MARGARETE ALICE FONTES SARAIVA
INHIBITORY SUBSTANCES PRODUCED BY Lactococcus lactis STRAINS ISOLATED FROM NATURALLY FERMENTED SAUSAGE
Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Microbiologia Agrícola, para obtenção do título de Doctor Scientiae.
VIÇOSA
Ficha catalográfica preparada pela Seção de Catalogação e Classificação da Biblioteca Central da UFV
T
Saraiva, Margarete Alice Fontes, 1973-
S243i Inhibitory substances produced by Lactococcus lactis strains 2012 isolated from naturally fermented sausage / Margarete Alice
Fontes Saraiva. – Viçosa, MG, 2012. xii, 102f. : il. ; 29cm.
Orientador: Célia Alencar de Moraes.
Tese (doutorado) - Universidade Federal de Viçosa. Inclui bibliografia.
1. Bactéria do ácido lático. 2. Bacteriocinas. 3. Micro-organismos. 4. Alimentos - Conservação. I. Universidade Federal de Viçosa. II. Título.
MARGARETE ALICE FONTES SARAIVA
INHIBITORY SUBSTANCES PRODUCED BY Lactococcus lactis STRAINS ISOLATED FROM NATURALLY FERMENTED SAUSAGE
Tese apresentada à Universidade Federal de Viçosa, como parte das exigências do Programa de Pós-Graduação em Microbiologia Agrícola, para obtenção do título de Doctor Scientiae.
APROVADA: 13 de abril de 2012.
Marta Cristina Teixeira Leite Maria Aparecida Scatamburlo Moreira
Hilário Cuquetto Mantovani Maria Cristina Baracat Pereira (Coorientadora)
iii
AGRADECIMENTOS
Ao Deus Pai, Filho e Espírito Santo pela constante presença em minha vida. À Universidade Federal de Viçosa e ao Departamento de Microbiologia, pela oportunidade de realização do curso e ao Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) pela concessão da bolsa de estudos.
À Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) pelo apoio financeiro durante o período de estágio no exterior (Doutorado sanduíche).
À orientadora Profa. Célia Alencar de Moraes, pelo apoio, incentivo em todos os momentos e confiança em mim depositada. Serei eternamente grata por todas as vezes que não mediu esforços para ajudar-me.
Ao Dr. Ingolf Figved Nes pela oportunidade, pelo carinho com que me recebeu no laboratório e pela relevante participação em meu trabalho.
Ao pesquisador Dag Brede Anders pelas valiosas contribuições.
Às professoras Maria Cristina Baracat Pereira e Marisa Vieira de Queiroz pelas disponibilidades e contribuições.
Ao prof. Hilário Cuquetto Mantovani, a profa. Marta Cristina Teixeira Leite e a profa Maria Aparecida Scatamburlo Moreira por terem aceitado participar da banca de defesa e pelos aconselhamentos.
A todos os professores do Departamento de Microbiologia, pelos ensinamentos.
A Fernanda Godoy pela sincera e maravilhosa amizade, pelo incentivo em todos os momentos, ajuda constante e agradável convivência.
A Andressa Pinheiro, Adriana dos Reis Ponce e Lygia de Fátima pela sincera amizade e apoio sempre nessa etapa importante da minha vida.
iv A Ana Paula do Carmo, Vanessa Di Raimo, José Carlos Filho, Fernanda Freitas, Marcelo Nagem e Alessandra Barbosa pelo agradável convívio e consideração sempre no laboratório.
Aos meus queridos irmãos e irmãs, meu pai e sobrinhos, pelo carinho e pela torcida para que eu atingisse esse objetivo importante.
Aos colegas dos Laboratórios de Microbiologia Industrial, Fisiologia de Micro-organismos, Microbiologia de Alimentos, Anaeróbios, Associações Micorrizas, Genética de Microrganismos, Petróleo e Ecologia pela boa convivência.
Aos colegas do Laboratory Microbial of the Gene technology, Department of Chemistry, Biotechnology and Food Science at Norwegian University of Life Sciences pelo apoio e agradável convivência.
v
BIOGRAFIA
MARGARETE ALICE FONTES SARAIVA, filha de Joaquim da Silva Saraiva e Lúcia da Silva Fontes, nasceu em Viçosa, Estado de Minas Gerais, no dia 09 de julho de 1973.
Em agosto de 1997 graduou-se em Economia Doméstica pela Universidade Federal de Viçosa. No período de julho de 1996 a janeiro de 1998, foi bolsista de iniciação científica pela FAPEMIG no Departamento de Microbiologia.
Em março de 1998, iniciou o Programa de Pós-Graduação em Microbiologia Agrícola, no nível de Mestrado com defesa da tese realizada em 17 de dezembro de 2000. No período de 2001 a 2007 dedicou-se a experiência profissional.
vi
SUMÁRIO
RESUMO ... ix
ABSTRACT... xi
INTRODUCTION... 1
CHAPTER 1 ... 3
Lactococcus lactis: a versatile lactic acid bacteria producer of antimicrobial peptides ... 3
1. Lactococcus lactis... 3
2. Bacteriocins: general aspects ... 5
2.1. Bacteriocins of Lactococcus lactis... 11
3. Perspectives of new antimicrobial compounds produced by Lactococcus lactis... 18
4. References... 19
CHAPTER 2 ... 34
Nisin Z production by wild strains of Lactococcus lactis isolated from naturally fermented sausage ... 34
Abstract... 34
1. Introduction... 35
2. Materials and methods ... 36
2.1. Bacterial strains and culture conditions ... 36
2.2. Genetic identification of bacteriocin-producing strains ... 36
2.3. Sequencing of nisin genes and nisin controlled promoters ... 37
2.4. Detection and curing of plasmids ... 37
2.5. Bacteriocin antimicrobial assay... 38
2.6. Purification and estimation of the molecular size of bacteriocins ... 38
2.7. Effect of carbon and nitrogen sources on bacteriocin production ... 39
vii
2.9. Statistics ... 40
3. Results... 40
3.1. Identification of bacteriocin-producing strains... 40
3.2. Sequencing of nisin genes and nisin controlled promoters ... 40
3.3. Detection and curing of plasmids ... 41
3.4. Purification and mass spectrometry analysis of bacteriocin... 41
3.5. Effect of carbon and nitrogen source on bacteriocin production... 42
3.6. Effect of aeration, initial medium pH and incubation temperatures on bacteriocin production... 43
4. Discussion ... 44
5. References... 51
CHAPTER 3 ... 66
Detection and activity of two new cell-bound antimicrobial compounds produced by Lactococcus lactis ID1.5 ... 66
Abstract... 66
1. Introduction... 67
2. Material and methods... 68
2.1. Bacterial strains and culture conditions ... 68
2.2. Antimicrobial activity assay... 69
2.3. Extraction and purification of the cell-bound active compounds... 69
2.4. Effect of heat, proteolytic enzymes and tween 80 on stability of antimicrobial compounds ... 70
3. Results... 71
3.1. Extraction and Purification of the cell-bound active compounds ... 71
3.2. Effect of heat, proteolytic enzymes and tween 80 on stability of antimicrobial compounds ... 72
3.3. Determination of inhibitory spectrum ... 72
viii
5. References... 76
CHAPTER 4 ... 85
Identification of the Natural Variant Nisin Z Produced by Lactococcus lactis PD6.9: a Potencial Anti-Staphylococcus aureus Bacteriocin ... 85
Abstract... 85
1. Introduction... 86
2. Materials and methods ... 87
2.1. Bacterial strains and culture conditions ... 87
2.2. Assay of bacteriocin activity... 87
2.3. Sequencing of nisin genes ... 87
2.4. Plasmid curing... 88
2.5. Purification of bacteriocin... 88
2.6. Mass spectrometry ... 89
2.7. Results ... 89
3. Discussion ... 91
4. References... 94
ix
RESUMO
SARAIVA, Margarete Alice Fontes, D.Sc., Universidade Federal de Viçosa, abril de 2012. Substâncias inibidoras produzidas por culturas de Lactococcus lactis
isoladas de salame fermentado naturalmente. Orientadora: Célia Alencar de Moraes. Coorientadores: Marisa Vieira de Queiroz e Maria Cristina Baracat Pereira.
xi
ABSTRACT
SARAIVA, Margarete Alice Fontes, D.Sc., Universidade Federal de Viçosa, April, 2012. Inhibitory substances produced by Lactococcus lactis strains isolated from
naturally fermented sausage. Adviser: Célia Alencar de Moraes. Co-advisers: Marisa Vieira de Queiroz and Maria Cristina Baracat Pereira.
1
INTRODUCTION
For many years consumers have requested alternative food grade preservatives without chemical additives. The use of antimicrobial produced by lactic acid bacteria (LAB) instead of chemical additive would enable the food industry to grant this request. The studies of diverse bacteriocins have been focused on their use as food preservatives, because they can inhibit food-born spoilage and pathogenic microorganisms. Furthermore, bacteriocins have demonstrated a remarkable potential as therapeutics for medical or veterinary uses, alone or in combination with other antimicrobial substances. A number of bacteriocins are produced by Lactococcus lactis and they have attracted particular attention for commercial applications.
