ISSN 0104-6632 Printed in Brazil
www.abeq.org.br/bjche
Vol. 24, No. 01, pp. 61 - 71, January - March, 2007
Brazilian Journal
of Chemical
Engineering
THE IMPACT OF KAPPA NUMBER
COMPOSITION ON EUCALYPTUS KRAFT
PULP BLEACHABILITY
M. M. Costa
1*and J. L. Colodette
21
Researcher at North Carolina State University, NCSU,
Department of Wood and Paper Science, Phone: 919-515-5795, North Carolina, USA E-mail: mcosta@ncsu.edu
2
Full Professor at Federal University of Viçosa E-mail: colodett@mail.ufv.br
(Received: March 12, 2005 ; Accepted: November 10, 2006)
Abstract - Consumption of chemicals during ECF bleaching of kraft pulp correlates reasonably well with kappa number, which measures with KMnO4 the total amount of oxidizable material in the pulp. However, the
method does not distinguish between the oxidizable material in residual lignin and other structures susceptible to oxidation, such as hexenuronic acids (HexAs), extractives and carbonyl groups in the pulp. In this study an attempt is made to separate the main contributors to the kappa number in oxygen - delignified eucalyptus Kraft pulps and evaluate how these fractions behave during ECF bleaching using chlorine dioxide as the sole oxidant (DEDD sequence). Residual lignin and HexAs proved to be the main fractions contributing to the kappa number and chlorine dioxide consumption in ECF bleaching. Pulp bleachability with chlorine dioxide increases with increasing HexAs content of the pulp but chlorine dioxide per se does not react with HexAs. Reduction of pulp with sodium borohydride under conditions for removing carbonyl groups has no impact on bleachability. No correlation was found between the pulp of the extractive content and pulp bleachability. The removal of HexAs prior to ECF bleaching significantly decreases the formation of chlorinated organics in the pulp (OX) and filtrates (AOX) as well as of oxalic acids in the filtrates.
Keywords: ECF; Bleachability; Extractives; Carbonyl groups; Hexenuronic acids; Chlorinated organics;
Eucalyptus.
INTRODUCTION
In the days of conventional bleaching based on elemental chlorine, consumption of chemicals was found to correlate reasonably well with kraft pulp kappa number. However, in ECF bleaching this correlation no longer holds true. Kappa number measures the total amount of material in the pulp that
is oxidizable with KMnO4. However, the method
does not distinguish between oxidizable material in residual lignin and in other structures, such as hexenuronic acids (HexAs), extractives and carbonyl groups in the pulp. These structures react readily
with elemental chlorine but show different reaction rates for ECF bleaching reagents such as chlorine dioxide and hydrogen peroxide. Hence, it is paramount to determine the kappa number composition (fractions) in order to understand pulp bleachability with these chemicals.
The two main fractions of the kappa number in kraft pulp are residual lignin and HexAs [1,2]. The
HexA fraction is formed of 4-O
-methylglucuronoxylan under the conditions
predominant in Kraft pulping [3]. The HexAs
interfere with pulp determination of residual lignin
62 M. M. Costa and J. L. Colodette
consume a portion of the bleaching reagents. Oxygen and hydrogen peroxide in alkaline medium do not
react with HexAs [4,5]. However, HexAs are
destroyed by some electrophylic bleaching reagents such as chlorine, hypochlorous acid, ozone and
peroxy acids [6]. HexAs are degraded indirectly in
chlorine dioxide bleaching by hypochlorous acid and/or chlorine which are generated "in situ" [6,7].
Only a small fraction of the kappa number is due to unsaturated structures derived from carbohydrates and extractives in the pulp. It has been demonstrated that oxidation of these structures consumes different amounts of KMnO4. The residual kraft lignin fraction
consumes about 11.6 equivalents of KMnO4/mol under
controlled consumption conditions [2]. In previous work a consumption of 8.4 to 8.6 equivalents of KMnO4/mol
HexAs was measured [1], and therefore one kappa
number unit is equivalent to about 11.9 mmol of HexAs /kg of pulp [1,4]. Another study cited a similar value of 10 mmol of HexAs/kg of pulp [8]. The double bond in the HexA molecule contributes from 1 to 7 kappa number units, depending on the type of pulp and stage of pulping [8,9].
