles arranged around microvesicles [25], controls the growth direction of the hyphae [26]. Zygomycota are usually described to be devoid of Spitzenkörper, and instead the vesicles are distributed more loosely in the apical dome [27]. A little behind the tip of the hypha is a region containing mitochondria and endoplasmic reticulum and responsible for a significant biosynthetic activity and energy production. Nuclei and vacuoles, which can occupy a significant part of the mature hypha, appear just behind [27].
Figure 2 a) Nonseptate hypha of Syncephalastrum racemosum (phylum Zygomycota) b) Septate hypha of Fusarium equiseti (phylum Ascomycota) (from Pitt and Hocking [5])
The process of polarized hyphae growth is complex and involves localized cell wall synthesis, secretory vesicles transport and exocytosis, turgor pressure, organelle positioning and cytoplasmic migration. Both cytoskeleton microtubules and F-actin cooperate in delivery of secretory vesicles to the expanding tip [26]. Hyphae can differentiate into specialised structures, e.g. appresoria and haustoria, and also function as gametes in sexual reproduction [24].
The fungal cell wall mediates the interaction of the cell with its surroundings in processes such as cell adhesion and fusion, nutrients uptake, secretion and cell signaling. It protects the cell from environmental stresses, like osmotic pressure [23] and resists against internal turgor pressure, being responsible for the shape of the fungal cell. Although fungal cell walls vary in composition among the fungal orders [31], it is usually made of glycoproteins and polysaccharides, mainly glucan and chitin, extensively cross-linked [32]. Glucan extends throughout the fungal cell wall, being its major structural polysaccharide.
It consists of a branched structure of glucose monomers, usually linked by β-1,3, sometimes β-1,6, glycosidic bonds. Chitin is a polymer of β-1,4 linked N-acetyl-glucosamine, localized immediately adjacent to the plasma membrane [32], and is mainly responsible for the strength of the wall [26]. In Zygomycota, chitin is usually modified after its synthesis by deacetylation, producing chitosan [33]. Both chitin and glucan are synthesized on the plasma membrane and are extruded during synthesis into the cell wall space [32]. Proteins present in the cell wall may be covalently or non-covalently bound, and consist of enzymes involved in cell wall synthesis, lysis or extracellular digestion and structural proteins [27]. Many cell wall proteins are highly glycosylated. Some protein at the surface of the cell wall are involved in cell adhesion and recognition [34]. Most cell wall glycoproteins have a typical N-terminal signal peptide and hence pass through the secretory pathway to the cell wall. Interestingly, several studies in different fungi have revealed the presence of mitochondrial and cytosolic proteins associated with the cell wall [35-38]. These proteins that do not have the typical secretory signal motif, are called “moonlighting” proteins, i.e. multifunctional, displaying functions which differ according to cell location [39].
The fungal cell wall is very dynamic, allowing active construction and remodeling. The collaborative action of the different proteins must be appropriately balanced to ensure cell dynamics, allowing for an adequate elasticity which permits growth and at the same time sufficient strength to avoid cell lysis [32].
1.1.1. Mucor spp.
species belongs to the Mucorales, one of the most prominent orders among the traditional phylum Zygomycota.
The genus Mucor is widespread in nature, particularly in soils, decaying vegetation, dung and others moist habitats [5]. Their members, including M. plumbeus, frequently appear as food spoilage agents [5, 40]. The characteristic fast growth of the non-septate hypha provides a selective advantage over septate hyphae of more advanced fungi [5]. Although Mucor spp. are usually not human pathogens, some species may cause severe opportunistic infections, potentially fatal [40]. Some biotechnology applications of Mucor spp. include the production of oriental foods [41] and their utilization as biotransformation agents [42], in particular, the potential of M. plumbeus as biocatalyst in synthetic chemistry has been often explored [43-45].
The property of dimorphism is one of the most interesting characteristics of several Mucor spp.. The yeast or hyphal growth depends essentially on the environmental conditions, where the gaseous atmosphere is crucial. While aerobic conditions favor hyphal growth, anaerobiosis is normally required for the development and maintenance of the yeast form [46]. The presence of a fermentable hexose is normally important for the fungal growth in the yeast form [40]. In addition to the mentioned conditions, a variety of synthetic compounds have been also reported to alter the cell morphology in Mucor spp.. The morphological forms are interconvertible resulting in a dimorphic shift from one form to the other when culture conditions change.
genetic recombination and may stay dormant for long periods of time. Asexual spores produced by Mucor spp. include arthrospores, chlamydospores and sporangiospores. Arthrospores and chlamydospores are particularly important due to its resistance to adverse environmental conditions, ensuring survival. These spores derive from hyphal structures, through septation of normally coenocytic phyphae [5, 40]. Chlamydospores from M. plumbeus are shown in Figure 3. The most widespread mode of asexual sporulation in the Mucorales is through production of sporangiospores, which, although non-motile, serve as a dispersal mechanism, relying on wind, insects and raindrops. They are formed inside globose sporangia, closed sacs borne at the tip of aerial sporangiophores [27] (Figure 4 a, c). A collumella is present in the central core of the sporangium, surrounded by the spores. In age, sporangial walls disintegrate, releasing the sporangiospores [5] (Figure 4 b, d).
Figure 4 Asexual reproductive structures from M. plumbeus. Images a and c: sporangia; images b and d: exposed columellae. Images a and b are from Webster and Weber [27]; images c and d are microscopic images (40× magnification) captured by Diego Hartmann, at Applied and Environmental Mycology Laboratory, ITQB, from agar medium cultures of the strain Mucor plumbeus Bonord (DMS 16513)
When placed on a suitable medium, sporangiospores germinate. The spores swell and a new inner wall is synthesized beneath the original spore wall [20]. A germ-tube emerges, breaking through the old spore wall, while the cell wall of the germ-tube is continuous with the new inner wall of the spore. Sometimes, more than one germ-tube emerges from the spore [48]. The germ-tube grows and branches, originating, in agar medium, a circular colony of vegetative mycelium [20].