L. lactis is quite desirable for industrial application because it is homofermentative, highly productive and generally recognized as safe. These bacteria are used in food production, and their antimicrobial metabolites may be considered safe agents for preventing growth of undesirable microorganisms. In addition, the incorporation of bacteriocin producing lactococci as starter or adjunct cultures in the manufacture of fermented foods provides an attractive and economic alternative to the addition of purified bacteriocins, indeed, metabolic compounds produced during fermentation are no longer considered as additives. Bacteriocin formation in situ may also contribute to the dominance of the producing strains over other bacteria during food fermentation.
Considering the growing interest for commercial application of bacteriocins, many aspects have been studied extensively in order to improve their production rate and productivity. Thereby, the understandings of factors that influence bacteriocin production as well as, the selection of good producer strains are important aspects to enhance peptide yield and to reduce production costs.
2 The aim of the present work was to identify the bacteriocin produced by strains of L. lactis isolated during natural fermentation of Italian type sausage in Brazil and investigate production in bach culture. Efforts were also made to characterize two cell-bound antimicrobial compounds produced by L. lactis ID1.5, with inhibitory proprieties against Gram positive and Gram negative bacteria. This work was carried out in the Laboratory of Industrial Microbiology, Department of Microbiology at Federal University of Viçosa and in the Laboratory Microbial of the Gene Technology, Department of Chemistry, Biotechnology and Food Science at Norwegian University of Life Sciences, in Aas, Norway.
3
CHAPTER 1
Lactococcus lactis: a versatile lactic acid bacteria producer of antimicrobial
peptides
1. Lactococcus lactis
4 liberation of health-enhacing bioactive peptides from milk (Leroy and De Vuyst, 2004).
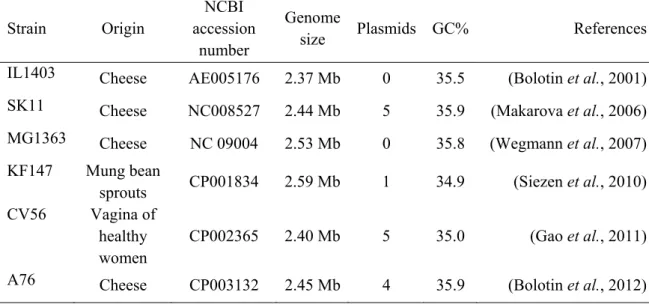
The genomes of several L. lactis strains have been sequenced (Table 1), but strains IL1403 (Bolotin et al., 2001) and MG1363 (Wegmann et al., 2007) are most commonly used in laboratories. Sequencing of some L. lactis plasmids reveled important traits including lactose catabolism, proteolysis, peptide and amino acid uptake, exopolisaccharide, bacteriocin production, bacteriophage resistance and citrate permease (Mills et al., 2006). The success of metabolic engineering approaches in L. lactis together with its high transformation efficiency indicates that this lactic acid bacteria (LAB) is a promising candidate for synthetic biology applications (Holo and Nes, 1989). In addition, some of the first synthetic promoters were designed for L. lactis (Morello et al., 2008).
5 L. lactis is also good candidate for the production and delivery of heterologous proteins and peptides that have potential therapeutic activity, because it is acid and bile resistant and well adapted to function as vehicles for oral delivery of vaccine antigens (Teusink and Smid, 2006).
Table 1. Sequenced Lactococcus lactis genomes
Strain Origin NCBI accession
number
Genome
size Plasmids GC% References
IL1403 Cheese AE005176 2.37 Mb 0 35.5 (Bolotin et al., 2001)
SK11 Cheese NC008527 2.44 Mb 5 35.9 (Makarova et al., 2006)
MG1363 Cheese NC 09004 2.53 Mb 0 35.8 (Wegmann et al., 2007)
KF147 Mung bean
sprouts CP001834 2.59 Mb 1 34.9 (Siezen et al., 2010)
CV56 Vagina of
healthy women
CP002365 2.40 Mb 5 35.0 (Gao et al., 2011)
A76 Cheese CP003132 2.45 Mb 4 35.9 (Bolotin et al., 2012)
2. Bacteriocins: general aspects
6 function as agents to manipulate microbial populations in food systems (i. e., promote the growth of strains with desirable properties by inhibiting competing strains) (Cotter et al., 2005). Promising results have been reported recently for combined treatments (such as HHP, bacteriocin, or moderate heat) on the inactivation of Clostridium botulinum and Bacillus cereus spore (Black et al., 2008; Gao and Ju, 2008). Such combined treatments could improve food safety and decrease the impact of the intense heat treatments required for endospore inactivation. In addition, the residual bacteriocin in the finished product affords natural protection against bacterial growth and toxin production during the product shelf life (Galvez et al., 2010).
In the medical field there is a great interest in novel antimicrobial compounds, especially in the light of the ever-increasing antibiotic resistance among pathogenic bacteria. Different bacteriocins are known to target many Gram-positive pathogens in vitro, including emerging antibiotic resistant bacteria such as methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococcus (VRE) (Arnusch et al., 2008; Piper et al., 2010). From a health perspective, a study has shown that the bacteriocin Abp118, produced by Lactobacillus salivarius UCC118, is effective in reducing L. monocytogenes infections in mice, suggesting a role for bacteriocins as anti-infective agents (Corr et al., 2007). This study has led to increased interest in the use of bacteriocin-producing strains as probiotic bacteria.
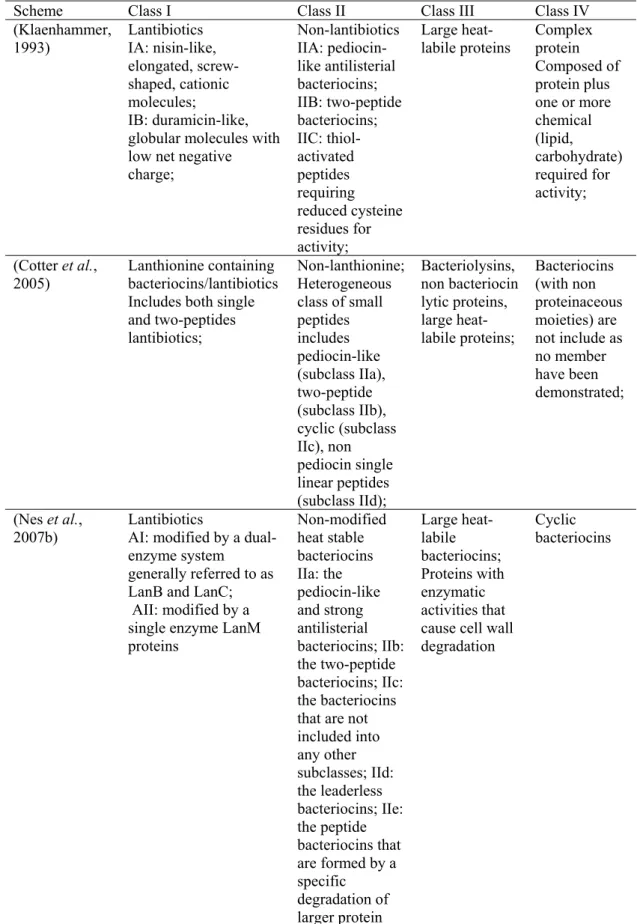
7 2005; Rink et al., 2007; Bierbaum and Sahl, 2009). Because of large structural variations, 11 subclasses have been suggested to lantibiotics (Bierbaum and Sahl, 2009). Nisin, Subtilin, Lacticin 481 and Lacticin 3147 are the best known member of the lantibiotics. The other main group is non-lantibiotic bacteriocins, Class II. They are small heat-stable peptides with no modified amino acids (except formation of disulphide bridges and circularization of cyclic peptides) (Cotter et al., 2005; Nes et al., 2007b). Many excellent reviews have described the bacteriocins in detail (Nes and Holo, 2000; Chatterjee et al., 2005; Cotter et al., 2005; Nes et al., 2007b; Maqueda et al., 2008; Asaduzzaman and Sonomoto, 2009; Bierbaum and Sahl, 2009; Papagianni and Anastasiadou, 2009) and the classification scheme for these bacteriocins proposed by (Klaenhammer, 1993; Cotter et al., 2005; Nes et al., 2007b) is shown (Table 2).
The typical mode of action used by bacteriocins to kill target cells is creating pores in the membrane by similar mechanisms as positively charged eukaryotic antimicrobial peptides: bacteriocins bind to anionic lipids and insert unspecifically into the phospholipid bilayer, where in aggregation of peptides leads to the formation of short-lived pore-like structure (Hechard and Sahl, 2002). Pore formation causes leakage of low molecular weight compounds (e.g., ions K+, PO42-, H+) leading to dissipation of the proton motive force (the transmembrane electric potential, ΔΨ, and pH gradient, ΔpH) that is deleterious to cells (Christensen and Hutkins, 1992; Hechard and Sahl, 2002). Pore size, stability and selectivity of transferable molecules vary between different bacteriocins. Not all bacteriocins bind to a receptor in the cell membrane. Nisin and some other lantibiotics (class I) specifically bind to the cell wall precursor molecule lipid II, to form lethal pores and/or inhibit cell wall synthesis in sensitive cells (Wiedemann et al., 2001; Asaduzzaman and Sonomoto, 2009). In addition, the membrane proteins belonging to a sugar transporter, the mannose phosphotransferase system (man-PTS) is the target receptor for bacteriocins pediocin-like and lactococcins A and B (Diep et al., 2007; Kjos et al., 2011).
