BIODISTRIBUIÇÃO DO RADIOFÁRMACO PERTECNETATO DE
SÓDIO (Na
99mTcO
4) EM RATOS SUBMETIDOS À RESSECÇÃO
EXTENSA DO INTESTINO DELGADO
Tese apresentada ao Programa de
Pós-graduação em Ciências da Saúde do Centro de
Ciências da Saúde da Universidade Federal do
Rio Grande do Norte, para a obtenção do título
de Doutor em Ciências da Saúde.
BIODISTRIBUIÇÃO DO RADIOFÁRMACO PERTECNETATO DE
SÓDIO (Na
99mTcO
4) EM RATOS SUBMETIDOS À RESSECÇÃO
EXTENSA DO INTESTINO DELGADO
Tese apresentada ao Programa de
Pós-graduação em Ciências da Saúde do Centro de
Ciências da Saúde da Universidade Federal do
Rio Grande do Norte, para a obtenção do título
de Doutor em Ciências da Saúde.
Orientador: Prof. Dr. Aldo da Cunha Medeiros
Catalogação da Publicação na Fonte. UFRN / Biblioteca Setorial do CCS C431b Chacon, Damaso de Araújo.
Biodistribuição do pertecnetato de sódio (Na99mTcO4) em ratos submetidos à ressecção extensa de intestino delgado / Damaso de Araújo Chacon. – Natal, RN, 2007.
68 f. : il.
Orientador: Aldo da Cunha Medeiros.
Tese (doutorado) – Universidade Federal do Rio Grande do Norte. Centro de Ciências da Saúde. Programa de Pós-Graduação em Ciências da Saúde.
1. Síndrome do intestino curto - Tese. 2. Cirurgia - Tese. 3. Intestinos - Tese. 4. Pertecnatato Tc 99m de sódio – Tese. 5. Ratos Wistar – Experiência – Tese. I. Medeiros, Aldo da Cunha. II. Título.
PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS DA SAÚDE
Prof. Dr. Aldo da Cunha Medeiros
BIODISTRIBUIÇÃO DO RADIOFÁRMACO PERTECNETATO DE
SÓDIO (Na
99mTcO
4
) EM RATOS SUBMETIDOS À RESSECÇÃO
EXTENSA DO INTESTINO DELGADO
Banca Examinadora:
Prof. Dr. Aldo da Cunha Medeiros – UFRN - Presidente
Prof. Dr. Adenilson de Souza da Fonseca – UERJ
Prof. Drª. Maria Teresa Jansem de Almeida Catanho - UFPE
Prof. Dr. Eryvaldo Sócrates Tabosa do Egito - UFRN
Prof. Drª. Lúcia de Fátima Campos Pedrosa - UFRN
v Para Leila,
Gustavo e Paulo Eduardo
que acompanharam com compreensão
vi
Gostaria de agradecer a todas as pessoas que contribuiram para a
conclusão deste trabalho.
Primeiramente, ao Professor Doutor Aldo da Cunha Medeiros pelo
apreço e inestimável orientação, cujos ensinamentos foram pautados por
competência, respeito, amizade e sobretudo atenção. Seu entusiasmo pela
pesquisa contagia a todos que têm o privilégio de ser seus alunos. Seu perfil de
pesquisador marca compromissos com a ética e a verdade científica.
Ao Professor Doutor José Brandão Neto, pela perseverança de
implantar um Programa de Pós-graduação em Ciências da Saúde na UFRN,
sonho alentado há tempo por professores desta instituição. Sua competência,
dedicação e esforço permitiram ultrapassar as inúmeras dificuldades na
consolidação de uma pós-graduação deste porte.
Ao Programa de Pós-graduação em Ciências da Saúde – PPgCSa da
Universidade Federal do Rio Grande do Norte pelas condições oferecidas
durante o doutorado.
Ao Professor Doutor Mário Bernardo Filho (UERJ) pelo respaldo
científico na área do nosso estudo e pelas informações valiosas sempre que
solicitadas.
À Professora Doutora Maria Teresa Jansem de Almeida Catanho
(UFPE), pela disponibilidade e entusiasmo de nos iniciar nas primeiras noções
vii
sempre encontrava tempo para colaborar ao longo de todo o trabalho.
Agradeço a valiosa e criteriosa colaboração de Ítalo Medeiros Azevedo
sempre disponível nos experimentos, nas análises estatísticas e no manuseio
do contador gama.
Também devo agradecimentos ao Doutor Arthur Villarin Neto por
disponibilizar material indispensável à minha pesquisa e pelos conselhos
valiosos em medicina nuclear.
A bióloga Kércia Regina Santos Gomes Pereira e a biomédica Maria
Kádja Menezes Tôrres Açucena, pelo apoio prestado e competência no
preparo do material radioativo.
A Francisca Vieira de Almeida pela responsabilidade e dedicação no
trato dos animais.
Às funcionárias da Secretaria da Pós-graduação em Ciências da Saúde,
Danielli Queiroz Lopes, Patrícia de Sousa Campos e Leilane Aquino de Paiva
pela eficiência no atendimento.
Ao amigo Ronaldo Pinheiro Gomes Mafra que dedicou seu precioso
tempo na leitura e correção do português.
E finalmente a todos os amigos que comentaram o manuscrito de forma
viii
Dedicatória ... v
Agradecimentos ... vi
Listas... ix
Resumo...xiii
1 INTRODUÇÃO ... 1
2 REVISÃO DA LITERATURA ... 3
2.1. Medicina Nuclear ... 3
2.2. Síndrome do Intestino Curto ... 5
3 ANEXAÇÃO DOS ARTIGOS ... 8
3.1. ARTIGO I – Biodistribuição do radiofármaco pertecnetato de sódio (Na99mTcO4) em ratos submetidos a ressecção extensa de intestino delgado ... 8
3.2. ARTIGO II – Glucan and Glutamine Reduce Bacterial Translocation in Rats Subjected to Intestinal Ischemia – Reperfusion... 20
3.3. ARTIGO III - Efeitos das vitaminas A e C em anastomoses intestinais de ratos tratados com corticosteróide1... 32
3.4. ARTIGO IV - Achados da fundoscopia e alterações do pé diabético em pacientes do Hospital Universitário Onofre Lopes/UFRN1... 41
4 COMENTÁRIOS, CRÍTICAS E CONCLUSÕES ... 50
4.1. Identificação do Tema... 50
4.2. Comentário sobre a Metodologia ... 51
4.3. Méritos e Contribuições... 52
4.4. Metas Futuras ... 53
5 REFERÊNCIAS... 55 Abstract
ix
Figura 1 - Apresentação esquemática do gerador 99Mo/99mTc ... 04
Gráficos 1 a 14 - Box plots comparativos da biodistribuição de Tc99m para cada
x
xi
%ATI Percentagem de radioatividade
%ATI/g Percentagem de radioatividade por grama
11C Carbono com número de massa 11
CPM Contagem por minuto
99Cu Cobre com número de massa 99
DMSA Ácido dimercaptosuccínico
DP Desvio padrão
DTPA Ácido dietilenotriaminopentacético
EGF Fator de crecimento epidérmico
18F Flúor com número de massa 18
67Ga Gálio com número de massa 67
GH Hormônio do crescimento
GLP-2 Glucagon tipo peptídeo 2
HIDA Ácido imunodiacético hepático
123I Iodo com número de massa 123
124I Iodo com número de massa 124
131I Iodo com número de massa 131
IC Intestino curto
111 In Isótopo instável do índio com número de massa 111
KeV Quiloeletrovolt
MBq Megabequerel
MDP Ácido metilenodifosfônico
99Mo Molibdênio com número de massa 99
xii NaCl Cloreto de sódio
Na99mTcO4 Pertecnetato de sódio
15O Oxigênio com número de massa 15
PYP Pirofosfato
Sn+2 Íon estanoso
Tc Tecnécio
99Tc Tecnécio com número de massa 99
99mTc Tecnécio metaestável com número de massa 99
VC - 99mTc Veneno de Crótalus marcado com tecnécio
DMSA -99mTc Ácido dimercaptosuccínico marcado com tecnécio
E.coli -99mTc Escherichia coli marcada com tecnécio
SAH -99mTc Sôro de albumina humana marcado com tecnécio
MDP -99mTc Ácido metilenodifosfônico marcado com tecnécio 99mTcO4- Íon pertecnetato
201Tl Tálio com número de massa 201
xiii
A ressecção extensa do intestino delgado resulta na síndrome do intestino
curto com repercussão desabsortiva importante. Uma avaliação
morfo-funcional poderá ser necessária aos órgãos envolvidos no processo. O objetivo
desse estudo foi avaliar a biodistribuição do pertecnetato de sódio em órgãos
de ratos submetidos a ressecção extensa do intestino delgado, a capacidade
adaptativa da mucosa intestinal remanescente e o comportamento da curva
ponderal pós-operatória. Foram utilizados 21 ratos Wistar alocados
aleatoriamente em três grupos (n=7). O grupo tratado, denominado intestino
curto (IC), foi submetido a anestesia e ressecção ampla do intestino delgado, o
grupo controle (C) e o grupo sham que submeteu-se à leve manipulação
cirúrgica das alças intestinais. Após observação por trinta dias administrou-se,
em todos os grupos, 0,l mL de pertecnetato de sódio via plexo venoso orbital.