As an example, the life cycle of one Mucor sp. is schematized in Figure 5 [20]. Both sexual and asexual reproduction is shown. Hyphae of
a
b
c
different mating type may come into proximity and form gametangia. Cell and nuclear fusion result in the formation of a diploid zygospore (sexual reproduction). After meiosis takes place, germination occurs, and sporangiophores and sporangia may be formed, resulting in the production of haploid sporangiospores. Germinated sporangiospores form mycelia, which can follow both sexual and asexual reproduction.
Figure 5 – Schematic representation of the life cycle of Mucor mucedo (from Carlile et al. [20])
1.2. Fungi as bioremediation agents
strategies [49]. In the environment, indigenous microorganisms can play key roles in the biodegradation of hazardous chemicals [50]. The exploitation of the catabolic capability of microorganisms to treat wastes and pollutants, i.e. bioremediation, is very promising due to microbial versatility in extreme conditions and their great ability to adapt by modifying the expression profile of their enzymatic machinery [49]. Bioremediation, either involving naturally occurring or newly introduced organisms, has emerged as a clean and low-cost methodology to clean up contaminated environments [50]. Particularly important in bioremediation technology, and often overlooked, is the toxicity of the products generated, which should not exceed the toxicity of the transformed compounds. McGrath and Singleton reported the production of toxic intermediates during fungal bioremediation of pentachlorophenol in soil [51].
Filamentous fungi are known decomposers of a considerable array of organic molecules, often being responsible for severe economic losses, due to food spoiling, wood rotting, and crops devastation. Notwithstanding these harmful effects, fungal degradation potential is of prime importance in nature. The utilization of cellulose and lignin (see below) by saprobic fungi contribute significantly to global nutrient cycling [1].
fungi can easily colonize insoluble substrates [6], which is important since many chemicals tend to precipitate, adsorb to surfaces or accumulate in organic matter and in tiny pores of solid matrices, leading to a significant decrease in their bioavailability [1]. Most fungi are robust organisms and may tolerate higher concentrations of pollutants than bacteria [54]. In addition, fungi possess a unique nutritional strategy, i.e. the secretion of extracellular enzymes to breakdown food resources, whose products are then absorbed by fungal hyphae. Importantly, some of these extracellular enzymes are associated with the degradation of lignin, a complex plant polymer consisting of nonrepeating phenyl propanoid units linked by various carbon-carbon and ether bonds and very resistant to attack by microorganisms [55]. The nature of the lignin degrading enzymes is essentially non-specific considering their substrate range, which allows fungal transformation and mineralization of a wide range of highly recalcitrant organopollutants that have similar structural composition to lignin [52]. Noticeably, the strategy of extracellular enzymatic action enhances bioavailability of pollutants [52].
An interesting feature of bioremediation by fungi is that an additional source of carbon and nitrogen is normally required, since pollutant degradation often occurs during secondary metabolism, and the pollutants are not usually used as nutrient source [6]. This strategy is called cometabolism, and differs from bacteria bioremediation, in which the pollutant is often used as carbon or/and nitrogen source [52]. However, one can also find reports in the literature where pollutants where used as fungal carbon and energy source [56].
the risks associated with the presence of pollutants in the environment, due to the lower bioavailability.
Interestingly, fungi also have an important role in facilitating pollutant degradation by bacteria in soil [1]. They were reported to facilitate the movement of pollutant degrading bacteria along the mycelia, by providing a continuous network of water-paths, which allow them to reach hydrophobic pollutants in the soil, that otherwise would be inaccessible [60].
1.2.1. Role of fungal enzymatic systems in biodegradation of pollutants
When considering their degradation ability, fungi are usually classified according to the appearance left on wood after fungal attack. This is related to their own enzymatic machinery. White rot fungi (WRF) are capable of lignin degradation, besides cellulose, leaving a whitish appearance on wood [20]. Taxonomically, WRF are mainly basidiomycetes, but a few ascomycetes are also responsible for white rot decay [61]. Brown rot fungi, can degrade cellulose, but are unable to degrade lignin [20]. Finally, soft rot species decompose only cellulose, but they attack damper wood and decay usually occurs only near the wood surface [20]. Theygrow essentially within the secondary wood cell wall [62]. The fungal species utilized in experimental work presented in this thesis are most often associated with soft rot. Noticeably, most soft rot species are common soil species and not true wood inhabiting fungi [20]. WRF are the most investigated fungi in bioremediation applications, due to the non-specific nature of their lignin degrading system (LDS) which is able to degrade and mineralise a wide range of xenobiotic compounds.
Phanerochaete chrysosporium is the most intensively studied WRF [63].
investigated with nutrient limitation and secondary metabolism (idiophasic growth) [1, 64].
1.2.1.1.Extracellular enzymes
To degrade lignin within wood, filamentous fungi, particularly WRF, must possess oxidants strong enough to attack non-phenolic (ether-linked) lignin structures, more difficult to oxidize than the phenolic content [65]. The latter constitutes only about 10% of lignin. In addition, these oxidants must be small enough to be able to penetrate the secondary lignified cell wall. The LDS enzymes of WRF can normally accomplish one-electron abstractions from organic substrates, producing diffusible organic radicals, which can perform spontaneous follow-up reactions [1]. Also, iron-reducing systems might use Fe2+ to generate biodegradative hydroxyl radicals [65].