8 Table 2. Classification schemes previously proposed for the bacteriocins produced by Gram-positive bacteria
Scheme Class I Class II Class III Class IV
(Klaenhammer, 1993) Lantibiotics IA: nisin-like, elongated, screw-shaped, cationic molecules; IB: duramicin-like, globular molecules with low net negative charge; Non-lantibiotics IIA: pediocin-like antilisterial bacteriocins; IIB: two-peptide bacteriocins; IIC: thiol-activated peptides requiring reduced cysteine residues for activity; Large heat-labile proteins Complex protein Composed of protein plus one or more chemical (lipid, carbohydrate) required for activity;
(Cotter et al., 2005)
Lanthionine containing bacteriocins/lantibiotics Includes both single and two-peptides lantibiotics;
Non-lanthionine; Heterogeneous class of small peptides includes pediocin-like (subclass IIa), two-peptide (subclass IIb), cyclic (subclass IIc), non pediocin single linear peptides (subclass IId); Bacteriolysins, non bacteriocin lytic proteins, large heat-labile proteins; Bacteriocins (with non proteinaceous moieties) are not include as no member have been demonstrated;
(Nes et al., 2007b)
Lantibiotics
AI: modified by a dual-enzyme system generally referred to as LanB and LanC; AII: modified by a single enzyme LanM proteins Non-modified heat stable bacteriocins IIa: the pediocin-like and strong antilisterial bacteriocins; IIb: the two-peptide bacteriocins; IIc: the bacteriocins that are not included into any other subclasses; IId: the leaderless bacteriocins; IIe: the peptide bacteriocins that are formed by a specific degradation of larger protein Large heat-labile bacteriocins; Proteins with enzymatic activities that cause cell wall degradation
9 The genes that are related to the bacteriocin production are usually clustered in operons or regulons. Generally, these clusters include structural genes proper and genes for immunity, processing, transport and of the regulatory systems (Nes and Johnsborg, 2004). Based on the data obtained, there is a tendency that in general the lantibiotics operons are more complex than those encoding no-lantibiotics because they need additional genes encoding enzymes for posttranslational modifications (Kotel'nikova and Gel'fand, 2002; Dimov et al., 2005). An unusual genomic organization is described for the bacteriocin carnobacteriocin BM1 produced by Carnobacterium piscicola. While its structural gene is located on the bacterial chromosome, its expression is dependent on the presence of a 61 kb plasmid which carries some of the genes required for the export and the immunity (Quadri et al., 1994).
Bacteriocins are usually synthetized as an inactive pre-peptide that includes an N-terminal leader sequence. The leader sequence presumably maintains the bacteriocin in an inactive form within the producer cell, facilitates interaction with the transporter (Oman and Van Der Donk, 2010). Subsequent cleavage of the pre-peptide at a sequence specific processing site removes the leader sequence from the antimicrobial molecule concomitantly with its export to the outside of the cell (Ennahar et al., 2000). The export of bacteriocins is usually achieved by a dedicated membrane-associated ATP-binding cassete (ABC) transporter that can also contain a proteolytic N-terminal domain belonging to the family of cysteine proteases that is responsible for cleavage of the leader peptide, or a sec-independent transporter system (Ennahar et al., 2000; Cotter et al., 2005).
10 expression of genes takes place, and mass production of bacteriocin results (Nes et al., 2007a).
Several bacteriocins can be produced in much higher amounts during in vitro fermentations under optimal physical and chemical conditions. Due to the complexity of the food matrix and the difficulty of quantifying bacteriocin activities in foods, in vitro studies can be performed to stimulate and study the in situ functionality of bacteriocinogenic starter (De Vuyst and Leroy, 2007; Trmčić et al., 2010). However, even during fermenter experiments, considerable differences in activity yields are obtained, and an influence of the environmental process conditions on the obtained bacteriocin activity can be seen (De Vuyst and Vandamme, 1992; Simsek et al., 2009). In most cases, bacteriocin production appears to be regulated and is consequently produced only under suitable growth conditions. The cultivation conditions directly affect bacteriocin production. Several factors including carbon and nitrogen sources, and temperature, pH and agitation seem to play a crucial role in bacteriocin production (Cheigh et al., 2002; Todorov and Dicks, 2009). Defining optimal growth conditions therefore represents a major hurdle in the bacteriocin screening system used today.
11
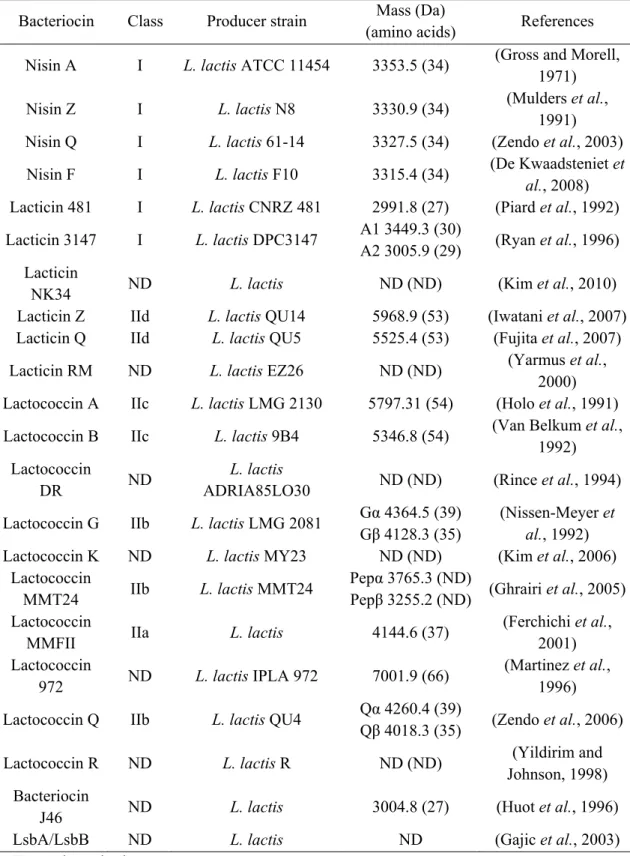
2.1.Bacteriocins of Lactococcus lactis
All lactococcal bacteriocins characterized thus far belong to either class I or class II (Table 3). Members of the class I (lantibiotics) include three lactococcal bacteriocins: nisin, lacticin 481 and lacticin 3147 (Table 3).
Nisin is the most characterized bacteriocin among the antimicrobial peptides produced by lactic acid bacteria. The inhibitory effects of nisin cells in food matrices are well described and have been shown to inhibit the growth of a wide range of gram positive bacteria including food spoilage and pathogenic bacteria such as Bacillus cereus, Clostridium perfringens, S. aureus and L. monocytogenes (Hampikyan, 2009; Mitra et al., 2011). Nisin has been commercially exploited on a large scale, due to its low toxicity, stability during processing and storage, efficacy at low concentration, economic viability, and the absence of deleterious effects on the food (Hurst et al., 1981; And and Hoover, 2003). Moreover, this safe and natural food additive has been utilized recently in clinical applications as an antimicrobial agent against the causative bacteria of bovine mastitis, and therefore it has been incorporated into commercial products that are used as an alternative treatment to antibiotics (Sears et al., 1992; Wu et al., 2007).