Decorridos trinta minutos os animais foram sacrificados com superdose de
anestésico para retirada de fragmentos do fígado, baço, pâncreas, estômago,
duodeno, intestino delgado, tireóide, pulmão, coração, rim, bexiga, músculo,
fêmur, e cérebro. As amostras foram levadas ao Contador Gama Automático
1470, WizardTM, Perkin-Elmer para contagem da radioatividade e posterior
cálculo do percentual de atividade radioativa por grama (%ATI/g) em cada
órgão. Segmentos de 3cm do jejuno foram retirados para análise histológica
da mucosa. Utilizou-se avaliação estatística paramétrica (ANOVA) e teste de
Tukey, considerando p<0,05 como significante. Não houve diferenças
significantes da %ATI/g nos órgãos dos grupos estudados. Verificou-se
acentuada redução inicial de peso, em seguida um aumento do peso dos
xiv
conclusão, a ressecção ampla do intestino delgado não alterou a
biodistribuição do radiofármaco nos órgãos avaliados entre os animais
estudados. A adaptação da espessura da mucosa pode ter contribuido para
reversão na perda de peso pós-operatória e para que a biodistribuição do
pertecnetato de sódio não fosse afetada pela intervenção cirúrgica. O trabalho
teve um caráter multidisciplinar com a participação de vários departamentos e
laboratórios, como Núcleo de Cirurgia, Departamento de Cirurgia, Laboratório
de Radiobiologia, Departamento de Patologia e Serviço de Medicina Nuclear,
atestando o caráter multidisciplinar da pesquisa.
No final do Século XIX, Becquerel e o casal Curie iniciaram estudos
sobre os efeitos biológicos das radiações. Em 1905 o rádio foi usado para
destruir células cancerosas da tireóide. A partir daí os avanços dos estudos
sobre a energia nuclear ocorreram nos mais diversos campos, na medicina, na
indústria, e na agricultura.1-3
Em 1937 o Tecnécio (99mTc) foi descoberto por Perrier e Segre,
tornando-se um dos isótopos mais usados para diagnóstico em medicina
nuclear. Nos anos setenta vários fármacos foram marcados com o 99mTc,
estabelecendo maior afinidade por determinados órgãos ou tecidos. O
Metilenodifosfonato de Sódio (MDP), tem sido usado para cintilografia óssea, o
Ácido Dimercaptosuccínico (DMSA), para estudo renal, o Ácido
Dietilenotriamino pentacético (DTPA), para estudo renal e cerebral, o Ácido
Iminodiacético Hepático e derivados (HIDA), para avaliação hepatobiliar.
Surgiram ainda, o Mercaptoacetiltriglicina (MAG3) com maior especificidade
para o diagnóstico dinâmico renal, o Sestamibi, para diagnóstico oncológico e
do miocárdio, assim como o Teboroxime e o Tetrofosmin para a avaliação da
perfusão cardíaca. Hoje, se dispõe de mais de trinta radiofármacos.1,4
Desde então, trabalhos experimentais foram publicados, especialmente
na área clínica, onde a marcação de hemácias com 99mTc merece destaque.5,6
Também foram identificadas várias interações medicamentosas tanto de
drogas naturais como sintéticas, que podem alterar a biodistribuição dos
radiofármacos.7-11 Holanda e colaboradores estudaram recentemente a
Um estudo realizado com extrato de Guava (Psidium guajava) descreveu
modificação na biodistribuição do 99mTc usandose hamáceas marcadas.13
Recentemente, outro trabalho demonstrou que o extrato de Ginkgo biloba
promovia diminuição da absorção do Na99mTcO4 no duodeno.14
Entretanto, trabalhos sobre a biodistribuição do Na99mTcO4 na área
cirúrgica ainda são incipientes. O presente estudo analisou o comportamento
do Na99mTcO4 em modelo animal, após uma intervenção cirúrgica abdominal de
grande porte, com a ressecção extensa do intestino delgado, que resultou na
síndrome do intestino curto.
Nestas condições, tornou-se relevante identificar a biodistribuição do
Na99mTcO4 em diversos órgãos que necessitem de uma avaliação
morfo-funcional pós-operatória através de exames de imagem da medicina nuclear.
Analisar a evolução ponderal dos animais no pós-operatório, bem como a
morfologia da mucosa do intestino delgado remanescente também constituíram
2 REVISÃO DA LITERATURA
2.1. Medicina Nuclear
A medicina nuclear utiliza cada vez mais radioisótopos que têm
afinidades para determinados órgãos ou tipos celulares.1-4 Esses radioisótopos
podem ser produzidos artificialmente em reatores nucleares, aceleradores de
partículas ou até mesmo em geradores portáteis.
São isótopos que sofrem decaimento, resultando em um elemento mais
estável com emissão de partículas alfa (α), beta (β-, β+), ou raios gama (γ). Este
fenômeno é conhecido como radioatividade e corresponde ao número de
desintegrações por segundo.2,15
O 99mTc, radioisótopo mais utilizado para diagnóstico em medicina
nuclear, destaca-se dos demais por apresentar características próximas de um
radioisótopo ideal, com meia vida de 6 horas, emissão de radiação γ, pouco
ionizante e de fácil captação por detector externo.3 (Tabela 1)
Tabela 1 - Radioisótopos para diagóstico
Radioisótopos Meia-vida Decaimento Energia (KeV) γ Abundância da Emissão γ (%)
99mTc 6 h
γ 140 89
131I 193 h β-,
γ 364 81
123I 13 h
γ 159 83
67Ga
78 h γ 93,185,300,394 37,20,17,5
111
In 67 h γ 171,245 90,94
201
Tl 73 h γ 135,167 3,2
11
C 20,4 min β+ 511 99,8
13
N 10 min β+ 511 100
15
O 2,07min β+ 511 99,9
18
F 110 min β+ 511 96,9
124
I 4,2 dias β+ 511 25
64
Cu 13 h β+ 511 38
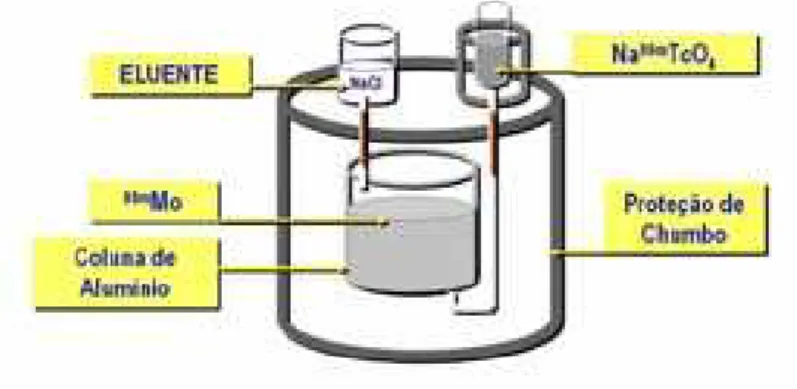
É obtido através do gerador 99Mo/99mTc composto por uma coluna
cromatográfica de óxido de alumínio (Al2O3), onde é depositado o molibdênio
que decai por emissão de partículas β-, formando os íons molibdato (99MoO4-2)
e pertecnetato (99mTcO4-). O 99MoO4-2 possui afinidade pelo alumínio.
Usando-se um eluente de cloreto de sódio a 0,9% (NaCl), o 99mTcO4- é separado na
forma de pertecnetato de sódio (Na99mTcO4).15 (Figura 1)
Figura 1 Apresentação esquemática do gerador 99Mo/99mTc.