Lignin peroxidase (LiP), manganese dependent peroxidase (MnP), versatile peroxidase (VP) and laccase are the major enzymes of the LDS of WRF, being involved in xenobiotic degradation [61].
enhances the activity of LiP, and it was suggested to protect LiP from inactivation by excess H2O2 [62], which at high concentrations inhibits and rapidly inactivates the enzyme [61]. LiPs have been implicated in the degradation of diverse pollutants, including several dyes [66, 67], chlorophenols [68, 69], polycyclic aromatic hydrocarbons (PAHs) [70], among several other compounds.
MnPs (EC 1.11.1.13) are heam-containing glycoproteins more widespread among WRF than LiP [65]. They are also strong oxidants but do not oxidize non-phenolic lignin-related structures. These enzymes have a manganese-binding site and oxidize Mn2+ to Mn3+, which mediates the oxidation of several phenolic substrates [54]. Before being released from the enzyme active site, Mn3+ must be stabilized by a physiologically generated oxalate or other chelator [61]. In P. chrysosporium, MnP is stimulated by both nutrient limitation and Mn2+ [65]. MnP activity has been associated with the degradation of diverse environmental pollutants and dyes [71-73]. Several isoenzymes of both LiP and MnP have been identified in fungi [74]. The peroxidase required H2O2 is endogenously produced from molecular oxygen by different enzymes such as glyoxal oxidase, glucose oxidase and aryl alcohol oxidase [62].
Versatile peroxidases (VP) (EC 1.11.1.16) are heam-containing structural hybrids between MnPs and LiPs [61]. They can oxidize Mn(II), but can also oxidize veratryl alcohol and phenolic and non-phenolic aromatic compounds in manganese-independent reactions.
Unlike peroxidases, laccases do not need the presence of cofactors. However, the presence of small molecules that work as mediators (e.g. 2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), gallic acid, syringaldazine and 1-hydroxybenzotriazole) enables the oxidation of compounds that are not normally oxidized by laccases, such as non-phenolic substrates, due to their low redox potential [75]. These mediators are oxidized by laccase to stable radicals, which may diffuse far away from the mycelium to sites that are inaccessible to the enzyme itself. Laccases have been reported to oxidize a wide range of xenobiotic compounds, such as chlorinated phenolics [76], synthetic dyes [77], PAHs [78] and pesticides [79].
Production of ligninolytic enzymes in non-ligninolytic fungi, particularly Ascomycota fungi, has been reported [80-82]. Although Zygomycota fungi are usually considered to be devoid of ligninolytic enzymes, a recent report indicates the production of LiP, MnP and laccase by a Mucor racemosus strain [83]. An extracellular LiP had been also reported and had been implicated in pentachlorophenol degradation by the Mucorales Rhizopus oryzae [84]. The question of whether the production of some of these enzymes, in other fungi besides WRF, has been fully investigated has been raised for example by Baldrian, in relation to laccase [75].
1.2.1.2.Cell-bound enzymes
The catalytic activity of the intracellular mixed-function cytochrome P-450 oxidase system (cyt P-450) has a significant role in the degradation of xenobiotic compounds [88, 90]. Cyt P-450 is often responsible for initiating the metabolism of pollutants, as an alternative to the extracellular oxidoreductases. These enzymes belongs to a superfamily of heam-thiolate proteins usually responsible for regio- and stereospecific transformations of lipophilic compounds into more hydrophilic derivatives, introducing an oxygen atom which originates from molecular oxygen [91]. The catalyzed reactions often result in the hydroxylation, epoxidation, dealkylation, sulfoxydation, deamination, desulphuration and dehalogenation of aliphatic, acyclic and aromatic compounds [91].
The activity of Cyt P-450 monooxigenases in xenobiotics degradation is also not exclusive of non-ligninolytic fungi. Several WRF have been reported to transform hazardous chemicals through the action of this enzymatic system [89, 92]. The genome of the fully sequenced P. chrysosporium contains 149 full-length cyt P-450 monooxigenases [88], which contribute to its high versatility towards xenobiotics [1]. Cyt P-450 versatility is even more important in fungal species lacking extracellular oxidoreductases.
mammalian organisms. In the non-ligninilytic genus Mucor, phase II conjugates of some chemical compounds have been identified, for example, in M. ramannianus [95] and M. circenelloides [96] and, in the work presented in this thesis, in M. plumbeus [97]. Interestingly, fungal phase II conjugation of chlorophenols had also been reported by Campoy et al. for the WRF Phlebia radiata [92].
Although cyt P-450 monooxigenases are most frequently reported to be involved in the initial fungal transformation of xenobiotics when it takes place intracellularly, other enzymatic processes may occur. The activity of intracellular tyrosinase produced by the Mucorales Rhizopus oryzae has been implicated in the degradation of PCP [84]. Tyrosinases (EC 1.14.18.1) are copper containing phenoloxidases which oxidize phenols including highly chlorinated ones [1], like PCP [84]. They catalyse the O-hydroxylation of monophenols (cresolase activity) and the subsequent oxidation of the resulting O-diphenols into reactive O-quinones (catecholase activity), both reactions using molecular oxygen [98]. These enzymes are mainly intracellular, although extracellular activity towards PCP was also suggested in another mucorales, Amylomyces rouxii [99].
3,5,6-trichlorohydroquinone by the soluble glutathione conjugate reductase [94].
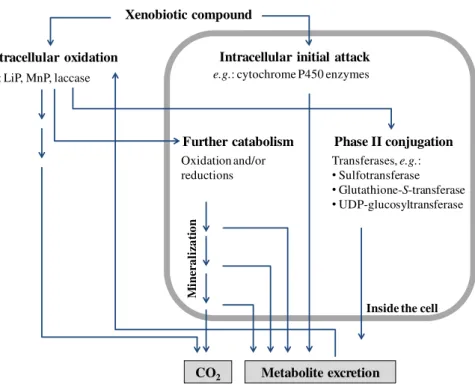
Figure 6 is a schematic representation of some of the different mechanisms presented in this section.