12
Fig. 1. Structure of nisin A and putative variants nisins Z, Q and F. Gray circles indicate
amino acid residues different among the natural nisin variants. Dha, dehydroalanine; Dhb,
dehydrobutyrine; Abu, 2-aminobutiric acid; Ala-S-Ala, lanthionine; Abu-S-Ala, β
13 Table 3. Peptide-bacteriocins isolated from Lactococcus lactis
Bacteriocin Class Producer strain Mass (Da)
(amino acids) References
Nisin A I L. lactis ATCC 11454 3353.5 (34) (Gross and Morell,
1971)
Nisin Z I L. lactis N8 3330.9 (34) (Mulders et al.,
1991)
Nisin Q I L. lactis 61-14 3327.5 (34) (Zendo et al., 2003)
Nisin F I L. lactis F10 3315.4 (34) (De Kwaadsteniet et
al., 2008)
Lacticin 481 I L. lactis CNRZ 481 2991.8 (27) (Piard et al., 1992)
Lacticin 3147 I L. lactis DPC3147 A1 3449.3 (30)
A2 3005.9 (29) (Ryan et al., 1996)
Lacticin
NK34 ND L. lactis ND (ND) (Kim et al., 2010)
Lacticin Z IId L. lactis QU14 5968.9 (53) (Iwatani et al., 2007)
Lacticin Q IId L. lactis QU5 5525.4 (53) (Fujita et al., 2007)
Lacticin RM ND L. lactis EZ26 ND (ND) (Yarmus et al.,
2000)
Lactococcin A IIc L. lactis LMG 2130 5797.31 (54) (Holo et al., 1991)
Lactococcin B IIc L. lactis 9B4 5346.8 (54) (Van Belkum et al.,
1992) Lactococcin
DR ND
L. lactis
ADRIA85LO30 ND (ND) (Rince et al., 1994)
Lactococcin G IIb L. lactis LMG 2081 Gα 4364.5 (39)
Gβ 4128.3 (35)
(Nissen-Meyer et
al., 1992)
Lactococcin K ND L. lactis MY23 ND (ND) (Kim et al., 2006)
Lactococcin
MMT24 IIb L. lactis MMT24
Pepα 3765.3 (ND)
Pepβ 3255.2 (ND) (Ghrairi et al., 2005)
Lactococcin
MMFII IIa L. lactis 4144.6 (37)
(Ferchichi et al.,
2001) Lactococcin
972 ND L. lactis IPLA 972 7001.9 (66)
(Martinez et al.,
1996)
Lactococcin Q IIb L. lactis QU4 Qα 4260.4 (39)
Qβ 4018.3 (35) (Zendo et al., 2006)
Lactococcin R ND L. lactis R ND (ND) (Yildirim and
Johnson, 1998) Bacteriocin
J46 ND L. lactis 3004.8 (27) (Huot et al., 1996)
LsbA/LsbB ND L. lactis ND (Gajic et al., 2003)
15
Fig 2. Nisin gene cluster and model of biosynthesis and regulation, i.e. nisABTCIPRKFEG.
Black and white triangles indicate nisin-inducible and constitutive promoters, respectively.
(1) Formation of prepeptide (prebacteriocin); (2) The prepeptide is modified by NisB and
NisC, translocated through a ABC-transporter NisT and processed by NisP, resulting in the
release of mature bacteriocin; (3) Histidine Protein Kinase (HPK) senses the presence of
bacteriocin and autophosphorylates; (4) The phosphoryl group (Pi) is subsequently
transferred to the response regulator (RR); RR activates transcription of the regulated genes;
and (6) Producer immunity mediated by immunity proteins, NisI, and dedicated
ABC-transport proteins, NisFEG (adapted from Kleerebezem, 2004).
(1)
(2) (3)
(4)
(5)
16 (Dodd et al., 1990), Tn5307 (Buchman et al., 1988) and Tn5481 (Immonen and Saris, 1998).
Lacticin 3147 and lacticin 481 are other lantibiotics produced by L. lactis (Table 3). Lacticin 3147 is also a broad-spectrum lantibiotic with potential uses in the food industry and in medicine. It is a two-component lantibiotic (two separate peptides act in concert for full activity) produced by L. lactis subsp. lactis DPC3147 isolated from an Irish kefir grain (Ryan et al., 1996). The genes required for DPC3147 to produce lacticin 3147 are carried on a 60.2 kb conjugative plasmid. Lacticin 481 exhibits a medium spectrum of activity, inhibiting a broad range of other LAB and Clostridium tyrobutyricum. This peptide can have a bacteriolytic effect on sensitive organisms (O'sullivan et al., 2002). It has been studied for its potential use in cheese ripening to induce lysis of starter strains and therefore deliver lactococcal enzymes during cheese manufacture to improve both flavor and quality (Guinane et al., 2005).
17 can have both a bactericidal and bacteriolytic mode of action on target cells. However, such bacteriocins inhibit other lactococci and therefore their associated applications are limited (Guinane et al., 2005). On the other hand, the lytic abilities of these bacteriocins may have an application in the acceleration of cheese ripening to induce starter cell lysis (Morgan et al., 2002). Lactococcin G, lactococcin Q and lactococcin MMT24 produced by L. lactis LMG 2081, L. lactis QU4 and L. lactis MMT24, respectively, are two-peptide bacteriocins (class IIb) with similar characteristics. They are cationic, contain between 30-50 residues long, hydrophobic and/or amphiphilic and both complementary peptides are required to obtain full acti- vity and the individual peptides display little or no antimicrobial activity (Nissen-Meyer et al., 2009).
These bacteriocins have a bactericidal mode of action and a narrow antimicrobial activity against closely related lactic acid bacteria (Nissen-Meyer et al., 1992; Ghrairi et al., 2005; Zendo et al., 2006). Lactococcin MMFII belongs to the class IIa bacteriocins figuring the first example of such a bacteriocin produced by a lactococcal strain described so far. It is one of the most active bacteriocin within these class IIa bacteriocins, rendering this bacteriocin attractive as an anti-Listeria compounds to protect food (Ferchichi et al., 2001). Lacticin Q and Z are highly cationic and tryptophan-rich bacteriocin, which have formylated N-terminal methionine residues and are synthesized without leader sequences. They inhibit a nanomolar range of MICs against several of Gram-positive bacteria, and have antimicrobial activity comparable to that of nisin A in terms of both intensity and spectrum. Unlike many of other LAB bacteriocins, the stability of lacticin Z and Q were emphasized under alkaline conditions rather than acidic conditions (Fujita et al., 2007; Iwatani et al., 2007).
18
3. Perspectives of new antimicrobial compounds produced by Lactococcus lactis
Some LAB also produce other low molecular weight compounds with antimicrobial activity such as reuterin and reutericyclin (Spinler et al., 2008), cyclic peptides (Schnurer and Magnusson, 2005) and biosurfactants (Moldes et al., 2007). Biosurfactants are amphiphilic compounds of microbial origin with a pronounced surface activity that exhibit a wide variety of chemical structures, such as glycolipids, lipopeptides, polysaccharide-protein complexes and fatty acids (Rodrigues et al., 2006). Biosurfactants can be produced by microorganisms in an extracellular position and/or associated with cell walls. Recently, the strain L. lactis CET-4434 has shown to be an interesting cell-bound biosurfactant producer, with inhibited the growth of C. piscicola used as target microorganisms (Rodríguez et al., 2010). Fractions of crude biosurfactant extract obtained from L. lactis 53 have shown antimicrobial activity against Staphylococcus epidermidis, S. aureus and Candida albicans (Rodrigues et al., 2006).
In addition, L. lactis CHD-28.3 showed antagonistic activity against several filamentous fungi, that was lost after enzymatic treatment with chymotrypsin, trypsin and pronase E (Roy et al., 1996).
19
4. References
And, H. C., Hoover, D. G. Bacteriocins and their Food Applications.
Comprehensive Reviews in Food Science and Food Safety, v.2, n.3, p.82-100. 2003.
Arnusch, C. J., Bonvin, A. M., Verel, A. M., Jansen, W. T., Liskamp, R. M., De Kruijff, B., Pieters, R. J., Breukink, E. The vancomycin-nisin (1-12) hybrid restores activity against vancomycin resistant Enterococci. Biochemistry, v.47, n.48, p.12661-3. 2008.
Asaduzzaman, S. M., Sonomoto, K. Lantibiotics: Diverse activities and unique modes of action. Journal of Bioscience and Bioengineering, v.107, n.5, p.475-487. 2009.
Bachmann, H., Starrenburg, M. J. C., Dijkstra, A., Molenaar, D., Kleerebezem, M., Rademaker, J. L. W., Van Hylckama Vlieg, J. E. T. Regulatory Phenotyping Reveals Important Diversity within the Species Lactococcus lactis. Applied and
Environmental Microbiology, v.75, n.17, p.5687-5694. 2009.
Basaran, P., Basaran, N., Cakir, I. Molecular differentiation of Lactococcus lactis subspecies lactis and cremoris strains by ribotyping and site specific-PCR. Current
Microbiology, v.42, n.1, p.45-8. 2001.
Bierbaum, G., Sahl, H. G. Lantibiotics: mode of action, biosynthesis and bioengineering. Current Pharmaceutical Biotechnology, v.10, n.1, p.2-18. 2009.
20 Bolotin, A., Quinquis, B., Ehrlich, S. D., Sorokin, A. Complete Genome Sequence of Lactococcus lactis subsp. cremoris A76. Journal of Bacteriology, v.194, n.5, p.1241-2. 2012.
Bolotin, A., Wincker, P., Mauger, S., Jaillon, O., Malarme, K., Weissenbach, J., Ehrlich, S. D., Sorokin, A. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Research, v.11, n.5, p.731-53. 2001.
Buchman, G. W., Banerjee, S., Hansen, J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. The Journal of
Biological Chemistry, v.263, n.31, p.16260-6. 1988.
Chatterjee, C., Paul, M., Xie, L., Van Der Donk, W. A. Biosynthesis and mode of action of lantibiotics. Chemistry Review, v.105, n.2, p.633-84. 2005.