Este pode ser utilizado em aplicações clínicas ou servir para preparar
radiofármacos mais complexos, marcados com substâncias químicas com
maior afinidade por determinados órgãos. Este procedimento requer a redução
de sua valência +7 através de agentes químicos como o íon estanoso.2
O Na99mTcO4 tem maior afinidade por glândulas salivares, tireóide,
estômago e rins. Liga-se inicialmente às proteínas plasmáticas com eliminação,
por via renal, de 30% nas primeiras 24 horas.2
Sua biodistribuição é estudada por diversas pesquisas básicas com
drogas naturais ou sintéticas.5,8,9
Um trabalho recente realizado com o VC-99mTc representou importante
com o extrato da erva ”Uncaria tomentosa” confirmou que em fitoterapia
continua-se a descobrir interações medicamentosas e biodistribuição de
radiofármacos.18
Na área cirúrgica, não há na literatura relato de pesquisas sobre
repercussão de intervenções cirúrgicas de grande porte na biodistribuição do
Na99mTcO4 para órgãos e tecidos.Um trabalho Investigou, em ratos, a
translocação bacteriana em modelo experimental de icterícia obstrutiva,
empregando Escherichia coli marcada com 99mTc (E.Coli - 99mTc). O estudo não
mostrou translocação da E.Coli - 99mTc.19
Ansari e colaboradores utilizaram a cintilografia com DMSA - 99mTc para
avaliar a função renal e consideraram satisfatória sua indicação em
procedimento laparoscópio de rim atrófico.20 Um estudo com esplenectomia total
em que usou-se a E. coli - 99mTc observou falha no sistema mononuclear
fagocitário, indicando a necessidade de técnicas cirúrgicas alternativas para
preservar o tecido esplênico e garantir a fagocitose bacteriana.21
2.2. Síndrome do Intestino Curto
A síndrome do intestino curto decorre por ressecção extensa do
intestino delgado e resulta na incapacidade da manutenção da absorção dos
nutrientes.22-25 Sua etiologia está associada a uma redução maior que 70% do
intestino delgado imposta por condições congênitas ou adquiridas, tais como:
isquemia mesentérica, volvo, trauma, doença de Crohn, e outras.26,27
desnutrição, perda de peso, anemia, hipersecreção gástrica, e litíases biliar e
renal.27
A adaptação intestinal a essa situação cirúrgica inclui um processo
complexo com a atuação de vários fatores, tendo como foco o aumento da
superfície absortiva por hiperplasia da mucosa intestinal remanescente.28-30
Técnicas cirúrgicas são utilizadas na tentativa de melhor adequar esse
segmento intestinal. Destacam-se os procedimentos para retardar o trânsito
intestinal com construção de válvulas, segmento intestinal interposto, construção
de segmentos anisoperistálticos e marcapasso intestinal reverso. Como métodos
que visam aumentar a superfície absortiva tem-se a enteroplastia longitudinal e
alongamento intestinal. Os transplantes do intestino delgado ou combinado de
fígado e intestino delgado estão reservados para pacientes com complicações
graves de falência intestinal.31-33
Nos trabalhos que envolvem a síndrome do intestino curto destacam-se
estudos sobre os mecanismos de adaptação do intestino, onde o aumento da
espessura e extensão da parede intestinal constituem fatores decisivos na
capacidade absortiva do segmento remanescente34. Outros, avaliaram as
alterações morfológicas da mucosa e identificaram mediadores da adaptação
intestinal, com destaque para a secreção pancreatobiliar e os hormônios.35-38
O hormônio do crescimento (GH), o glucagon tipo peptídeo-2 (GLP-2), e o fator
de crescimento epidérmico (EGF), têm sido identificados como os principais
promotores da adaptação.39-41 A Glutamina também é usada para minimizar o
dano da redução do intestino, promovendo a reabilitação da absorção
intestinal.42 Um modelo em ratos chama a atenção para o efeito da
e a morfometria da mucosa intestinal. Sua conclusão é que a dieta oral
acrescida de glutamina não contribuiu para reduzir a perda de peso dos
animais, mas foi importante no efeito da adaptação intestinal.43 Martin et al
demonstraram que o GLP-2 associado à nutrição parenteral total estimulou a
adaptação do intestino dos ratos submetidos à ressecção intestinal, e
observaram aumento das criptas de sua mucosa.44
O presente trabalho constitui o primeiro estudo sobre biodistribuição do
Na99mTcO4 em órgãos de ratos submetidos à ressecção extensa do intestino
delgado. Trata-se de um modelo experimental que pretendeu simular a
síndrome do intestino curto, problema que resulta em intensa repercussão
metabólica em diversos órgãos. No pós-operatório de pacientes portadores da
síndrome podem ser necessários exames complementares para
esclarecimento diagnóstico de complicações dela decorrentes ou de outras
doenças eventuais.A justificativa do trabalho prende-se ao fato de que,
havendo alterações na biodistribuição de radiofármacos para determinados
órgãos, resultados falso-positivos ou falso-negativos podem ocorrer, obrigando
3 ANEXAÇÃO DOS ARTIGOS
3.1. ARTIGO I – Biodistribuição do radiofármaco pertecnetato de sódio(Na99mTcO4) em ratos submetidos a ressecção extensa de intestino delgado
Aceito para publicação na Acta Cirúrgica Brasileira em 15.04.07
Biodistribution of the radiophamarceutical sodium pertechnetate
(Na
99mTcO
4) after massive small bowel resection in rats
1Dâmaso de Araújo Chacon2, Irami Araújo-Filho2, Arthur Villarim-Neto2, Amália
Cínthia Meneses Rêgo3, Ítalo Medeiros Azevedo4, Mário Bernardo-Filho5, José
Brandão-Neto6, Aldo Cunha Medeiros6
1. Research performed at Nucleus for Experimental Surgery, Federal University of Rio Grande do Norte (UFRN), Brazil.
2. Fellow PhD degree, Postgraduate Program in Health Sciences, UFRN, Brazil. 3. Graduate Student, Scientific Initiation Program, UFRN, Brazil.
4. Statistician, Department of Surgery, UFRN, Brazil.
5. PhD, Chairman, Biophysics and Biometry Department, UERJ, Brazil
6. PhD, Full Professor, Postgraduate Program in Health Sciences, UFRN, Brazil.
ABSTRACT
experimentally-produced short bowel syndrome, an adaptive response by the intestinal mucosa reduced weight loss. The biodistribution of Na99mTcO4 was not affected by massive intestinal resection, suggesting that short bowel syndrome is not the cause of misleading interpretation, if an examination using this radiopharmaceutical is indicated.
Key words: Short Bowel Syndrome. Sodium Pertechnetate Tc 99m. Pharmacokinetics.
Rats.
RESUMO
Objetivo: Avaliar em modelo animal com ressecção extensa do intestino delgado a
biodistribuição de pertecnetato de sódio (Na99mTcO4) em órgãos e tecidos, a evolução ponderal e a morfometria da mucosa do intestino delgado remanescente. Métodos: Vinte e um ratos Wistar foram aleatoriamente divididos em três grupos de sete animais cada. O grupo intestino curto (IC) foi submetido a ressecção extensa do intestino delgado, o grupo controle (C) não foi operado e o grupo sham foi submetido a leve manipulação cirúrgica das alças intestinais.Todos foram pesados semanalmente. No 30º dia pós-operatório foi administrado 0,l mL de Na99mTcO4 aos animais dos três grupos, IV no plexo orbital, com atividade radioativa média de 0,66MBq. Após 30 minutos os ratos foram mortos e retirados fragmentos do fígado, baço, pâncreas, estomago, duodeno, intestino delgado, tireóide, pulmão, coração, rim, bexiga, músculo, fêmur, e cérebro. As amostras foram lavadas com solução de NaCl 0,9%.A radioatividade foi contada peloContador Gama 1470, WizardTM Perkin-Elmer e calculado o percentual de atividade radioativa por grama (%ATI/g) de cada órgão. Biópsias do jejuno foram submetidas a análise da espessura da mucosa (coloração HE). Utilizou-se avaliação estatística paramétrica (ANOVA) e teste de Tukey, considerando p<0,05 como significante. Resultados: Não houve diferenças significantes da %ATI/g nos órgãos dos grupos estudados (p>0,05). Verificou-se acentuada redução inicial de peso, em seguida um aumento do peso dos animais tratados a partir da segunda semana de observação e aumento da espessura da mucosa jejunal do grupo IC, comparado com os demais.
Conclusão: Em ratos com síndrome do intestino curto, uma adaptação na espessura da
mucosa contribuiu para reversão na perda de peso inicial e para que a biodistribuição do Na99mTcO4 não fosse afetada pela ressecção extensa do intestino, sugerindo que o intestino curto não é causa de interpretações duvidosas, quando exame cintilográfico com este radiofármaco estiver indicado.
Descritores: Síndrome do Intestino Curto. Pertecnetato Tc99m de Sódio.
Farmacocinética. Ratos.
Introduction
Radioisotopes are used in nuclear medicine for diagnostic and therapeutic purposes. The labelling capacity of these isotopes for the plasmatic proteins is well known, and their bioavailability and pharmacokinetics can be modified by drugs and diseases1,2.
intestines and other organs3. It is rapidly eliminated by the urine and when incorporated to specific substances, produces organ images of different densities and functions4. Experimental studies carried out with the labelling of red blood cells with 99m
Tc identified important biological effects, in addtion to alterations in the labelling process5,7. Gomes et al carried out a study with mitomicin-C that described alterations in the labelling of red blood cells6. The literature reports that several natural drugs reduce the efficiency of labelling red blood cells with 99mTc7. An experimental study with Vincristin, used in oncology protocols, showed an interaction of this drug with 99m
Tc in several organs8.
However, there are no reports of research studying the biodistribution of radiopharmaceuticals after surgical procedures. In the present study, we used an experimental model of massive resection of the small intestine, characterizing the short bowel syndrome, which results in unsuitable water and nutrient absorption, causing malnutrition9,10.
In spite of the short bowel syndrome, the intestine can be adapted through physiologic, cellular and moleculares mechanisms9. In some patients, dilation and lengthening of the remnant small intestine occur as a phenomenon of functional adaptation. Surgical techniques have been reported that attempt to lengthen this intestinal segment. Such procedures are complex and frequently ineffective, and call for assessments of their efficacy11. Recently, new therapeutic methods, such as isolated small intestine transplantation or combined with liver transplantation, have been an alternative for cases of hepatic failure due to total parenteral nutrition in the treatment of short bowel syndrome12.
Given the antiabsorptive effect of the operation, with great repercussions on the metabolism, radioisotope images may be necessary in the postoperative, in order to control the series of pathological conditions resulting from short bowel syndrome. Scintigraphy can be used in the postoperative of intestinal resections to assess the morphology and metabolism of several organs. Under these conditions, it becomes relevant to study the biodistritubion of Na99mTc04 in specific organs and tissues. Therefore, the present paper aims to study, in an animal model of massive resection of the small intestine, the biodistribution of Na99mTcO4 in several organs by means of radiation counting in organs and tissues in the postoperative period. We also evaluated the ponderal evolution of the animals after the operation, as well as the mucosal morphometry of the remnant small intestine.