Intracellular initial attack
Phase II conjugation Xenobiotic compound
Extracellular oxidation
e.g.: LiP, MnP, laccase e.g.: cytochrome P450 enzymes
Transferases, e.g.:
•Sulfotransferase
•Glutathione-S-transferase
•UDP-glucosyltransferase Further catabolism
Oxidation and/or reductions
Metabolite excretion
M
inera
li
za
ti
o
n
CO2
Inside the cell
Figure 6 A schematic diagram of the principal mechanisms used by fungi to degrade organic chemicals. Adapted from Harms et al. [1]
1.3. Pentachlorophenol (PCP)
Figure 7− Chemical structure of Pentachlorophenol
PCP was first introduced into the environment, as a preservative for timber and lumber, in 1936 [102, 103]. During several decades, PCP, together with its salts and esters, particularly sodium pentachlorophenate (NaPCP) and sodium pentachlorophenyl laurate (PCPL), were widely used in agricultural, industrial and domestic applications [102, 104]. Examples of such applications include defoliants, wood preservatives and biocides (e.g. insecticide, fungicide, bactericide, herbicide, algicide and molluscicide) [102, 104, 105].
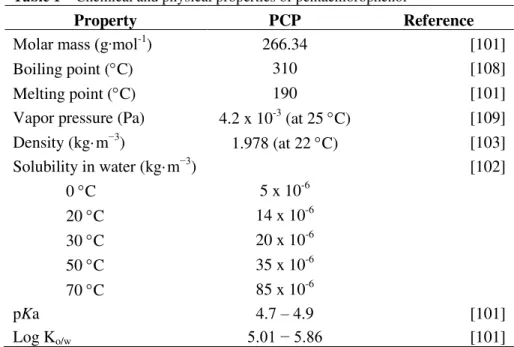
Table 1–Chemical and physical properties of pentachlorophenol
Property PCP Reference
Molar mass (g∙mol-1) 266.34 [101]
Boiling point (C) 310 [108]
Melting point (C) 190 [101]
Vapor pressure (Pa) 4.2 x 10-3 (at 25 C) [109]
Density (kg·m−3) 1.978 (at 22 C) [103]
Solubility in water (kg·m−3) [102]
0 C 5 x 10-6
20 C 14 x 10-6
30 C 20 x 10-6
50 C 35 x 10-6
70 C 85 x 10-6
pKa 4.7 – 4.9 [101]
Log Ko/w 5.01 − 5.86 [101]
Concerns over the toxicity of PCP and its potential to cause adverse effects in humans and animals (see below), associated with its stability and persistence in the environment, resulted in the restriction or ban of the production and use of PCP in many countries [102, 110, 111]. Commercial grade PCP contains approximately 10% impurities, which are formed during the manufacturing process [103] and can be more hazardous and more stable in the environment than PCP itself. These impurities are mainly other polychlorinated phenols, polychlorinated dibenzo-p-dioxins (PCDD), and polychlorinated dibenzofurans (PCDF) [103]. Hexachlorobenzene and chlorinated ethers may also be formed in the manufacturing process of PCP [104].
professional and industrial use was permitted, but with several restrictions [105, 107]. In particular, the marketing and use of PCP, its salts and esters in concentrations equal or higher than 0.1% by mass was forbidden, with a few particular exceptions. These exceptions include, for example, the use for wood treatment in industrial installations and use of PCP as a synthesising and/or processing agent in industrial processes The production of PCP within the EU ceased in 1992 [104], with the remaining countries that still used PCP, particularly France, Spain, Portugal and the United Kingdom, relying on imports from the USA [105, 107]. The EU-wide consumption of PCP, its salts and esters were significantly reduced from an estimated consumption of 2,500 t in the mid-80s to 426 t in 1996 ([107] and references herein). In the more recent years, PCP-containing chemicals were mainly used in the treatment of wood as a sapstain control agent [104]. PCPL is used in textiles, particularly those for military use, as a preservative against attack by fungi and bacteria [104]. Indoor use was forbidden in several countries due to cases of suspected PCP intoxication following exposure during product application [104]. The use of PCP, NaPCP and PCPL ceased in all EU Member States at the end of 2008 [104].
1.3.1. Environmental contamination of PCP
industries have changed methodologies to reduce contamination by chlorophenolic compounds [109]. In addition, PCP may be produced as a metabolite from the degradation of chemical compounds such as hexachlorobenzene (HCB), pentachlorobenzene (PeCB) and pentachloronitrobenzene [113]. The production of PCP as a metabolite of degradation of PeCB and HCB was suggested by Carrizo et al., who detected PCP in blood serum collected between 2001 and 2003 from 4-year-old children living near a large factory of organochlorine solvents [114]. Both PeCB and HCB are widespread environmental pollutants often produced as by-products in the manufacture of organochlorine solvents.
Hattemer-Frey and Travis estimated the partition of PCP to be 96.5% to soil, 2.5% to water, 1% to sediment and less than 1% to air, suspended sediment and biota [120]. In soil, the presence of chlorophenols may originate directly from industrial residues, the biodegradation of herbicides and pesticides, and from atmospheric deposition [101]. The transport in soils depends on the pH, the content of organic matter, texture and permeability of the soil, total precipitation, evaporation rate and microbial degradation. The uptake of PCP by plants and animals can also contribute to PCP removal from soil [110].
Leaching of chlorophenols through soil permits PCP to enter aquatic systems, spreading more widely to other parts of the environment [110]. Levels of PCP in freshwater bodies in Belgium, Germany and Netherlands in the 1990s ranged between 0.01 and 0.17 µg∙L-1, with a maximum concentration of 1.5 µg∙L-1, found in the Scheldt river, in Belgium [107]. Levels in marine waters were usually much lower, with reported levels in Belgium and Netherlands close or below the detection limit of ~ 0.01 µg∙L-1.