Cheigh, C. I., Choi, H. J., Park, H., Kim, S. B., Kook, M. C., Kim, T. S., Hwang, J. K., Pyun, Y. R. Influence of growth conditions on the production of a nisin-like bacteriocin by Lactococcus lactis subsp. lactis A164 isolated from kimchi. Journal
Biotechnology, v.95, n.3, p.225-35. 2002.
Christensen, D. P., Hutkins, R. W. Collapse of the proton motive force in Listeria monocytogenes caused by a bacteriocin produced by Pediococcus acidilactici.
Applied and Environmental Microbiology, v.58, n.10, p.3312-5. 1992.
Cocaign-Bousquet, M., Garrigues, C., Loubiere, P., Lindley, N. D. Physiology of pyruvate metabolism in Lactococcus lactis. Antonie Van Leeuwenhoek, v.70, n.2-4, p.253-67. 1996.
21 UCC118. Proceedings of the National Academy of Sciences, v.104, n.18, p.7617-21. 2007.
Cotter, P. D., Hill, C., Ross, R. P. Bacteriocins: developing innate immunity for food.
Nature Reviews Microbiology, v.3, n.10, p.777-88. 2005.
Crow, V. L., Pritchard, G. G. Fructose 1,6-diphosphate-activated L-lactate dehydrogenase from Streptococcus lactis: kinetic properties and factors affecting activation. Journal of Bacteriology, v.131, n.1, p.82-91. 1977.
Crow, V. L., Thomas, T. D. D-tagatose 1,6-diphosphate aldolase from lactic streptococci: purification, properties, and use in measuring intracellular tagatose 1,6-diphosphate. Journal of Bacteriology, v.151, n.2, p.600-8. 1982.
De Kwaadsteniet, M., Ten Doeschate, K., Dicks, L. M. Characterization of the structural gene encoding nisin F, a new lantibiotic produced by a Lactococcus lactis subsp. lactis isolate from freshwater catfish (Clarias gariepinus). Applied and
Environmental Microbiology, v.74, n.2, p.547-9. 2008.
De Vuyst, L., Leroy, F. Bacteriocins from lactic acid bacteria: production, purification, and food applications. Journal Molecular Microbiology
Biotechnology, v.13, n.4, p.194-9. 2007.
De Vuyst, L., Vandamme, E. J. Influence of the carbon source on nisin production in Lactococcus lactis subsp. lactis batch fermentations. Journal of General
Microbiology, v.138, n.3, p.571-8. 1992.
Diep, D. B., Skaugen, M., Salehian, Z., Holo, H., Nes, I. F. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proccedings of the
22 Dimov, S., Ivanova, P., Harizanova, N. Genetics of bacteriocins biosynthesis by lactic acid bacteria. Biotechnology and Biotechnological Equipment, v.19, n.2, p.4-10. 2005.
Dodd, H. M., Horn, N., Gasson, M. J. Analysis of the genetic determinant for production of the peptide antibiotic nisin. Journal of General Microbiology, v.136, n.3, p.555-66. 1990.
Eec. European Economic Community Commission Directive. Official Journal
European Union 255: 1-6 p. 1983.
Ennahar, S., Sashihara, T., Sonomoto, K., Ishizaki, A. Class IIa bacteriocins: biosynthesis, structure and activity. FEMS Microbiology Reviews, v.24, n.1, p.85-106. 2000.
Espeche, M. C., Otero, M. C., Sesma, F., Nader-Macias, M. E. Screening of surface properties and antagonistic substances production by lactic acid bacteria isolated from the mammary gland of healthy and mastitic cows. Veterinary Microbiology, v.135, n.3-4, p.346-57. 2009.
Even, S., Garrigues, C., Loubiere, P., Lindley, N. D., Cocaign-Bousquet, M. Pyruvate metabolism in Lactococcus lactis is dependent upon glyceraldehyde-3-phosphate dehydrogenase activity. Metabolic Engineering, v.1, n.3, p.198-205. 1999.
Ferchichi, M., Frere, J., Mabrouk, K., Manai, M. Lactococcin MMFII, a novel class IIa bacteriocin produced by Lactococcus lactis MMFII, isolated from a Tunisian dairy product. FEMS Microbiology Letter, v.205, n.1, p.49-55. 2001.
23 Fujita, K., Ichimasa, S., Zendo, T., Koga, S., Yoneyama, F., Nakayama, J., Sonomoto, K. Structural analysis and characterization of lacticin Q, a novel bacteriocin belonging to a new family of unmodified bacteriocins of gram-positive bacteria. Applied and Environmental Microbiology, v.73, n.9, p.2871-7. 2007.
Gajic, O., Buist, G., Kojic, M., Topisirovic, L., Kuipers, O. P., Kok, J. Novel mechanism of bacteriocin secretion and immunity carried out by lactococcal multidrug resistance proteins. Journal Biology Chemistry, v.278, n.36, p.34291-8. 2003.
Galvez, A., Abriouel, H., Benomar, N., Lucas, R. Microbial antagonists to food-borne pathogens and biocontrol. Current Opinion Biotechnology, v.21, n.2, p.142-8. 2010.
Gao, Y., Lu, Y., Teng, K. L., Chen, M. L., Zheng, H. J., Zhu, Y. Q., Zhong, J. Complete genome sequence of Lactococcus lactis subsp. lactis CV56, a probiotic strain isolated from the vaginas of healthy women. Journal of Bacteriology, v.193, n.11, p.2886-7. 2011.
Gao, Y. L., Ju, X. R. Exploiting the combined effects of high pressure and moderate heat with nisin on inactivation of Clostridium botulinum spores. Journal
Microbiology Methods, v.72, n.1, p.20-8. 2008.
Ghrairi, T., Frere, J., Berjeaud, J. M., Manai, M. Lactococcin MMT24, a novel two-peptide bacteriocin produced by Lactococcus lactis isolated from rigouta cheese.
International Journal Food Microbiology, v.105, n.3, p.389-98. 2005.
Gross, E., Morell, J. L. The structure of nisin. Journal of the American Chemical
Society, v.93, n.18, p.4634-5. 1971.
Guinane, C. M., Cotter, P. D., Hill, C., Ross, R. P. Microbial solutions to microbial problems; lactococcal bacteriocins for the control of undesirable biota in food.
24 Hampikyan, H. Efficacy of nisin against Staphylococcus aureus in experimentally contaminated sucuk, a Turkish-type fermented sausage. Journal Food Protection, v.72, n.8, p.1739-43. 2009.
Hechard, Y., Sahl, H. G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie, v.84, n.5-6, p.545-57. 2002.
Holo, H., Nes, I. F. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Applied and Environmental Microbiology, v.55, n.12, p.3119-23. 1989.
Holo, H., Nilssen, O., Nes, I. F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene.
Journal of Bacteriology, v.173, n.12, p.3879-87. 1991.
Huot, E., Meghrous, J., Barrena-Gonzalez, C., Petitdemange, H. Bacteriocin J46, a New Bacteriocin Produced by Lactococcus lactis subsp. cremoris J46: Isolation and Characterization of the Protein and Its Gene. Anaerobe, v.2, n.3, p.137-145. 1996.
Hurst, A., Perlman, D., Laskin, I. A. Nisin. In: (Ed.). Advances in Applied
Microbiology: Academic Press, v.Volume 27, 1981. Nisin, p.85-123
Immonen, T., Saris, P. E. Characterization of the nisFEG operon of the nisin Z producing Lactococcus lactis subsp. lactis N8 strain. DNA Sequence, v.9, n.5-6, p.263-74. 1998.
Iwatani, S., Zendo, T., Yoneyama, F., Nakayama, J., Sonomoto, K. Characterization and structure analysis of a novel bacteriocin, lacticin Z, produced by Lactococcus lactis QU 14. Bioscience Biotechnology Biochemistry, v.71, n.8, p.1984-92. 2007.
25 purified lantibiotic lacticin NK34 against infection by Staphylococcus species isolated from bovine mastitis. Journal Dairy Science, v.93, n.8, p.3610-5. 2010.
Kim, Y. S., Kim, M. J., Kim, P., Kim, J. H. Cloning and production of a novel bacteriocin, lactococcin K, from Lactococcus lactis subsp. lactis MY23.
Biotechnology Letters, v.28, n.5, p.357-62. 2006.
Kjos, M., Borrero, J., Opsata, M., Birri, D. J., Holo, H., Cintas, L. M., Snipen, L., Hernandez, P. E., Nes, I. F., Diep, D. B. Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiology, v.157, n.Pt 12, p.3256-67. 2011.
Klaenhammer, T. R. Genetics of bacteriocins produced by lactic acid bacteria.
FEMS Microbiology Reviews, v.12, p.39-85. 1993.
Kleerebezem, M. Quorum sensing control of lantibiotic production; nisin and subtilin autoregulate their own biosynthesis. Peptides, v.25, n.9, p.1405-14. 2004.
Kotel'nikova, E. A., Gel'fand, M. S. Production of bacteriocins by gram-positive bacteria and the mechanisms of transcriptional regulation. Genetika, v.38, n.6, p.758-72. 2002.