Methods
(20mg/Kg intraperitoneal) and ketamine (20mg/Kg intramuscular); they were operated on under sterile conditions.
A 3cm midline laparotomy was performed and intestinal transections were done 5 cm above the ileocecal junction and 5 cm from the duodenojejunal transition. With the aid of a surgical microscope (DF Vasconcelos, São Paulo, Brazil), interrupted sutures of 6-0 prolene (Ethicon®, Brazil) were used for bowel anastomosis. The animals typically have a small bowel length of 100 cm, and accordingly, residual length was 5 cm of jejunum and 5 cm of ileum (10 cm), corresponding to 90% of resection. After surgery, the abdomen was closed with interrupted sutures of 4-0 nylon suture (Ethicon®). The animals were allowed water immediately after surgery and food on the second postoperative day. The sham rats were subjected to a 3 cm medium laparotomy and mild manipulation of the small bowel. The rats were weighed weekly with a digital scale (Filizola® São Paulo, Brazil) and observed for 30 days.
On the 30th day all the animals were anaesthetized again, and injected with 0.lmL of Na99mTcO4 in the venous orbital plexus, corresponding to radioactive activity of 0.66MBq. After 30 minutes, the animals were killed by lethal dose of anesthetic. Samples of the liver, spleen, pancreas, stomach, duodenum, small intestine, thyroid, lung, heart, kidney, bladder, muscle, femur and brain were harvested. The samples were washed in 0.9% NaCl , weighed on a high-precision digital scale (Bel-Mark 160-II Itália®) and subjected to radioactivity detection using a 1470 WizardTM Gamma Counter- Perkin-Elmer, with automatic correction of radiation decline. The percentage of radioactive activity/g (%ATI/g) of each organ was calculated by dividing the activity/g of the tissue by the total activity administered to each animal.
Samples with 2cm of jejunum were harvested 2 cm below the anastomosis. After washed in 0.9% saline, the excised tissues were fixed in 10 % buffered formalin for 48 h, dehydrated and embedded in paraffin. Sections cut at 5µm thickness were stained with hematoxilyn and eosin and morphology was assessed by an observer, who was unaware of the tissue origin. For the morphometric study of intestinal mucosa, Media Cybernetics – LP, USA, Image Pro-Plus software was used with an Olimpus BX-50 microscope fitted with a digital (Samsung®) video camera. The video camera transferred the image from the microscope to the computer screen. The measurement of the mucosal thickness was delimited with a computer mouse from the apex of the villus to the muscularis mucosae and was expressed in microns (µm). The analysis was made under 40x magnification using specimens in which the villi and the crypts were perpendicular to the muscularis mucosae.
For the analysis of the different data related to postsurgical weight loss, to the measurements of total mucosal thickness, and to the biodistribution of sodium pertechnetate of the different groups, parametric variance (ANOVA) was used. For the multiple comparisons, the Tukey test was used. A significance level of 5% (p<0.05) was established.
Results
radioactive activity (%ATI/g) had very similar values among the groups, without significant differences (Table 1).
TABLE 1 – Biodistribution of Na99mTcO4 in the organs of the respective groups
%ATI/g Organs
SB C Sham
ANOVA (1)
Liver 0.35 ± 0.089 0.36 ± 0.079 0.39 ± 0.113 0.794400
Spleen 0.22 ± 0.090 0.18 ± 0.031 0.19 ± 0.044 0.565470
Estomach 2.58 ± 0.730 2.72 ± 0.614 4.11 ± 1.793 0.116180
Small bowel 0.28 ± 0.107 0.20 ± 0.052 0.28 ± 0.130 0.690700
Duodenum 1.73 ± 1.814 0.41 ± 0.062 1.13 ± 1.719 0.378723
Pancreas 0.16 ± 0.063 0.14 ± 0.055 0.18 ± 0.125 0.811183
Kidney 0.41 ± 0.086 0.42 ± 0.082 0.36 ± 0.187 0.738872
Heart 0.17 ± 0.075 0.27 ± 0.057 0.17 ± 0.084 0.076831
Lung 0.35 ± 0.105 0.38 ± 0.125 0.31 ± 0.058 0.581337
Thyroid 5.35 ± 1.979 3.71 ± 1.256 3.80 ± 1.058 0.187603
Bladder 0.39 ± 0.114 0.33 ± 0.109 0.27 ± 0.139 0.309546
Muscle 0.07 ± 0.028 0.06 ± 0.019 0.05 ± 0.035 0.570391
Femur 0.16 ± 0.055 0.14 ± 0.036 0.15 ± 0.050 0.760950
Brain 0.02 ± 0.013 0.01 ± 0.003 0.03 ± 0.027 0.482193
Mean ± Standard deviation
(1) P- from analysis of variance (ANOVA).
The results of the test did not show statistically significant differences (p>0.05), for all the variables. %ATI/g, percent radioacvivity per gram of tissue.
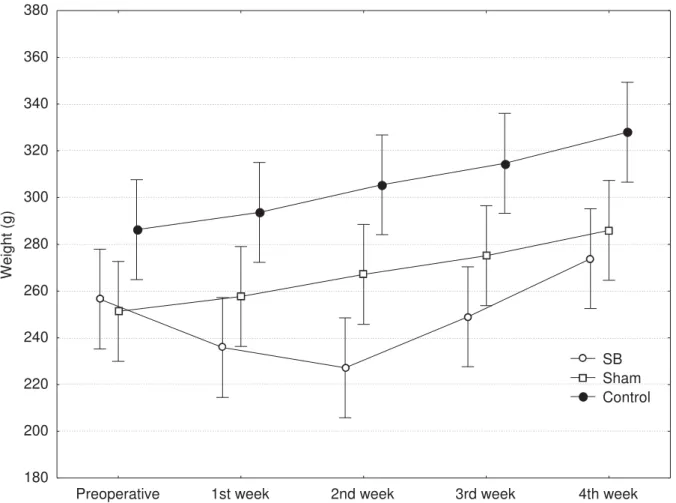
Preoperative 1st week 2nd week 3rd week 4th week 180
200 220 240 260 280 300 320 340 360 380
Wei
ght (g)
SB Sham Control
FIGURE 1 – Mean weight of rats in each group and postoperative period
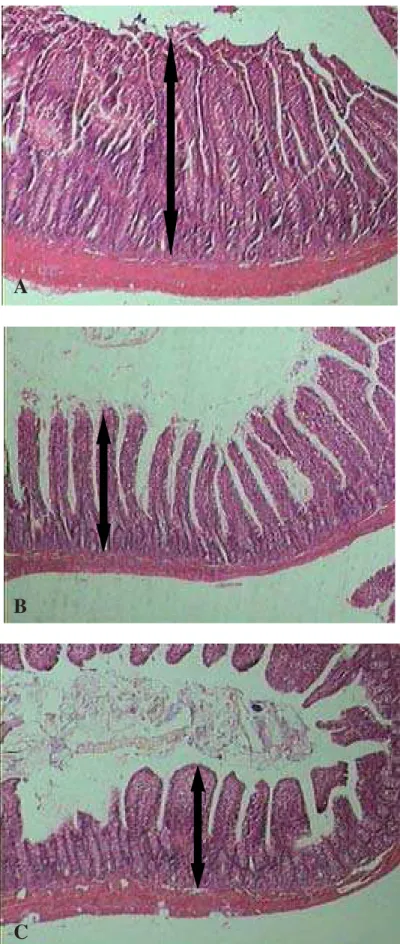
The presence of an increase in intestinal mucosa thickness was detected in all IC rats, when compared to C and SHAM rats until the end of the observation period, as seen in Table 2 (p<0.05) and Figure 2.
TABLE 2 – Jejunal mucosa thickness of rats and their respective groups
Groups Variable
SB C Sham
ANOVA (1)
Mucosal thickness in µm(2)
334.34 ± 25.9ab 194.40 ± 39.0a 194.22 ± 33.2b 0.0000
Values expressed in Mean ± Standard deviation (1) p-value of analysis of variance (ANOVA).
(2) Groups identified with the same letter differ significantly at a level of 5% (Tukey test).
FIGURE 2 - Small intestine morphology and mucosal cell proliferation on day 30 in short bowel (A) control (B) and sham (C) animals. Massive intestinal resection (A) induced significant increases in total mucosal thickness (arrows), when compared to control and sham animals (B, C) (see Table 2; p<0.05). HE, 40x
A
B
Discussion
The alterations in the biodistribution of Na99mTcO4 in organs and tissues are very well identified in several studies that used no experimental surgical models 1,,6,,8,13,14. However, in the postoperative of major surgical procedures, there are no reports concerning the biodistribution of radiopharmaceuticals. The present experimental model of the short bowel syndrome in rats submitted to massive resection of the small intestine was used to determine the biodistribution profile of Na99mTcO4 in several organs and tissues.
Intestinal failure is characterized by malnutrition and/or dehydration as a result of the inadequate digestion and absorption of nutrients. The most common cause of intestinal failure is short bowel syndrome, which occurs when the functional small bowel mass is reduced below the level necessary for adequate nutrient and water absorption. This condition frequently results from a massive resection of the small bowel. Following resection, the intestine is capable of adaptating in response to enteral nutrients as well as other trophic stimulation. Rodents are commonly used in well-characterized models to assess the process of intestinal adaptation16. Following small bowel resection in the rat, the remnant intestinal mucosa undergoes compensatory alterations in an attempt to restore normal absorptive capacity. Morphologic and functional changes include increases in mucosal length, enterocyte proliferation, as well as increased electrolyte, glucose and amino acid uptake16,17.