In water, PCP exists as dissociated, non-dissociated or adsorbed onto suspended matter, depending on the pH [101]. In sediments, PCP, NaPCP and PCPL can be strongly adsorbed [107]. When the concentration of PCP in the overlying waters falls, desorption usually occurs. The residence time of PCP in sediments is likely to be high due to low rates of biological and photochemical degradation. Therefore, sediments are considered a source of chronic pollution in aquatic environments. Analysis of river sediments in Pearl River Delta in China, collected in 2000 and 2001, indicated levels of PCP ranging from 1.4 to 34 µg∙kg-1, with an average concentration of 7.9 µg∙kg-1, dry weight [122].
Transfer of PCP into air occurs mainly by volatilisation from soil, water surfaces or treated wood [120]. In general, the concentration of chlorophenols in air is linked to local sources of emission [101]. Once in air, PCP can be washed out during precipitation, while photochemical degradation is assumed to be negligible [120]. Concentrations ranging from a few to 500 mg∙m-3 were detected in air in the vicinity of a wood impregnation plant, while levels of 5.7 to 7.8 ng∙m-3 were reported in 1977 for urbanized areas in Antwerp ([101]). Concentrations up to 0.8 ng∙m-3 were detected in air samples from indoor environments in Japan, in 2003 [123]. In this study, PCP was detected in 17 of the 26 samples analysed, with a median concentration of 0.1 ng∙m-3. In the two samples from outdoor environments analysed, only in one PCP could be detected in a concentration of 0.1 ng∙m-3.
1.3.2. Bioaccumulation of PCP and human exposure
et al. observed concentrations of PCP in house dust in concentrations up to 32.2 mg∙kg-1 [127].
Due to its lipophilicity, PCP may accumulate in living organisms [108, 109] at different trophic levels, leading to human exposure through ingestion of contaminated food [101, 128]. Biomagnification is particularly acute for aquatic organisms, which can bioaccumulate organic pollutants after transport through the cell membrane [108] and ingestion of contaminated food. Ge et al. measured the concentration of PCP in different freshwater organisms from the Jiangsu Province in China and detected levels up to 61 µg∙kg-1 PCP (detected in carp), while the mean concentration found was 5.2 µg∙kg-1 PCP [128]. In this study, however, the risk to humans through dietary exposure was considered relatively small.
Reports of the detection of PCP in human tissues (e.g. liver, kidney, testis, prostate, adipose tissue, brain, spleen, lung and bile [129, 130]) and fluids (e.g. blood, urine, seminal fluid and breast milk [114, 122, 127, 130-133]) across general populations can be found in the literature. The concentration of PCP found in urine in the general population is usually a few µg per litre [127, 134]. Cline et al. compared PCP levels in those exposed occupationally to non-occupationally exposed individuals and reported serum PCP values ranging from 15-75 µg∙kg-1 in non-occupational exposure to 69-1,340 µg∙kg-1 in residents of PCP-treated log homes [135].
regarded as a reliable indicator to estimate the environmental exposure to toxicants.
1.3.3. Toxicity of PCP
Although PCP toxicity has been extensively studied [137-142], its molecular basis is still not completely understood. Both chronic and acute intoxication have been reported, with chronic intoxication usually associated with occupational exposure. Sawmill workers or people living in log homes, treated with PCP containing formulations have often been reported to suffer from chronic intoxication, with a suspected increased risk of developing a number of diseases [124, 130] (see below). It is very difficult to quantify the level of exposure causing both chronic and acute intoxication, particularly when inhalation from treated wood is considered [130]. It is also very difficult to establish a causal relationship between the levels of exposure and toxicity because of concomitant exposure to other contaminants, including the ones present in PCP formulations [130] (see above, section 1.3).
Chronic intoxication may cause skin, blood and kidney diseases, disorders of the gastrointestinal and respiratory tracts and female infertility, among others [124, 130]. In the case of acute intoxication, skin lesions, headaches, conjunctivitis, sore throat and nasal discharges are often reported. More severe symptoms include increased body temperature, weakness, tachycardia, nausea and convulsions, which may ultimately lead to death (reviewed by Proudfoot [130]).
damage to the cell, especially if alternative energetic processes are not available [143]. A significant decrease in intracellular ATP level was observed in human lymphocytes [145] and hepatocytes of the fish Carassius carassius [139] exposed to PCP, at concentrations of 1 mg∙Kg-1 and 1 µM, respectively. PCP has also been reported to alter the activity of several enzymes of glycolysis and the citric acid cycle in the liver of the eel Anguilla anguilla [146].
Due to its lipophilic nature, PCP is known to affect the lipid matrix in the membranes, increasing membrane permeability and destroying the internal hydrogen ion concentration gradients [147]. Duxbury and Thompson reported ability of 282 µM PCP to significantly alter the molecular organisation and physical properties of membrane bilayers [148]. In their study, PCP was also reported to bind the hydrophobic interior of the lipid bilayers. Fernández Freire et al. have reported lysosomal membrane damage in Vero cells exposed to 10 µM PCP for 3h and suggested it as an early event associated with PCP toxicity in these cells, preceding mitochondrial disfunction, ultimately responsible for the uncoupling of oxidative phosphorylation [137]. An apoptotic response to PCP was also observed in this study. Cell death, by necrosis, apoptosis, or both, has been often reported in response to PCP [139-142, 149, 150].
demonstrates association between hematopoietic cancer and PCP exposure [154].