The HexA content of the pulp can be selectively hydrolyzed with Hg2+ and quantified by the decrease in kappa number [4]. On an industrial scale, a hot acid stage (Ahot) has been proposed for selective removal
of a portion of the HexAs [10]. This stage significantly reduces the consumption of bleaching reagent, especially for hardwood pulps. In ECF bleaching of kraft-O2 (oxygen - delignified Kraft) birch pulp, the
inclusion of a hot acid stage (Ahot) without washing
(sequences ODED versus O/AhotDED) resulted in a
savings of 5.7 kg ClO2/t of pulp
[8]
. For eucalyptus kraft-O2 pulp, inclusion of Ahot as the first stage with
interstage washing (DEDED vs AhotDEDED) saved
8.87 kg ClO2/t
[11]
. Savings of about 6.0 kg ClO2/t of
pulp has also been observed in ECF bleaching of
Eucalyptus globulus kraft-O2 pulp through inclusion
of an Ahot stage followed by washing (DEDED vs
AhotDEDED)
[12]
.
The bleachability of kraft pulps may vary significantly depending upon the raw material and manufacturing process. The difference in bleachability between several pulps become from the
same raw material is not easy to rationalize, particularly when they have the same kappa numbers and brightnesses. It is expected that HexAs and other carbohydrate structures have different bleachabilities for different bleaching reagents from residual ligninº. It was thus the objective of this study to determine why kraft pulps with similar characterizing respond differently to a given bleaching process. An attempt was made to separate the main kappa number fractions of oxygen - delignified eucalyptus Kraft pulps from different sources and to determine how these fractions behave during ECF bleaching using chlorine dioxide as sole oxidant – DEDD bleaching. The reactivity of HexAs in the chlorine dioxide stage and the bleachability of kraft-O2 pulp after the Ahot
stage were also evaluated.
EXPERIMENTAL
Two oxygen – delignified eucalyptus Kraft pulp
(Kraft-O2) samples (Table 1) obtained by modified
Kobudomari cooking (Kvaerner proprietary cooking
technologies) were used in this study.
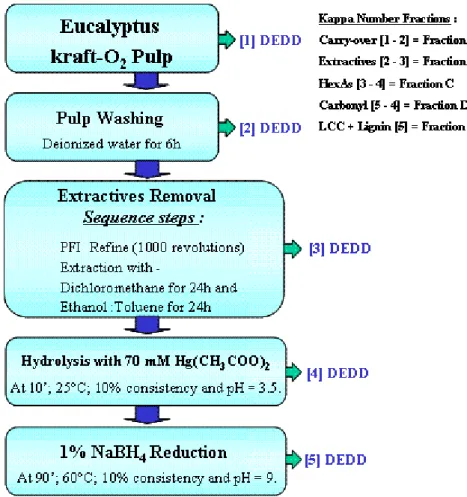
Pulp Kappa Number Fractions
Pulp sample G (Table 1) was used to evaluate the effect of the main kappa number fractions on bleachability. Figure 1 shows the procedure used to separate the kappa number fractions. With this procedure it was possible to quantify by difference the chlorine dioxide consumption due to “carry – over” (A fraction), extractives (B fraction), HexAs (C fraction), pulp carbonyl groups (D fraction) and lignin-HexAs complex (lignin carbohydrate complex - LCC) plus the residual lignin structures and other unsaturated structures (E fraction). The bleachability of each fraction was calculated as the ratio between the drop in kappa number and equivalent chlorine dioxide consumption in DEDD bleaching to 88% ISO brightness. The most important bleaching conditions are shown in Table 2. The efficiency of HexA removal by chlorine dioxide was also evaluated for sample J.
Table 1: Characteristics of the samples from the Kobudomari pulping process used in this study.
kraft-O2 Kappa Viscosity, Brightness, Pulp COD, Alkaline Loss, Ash, Metals, g/t
-pulp Number mPa*s % ISO kg O2/t kg NaOH/t % Ca Mg Mn Fe Cu
G 9.7 29.0 56.0 15.0 6.6 0.53 735 50 12 12 0.49
Figure 1: Scheme for separation of kraft-O2 pulp kappa number fractions.
Table 2: Conditions used for HexAs removal and pulp bleaching.
Bleaching stage Conditions
D0 E D1 D2
Consistency, % 10 10 10 10
Time, min 40 85 150 150
Temperature, ºC 50 85 75 75
Final pH, (± 0.2)♦ 2.5 - 2.8 11.0 3.5 4.0
ClO2, %♠ 0.7 - 0.21 - 0.5 - 0.2 0.28 - 0.75
♦
Final pH controlled with NaOH and H2SO4 when necessary. For the experiments using dimethylsulfoxide reaction pH was controlled at
2.8 with a buffer of sodium biphosphate and citric acid (1g NaHPO4 per 5.53g C6H8O7).
♠ D
1: D2 ratio between stages around 70: 30 to 80: 20.