Kuipers, O. P., Beerthuyzen, M. M., Siezen, R. J., De Vos, W. M. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis. Requirement of expression of the nisA and nisI genes for development of immunity. European
Journal Biochemistry, v.216, n.1, p.281-91. 1993.
26 Leroy, F., De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends in Food Science and Technology, v.15, n.2, p.67-78. 2004.
Li, H., O'sullivan, D. J. Identification of a nisI promoter within the nisABCTIP operon that may enable establishment of nisin immunity prior to induction of the operon via signal transduction. Journal of Bacteriology, v.188, n.24, p.8496-503. 2006.
Lubelski, J., Rink, R., Khusainov, R., Moll, G. N., Kuipers, O. P. Biosynthesis, immunity, regulation, mode of action and engineering of the model lantibiotic nisin.
Cellular and Molecular Life Sciences, v.65, n.3, p.455-76. 2008.
Makarova, K., Slesarev, A., Wolf, Y., Sorokin, A., Mirkin, B., Koonin, E., Pavlov, A., Pavlova, N., Karamychev, V., Polouchine, N., Shakhova, V., Grigoriev, I., Lou, Y., Rohksar, D., Lucas, S., Huang, K., Goodstein, D. M., Hawkins, T., Plengvidhya, V., Welker, D., Hughes, J., Goh, Y., Benson, A., Baldwin, K., Lee, J. H., Diaz-Muniz, I., Dosti, B., Smeianov, V., Wechter, W., Barabote, R., Lorca, G., Altermann, E., Barrangou, R., Ganesan, B., Xie, Y., Rawsthorne, H., Tamir, D., Parker, C., Breidt, F., Broadbent, J., Hutkins, R., O'sullivan, D., Steele, J., Unlu, G., Saier, M., Klaenhammer, T., Richardson, P., Kozyavkin, S., Weimer, B., Mills, D. Comparative genomics of the lactic acid bacteria. Proccedings of the National Academy of
Sciences, v.103, n.42, p.15611-6. 2006.
Maqueda, M., Sánchez-Hidalgo, M., Fernández, M., Montalbán-López, M., Valdivia, E., Martínez-Bueno, M. Genetic features of circular bacteriocins produced by Gram-positive bacteria. FEMS Microbiology Reviews, v.32, n.1, p.2-22. 2008.
Martinez, B., Suarez, J. E., Rodriguez, A. Lactococcin 972 : a homodimeric lactococcal bacteriocin whose primary target is not the plasma membrane.
27 Mills, S., Mcauliffe, O. E., Coffey, A., Fitzgerald, G. F., Ross, R. P. Plasmids of lactococci – genetic accessories or genetic necessities? FEMS Microbiology
Reviews, v.30, n.2, p.243-273. 2006.
Mitra, S., Mukhopadhyay, B. C., Biswas, S. R. Potential application of the nisin Z preparation of Lactococcus lactis W8 in preservation of milk. Letters in Applied
Microbiology, v.53, n.1, p.98-105. 2011.
Moldes, A. B., Torrado, A. M., Barral, M. T., Domínguez, J. M. Evaluation of Biosurfactant Production from Various Agricultural Residues by Lactobacillus pentosus. Journal of Agricultural and Food Chemistry, v.55, n.11, p.4481-4486. 2007.
Morello, E., Bermúdez-Humarán, L. G., Llull, D., Solé, V., Miraglio, N., Langella, P., Poquet, I. Lactococcus lactis, an Efficient Cell Factory for Recombinant Protein Production and Secretion. Journal of Molecular Microbiology and Biotechnology, v.14, n.1-3, p.48-58. 2008.
Morgan, S. M., O'sullivan, L., Ross, R. P., Hill, C. The design of a three strain starter system for Cheddar cheese manufacture exploiting bacteriocin-induced starter lysis.
International Dairy Journal, v.12, n.12, p.985-993. 2002.
Mulders, J. W., Boerrigter, I. J., Rollema, H. S., Siezen, R. J., De Vos, W. M. Identification and characterization of the lantibiotic nisin Z, a natural nisin variant.
European Journal of Biochemistry, v.201, n.3, p.581-4. 1991.
Nes, I. F., Diep, D. B., Holo, H. Bacteriocin diversity in Streptococcus and Enterococcus. Journal of Bacteriology, v.189, n.4, p.1189-98. 2007a.
Nes, I. F., Holo, H. Class II antimicrobial peptides from lactic acid bacteria.
28 Nes, I. F., Johnsborg, O. Exploration of antimicrobial potential in LAB by genomics.
Current Opinion in Biotechnology, v.15, n.2, p.100-104. 2004.
Nes, I. F., Yoon, S. S., Diep, D. B. Ribosomally Synthesiszed Antimicrobial Peptides (Bacteriocins) in Lactic Acid Bacteria: A Review. Food Science and
Biotechnology, v.16, n.5, p.675-690. 2007b.
Neves, A. R., Pool, W. A., Kok, J., Kuipers, O. P., Santos, H. Overview on sugar metabolism and its control in Lactococcus lactis - the input from in vivo NMR.
FEMS Microbiology Reviews, v.29, n.3, p.531-54. 2005.
Nissen-Meyer, J., Holo, H., Havarstein, L. S., Sletten, K., Nes, I. F. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. Journal of Bacteriology, v.174, n.17, p.5686-92. 1992.
Nissen-Meyer, J., Rogne, P., Oppegard, C., Haugen, H. S., Kristiansen, P. E. Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by gram-positive bacteria. Current Pharmaceutical
Biotechnology, v.10, n.1, p.19-37. 2009.
O'sullivan, L., Morgan, S. M., Ross, R. P., Hill, C. Elevated enzyme release from lactococcal starter cultures on exposure to the lantibiotic lacticin 481, produced by Lactococcus lactis DPC5552. Journal Dairy Science, v.85, n.9, p.2130-40. 2002.
Oman, T. J., Van Der Donk, W. A. Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nature Chemical Biology, v.6, n.1, p.9-18. 2010.
Papagianni, M., Anastasiadou, S. Pediocins: The bacteriocins of Pediococci. Sources, production, properties and applications. Microbial Cell Factories, v.8, p.3. 2009.
29 Produced by Lactococcus lactis subsp. lactis CNRZ 481. Applied and
Environmental Microbiology, v.58, n.1, p.279-84. 1992.
Piper, C., Hill, C., Cotter, P. D., Ross, R. P. Bioengineering of a Nisin A-producing Lactococcus lactis to create isogenic strains producing the natural variants Nisin F, Q and Z. Microbiology Biotechnology, v.4, n.3, p.375-82. 2010.
Quadri, L. E., Sailer, M., Roy, K. L., Vederas, J. C., Stiles, M. E. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. The Journal of Biological Chemistry, v.269, n.16, p.12204-11. 1994.
Quadri, L. E. N. Regulation of Class II Bacteriocin Production by Cell-Cell Signaling. The Journal of Microbiology, v.41, n.3, p.175-182. 2003.
Ramos, A., Neves, A. R., Ventura, R., Maycock, C., Lopez, P., Santos, H. Effect of pyruvate kinase overproduction on glucose metabolism of Lactococcus lactis.
Microbiology, v.150, n.4, p.1103-11. 2004.
Rauch, P. J., De Vos, W. M. Characterization of the novel nisin-sucrose conjugative transposon Tn5276 and its insertion in Lactococcus lactis. Journal of Bacteriology, v.174, n.4, p.1280-7. 1992.
Rince, A., Dufour, A., Le Pogam, S., Thuault, D., Bourgeois, C. M., Le Pennec, J. P. Cloning, expression, and nucleotide sequence of genes involved in production of lactococcin DR, a bacteriocin from Lactococcus lactis subsp. lactis. Applied and
Environmental Microbiology, v.60, n.5, p.1652-7. 1994.
30 Rodrigues, L. R., Teixeira, J. A., Van Der Mei, H. C., Oliveira, R. Physicochemical and functional characterization of a biosurfactant produced by Lactococcus lactis 53.
Colloids and Surfaces B: Biointerfaces, v.49, n.1, p.79-86. 2006.
Rodríguez, N., Salgado, J. M., Cortés, S., Domínguez, J. M. Alternatives for biosurfactants and bacteriocins extraction from Lactococcus lactis cultures produced under different pH conditions. Letters in Applied Microbiology, v.51, n.2, p.226-233. 2010.
Roy, U., Batish, V. K., Grover, S., Neelakantan, S. Production of antifungal substance by Lactococcus lactis subsp. lactis CHD-28.3. International Journal of
Food Microbiology, v.32, p.27-34. 1996.
Ruyter, P. G. G. A. D., Kuipers, O. P., Beerthuyzen, M. M., Alen-Boerrigter, I. J. V., Vos, W. M. D. Functional analysis of promoters in the gene cluster of Lactococcus lactis. Journal of Bacteriology,v.178, p.3434-3439. 1996.
Ryan, M. P., Rea, M. C., Hill, C., Ross, R. P. An application in cheddar cheese manufacture for a strain of Lactococcus lactis producing a novel broad-spectrum bacteriocin, lacticin 3147. Applied and Environmental Microbiology, v.62, n.2, p.612-9. 1996.