The endocrine system, particularly the normal functioning of the thyroid hormone, is known to be affected by PCP [117, 155]. PCP was reported to compete for the thyroxine (T4) binding site of transthyretin (TTR), a carrier of T4 [155]. In crucian carp (Carassius carassius), concentrations of PCP between 2.0 and 20.4 µg∙L-1 contributed to endocrine disruption, significantly altering the concentration of serum testosterone, which is thought to affect reproduction [156]. In this study, significant changes were also observed in the activities of hepatic enzymes responsible for both steroid and xenobiotic metabolism, such as glutathione S-transferase and ethoxyresorufin O-deethylase (EROD), a cyt P-450 monooxigenase. These changes suggest that modulation of these enzymes by PCP could potentially alter serum testosterone concentrations.
In addition, PCP is neurotoxic [157] and affects the immune system of humans and animals [138, 158]. Taylor et al. analysed the PCP capacity to affect human natural killer (NK) cell function and observed a progressive loss of lytic function (greater than 80%) within 6 days after exposure to 10 µM for 1h [159]. NK cells have an important role in the defence against tumour cells and virally infected cells. Further analysis indicated that PCP affected the binding of NK cells to tumour cells, decreasing the expression of cell-surface proteins needed for binding [160].
collapse and nucleus shrinkage [140], while an increase in size and granularity was reported in lymphocytes exposed to 125 mg∙Kg-1 [145]. Michałowicz has also observed protein and DNA damage in human lymphocytes due to PCP exposure at concentrations higher than 0.04 and 0.2 mg∙Kg-1, respectively [145].
PCP is also toxic to algae and plants. In algae, PCP affects photosynthesis [161, 162] and decreases the concentration of photosynthetic pigments [163, 164]. In plants, it was reported to cause oxidative stress and damage [165, 166]. Studies on wheat (Triticum aestivum L.) and reed canary grass (Phalaris arudinacea L.) showed an increased level of free phenols, ascorbic acid and both oxidised and reduced glutathione, induction of lipid peroxidation and alterations in the activity of several antioxidative enzymes, such as catalase, superoxide dismutase, glutathione reductase and glutathione S-transferase. Genotoxicity was reported in onion (Allium cepa L.), supported by a micronuclei increase of 180 fold after 24h exposure to 10 µM PCP [167]. Aquatic organisms are also particularly sensitive to PCP. In addition to the endocrine disruption reported in crucian carp (see above) [156], PCP toxicity has been reported, for instance, in Mediterranean sea urchin Paracentrotus lividus embryos. In this species, 500 µg∙L-1 PCP seriously affected cytoskeleton assembly [168].
Based on the previously described, it is clear that PCP affects a large range of organisms and has multiple potential cellular targets.
1.4. Fungal degradation of PCP
the investigations on this topic, with particular attention given to P. chrysosporium [69, 94, 171-174].
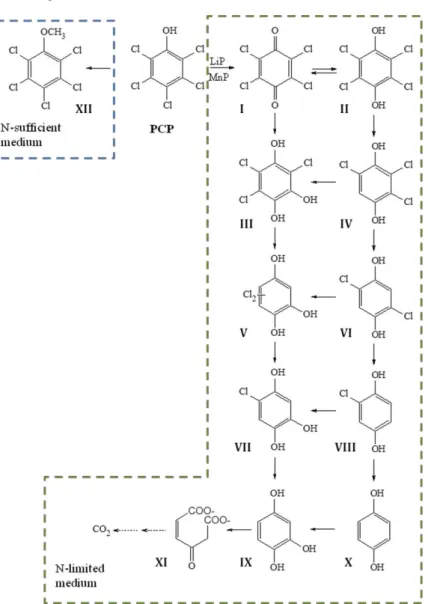
Reddy and Gold have described the full degradation pathway of PCP in P. chrysosporium [69, 94]. To date, this is the best characterised pathway of PCP degradation by a fungal species and is depicted in Figure 8. The initial dechlorination of PCP to tetrachlorobenzoquinone (TeCBQ, I) is catalysed extracellularly by ligninolytic peroxidases, while the remaining chlorine atoms are removed by successive reductive dechlorination reactions inside the cell [69]. The initial conversion occurs only under ligninolytic conditions, i.e. nitrogen-limiting, secondary metabolic conditions. The remaining degradation reactions occur in both primary and secondary metabolic growth, indicating that they are not dependent on the LDS (see section 1.2.1). Once formed, TeCBQ (I) can be reduced to tetrachlorohydroquinone (TeCHQ, II), either enzymatically or non-enzimatically. Both compounds undergo successive reductive dechlorination reactions in parallel pathways, leading to the production of 1,2,4-trihydroxybenzene (1,2,4-THB, IX). The metabolic flow can be redirected from one pathway to the other by hydroxylation reactions. Ring cleavage of 1,2,4-THB, catalysed by a dioxygenase [69, 175], leads to the production of maleyl acetate (XI), with subsequent degradation to CO2 [69].
dichlorohydroquinone (DCHQ, VI) and chlorohydroquinone (CHQ, VIII), removing all the chlorine atoms from TeCHQ (II) [94].
Figure 8 PCP degradation pathway proposed for Phanerochaete chrysosporium
by Reddy and Gold [69]. I) TeCBQ, II) TeCHQ,
Under nitrogen sufficient conditions, PCP is slowly methylated by P. chrysosporium to pentachloroanisole (PCA, XII) as the sole product formed [69]. Fungal methylation of chlorophenols is recognized as an important detoxification reaction [105, 176] and has been often reported in the literature [177, 178]. These reactions are particularly relevant for fungi able to colonise cork during the manufacturing process of stoppers [176, 179, 180], since chloroanisoles have been thought to be responsible for the “cork taint” defect in wine [179] (see below, section 1.5). In a study with cork-isolated strains, the metylation of 2,4,6-trichlorophenol (2,4,6-TCP) by Trichoderma longibrachiatum was reported to be catalysed by a mycelium-associated S-adenosyl-L-methionine (SAM)-dependent methyltransferase. The enzyme was strongly induced by 2,4,6-TCP [176].