Analytical Methods
Except where otherwise stated, the standard analytical methods from TAPPI [13], ISO [14], PTS [15],
DIN [16], SCAN [17] and STANDARD METHODS [18]
were used. For hexenuronic acids (HexAs) and oxalate contents, high – performance liquid
chromatography [19] and ion chromatography [18]
procedures were used, respectively.
RESULTS AND DISCUSSION
Kraft-O2 Pulp Kappa Number Fractions
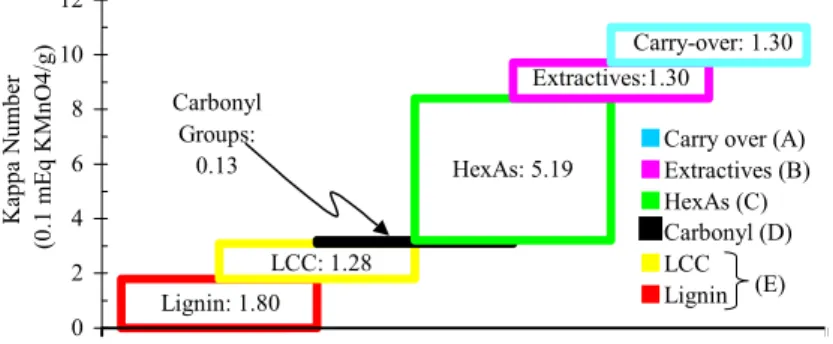
The main kappa number fractions of sample G (Table 1) are shown in Figure 2.
64 M. M. Costa and J. L. Colodette
with previously reported results [2]. It should be noted that the lignin content in this oxygen – delignified
Eucalyptus spp. Kraft pulp was very low.
According to the mechanism proposed, a selective hydrolysis of the glucosidic linkage between HexA groups and the xylan chain occurs
with Hg2+ (mercuric acetate) [4]. The HexAs and
mercury catalyzer are recovered at the end of this reactionº. Another hypothesis is that a portion of the HexA groups are covalently linked to the residual lignin [20, 21] and this fraction tends to remain in the
pulp bound only to residual lignin after Hg2+
hydrolysis. The fraction of the HexA groups bound
to lignin (lignin-carbohydrate complex - LCC) was degraded under severe acid hydrolysis conditions, such as those used in the determination of Klason ligninº. About 12.7 mmol HexAs/kg of pulp (Table 3) were found in the acid filtrate produced by determination of Klason lignin after Hg2+ hydrolysis
and NaBH4 reduction (E fraction – Figure 1). The
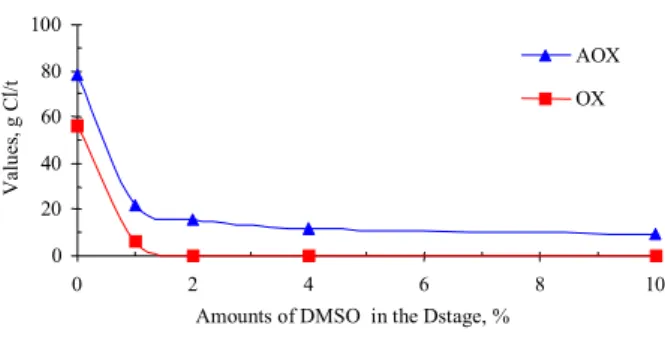
HexAs that may be complexed with lignin (Figure 2) are equivalent to 1.28 kappa number units, since 9.94 mmol HexAs/kg of pulp are equal to one kappa number unit (Table 3). Previous reports cite values in the range of 10 to 11.9 mmol HexAs/kg of pulp as corresponding to one kappa number unit [1,8].
Lignin: 1.80 LCC: 1.28 Carbonyl
Groups:
0.13 HexAs: 5.19
Extractives:1.30
Carry-over: 1.30
0 2 4 6 8 10 12
1
Kraft-O2pulp Kappa # Fractions
Ka
p
p
a Nu
mb
er
(
0
.1 m
E
q K
M
nO
4/g)
Carry over (A) Extractives (B) HexAs (C) Carbonyl (D) LCC Lignin (E)
Figure 2: Contributors to Kappa number in an oxygen - delignified eucalyptusKraft pulp (sample G - initial kappa # = 9.7).
Table 3: Relationship between kappa number and residual lignin and HexA contents of a kraft-O2 pulp (sample G) after different treatments.