In humans, the alterations of intestinal absorption due to massive resection of the small intestine usually cause significant weight loss15. However, in rodents, there is a rapid adaptation of the intestinal mucous membrane, which minimizes weight loss16. These mechanisms of intestinal adaptation take place at physiologic, cellular and molecular levels and they do not correspond to what occurs in the human intestine 17. Nutrients, electrolytes, hormones, cytokines and other elements take part in the process, which involves mainly the intestinal mucous membrane. The process begins with apoptosis and continues with an increase in epithelial cells, vilosities and mucosal crypts, and a consequent remodeling of their architecture. Functionally, this allows for increased substance transport through the intestinal mucosa17.
In the present study a significant decrease was observed in the weights of rats submitted to massive intestinal resection, in the immediate postoperative period, and weight recovery begining at the end of the second week. These data coincide with a classic study on the subject, where morphological and functional adaptations of the jejunum were observed between the first and second postoperative weeks18. This phenomenon was also shown in the morphometry of the jejunal mucous membrane of the animals subjected to massive resection of the present study. Therefore, the mucous membrane hyperplasia observed in the jejunal mucosa of the SB rats of the present experiment, likely contributed to the rapid weight recovery of the animals, starting from the second postoperative week. Welters et al verified that intestinal function recovery begins with the hyperplasia of the intestinal mucosa and that absorptive function depends on the maturity of the enterocytes, a fundamental factor for nutrient metabolism19.
malnutrition-inducing diets, Passos et al20 showed that malnutrition affected the biodistribution of Na99mTcO4 in different organs such as the thyroid, brain, stomach and heart. In their study, the intestine was not surgically manipulated.
Studies in animals have been investigating substances that regulate the absorptive function of the intestine21. These mechanisms are mediated by multiple factors, including enteral or parenteral nutrition, hormones and growth factors22. Recently, studies on the use of the human growth hormone (GH), the epidermal growth factor (EGF) and the glucagons-like peptide-2 (GLP-2), produced in the L-cells of the small intestine, have confirmed them as agents that increase intestinal adaptation after massive resection23. The study suggests that, whereas GLP-2 is important in controlling adaptation, there are spatial or regional systems in place that use varying pathways. The significant increase in nutrient-stimulated GLP-2 secretion suggests that GLP-2 is involved not only in the initiation, but also in maintaining the ongoing adaptive process. The increases in mucosal proliferation that are temporally associated with a maintained GLP-2 release, suggest that GLP-2 is important in initiating and maintaining the small intestine’s adaptive response to resection24.
Curtis et al studied rats submitted to massive resection of the small intestine using marker 51mCrl3mC and protein, and observed the animals for one week. They concluded that the rats had no alteration in absorption and digestion time when compared to the treated group and the control; this demonstrated the fast physiological adaptation of the animals25. A growing number of tissue factors are being investigated for having great potential in promoting intestinal adaptation in animals and humans with short bowel syndrome, in the hope of obtaining effective therapies for the syndrome in the future23,26.
In summary, massive intestinal resection in the current study did not interfere significantly with the biodistribution of the radiopharmaceutical Na99mTcO4 in the organs studied. Certainly the mucosal hyperplasia of the remnant intestine was a preponderant factor for the quick weight loss reversal of the animals, and consequent preservation of their healthy metabolism.
The present study does not allow us to comment on the mechanisms by which intestinal resection results in the stimulation of trophic effects and mucosal adaptation, allowing normal biodistribution of Na99mTcO4 in rats. Identifying factors that may enhance the process of intestinal adaptation is an exciting area of research with important potential clinical applications. This area will require further studies.
Conclusion
In rats with experimentally-induced short bowel syndrome, an adaptive response by the intestinal mucosa reduced weight loss. The biodistribution of Na99mTcO4 was not affected by massive intestinal resection, suggesting that short bowel syndrome is not the cause of misleading scintigraphy interpretation when an examination with this radiopharmaceutical is indicated.
References
2. Holanda CMCX, Leite RCH, Catanho MTJ, Souza GML, Bernardo-Filho M The effect of glucantime™ on the labeling of blood constituents with technetium-99m. Acta Cir Bras. 2005; 20 (suppl.1):126-30.
3. Kuni CC. Manual of Nuclear Medicine Imaging. New York: Thieme Medical Publishers; 1997.
4. Castro LF, Silva ATA, Chung MC, Ferreira AG, Ferreira EI. Biofosfatos (BFs) como transportadores osteotrópicos no planejamento de fármacos dirigidos. Quim Nova. 2004;27:456-60.
5. Gomes ML, Oliveira MBN, Bernardo-Filho M. Drug interaction with radiopharmaceuticals: effect on the labeling of red blood cells with technetium-99m and on the bioavailability of radiopharmaceuticals. Braz Arch Biol Technol. 2002; 45: 143-9.
6. Gomes ML, Mattos MMD, Freitas RS, Diré GF, Lima EAC, Souza SMS, Bernardo-Filho M. Evaluation of the effect of mitomicin-C on the bioavailability of technetium-99m-labelled sodium pyrophosphate in mice. Cell Mol Biol. 2002; 48:757-9.
7. Oliveira JF, Avila AS, Braga ACS, de Oliveira MB, Boasquevisque EM, Jales RL, Cardoso VN, Bernardo-Filho. Effect of extract of medicinal plants on the labeling of blood elements with Technetium-99m and the morphology of red blood cells: a study with Paullinia cupana. Fitot. 2002; 73:305-12.
8. Mattos D M M, Gomes M L, Freitas R S, Boasquevisque EM, Cardoso VN, Paula EF, Bernardo-Filho M. The effect of vincristine on the biodistribution of technetium-99m-DTPA, GHA and DMSA in balb/c female mice. J Nucl Med Technol. 2000; 28:271-4.
9. Zhou X, Li YX, Li N,Li JS. Effect of bowel rehabilitative therapy on structural adaptation of remnant small intestine: animal experiment.World J Gastroenterol. 2001;7:66-73.
10. Tannuri U. Síndrome do intestino curto na criança - tratamento com nutrição parenteral domiciliar. Rev Assoc Med Bras. 2004;50:330-7.
11. Bianchi A. Intestinal loop lengthening: a technique for increasing small intestinal length. J Pediatr Surg. 1980; 15:145-51.
12. Iyer K, Kaufman S, Sudan D, Horslen S, Shaw B, Fox I, Langnas A. Long-term results of intestinal transplantation for pseudo-obstruction in children. J Pediatr Surg. 2001; 36:174-7.
14. Holanda CMCX, Leite CH, Nunes RAS, Oliveira HA, Catanho MTJ, Souza GM, Bernardo-Filho M. Effect of antimalarial drugs on the bioavailability of the methylenediphosphonic acid labeled with technetium99m (99mTc-MDP) in Wistar rats. Braz Arch Biol Technol. 2006; 49:207-14.
15. Goulet O, Baglin-Gobet S, Talbotec C, Fourcade L, Colomb V, Sauvat F, Jais JP, Michel JL, Jan D, Ricour C. Outcome and long-term growth after extensive small bowel resection in the neonatal period: a survey of 87 children. Eur J Pediatr Surg. 2005; 15:95-101.
16. O'Connor TP, Lam MM, Diamond J. Magnitude of functional adaptation after intestinal resection. Am J Physiol Regul Integr Compar Physiol. 1999; 276: 1265-75.
17. Drozdowski L, Thomson ABR. Intestinal mucosal adaptation. World J Gastroenterol. 2006; 12: 4614-27.
18. Hanson WR, Osborne JW, Sharp JG. Compensation by the residual intestine after intestinal resection in the rat. II. Influence of postoperative time interval. Gastroenterology. 1977; 72:701-5.
19. Welters CFM, Dejong CHC, Deutz NEP, Heineman E. Intestinal adaptation in short bowel syndrome. ANZ J Surg. 2002; 72: 229–36.
20. Passos MC, Ramos CF, Bernardo-Filho M, Mattos DMM, Moura EG. The effect of protein and energy restriction on the biodistribution of Na99TcmO4 in Wistar rats. Nucl Med Commun. 2000;21:1069-62.
21. DiBaise JK, Young RJ, Vanderhoof JA. Intestinal rehabilitation and the short bowel syndrome: Part 2. Am J Gastroenterol. 2004;99:1823–32.
22. Spadoni JM, Aguilar-Nascimento JE, Silva MHGG, Spadoni-Neto B, Costa PATFB, Aléssio DMT. Effects of the combined use of glutamine and growth hormone in the intestinal adaptation after massive resection of the small bowel in rats. Acta Cir Bras. 2005; 20:382-9.
23. Cisler JJ, Buchman AL. Intestinal adaptation in short bowel syndrome. J Invest Med. 2005; 53:402-13.
25. Curtis KJ, Sleisenger MH, Kim YS. Protein digestion and absorption after massive small bowel resection. Dig Dis Sci. 1984; 29:834-40.