Figure 9 Pathway of biodegradation of 2, 4, 6-trichloroanisole (2, 4, 6-TCA) by
Phlebia radiata propose by Campoy et al. [92].
1.4.1. Degradation of PCP by Mucorales
Although most of the investigation on the fungal degradation of chlorophenols has been focused on WRF, biodegradation of PCP by Zygomycota, particularly Mucorales, has often been reported [13, 84, 170, 181, 182]. An investigation of the ability of 70 Zygomycota fungal strains for NaPCP degradation in liquid cultures revealed high degradation ability [170]. Phenoloxidase activity was not detected in Mucor plumbeus strains, neither in most of the strains investigated, suggesting the participation of other enzymes. Degradation rate of Mucor plumbeus strains ranged from 32 to 63% in 5 days, when 100 mg∙L-1 NaPCP was added to culture media with glucose. Interestingly, a previous study by the same research group and involving fungal strains from diverse taxonomic groups had indicated Zygomycota fungi as the best PCP degraders [183].
grown for 24h [84]. It was suggested this strain produced extracellular LiP and both intra- and extracellular tyrosinase. Immobilization in nylon fiber increased its degradation ability [184]. Extracellular tyrosinase was also proposed to participate in PCP degradation by Amylomyces rouxii, being induced by PCP and/or tyrosine [99]. Enzymatic activity could not be found in the absence of both compounds. The heterologous transformation of A. rouxii with peroxidase genes from P. chrysosporium doubled the amount of PCP removed in liquid media without tyrosine, in one transformant showing MnP activity when compared with the parental strain [185]. The aim of the transformation was to couple both enzymatic processes (phenoloxidases and peroxidases) used in nature for PCP removal in one single strain. In the presence of tyrosine, however, no significant increase in PCP degradation rate was observed.
Some Mucorales, such as Rhizopus nigricans and Rhizopus oryzae, are reported to be unable to germinate when incubated in the presence of 12.5 mg∙L-1 PCP in liquid media [84, 182]. To overcome this inhibition, studies on PCP degradation involving these species usually make use of previously grown mycelium, instead of fungal spores. Inhibition of spore germination had been previously reported by Mileski et al. for the Basidiomycota P. chrysosporium, incubated with 4 mg∙L-1 PCP [171]. Besides PCP degradation, a few studies involving Mucorales, report PCP adsorption to fresh mycelia. Adsorption of PCP to A. rouxii (formerly reported as Rhizopus nigricans) mycelia ranged between 80 and 90% [182], while 20% of the residual PCP found in the media after incubation was detected in the sonicated biomass extract [84].
dechlorination of PCP was suggested to involve cytochrome P-450 activity [181]. Other metabolites formed in nutrient-rich Sabouroud medium, although in relatively low concentrations, were PCA, pentachloroethoxybenzene (PCEB), 2,3,4,6- and 2,3,5,6-tetrachlorophenols, and 2,3,4,6-tetrachloroanisole (TeCA) (Figure 10). Despite the production of PCA and TeCA by M. racemosissimus, the investigation of 2, 4, 6-TCA producers amongst cork isolated fungal strains, concluded that M. plumbeus did not produce 2, 4, 6-TCA from TCP [176].
Figure 10 Schematic representation of PCP transformation pathway of
Mucor ramosissimus suggested by Szewczyk and Długoński [181].
1.5. Cork colonising fungi
suberin, a complex cross-linked polymer composed of aromatic and aliphatic domains with the aromatic domain structurally related to lignin. In a previous study, Silva Pereira et al. observed perforation of cork cell wall by cork colonising fungal strains and suggested fungal ability to degrade both suberin and lignin of cork [188]. As mentioned before (see section 1.2), the degradation of lignin by fungi is usually associated with the secretion of extracellular enzymes, which have a role in both lignin and organic pollutants degradation
The use of cork stoppers in bottled wine has been often blamed for the presence of a defect in wine commonly reported as “cork taint” and characterized by an earthy, mouldy flavour or aroma [179]. This defect, reported in 2 to 7% of wines, is associated with the presence of chloroanisoles, particularly 2,4,6-TCA, but also to 2,3,4,6-TeCA and PCA [176]. As previously referred (see section 1.4), chloroanisoles are produced from the corresponding chlorophenols after a methylation reaction normally considered a detoxification process. Therefore, the presence of chloroanisoles in cork stoppers suggests PCP contamination of cork. Interestingly, both chlorophenols and chloroanisoles have been reported in cork slabs collected from cork oak trees in Portuguese forests [105].
1.6. Aims of the thesis
The work presented here investigates the potential of cork colonising fungal strains for PCP degradation and aims to increase our understanding of the biotransformation of PCP by filamentous fungi.
An initial screening study, presented in Chapter II, aimed to select the most interesting fungal strains, based on the PCP degradation yields in both glucose and glucose-free liquid mineral media.
The subsequent investigation of the production of degradation metabolites and the enzymatic reactions involved in PCP degradation by M. plumbeus (Chapter III), aimed to unravel the PCP degradation pathway by this fungal species.
References
[1] H. Harms, D. Schlosser, L.Y. Wick, Untapped potential: exploiting fungi in bioremediation of hazardous chemicals, Nat. Rev. Microbiol. 9 (2011) 177-192.
[2] K. Kavanagh, Fungi Biology and Applications, 2nd ed., Wiley-Blackwell, Oxford, 2011.
[3] J.E. Stajich, M.L. Berbee, M. Blackwell, D.S. Hibbett, T.Y. James, J.W. Spatafora, J.W. Taylor, The Fungi, Curr. Biol. 19 (2009) R840-R845.
[4] I.V. Grigoriev, H. Nordberg, I. Shabalov, A. Aerts, M. Cantor, D. Goodstein, et al., The Genome Portal of the Department of Energy Joint Genome Institute, Nucleic Acids Res. 40 (2012) D26-D32.