Schnurer, J., Magnusson, J. Antifungal lactic acid bacteria as biopreservatives.
Trends in Food Science & Technology, v.16, p.70-78. 2005.
Sears, P. M., Smith, B. S., Stewart, W. K., Gonzalez, R. N., Rubino, S. D., Gusik, S. A., Kulisek, E. S., Projan, S. J., Blackburn, P. Evaluation of a Nisin-Based Germicidal Formulation on Teat Skin of Live Cows. Journal of Dairy Science, v.75, n.11, p.3185-3190. 1992.
31 lactis KF147, a plant-associated lactic acid bacterium. Journal of Bacteriology, v.192, n.10, p.2649-50. 2010.
Simsek, O., Con, A. H., Akkoc, N., Saris, P. E., Akcelik, M. Influence of growth conditions on the nisin production of bioengineered Lactococcus lactis strains.
Journal Industrial Microbiology Biotechnology, v.36, n.4, p.481-90. 2009.
Smit, G., Smit, B. A., Engels, W. J. M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiology Reviews, v.29, n.3, p.591-610. 2005.
Spinler, J. K., Taweechotipatr, M., Rognerud, C. L., Ou, C. N., Tumwasorn, S., Versalovic, J. Human-derived probiotic Lactobacillus reuteri demonstrate antimicrobial activities targeting diverse enteric bacterial pathogens. Anaerobe, v.14, n.3, p.166-171. 2008.
Takala, T. M., Saris, P. E. C terminus of NisI provides specificity to nisin.
Microbiology, v.152, n.Pt 12, p.3543-9. 2006.
Tauber, M., Geis, A. The Genus Lactococcus. In: M. Dworkin, S. Falkow, E. Rosenberg, E. Stackerbrant (Ed.). The Prokaryotes: a handbook on the biology of
bacteria. New York, USA: Springer Science, v.4, 2006. The Genus Lactococcus, p.205-228
Teusink, B., Smid, E. J. Modelling strategies for the industrial exploitation of lactic acid bacteria. Nature Review Microbiology, v.4, n.1, p.46-56. 2006.
Todorov, S. D., Dicks, L. M. Effect of modified MRS medium on production and purification of antimicrobial peptide ST4SA produced by Enterococcus mundtii.
32 Trmčić, A., Monnet, C., Rogelj, I., Matijašić, B. Expression of nisin genes in cheese-a qucheese-antitcheese-ative recheese-al-time polymercheese-ase chcheese-ain recheese-action cheese-approcheese-ach. Journal Dairy Science, v.94, p.77-85. 2010.
Van Belkum, M. J., Kok, J., Venema, G. Cloning, sequencing, and expression in Escherichia coli of lcnB, a third bacteriocin determinant from the lactococcal bacteriocin plasmid p9B4-6. Applied and Environmental Microbiology, v.58, n.2, p.572-7. 1992.
Van Belkum, M. J., Kok, J., Venema, G., Holo, H., Nes, I. F., Konings, W. N., Abee, T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. Journal
of Bacteriology, v.173, n.24, p.7934-41. 1991.
Van Den Berg Van Saparoea, H. B., Bakkes, P. J., Moll, G. N., Driessen, A. J. Distinct contributions of the nisin biosynthesis enzymes NisB and NisC and transporter NisT to prenisin production by Lactococcus lactis. Applied and
Environmental Microbiology, v.74, n.17, p.5541-8. 2008.
Wegmann, U., O'connell-Motherway, M., Zomer, A., Buist, G., Shearman, C., Canchaya, C., Ventura, M., Goesmann, A., Gasson, M. J., Kuipers, O. P., Van Sinderen, D., Kok, J. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. Journal of Bacteriology, v.189, n.8, p.3256-70. 2007.
Wiedemann, I., Breukink, E., Van Kraaij, C., Kuipers, O. P., Bierbaum, G., De Kruijff, B., Sahl, H. G. Specific binding of nisin to the peptidoglycan precursor lipid II combines pore formation and inhibition of cell wall biosynthesis for potent antibiotic activity. Journal Biology Chemistry, v.276, n.3, p.1772-9. 2001.
33 Yarmus, M., Mett, A., Shapira, R. Cloning and expression of the genes involved in the production of and immunity against the bacteriocin lacticin RM. Biochimistry
Biophysical Acta, v.1490, n.3, p.279-90. 2000.
Yildirim, Z., Johnson, M. G. Detection and characterization of a bacteriocin produced by Lactococcus lactis subsp. cremoris R isolated from radish. Letter
Applied Microbiology, v.26, n.4, p.297-304. 1998.
Zendo, T., Fukao, M., Ueda, K., Higuchi, T., Nakayama, J., Sonomoto, K. Identification of the lantibiotic nisin Q, a new natural nisin variant produced by Lactococcus lactis 61-14 isolated from a river in Japan. Bioscience Biotechnology
and Biochemistry, v.67, n.7, p.1616-9. 2003.
Zendo, T., Koga, S., Shigeri, Y., Nakayama, J., Sonomoto, K. Lactococcin Q, a novel two-peptide bacteriocin produced by Lactococcus lactis QU 4. Applied and
34
CHAPTER 2
Nisin Z production by wild strains of Lactococcus lactis isolated from naturally
fermented sausage
Abstract
35
1. Introduction
Bacteriocin producing lactic acid bacteria (LAB) are used as starter or protective cultures in the production of foods. In particular, Lactococcus lactis, a nisin-producing, has been utilized in fermented foods and it is generally recognized as a safe (GRAS). Hence, L. lactis-derived nisin has been extensively studied as a model bacteriocin in several applications. Nisin A, a ribosomally synthesized lantibiotic is the first bacteriocin approved and employed as food preservative commercially (Cotter et al., 2005). The nisin A operon has been studied in detail and consists of eleven genes, i.e., nisABTCIPRKFEG (Trmčić et al., 2011). The prepeptide is encoded by the structural gene nisA (Kuipers et al., 1993). Transcription of nisin genes is accomplished by three major promoter sites of which the promoter preceding nisR is constitutive, whereas the nisA and nisF promoters are controlled by the two component regulatory system NisRK (De Ruyter et al., 1996). The NisRK-mediated regulatory system, responds to changes in environmental factors (Kleerebezem, 2004). In addition, to the nisR promoter, another constitutive promoter has been identified before nisI. nisI gene encodes an immunity protein (Takala and Saris, 2006) that together with, nisF, nisE, and nisG encode ATP-binding cassete transporters are responsible for immunity of the producing strain (Immonen and Saris, 1998). In view of the widespread use of this bacteriocin, an important factor to consider for its application is the cost of nisin production. It is well known that nisin production at fermentation systems is influenced by many factors such as type and the level of carbon and nitrogen sources, pH, and temperature (De Vuyst and Vandamme, 1992; Matsusaki et al., 1996; Cheigh et al., 2002), optimization of bacteriocin production and enhancement of its activity may have economic significance. Moreover, nisin production abilities of the producer strains can also differ (Kim et al., 1997).
36 fermented meat products (Nieto-Lozano et al., 2002; Albano et al., 2007; Héquet et al., 2007; Müller et al., 2009).
In our previous study on bacteriocin production by lactic acid bacteria isolated from fermented sausage in Brazil (unpublished data), several Gram-positive bacteria were screened for the ability to produce bacteriocin against food-borne pathogens. The purpose of this study was to characterize the bacteriocin secreted by L. lactis strains ID1.5, ID3.1, ID8.5, PR3.1 and PD4.7 isolated from Italian type fermented sausage and investigate production in batch culture.
2. Materials and methods
2.1. Bacterial strains and culture conditions
Bacteria used in this study were Lactococcus lactis ID1.5, ID3.1, ID8.5, PR3.1 and PD4.7 isolated from Italian type fermented sausage and maintained as a part of our culture collection. All LAB used were grown at 30 °C in LAPTg broth containing (per liter): 10g glucose, 10g tryptone, 10g yeast extract, 15g animal peptone and 1g tween 80 (Juarez Tomas et al., 2004). The susceptible strain Micrococcus luteus ATCC 10240 was used as the indicator strain in biological assay for bacteriocin quantification. It was grown at 30 °C in basal media containing (per liter): 10g glucose, 10g animal peptone, 8g meat extract, 4g yeast extract, 5g NaCl and 2,5g Na2HPO4.
2.2.Genetic identification of bacteriocin-producing strains
37 of 2 min at 95 °C, 30 s at 58 °C, and 2 min at 72 °C; with a final 5 min at 72 °C. Amplicons were purified and ligated to pGEN-T Easy (PROMEGA) and cloned. Plasmids DNA from the clones were isolated by EZ.N.A. TM Plasmid Spin Protocol (Omega, USA), analyzed by PCR and the cloned fragment was sequenced. Sequences were aligned using BLAST software provided online by National Center for Biotechnology Information (USA) to determine the closest known relatives of the partial 16S sequence obtained.