26. Drucker D, Ehrlich P, Asa SL, Brubaker P. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996; 93:7911–6.
Conflict of interest: none Financial source: CNPq
Correspondence:
Dâmaso de Araújo Chacon
3.2. ARTIGO II – Glucan and Glutamine Reduce Bacterial Translocation in Rats Subjected to Intestinal Ischemia – Reperfusion.
Publicado no Journal of Investigative Surgery 2006; 19:39 – 46.
GLUCAN AND GLUTAMINE REDUCE BACTERIAL TRANSLOCATION IN RATS SUBJECTED TO INTESTINAL ISCHEMIA-REPERFUSION
Aldo Cunha Medeiros , PhD* ; Dâmaso Araújo Chacon, MD ; Valéria Soraya Farias Sales, PhD ; Eryvaldo Sócrates Tabosa Egito, PhD ; José Brandão-Neto, PhD; Laíza Araújo Mohana Pinheiro; Mariana Rego Carvalho.
Postgraduate Program in Health Sciences – Federal University of Rio Grande do Norte, Brazil. Av. Gustavo Cordeiro de Farias, s/n – 59010-180 - Natal/RN, Brazil
Corresponding author:
Dr. ALDO CUNHA MEDEIROS
Av. Miguel Alcides Araújo 1889 Natal-RN 59078-270 Brazil Fax: +55-84-2176075; e-mail: aldo@ufrnet.br
Abstract
Intestinal ischemia/reperfusion (I/R) may induce bacterial translocation (BT). Glutamine (GLN)-enriched nutrition decreases BT. However, little is known about the effect of glucan (GL) in BT. This study investigated the combined effect of GL/GLN on BT, intestinal damage and portal blood cytokines in animals under I/R. Four groups of 10 rats each were subjected to 60 min of intestinal ischemia and 120 min of reperfusion. Control group (1) received only rat chow/water, group 2 received glutamine via gavage, group 3 received subcutaneuos soluble 1-3-(D)-glucan, and group 4 received GL+GLN, respectively. A fifth sham group (5) served as a normal control. Bacterial cultures of ileum, mesenteric lymph nodes (MLN), liver and lung biopsies, histological changes of ileum and serum cytokines variables were examined after I/R. Data were analyzed by ANOVA and Newman-Keuls test. Results showed that GLN, GL and GL/GLN significantly reduced BT to MLN, liver and lung. BT was more attenuated after GL treatment than GLN (p<0.05). Rats treated with both GL and GLN exhibited lower bacterial colony counts than the ones treated only with GLN or GL. Severe mucosal damage on histological findings was shown in group 1, but these findings were significantly ameliorated p<0.05) in groups 3 and 4. TNF-α and IL-6 levels in portal serum were significantly reduced and IL-10 was increased by GL and GLN treatment. Conclusions: the use of GL was more effective than GLN in reducing BT, intestinal damage and cytokine levels after I/R. Additionally, the combination of GL and GLN improved results.
Introduction
Bacterial translocation (BT) is the passage of viable indigenous bacteria from the gastrointestinal tract to normally sterile extraintestinal sites, such as the mesenteric lymph nodes (MLN), liver, lungs and other tissues[1]. BT is characterized by the appearance of enteric bacteria in the MLN, liver, and spleen in several conditions, including burns, endotoxemia, hemorrhagic shock and intestinal ischemia/reperfusion (I/R) [2,3]. I/R injury of the small intestine induces marked changes in the mucosa [4]; therefore, impairment of the mucosal barrier function leads to increased BT and endotoxemia [5].
Glucans are a heterogeneous group of glucose polymers found in the cell walls of plants and fungi. They are natural biological response modifiers and their effects are numerous, including anti-tumor response, antibacterial and antiviral activity, hematopoiesis stimulation and wound healing [6,7].
Soluble (1,3)-D-glucan has been demonstrated to be active against infection and to prevent shock in rats and mice [8,9]. Clinical studies suggest that its administration to trauma/surgical patients not only stimulates conversion from leukocyte anergy, but also decreases septic complications and improves the survival rate [10]. However, little is known about the effect of soluble (1,3)-D-glucan on BT.
On the other hand, it has been shown that the supplementation of enteral and parenteral feeds with glutamine (GLN) is able to decrease the frequency of pneumonia, sepsis, and bacteremia in trauma patients [11], to improve T-cell function [12] and to enhance the bactericidal function of neutrophils [13]. Such GLN supplementation preserves the intestinal morphology and function, and may reduce BT as well [14].
The purpose of the present investigation was to study the effect of a soluble (1,3)-D-glucan alone, or in combination with GLN, on BT, gut damage and portal blood cytokines in rats with intestinal I/R.
Materials and Methods
Animals
Experimental design
The rats were randomly assigned to 5 groups (n=10 in each group) as described below. The control group (nº 1) received only chow and water; group 2 received 1.5mg/Kg/day of 5% glutamine (Glutamin, Support, Brazil) via metal
tube gavage every morning; Group 3 received subcutaneous injection of 2 mg/Kg of glucan (Immunoglucan, Hebron, Brazil); and Group 4 received 2
mg/Kg of GL and 1.5mg/Kg/day of 5% GLN. These treatments were administered for 5 days before surgery. Group 5 (sham) served as a normal control and the rats received only chow and water.
Animals were fasted 12 hr before the experiment and anesthetized with an intramuscular injection of 100mg of ketamine/Kg of body weight. In groups 1,2,3 and 4, under sterile conditions, a laparotomy was done and the superior mesenteric artery (SMA) was occluded with a microvascular clamp for 60 minutes. In order to block any collateral blood supply, the right colic and proximal jejunal arteries were also clamped. The laparotomy incision was then closed, to be opened later for removal of the clamps after 60 minutes of ischemia. Reperfusion was confirmed by the return of pulsation to the mesenteric arcade. The incision was again closed and the animals were sacrificed by anesthetic overdose 120 minutes later. The sham-operated rats received the same surgical procedure as the other groups without being subjected to the ischemia-reperfusion protocol.
Measurement of bacterial translocation
At the end of the procedures (time = 180 minutes), a midline laparotomy was performed under aseptic conditions and biopsies were aseptically obtained for bacterial colony counts. A 1.5 cm long sample of the terminal ileum was excised, opened on its antimesenteric border, and washed in sterile 0.9% saline solution, for quantitation of bacterial population. One gram of MLN complex, liver and lung was removed for culture. Tissues were homogenized and aseptically solubilized after addition of 0.5 mL of 0.9% saline. Aliquots of 0.2mL were processed and cultured on selective MacConkey's agar and blood agar for detection of gram-negative and gram-positive bacteria, respectively. The agar plates were incubated at 37 oC and examined for growth after 24 and 48 hours. Any growth in the plates of bacteria of the same biotype as cultured in the ileum was considered positive and expressed as colony-forming units per gram of tissue (GFU/g). All procedures were performed under laminar air flux.
Histological study
manner by an experienced pathologist according to microscopic criteria for degree of damage based on a grading system previously described [17]: normal mucosa, 0; subepithelial space at the viluus tip, 1; more extended subepithelial space, 2; epithelial lifting along villus, 3; denuded villi, 4; loss of villus tissue, 5; crypt layer infarction, 6; transmucosal infarction, 7; transmural infarction, 8.
Cytokine assays
Portal blood samples were collected and used for measurement of tumor necrosis factor-alfa (TNFα), interleukin-6 (IL-6) and interleukin-10 (IL-10), determined using enzyme-linked immunoassay kits (all from PeproTech, Rocky Hill, NJ, USA), according to the manufacturer’s recommended protocols. The fluorescence was measured by a Bio-Tec Instruments EL 808 ultra microplate reader, using KC4-V3.0 analysis software. Sensitivity of detection was 30 pg/ml for cytokines.
Statistics
Data analysis was performed using the BioEstat 2.0 program. Differences between the microbiological samples as measured by positive cultures were evaluated by a test for differences between proportions. The results were tabulated and compared by ANOVA using post hoc analysis with Newman-Keuls test. P<0.05 was considered significant.
Results
Body weight
At the beginning of the experiment the body weight of the animals was 240±37g. After 5 days of GL and GLN supplementation they weighed 248±25g and the difference was not significant (p>0.05).
Bacterial translocation
BT of enteric microrganisms to the MLN, liver and lung occurred in all animals. In the Group 1 the incidence of positive cultures and BT to MLN, liver and lung was 100%, 80% and 90%, respectively (Table I). In mesenteric lymph nodes, after treatment with GLN, GL and GL+GLN, the BT was 70%, 40% and 20%, respectively, which was significant when compared to control animals (p<0.01 vs. control). (Table I). So, Group 1 was the only one where the incidence of bacteria isolated from MLN, liver and lung was from 80% to 100%. The incidence of BT to MLN, liver and lung was significantly reduced in the groups supplemented with GL and GLN, when compared with the control (p<0.05). The incidence of BT differed between the groups 2 and 3 in MLN and liver (p<0.05). Nevertheless, the combined treatment with GL and GLN (Group 4) reduced BT to MLN, liver and lung, compared to groups 1, 2 and 3 (p<0.05), but it was not different from the sham (5) group (p>0.05).