Treatment (Figure 1) Kappa # KL, %♣ RL, mmol/kg♣ HexAs, mmol/kg♦
Washed 9.70 0.57 28.9 69.2
Extracted 8.40 0.40 20.3 64.3
Hg2+ Hydrolyzed 3.21 0.33 16.8 12.7
NaBH4 Reduced
1.28 (LCC)
1.80 (RL)♦ 0.30 15.2 12.7
♣
Residual lignin (RL) was calculated from the acid an – insoluble Klason lignin (KL) with an estimated molecular weight of 197g/mol [2];
♦
One kappa number unit was equivalent to 8.44 mmol RL/kg or 9.94 mmol HexAs/kg.
Besides the hexenuronic acid groups resistant to
Hg2+ catalyzed hydrolysis, the E fraction has an
insoluble Klason lignin content of about 15.2 mmol/kg of pulp (Table 3). The ratio between residual lignin content and kappa number (1.8 units) indicates that 8.44 mmol of residual lignin per kg of pulp is equivalent to one kappa number unit (Table 3). Authors in the literature cite values ranging from 7.94 to 10.75 mmol of residual lignin / kg of pulp as
equivalent to one kappa number unit [2, 22]. Thus,
residual lignin is almost entirely responsible for the
remaining KMnO4 consumption by the E fractionº.
However, this does not exclude the contribution of
other unsaturated chemical constituents. In fact, it confirms the possibility that a portion of the HexAs is bound to chemical components of the pulp other
than xylanº. Therefore, KMnO4 consumption of the
E Fraction is due to residual lignin content plus LCC content, the fractions resistant to the treatments listed in Figure 1.
Another important observation to be made Table 3 concerns the relation between Klason lignin and
kappa number for the kraft-O2 pulps. This relation
This interference is mostly due to the HexA content, as shown in Figure 2. Table 3 also shows that the residual lignin content (acid – insoluble Klason lignin) of the E fraction was about 0.30% odt, while the kappa number due to the residual lignin was about 1.8 units. Thus, the percentage ratio due to the residual lignin in oxygen – delignified eucalyptus Kraft pulp was 0.167%.
Bleachability of Kappa Number Fractions
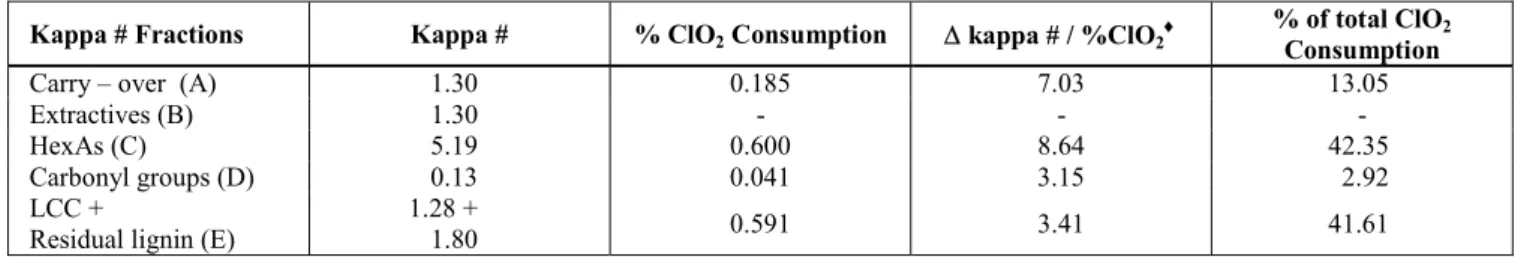
In Table 4 the chlorine dioxide consumption due to each kappa number fraction is presented. These values were obtained by the difference in chlorine dioxide consumption between successive treatments listed, as described in Figure 1. The bleachability
value (∆kappa #/%ClO2) for each fraction is also
presented in Table 5. Bleachabilities of the A and C fractions were higher than those of the D and E (LCC + residual lignin) fractions. Removal of
extractives (B fraction) resulted in a reduction of 1.3 kappa number units and in a 3.4% increase in ISO brightness. However, these benefits did not affect chlorine dioxide consumption, as already demonstrated in other studies [23]. On the other hand, it was clearly seen that removal of the C fraction resulted in a decrease in pulp bleachability. The LCC plus residual lignin components (E fraction) had a higher chlorine dioxide consumption per kappa number unit than the HexAs (C fraction), as seen in Table 4. This fact confirms for chlorine dioxide what
had already been demonstrated for KMnO4 oxidation
[2]
. It is therefore probable that pulps with a higher HexA content also have higher bleachability with chlorine dioxide alone, i.e. low chlorine dioxide consumption per kappa number unit. Note that the opposite has been shown for oxygen and peroxide bleaching. It can also be observed in Table 5 that the C and E fractions are responsible for most of the chlorine dioxide consumption (~ 84%).
Table 4: Chlorine dioxide consumption due to each kraft-O2 pulp (sample G) kappa number fractionº.