2.3.Sequencing of nisin genes and nisin controlled promoters
Nucleotide sequencing was performed with the PCR products obtained from the amplifications of genomic DNA with primers specific to nisA structural gene and nisA, nisF promoters (designed according to the nisin A regulon, GenBank: HM219853.1) listed in Table 1. The PCR conditions were different for each pair of primers. The PCR thermal cycle program included an initial denaturation at 94 °C for 2 min, followed by 35 cycles, with a denaturation step at 94 °C for 1 min, an annealing step of 30 s, at 40 °C, at 48 °C and at 68 °C for primers sets nqf/naqzr, pnisAf/pnisAr and pnisFf/pnisFr, respectively, followed by extension step during 1 min at 72 °C. Final extension was performed at 72 °C for 7 min. The desired bands after PCR amplification were cut from the gel and purified with Gel Extraction Kit (Nucleospin® Gel and PCR clean up, Machery-Nagel, Germany) and sequenced. The determined sequences were compared with the GenBank database using tools as metioned above.
2.4.Detection and curing of plasmids
38 overlaid with soft agar containing L. lactis IL1403 as indicator organism, and the presence of plasmids was checked.
2.5.Bacteriocin antimicrobial assay
Quantitative determination of the antimicrobial activity of the bacteriocin was performed by using agar well diffusion assay technique (Ryan et al., 1996). Preparations of the cell-free culture supernatant (boiled and neutralized) as well as purified bacteriocin were serially diluted and tested against indicator strain. One arbitrary unit (AU mL-1) was defined as the reciprocal of the highest dilution that showed a zone of inhibition with at least 5 mm in diameter.
2.6.Purification and estimation of the molecular size of bacteriocins
39 TOF/TOF (Bruker Daltonic GmBH, Bremen, Germany) by using a pulsed ion extraction setting of 40 ns and an acceleration voltage of 25 kV.
2.7.Effect of carbon and nitrogen sources on bacteriocin production
The carbon sources tested were glucose, lactose, sucrose and fructose at the concentration of 10 g L-1. Bath cultures (1% inoculum, v/v, standardized to OD600nm = 0.6) were grown in LAPTg broth with glucose; and LAPTg broth without glucose, supplemented with lactose, sucrose or fructose. Incubation was at 30 °C, without agitation, for 24 h. Samples were examined each hour for bacterial growth (OD600nm), changes in culture pH and bacteriocin production.
To study the effect of different nitrogen sources on bacteriocin production, the LAPTg medium added of glucose at 10 g L-1 was supplemented with each different nitrogen source (at 35 g L-1). The nitrogen sources separately added were tryptone, yeast extract, meat extract, animal peptone, soy peptone, and casein peptone. After 24 hours of incubation at 30 °C, the final optical densities, culture pH value and bacteriocin production (expressed as AU mL-1) were determined.
2.8.Effect of aeration, initial medium pH value and incubation temperatures on bacteriocin production
40
2.9.Statistics
All experiments with regard to bacteriocin production were carried out in triplicate and repeated twice. When error bars are given in the figures, they refer to the standard deviation of the mean.
3. Results
3.1.Identification of bacteriocin-producing strains
In order to study the phylogenetic position of lactic acid bacteria, 1500 nucleotideos of the 16S rDNA of the strains were amplified by PCR, sequenced, and subjected to 16S rDNA sequence analysis. The BLAST analysis showed 99% nucleotide identity of the strains ID1.5. ID3.1, PD4.7 and PR3.1 with the16S rDNA sequence of L.lactis CV56 and L.lactis SL3 16S ribosomal RNA gene (GenBank: CP002365.1 and AY675242.1, respectively). The 16S rDNA sequence of strain ID8.5 showed 99% nucleotide identity with L. lactis KLDS 16S ribosomal RNA gene (GenBank: DQ340068.1). The analysis indicate that all the isolates are strains of L. lactis presenting close similarity with the subspecies lactis.
3.2. Sequencing of nisin genes and nisin controlled promoters
The PCR products obtained from the amplifications of genomic DNA of L. lactis ID1.5, ID3.1, ID8.5, PR3.1 and PD4.7, with primers specific to nisin structural gene were subjected to nucleotide sequencing. Results indicated that the nisin gene sequences in all strains were identical to that of nisin Z (GenBank: AB375441). Homology was also observed with nisin A (GenBank: HM219853), except for a C-to-A that resulted in an asparagine (AAT) residue at position 27 of the nisin peptide, instead of histidine (CAT), as the deduced amino acid sequences shown in Fig. 1. This indicated that the bacteriocins produced by all strains are variants of nisin A (Fig. 1).
41 sequence recorded for the promoter region encoding nisin Z (GenBank: Y13384.1) and the promoter region encoding nisin A (GenBank: Z18947.1). They have a consensus promoter characterized by -35 and -10 sequences that are spaced by an average of 17 nucleotides (Fig 2a). The promoter region upstream of structural nisZ gene contains a TCT direct repeat with 8-bp spacer region at the positions -39 to -26, upstream of the transcription start site (Fig. 2a). It also contains a second TCT-N8-TCT motif present upstream of structural gene nisZ at positions -107 to -94 (Fig. 2a). Analysis of the amplified fragment containing the promoter region of nisF of all strains have 100% identity to the sequence recorded for the promoter region encoding nisF presented in the nisin A regulon (GenBank: HM219853). The sequence/upstream of the nisF transcription start site included a TCT-N8-TCT motif present at the same position, -39 to -26, upstream of the transcription start site of nisZ. All promoter sequences nisF and nisZ were identical among L. lactis ID1.5, ID3.1, ID8.5, PR3.1 and PD4.7 (Fig. 2b).
3.3.Detection and curing of plasmids
Plasmids were found in all strains of L. lactis (Fig. 3). Thus, the plasmid curing was conducted to examine whether the genetic determinants for bacteriocin production in L. lactis ID1.5, ID3.1, ID8.5, PR3.1 and PD4.7 are located on plasmid. It was observed that the strains ID1.5, ID3.1, ID8.5, PR3.1 and PD4.7cured derivatives were able to produce bacteriocin after growth in the presence of ethidium bromide (10 μg mL-1), suggesting that the genes encoding the bacteriocin are located on the chromosome.
3.4.Purification and mass spectrometry analysis of bacteriocin
42 were directly analyzed by mass spectrometry. The molecular masses of the purified fractions from L. lactis ID1.5, ID3.1, ID8.5, PD4.7 and PR3.1 were 3330.567, 3330.514, 3329.985, 3329.561 and 3329.591 Da, respectively (Fig. 4), which are similar to the molecular mass of the nisin Z, 3330. 93 Da (Piper et al., 2010). Through the MALDI/MS analysis, the bacteriocins produced by all strains were identified as nisin Z.
3.5.Effect of carbon and nitrogen source on bacteriocin production
The bacteriocin production kinetics of L. lactis ID1.5, ID 3.1, ID8.5, PR3.1 and PD4.7 were studied under different conditions. Batch fermentation profiles of microbial growth and bacteriocin production are presented in Fig. 5 and Fig. 6. The strains started producing bacteriocins at early exponential phase, their activity were already detectable after 2 hours of growth. Exponential growth took place during a period of about 1 to 6 hours for all strains. Maximum cell density was reached after 4 and 6 or 7 hours in the medium supplemented with glucose and with other carbon sources, respectively. The activities increase concomitantly with an increase in the growth and reached its maximal activity at the stationary phase when the pH of the medium was decreased to 4.3. However, when the strains were cultivated in medium added with lactose the final culture pH was approximately 5.2 (Fig. 6A). A decrease on bacteriocin production was observed after 8 hours incubation for all strains, except to L. lactis ID3.1, when it was grown in medium supplemented with fructose, the bacteriocin activity remained constant (Fig. 5Bb).
43 In general, the strains presented different values of bacteriocin activities. Maximal bacteriocin activity was recorded by L. lactis ID1.5 and ID8.5, their activities were approximately 34000 AU mL-1, 125000 AU mL-1, 136000 AU mL-1, 226000 AU mL -1
in medium supplemented with glucose, fructose, lactose and sucrose, respectively, as sole carbon source (Fig. 5 and Fig. 6).
When L. lactis ID1.5, ID 3.1, ID8.5, PR3.1 and PD4.7 were cultivated in LAPTg medium that lacked peptone, tryptone and yeast extract and was added of different nitrogen sources ( at 35 g L-1), a range of the growth and bacteriocin activities were obtained (Table 2). The strains were capable of growth in medium with each nitrogen source separately, but the highest bacteriocin activities were found in cells grown in medium with tryptone or casein peptone. When meat extract was used as single nitrogen source, all the strains grew well but produced little bacteriocin (Table 2).
3.6.Effect of aeration, initial medium pH and incubation temperatures on bacteriocin production
All strains displayed similar behaviors in terms of cell growth and bacteriocin production at each of the tested temperatures. Cell growth occurred at temperatures ranging from 20 °C to 40 °C (Table 2), with highest levels of bacteriocin production recorded at 25 °C and 30 °C. However, all strains showed a decrease of approximately 28% of bacteriocin production at 40 °C.