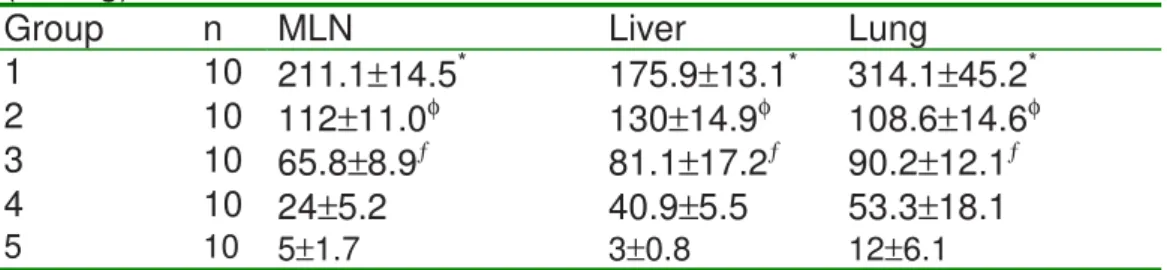
found. The magnitude (i.e., number of bacteria colonies/g of tissue) of translocation to the MLN and liver differed among the groups treated with GLN, GL and GL+GLN. A significant difference (p<0.01) was found when the three groups were compared with the control rats. The number of CFU/g isolated from the lung of Group 2 did not differ from Group 3 (p>0.05). There was a significant decrease in bacterial CFU/g in Group 4 compared with Groups 2 and 3 (p<0.01). (Table II). These results are approximately in agreement with that observed in Table I.
Ileal colonization
The quantitative analysis of bacteria colonizing the ileum showed a significant (p<0.05) Gram positive and Gram negative overgrowth in all control animals. This overgrowth was shown by high CFU/g in the ileum of Gram positive streptococci, and Gram negative E. coli, Proteus sp and enterococci strains. The ileum bacterial count decreased in groups supplemented with GL and GLN, compared with the control. A significant difference was observed (Table III) when the rats supplemented with GL+GLN were compared with groups 1,2,3 and the sham rats (p<0.05).
Macroscopic and histological studies
The ileum demonstrated gross dilatation and intramural hemorrhage after I/R, mainly in control rats. The microscopic findings of the ileum reveled marked mucosal damage after I/R and it was more severe in group 1 than in other groups. The damages included denudation of mucosa, transmural infarction, and neutrophil infiltration of the lamina propria and mucosal layer (Figure 1and Table V). Although mucosal damage was also observed in Group 4, intestinal tissue injury was less severe than in the groups 2 and 3 (p<0.05).
Table I - Incidence of bacterial translocation after intestinal ischemia/reperfusion in rats treated with glucan and glutamine. [n infected/total n (%)].
Group n MLN Liver Lung
1 10 10/10* (100) 8/10* (80) 9/10* (90)
2 10 7/10§ (70) 5/10§ (50) 6/10 (60)
3 10 4/10 (40) 3/10 (30) 5/10 (50)
4 10 2/10ƒ (20) 1/10ƒ (10) 2/10ƒ (20)
5 10 1/10 (10) 0/10 (0) 0/10 ( 0)
*p<0.05 vs. 2,3,4,5; ƒp<0.05 vs. 1,2,3; § p<0.05 vs. 3.
Groups: 1, control; 2, glutamine; 3, glucan; 4, glutamine + glucan; 5, sham; MLN, mesenteric lymph node.
Table II - Bacterial colony counts obtained from MLN, liver and lung cultures. (CFU/g).
Group n MLN Liver Lung
1 10 211.1±14.5* 175.9±13.1* 314.1±45.2*
2 10 112±11.0φ 130±14.9φ 108.6±14.6φ
3 10 65.8±8.9ƒ 81.1±17.2ƒ 90.2±12.1ƒ
4 10 24±5.2 40.9±5.5 53.3±18.1
*p<0.01 vs. 2,3,4,5; φp<0.01 vs. 3,4,5; ƒp<0.01 vs.4,5.
Groups: 1, control; 2, glutamine; 3, glucan; 4, glutamine + glucan; 5, sham; MLN, mesenteric lymph node.
Table III -Incidence of ileum colonization in treated and untreated rats. Ileum
GFU(log10) 1 2 3 4 5
Gram + 5.5±1.4 3.6±0.3φ 4.0±0.5 2.2±0.7* 4.4±1.1 Gram - 6.8±0.3 4.1±0.9φ 3.4±1.3 1.9±0.8* 4.7±0.3
* p<0.05 vs. groups 1, 2, 3, 5; φ p<0.05 vs. groups 1, 4.
Groups: 1, control; 2, glutamine; 3, glucan; 4, glutamine + glucan; 5, sham.
Table IV - Portal serum levels of cytokines comparing groups untreated and treated with glutamine and glucan.
Groups n TNF-α (pg/mL) IL-6 (pg/mL) IL-10 (pg/mL)
1 10 163.7±12* 341.1±46* 251.8±36*
2 10 148±23 290±31 264±47
3 10 81.7±9.3** 154±17** 322±21**
4 10 47±18 103±12.4 410±78
5 10 0 0 0
*p<0.05 vs. groups 3,4; ** p<0.05 vs. groups 2, 4.
Groups: 1, control; 2, glutamine; 3, glucan; 4, glutamine + glucan; 5, sham.
Table V - Histological grade of mucosal injury according to classification previously described [17].
1 control x x x x x x x x x x
2 glutamine x x x x x x x x x x
3 glucan x x x x x x x x x x
4 glutamine +
glucan x x x x x x x x x x
5 sham x x x x x x x x x x
GRADES 0 1 2 3 4 5 6 7 8
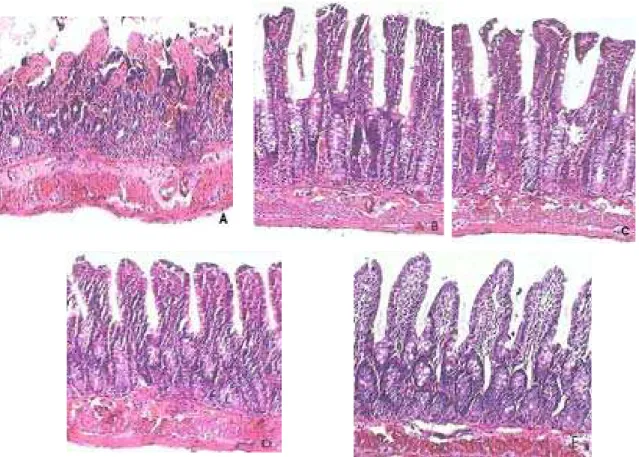
Figure 1. Photomicrographs of ileum segments (HE, 100x).
A. Control (1) group showing massive epithelial lifting, dilated capillaries, hemorrhage and ulceration; B. glutamine treatment (group 2) results in a decrease in epithelial injury. The villi are shorter than sham group and it is observed epithelial lifting; C. glucan pretreatment (group 3) results in lifting of epithelium and infiltration of leukocytes within lamina propria and epithelium; D. The treatment with glucan + glutamine (group 4) results in histology of ileal mucosa similar to that of sham group; E. Sham (group 5) showing normal histology.
Cytokines concentration
The concentrations of TNF-α, IL-6 and IL-10 in the serum of control operated rats were 163.7±12pg/mL, 341±46pg/mL and, 251.8±36pg/mL respectively. Significant decrease in serum level of TNF-α (81.7±9.3pg/ml) and IL-6 (154±17pg/ml) was observed in group 3, when compared with group 1 and group 2 rats (p<0.05). When the administration of GL was concomitant with GLN (group 4), the levels of TNF-α and IL-6 were significantly lower than in groups 1, 2 and 3 (p<0.05). Nevertheless, an inverse result was observed in the IL-10 levels. There was a significant increase (p<0.05) in the level of IL-10 in groups 3 and 4 when compared to groups 1and 2. TNF-α, IL-6 and IL-10 were not detected in the portal serum of the sham rats (Table IV).
Discussion
complications when patients suffer from intestinal blood supply damage and lack of nutrient support [ 3]. It has been discovered from animal models that BT may be associated with an insufficiency of perfusion and lack of essential nutrients in their mucosa [15,16]. This event may result in the release of endotoxins, because of the change in the gut barrier function, thus facilitating BT [18]. Soluble (1,3)-D-glucan has been demonstrated to actuate against shock and infection in rats and mice [8,9]. Clinical studies suggest that the administration of soluble glucans to trauma/surgical patients stimulates the conversion from leukocyte anergy to normal ones, decreases septic complications and improves the survival rate [10,19]. Soluble glucan reduced myocardial infarction in rat hearts subjected to ischemia followed by reperfusion. The induced cardioprotection occurred rapidly and did not require prolonged pretreatment time [20]. This effect seems to be receptor-mediated and several different receptors for 1,3-glucan have been described. Phagocytic non-CR3 receptor in monocytes/macrophages specific for 1,3-glucans [21,22], glycosphingolipid 1,3-glucan receptor in human neutrophils [23], and dectin-1 in macrophages were identified as potential glucan receptors [24]. Furthermore, toll-like receptors have been reported to coordinate macrophage activation in response to zymosan particles [25]. The results found in this study showed that isolated pretreatment with GL significantly reduced BT to MLN, liver and lung, and was more effective than simple GLN supplementation. The combined GL+GLN treatment was more effective in reducing bacterial colony counts than the GL treatment, indicating a synergistic effect. Based on published evidences, glutamine pretreatment for five days was used in the present study. A previous research considered that a minimum of 5 days of feeding was necessary for the GLN supplementation to show an effect on infectious morbidity [11]. As the experimental model used in this study included biopsies and dosages 60 minutes after I/R, we decided to use a pretreatment for five days.
control and sham, meant that it was more effective than GLN, which did not reduce bacterial overgrowth.