Kappa # Fractions Kappa # % ClO2 Consumption ∆ kappa # / %ClO2♦
% of total ClO2
Consumption
Carry – over (A) 1.30 0.185 7.03 13.05
Extractives (B) 1.30 - - -
HexAs (C) 5.19 0.600 8.64 42.35
Carbonyl groups (D) 0.13 0.041 3.15 2.92
LCC +
Residual lignin (E)
1.28 +
1.80 0.591 3.41 41.61
♦Bleachability of each kappa number fraction.
OX and AOX Generation by the Different Kappa Number Fractions
The contribution of each kappa number fraction to chlorinated organics generation during DEDD bleaching to 88% ISO is given in Table 5. The C and E fractions (see Fig 1) were the greatest contributors to OX generationº. These fractions were responsible for 35.5 (C fraction) and 40.4 % (E fraction) of total OX generated. The E fraction was the single most important contributor to OX formation, with a larger contribution to OX formation per kappa number unit than the C fractionº. On the other hand, AOX formation was mainly caused by the A and C fractions. These fractions were responsible for 31.5 and 44.3 % of total AOX generated, respectively. The contribution from residual lignin (E fraction) to AOX content was insignificant (Table 5), a fact that has been reported but not explained in other studies
[24, 25]
. The large contribution of HexAs to the AOX discharged in to the effluent may be related to the
higher solubility of the chlorinated organics derived from the HexAs than of those produced from ligninº. The chlorinated organics derived from lignin are more likely to stick to the pulp and appear as OX values in the bleached pulp; this is easily seen in Table 5. Carry – over (A fraction) on the pulp was the largest contributor to AOX formation per kappa number unit. The values referring to the D fraction should be disregarded because the kappa number fraction due to carbonyl groups is negligible.
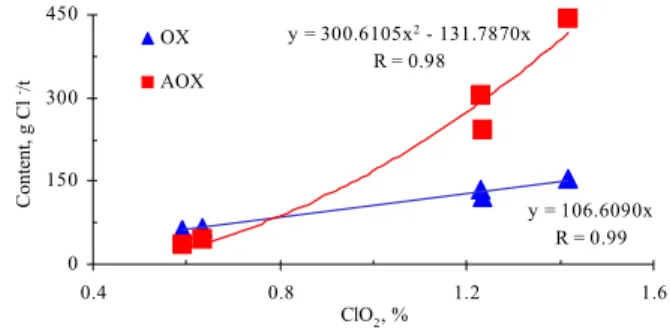
A good correlation was found between OX and AOX formation during the chlorine dioxide stage (Figure 3) as well as between these chlorinated organic compounds and the total chlorine dioxide consumption (Figure 4). This latter correlation can
be explained by the increased concentration of Cl2
66 M. M. Costa and J. L. Colodette
Table 5: Contribution of each kraft-O2 pulp (sample G) kappa number fraction to the generation of chlorinated organic compound in the pulp (OX) and filtrates (AOX).
Kappa # OX, AOX,
Fraction Kappa # g/t % of
Total value / # kappa g/t
% of
Total value / kappa #
Carry – over (A) 1.30 19.0 12.2 14.6 140 31.5 108
Extractives (B) 1.30 14.4 9.27 11.1 62.0 14.0 47.7
HexAs (C) 5.19 55.2 35.5 10.6 197 44.3 37.9
Carbonyl groups (D) 0.13 4.00 2.58 - 8.10 1.82 -
LCC +
Residual lignin (E)
1.28 +
1.80 62.7 40.4 20.4 37.4 8.43 12.2
y = 0.024336x2 - 0.982043x
R = 1
0 200 400 600
50 75 100 125 150 175
OX, g Cl-/t
AOX,
g
Cl
-/t
y = 106.6090x R = 0.99 y = 300.6105x2 - 131.7870x
R = 0.98
0 150 300 450
0.4 0.8 1.2 1.6
ClO2, %
C
ont
ent
, g C
l
-/t
OX
AOX
Figure 3: Correlation between OX content in the pulp (sample G) and AOX in the effluent
of the DEDD sequence after each kappa number fractionation stage
listed in Figure 1.
Figure 4: Correlation between chlorinated organic compounds (OX and AOX) and chlorine dioxide
consumed in the DEDD bleaching after each of the kappa number fractionation treatments
listed in Figure 1.