[5] J.I. Pitt, A.D. Hocking, Fungi and Food Spoilage, 3rd ed., Springer, New York, 2009.
[6] J.W. Bennet, K.G. Wunch, B.D. Faison, Use of Fungi in Biodegradation, in: C.J. Hurst (Ed.) Manual of environmental microbiology, American Society for Microbiology Washington DC, USA, 2002, pp. 960-971.
[7] G.M. Gadd, Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation, Mycol. Res. 111 (2007) 3-49.
[8] F. Carlsson, M. Edman, S. Holm, A.-M. Eriksson, B.G. Jonsson, Increased heat resistance in mycelia from wood fungi prevalent in forests characterized by fire: a possible adaptation to forest fire, Fungal Biol. 116 (2012) 1025-1031.
[10] F.H. Gleason, C.N. Daynes, P.A. McGee, Some zoosporic fungi can grow and survive within a wide pH range, Fungal Ecol. 3 (2010) 31-37. [11] F.J. Deive, A. Rodríguez, A. Varela, C. Rodrigues, M.C. Leitão, J. Houbraken, et al., Impact of ionic liquids on extreme microbial biotypes from soil, Green Chem. 13 (2011) 687-696.
[12] R. Kataoka, K. Takagi, I. Kamei, H. Kiyota, Y. Sato, Biodegradation of Dieldrin by a Soil Fungus Isolated from a Soil with Annual Endosulfan Applications, Environ. Sci. Technol. 44 (2010) 6343-6349.
[13] R. Szewczyk, P. Bernat, K. Milczarek, J. Długoński, Application of microscopic fungi isolated from polluted industrial areas for polycyclic aromatic hydrocarbons and pentachlorophenol reduction, Biodegradation 14 (2003) 1-8.
[14] M. Valix, J.Y. Tang, R. Malik, Heavy metal tolerance of fungi, Miner. Eng. 14 (2001) 499-505.
[15] J.W. Bennet, R. Davis, C. Raper, Introduction to Filamentous Fungi, History and Importance to Human Affairs, in: K.A. Borkovich, D.J. Ebbole (Eds.) Cellular and Molecular Biology of Filamentous Fungi, ASM Press, Washington, DC, 2010, pp. 3-7.
[16] R. Fischer, U. Kües, Asexual sporulation in mycelial fungi, in: U. Kües, R. Fischer (Eds.) The Mycota I, Growth, Differentiation and Sexuality, Springer-Verlag Berlin Heidelberg, 2006, pp. 263-292.
[17] R.H. Whittake, New Concepts of Kingdoms of Organisms, Science 163 (1969) 150-160.
[19] D.S. Hibbett, M. Binder, J.F. Bischoff, M. Blackwell, P.F. Cannon, O.E. Eriksson, et al., A higher-level phylogenetic classification of the Fungi, Mycol. Res. 111 (2007) 509-547.
[20] M.J. Carlile, S.C. Watkinson, G.W. Gooday, The Fungi, 2nd ed., Academic Press, New York, 2001.
[21] P.S. Dyer, C.M. O'Gorman, A fungal sexual revolution: Aspergillus and Penicillium show the way, Curr. Opin. Microbiol. 14 (2011) 649-654. [22] K.J. Boyce, A. Andrianopoulos, Morphogenesis: Control of cell types and shape, in: U. Kües, R. Fischer (Eds.) The Mycota I, Growth, Differentiation and Sexuality, Springer-Verlag Berlin Heidelberg, 2006, pp. 3-20.
[23] G. Steinberg, Hyphal growth: a tale of motors, lipids, and the Spitzenkörper, Eukaryot. Cell 6 (2007) 351-360.
[24] J.G.H. Wessels, Wall Growth, Protein Excretion and Morphogenesis In Fungi, New Phytol. 123 (1993) 397-413.
[25] R.J. Howard, Ultrastructural analysis of hyphal tip cell-growth in fungi: Spitzenkörper, cytoskeleton and endomembranes after freeze-substitution, J. Cell Sci. 48 (1981) 89-103.
[26] G. Steinberg, Motors in fungal morphogenesis: cooperation versus competition, Curr. Opin. Microbiol. 14 (2011) 660-667.
[27] J. Webster, R.W.S. Weber, Introduction to Fungi, 3rd ed., Cambridge University Press, New York, 2007.
![Figure 1 – Phylogeny and classification of fungi according to Hibbett et al. [19]](https://thumb-eu.123doks.com/thumbv2/123dok_br/15764174.640102/30.748.190.583.396.834/figure-phylogeny-classification-fungi-according-hibbett-et-al.webp)
![Figure 2 a) Nonseptate hypha of Syncephalastrum racemosum (phylum Zygomycota) b) Septate hypha of Fusarium equiseti (phylum Ascomycota) (from Pitt and Hocking [5])](https://thumb-eu.123doks.com/thumbv2/123dok_br/15764174.640102/33.748.126.641.105.389/nonseptate-syncephalastrum-racemosum-zygomycota-septate-fusarium-equiseti-ascomycota.webp)
![Figure 3 – Chlamydospores from Mucor plumbeus (from Webster and Weber [27])](https://thumb-eu.123doks.com/thumbv2/123dok_br/15764174.640102/36.748.144.606.567.796/figure-chlamydospores-mucor-plumbeus-webster-weber.webp)



![Figure 10 Schematic representation of PCP transformation pathway of Mucor ramosissimus suggested by Szewczyk and D ługoński [181]](https://thumb-eu.123doks.com/thumbv2/123dok_br/15764174.640102/65.748.195.536.374.705/figure-schematic-representation-transformation-ramosissimus-suggested-szewczyk-ługoński.webp)