GLN has been shown to be an important fuel used by gut mucosa cells as a regulator of cell proliferation and as an essential aminoacid for mucosal growth and function [30]. I/R injury of the small intestine originates marked alterations, mainly in the mucosal layer. They are produced not only during ischemia, but also after reperfusion [31]. In the present study, histological findings of intestinal damage were observed after I/R in the control group, but the damage was less severe in the groups 2, 3 and 4. Our results clearly showed that GL and GLN pretreatment protected the ileum from I/R injury, and the combined use of GL and GLN was the most effective in protecting mucosa.
In this study, increased serum levels of TNF-α, IL-6 and IL-10 refleted the ischemia/reperfusion injury, as demonstrated by other in vivo trials [3,32]. It has been suggested that IL-6 produced by intraepithelial lymphocytes is responsible for the loss of intestinal barrier function following hemorrhage, and the extent of loss can be correlated with plasma levels of this cytokine [33]. In rats treated with GL it was observed a significantly different cytokine response, which was characterized by decreased production of TNF-α and IL-6, compared with controls and rats treated with GLN, suggesting that immunomodulation with soluble glucan might act to depress the inflammatory cytokine response. The decrease in secretion of these pro-inflammatory cytokines coincided with the increase in IL-10 secretion and could, at least in part, be explained by the action of this cytokine, known to have anti-inflammatory activity. In fact, IL-10 has been shown to inhibit lipopolissacharide-induced monocyte tissue factor expression in whole blood [34], and to decrease TNF-α production in human monocytes [35]. It is known that the sole surgical insult produces cytokine expression under normal circumstances. In this study, cytokines expression was considered zero in sham animals because they were not detected in serum by the used methodology. By the way, the sensitivity of detection was 30 pg/mL to all cytokines.
Surgery leads to a transitory immunosuppression , which is associated with decreased GLN levels and increased susceptibility to infection and sepsis. A clinical trial in major abdominal surgery patients was performed with infusion of GLN. The treatment reduced serum TNF-α and immunossuppression [36]. Clinical administration of purified soluble glucan has a number of desirable effects on immune function, including the ability to confer resistance to tumors and various infectious conditions. These findings have stimulated interest in the development of glucan-based therapeutics [37,38].
In conclusion, the isolated use of subcutaneous GL or oral GLN significantly reduced BT in intestinal I/R rats, with GL showing more effectiveness than GLN. The combined administration of GL+GLN considerably attenuated BT, intestinal injury and cytokine production, compared with the isolated treatment. Therefore, the use of GL combined with GLN may be a therapeutic target for future clinical trials in trauma and immunossuppressed patients.
References
1. Berg R D, Garlington A W. Translocation of certain indigenous bacteria from the gastro-intestinal tract to the mesenteric lymph-nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403-11. 2. Deitch E A, Winterton J, Li M, Berg R. The gut as a portal of entry for
bacteremia - role of protein-malnutrition. Ann Surg. 1987;205:681-92. 3. Grotz M R W, Deitch E A, Ding J Y, Xu D Z, Huang Q H, Regel G.
Intestinal cytokine response after gut ischemia - Role of gut barrier failure. Ann Surg. 1999;229:478-86.
4. Ozturk C, Avlan D, Cinel I, Cinel L, Unlu A, Camdeviren H, et al. Selenium pretreatment prevents bacterial translocation in rat intestinal ischemia/reperfusion model. Pharmacol Res. 2002;46:171-7.
5. Beebe D S, Kim S, Belani K G, Fryer J, Benedetti E, Moon S, et al. Endotoxemia During Small-Bowel Transplantation in Pigs. Transplant Proc. 1995; 27:593-4.
6. Goldman R C. Biological response modification by h-D-glucans. Annu Rep Med Chem. 1995;30:129-38.
7. Ross G D, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999;42:61-74.
8. Vereschagin E I, van Lambalgen A A, Dushkin M I, Schwartz Y S, Polyakov L, Heemskerk A, et al. Soluble glucan protects against endotoxin shock in the rat: The role of the scavenger receptor. Shock. 1998;9:193-8.
9. Hetland G, Ohno N, Aaberge I S, Lovik M. Protective effect of beta-glucan against systemic Streptococcus pneumoniae infection in mice. FEMS Immunol Med Microbiol. 2000; 27:111-6.
10. Williams D L, Mueller A, Browder W. Preclinical and Clinical-Evaluation of Carbohydrate Immunopharmaceuticals in the Prevention of Sepsis and Septic Sequelae. J Endotoxin Res. 1995;2:203-8.
11. Houdijk A P J, Rijnsburger E R, Jansen J, Wesdorp R I C, Weiss J K, McCamish M A, et al. Randomised trial of glutamine-enriched enteral nutrition on infectious morbidity in patients with multiple trauma. Lancet. 1998; 352:772-6.
12. Oriordain M G, Fearon K C H, Ross J A, Rogers P, Falconer J S, Bartolo D C C, et al. Glutamine-Supplemented Total Parenteral-Nutrition Enhances T-Lymphocyte Response in Surgical Patients Undergoing Colorectal Resection. Ann Surg. 1994; 220:212-21.
13. Ogle C K, Ogle J D, Mao J X, Simon J, Noel J G, Li B G, et al. Effect of Glutamine on Phagocytosis and Bacterial Killing by Normal and Pediatric Burn Patient Neutrophils. J Parenter Enter Nutr. 1994; 18:128-33.
14. Buchman A L. Glutamine: Is it a conditionally required nutrient for the human gastrointestinal system? J Am Coll Nutr. 1996;15:199-205.
15. Foitzik T, Kruschewski M, Kroesen A J, Hotz H G, Eibl G, Buhr H J. Does glutamine reduce bacterial translocation? A study in two animal models with impaired gut barrier. Int J Colorectal Dis. 1999;14:143-9.
17. Park P O, Haglund U, Bulkley G B, Falt K. The Sequence of Development of Intestinal Tissue-Injury after Strangulation Ischemia and Reperfusion. Surgery. 1990;107:574-80.
18. Swank G M, Deitch E A. Role of the gut in multiple organ failure: Bacterial translocation and permeability changes. World J Surg. 1996;20:411-17.
19. Williams D L, Mueller A, Browder W. Glucan-based macrophage stimulators - A review of their anti-infective potential. Clin Immunother. 1996;5: 392-9.
20. Li C F, Ha T Z, Kelley J, Gao X, Qiu Y F, Kao R L, et al. Modulating Toll-like receptor mediated signaling by (1 -> 3)-beta-D-glucan rapidly induces cardioprotection. Cardiovasc Res. 2004;61:538-47.
21. Czop J K, Austen K F. A Beta-Glucan Inhibitable Receptor on Human-Monocytes - Its Identity with the Phagocytic Receptor for Particulate Activators of the Alternative Complement Pathway. J Immunol. 1985;134:2588-93.
22. Czop J K, Kay J. Isolation and Characterization of Beta-Glucan Receptors on Human Mononuclear Phagocytes. J Exp Med. 1991;173:1511-20.
23. Wakshull E, Brunke-Reese D, Lindermuth J, Fisette L, Nathans R S, Crowley J J, et al. PGG-Glucan, a soluble beta-(1,3)-glucan, enhances the oxidative burst response, microbicidal activity, and activates an NF-kappa B-like factor in human PMN: Evidence for a glycosphingolipid beta-(1,3)-glucan receptor. Immunopharmacology. 1999;41:89-107.
24. Brown G D, Gordon S. Immune recognition - A new receptor for beta-glucans. Nature. 2001;413:36-7.
25. Ozinsky A, Underhill D M, Fontenot J D, Hajjar A M, Smith K D, Wilson C B, et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766-71.
26. Zapatasirvent R L, Hansbrough J F, Ohara M M, Riceasaro M, Nyhan W L. Bacterial Translocation in Burned Mice after Administration of Various Diets Including Fiber-Enriched and Glutamine-Enriched Enteral Formulas. Crit Care Med. 1994;22:690-6.
27. Gianotti L, Alexander J W, Gennari R, Pyles T, Babcock G F. Oral Glutamine Decreases Bacterial Translocation and Improves Survival in Experimental Gut-Origin Sepsis. J Parenter Enter Nutr. 1995;19:69-74. 28. Harward T R S, Coe D, Souba W W, Klingman N, Seeger J M. Glutamine
Preserves Gut Glutathione Levels During Intestinal Ischemia/Reperfusion. J Surg Res. 1994;56:351-5.
29. Jung S E, Youn Y K, Lim Y S, Song H G, Rhee J E, Suh G J. Combined administration of glutamine and growth hormone synergistically reduces bacterial translocation in sepsis. J Korean Med Sci. 2003;18:17-22.
30. Van der Hulst RRWJ, van Kreel BK, von Meynfeldt MF. Glutamine and the prevention of gut integrity. Lancet. 1993;341:1363-1365.
31. Parks DA, Granger DN. Contribution of ischemia and reperfusion to mucosal lesion formation. Am J Physiol. 1986;250:G749-53