However, it can be seen in Figure 4 that the AOX content as a function of chlorine dioxide consumption is higher than the increase in OX content. This shows that OX formation depends not only on the amount of chlorine dioxide, but also on the available chemical contributors that remain bound to the
bleached pulp after reacting with Cl2 and HClO
formed in situ. In table 5 the quantity of chlorinated organic compounds (OX + AOX) generated per kg of chlorine dioxide consumed during bleaching is shown for from each kappa fractionº. It is readily observed that for an equivalent amount of chlorine dioxide, the formation of chlorinated organic compound decreases about 40% after hydrolysis of
the HexAs with Hg2+. This clearly shows that the
HexAs measured in the C fraction are one of the most important contributors to chlorinated organics formation during chlorine dioxide bleaching of
Eucalyptus spp. kraft-O2 pulp.
Organic Matter Generated in DEDD Effluent by Each Kappa Number Fraction
Table 6: Contribution of each kappa number fraction to COD and TOC values in the DEDD sequence filtrates
Kappa # fraction Kappa # COD,
Kg O2/t♣
% total COD
COD/ # kappa
TOC,
kg C/t♣
% total TOC TOC/
Kappa #
Carry – over(A) 1.30 1.10 5.80 0.85 0.18 4.01 0.14
Extractives (B) 1.30 1.45 7.64 1.12 0.78 17.6 0.60
HexAs (C) 5.19 7.57 39.9 1.46 1.73 38.8 0.33
Carbonyl groups (D) 0.13 0.36 1.90 2.77 0.04 0.82 0.28
LCC +
Residual lignin (E)
1.28 + 1.80
8.49 44.8 2.76 1.73 38.8 0.56
♣
Values measured in individual effluent stages. Where COD= 2.3918(TOC)2 + 0.4842(TOC) and R2= 0.97.
Oxalate Generation in Effluent from DEDD Bleaching of Pulps by the Different Kappa Number Fractionation Treatments
The contribution of each kappa number fraction to oxalate (C2O4
=
) values was calculated based on the oxalate formation in filtrates from DEDD bleaching to 88% ISO brightness (Table 7). Basically, the oxalate in the pulp originates in oxidative reactions occurring during bleaching [27, 28]. The C (35.3%) and E (30.1%) pulp fractions contributed the most to oxalate formationº. The largest source of oxalate formation during bleaching is HexAs [5, 29]. Residual lignin is also an important source of oxalate
formation [30]. Some authors have reported that
residual lignin is the main source of oxalate
formation during softwood bleaching [27,28].
However, this is not true for kraft-O2 eucalyptuspulps,
which have high HexA contents and low residual lignin contents (Table 3). In this study the kappa number contribution of HexAs + LCC (hexenuronoxylan - lignin complex) was about 6.47 units. Using the ratio given in Table 7 of 0.085 kg C2O4
=
/t per kappa number unit due to HexAs, one obtains a value of 0.55 kg C2O4
=
/t. This value corresponds to 44% of the total oxalate generation shown in Table 7 (1.25 kg C2O4
=
/t), which is greater than the individual contributions of the other kappa number fractions. This shows the importance of HexAs removal prior to the first chlorine dioxide stage to prevent calcium oxalate scale, especially in bleach plants with a high degree of water circuit recirculation [31].
Table 7: Oxalate formation due to each kappa number fraction in DEDD filtrate for a eucalyptus kraft-O2 pulp (sample G)
Kappa # Fraction Kappa # C2O4
=
, kg/t % totalC2O4
=
C2O4
=
, kg/t/#kappa
Carry – over(A) 1.30 0.176 14.2 0.136
Extractives (B) 1.30 0.212 17.1 0.163
HexAs (C) 5.19 0.439 35.3 0.085
Carbonyl groups (D) 0.13 0.043 3.47 0.332
LCC +
Residual lignin (E)
1.28 +
1.80 0.374 30.1 0.122
Efficiency of HexA Removal with Chlorine Dioxide
Figure 5 shows the efficiency of removal of HexAs with chlorine dioxide after extraction, i.e., after the DE stages. The conditions used in the D and E stages are given in Table 2. It was seen that the D stage (kappa factor = 0.18, pH 2.8) removed just 25.6% of the HexAs from the pulp. This indicates that efficiency of removal of HexAs in a single D stage is low. This efficiency fell even lower when
elemental chlorine (Cl2) and hypochlorous acid
(HOCl) generated in situ in the chlorine dioxide stage were deactivated during the reaction with pulp. The results in Figure 5 show that the efficiency of removal of HexAs in the DE stages was practically null after deactivation of these oxidants with DMSO [dimethylsulfoxide (CH3)2SO]. DMSO is an additive
that reacts quickly and selectively with Cl2 and/or
HOCl produced during the D stage [(CH3)2SO +
68 M. M. Costa and J. L. Colodette
43.0
40.5 41.6
42.8 42.4
32.0
30 35 40 45
kraft-O2 0 1 2 4 10
DMSO in the Dstage, %
H
exA
s,
mmol
/k
g
- 25.6%
0 20 40 60 80 100
0 2 4 6 8 10
Amounts of DMSO in the Dstage, %
Va
lu
es
, g
C
l/t
-AOX OX
Figure 5: HexAs content in kraft-O2 pulp (sample J)
after DE stages for different amounts of DMSO in the D stage.
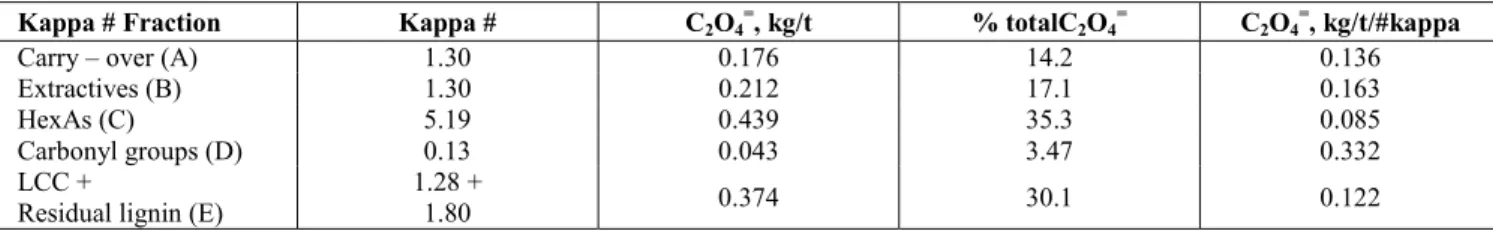
Figure 6: Chlorinated organic compounds in a kraft-O2 pulp (sample J) after the DE stages for
different amounts of DMSO in the D stage.
Therefore, it can be affirmed that chlorine dioxide per se does not effectively react with HexAs. The active species that react with HexAs in the D stage are by-products of chlorine dioxide reactions with pulp. These oxidizers are probably a mixture of
HOCl and Cl2 that are present in to a larger or
smaller extent depending on reaction pH.
It should be noted that deactivation by DMSO during the D stage results in a substantial decrease in the formation of chlorinated organic compound, as measured by OX in the pulp and AOX in the DE effluent (Figure 6). Complete inhibition of formation of chlorinated organic compounds bound to the pulp (covalently bonded) was observed at a 2% amount of DMSO. This demonstrates that OX formation in eucalyptus pulp is due exclusively to pulp reactions
with HOCl and Cl2. AOX was reduced up to 88.4%
after addition of DMSO, similarly to reductions
reported previously [26]. These results are in
accordance with the theory that chlorine dioxide per se reacts with the pulp only by oxidation, which does not result in the formation of chlorine-substituted products [32].
On the other hand, the Klason lignin content of the pulp, measured after the DE stages (Figure 7), fell about 71%, indicating that the D stage is much more effective in removal of lignin than of HexAs. Efficiency of HexA removal was only about 26% (Figure 5). Residual lignin in the kraft-O2 pulp may
contain etherified or phenolic structures. The reactions between chlorine dioxide and residual lignin result exclusively in the oxidation of phenolic
structures without formation of chlorine-substituted
products [32]. This explains why residual lignin
content (acid – insoluble Klason lignin) increased as a function of DMSO applied in the D stage, since this additive suppressed the delignification that would occur through in situ formation of elemental chlorine. In this context, the rate of delignification in the DE stages fell from 70.7% in the absence of DMSO to about 47.7% with 10% DMSO, while the kappa number rose from 3.6 to 5.3 units.
The decrease in the rate of delignification caused by addition of DMSO did not have a significant effect on brightness and viscosity (Figure 8), indicating that the gain in brightness in the D stage was due to the chlorine dioxide present, while the loss in viscosity, when it occurred, was caused by chlorine and hypochlorous acid as well as by chlorine dioxide.
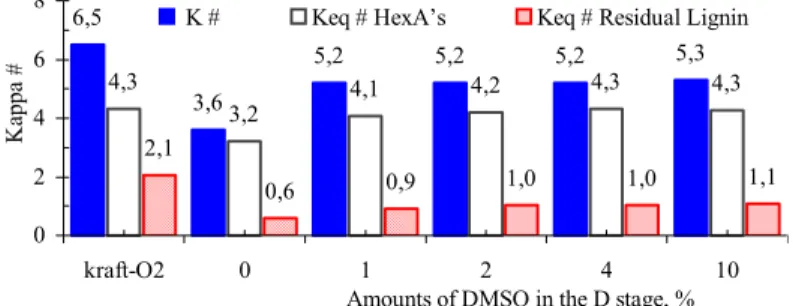
Figure 9 shows that even after the DE stages, carried out under normal process conditions (without addition of DMSO), a good portion of the kappa number is still the HexAs. This confirms the technical viability of implementing the Ahot stage for
17.4
5.1
7.6 8.6 8.6 9.1
0 4 8 12 16 20
kraft-O2 0 1 2 4 10
Amounts of DMSO in the D stage, %
L
ig
n
in
, m
m
o
l/k
g - 47.7%
- 70.7%
58,0
73,9 73,5 73,5 73,4 73,6
18,0 18,2 17,8 18,0 17,4
19,1
0 20 40 60 80
kraft-O2 0 1 2 4 10
Amount of DMSO in the D stage, % Viscosity , mPa*s Brightness, % ISO
Figure 7: Insoluble residual lignin content in kraft-O2
pulp (sample J) and after the DE stages for different amounts of DMSO applied in the D stage.
Figure 8: Brightness and viscosity of kraft-O2 pulp
(sample J) before and after the DE stages for different amounts of DMSO charges applied in the D stage.
6,5
3,6
5,2 5,2 5,2 5,3
4,3
3,2
4,1 4,2 4,3 4,3
2,1
0,6 0,9 1,0 1,0 1,1
0 2 4 6 8
kraft-O2 0 1 2 4 10
Amounts of DMSO in the D stage, %
K
appa #
K # Keq # HexA’s Keq # Residual Lignin
Figure 9: Pulp kappa number (K #) and kappa number equivalent to HexA content (Keq # HexA’s)
and to residual lignin (Keq # residual lignin) in kraft-O2 (sample J) before and after
the DE stages for different amount of DMSO charges applied in the D stage.
CONCLUSIONS
1. The main kappa number fractions of eucalyptus
kraft-O2 pulp are HexAs and lignin carbohydrate
complex (LCC) plus residual lignin.
2. Chlorine dioxide consumption in the DEDD
sequence is due mainly to HexAs and lignin carbohydrate complex (LCC) plus residual lignin, in this order.
3. The bleachability (chlorine dioxide consumption
per kappa number unit) of HexAs is greater than that of lignin carbohydrate complex (LCC) plus residual lignin.
4. One kappa number is equal to 8.44 mmol of
residual lignin or 9.94 mmol of HexAs per kg of eucalyptus kraft-O2 pulp.
5. OX in pulp and COD, TOC and oxalate in
effluents of DEDD bleaching are mainly generated by HexAs and lignin carbohydrate complex (LCC) plus residual ligninº.
6. AOX formation in effluents of DEDD bleaching was
mainly caused by the HexA and carry – over fractions.
7. Chlorine dioxide per se does not react effectively with HexAs.
8. The removal of eucalyptus kraft-O2 pulp carbonyl
groups and extractives has little impact on bleachability.
REFERENCES
Li, J., Gellerstedt, G. The contribution to kappa number from hexenuronic acid groups in pulp xylanº. Carbohydrate Research, vol.. 302, pp 213-218, 1997.
Li, J., Gellerstedt, G. On the structural significance of the kappa number measurement. Nordic Pulp and Paper Research Journal, vol. 13, nº. 2, pp. 153-158, 1998.
Jiang, Z., Van Lierop, B.Vol., Berry, R. Hexenuronic acid groups in pulping and bleaching chemistry. Tappi Journal, vol. 83, nº. 1, pp. 167-175, 2000. Gellerstedt, G., Li, J. An HPLC method for the
70 M. M. Costa and J. L. Colodette
groups in chemical pulps. Carbohydrate Research, vol. 294, pp. 41-51, 1996.
Henricson, K. Ahl Stage – A new bleaching stage for kappa reduction and metal profile control. In: International Emerging Technologies Conference and Exhibition, 1997, Orlando. Proceedings... Orlando, March 9-13, 1997.
Juutilainen, S., Vilpponen, A., Pikka, O., Vuorinen, T., Henricson, K. Combining chlorine dioxide bleaching of birch kraft pulp with an A-stage at high temperatures. Tappi Jounal, 1999.
Törngren, A., Gellerstedt, G. The nature of organic bound chlorine from ECF - bleaching found in kraft pulp. In: International Symposium Wood and Pulping Chemistry (ISWPC), 9., 1997, Montreal, Canada. Proceedings... Montreal: Oral presentation (M2-1 M2-4), June 9-12 1997. vol. 2, 901 pp.
Devenyns, J., Chauveheid, E., Märtens, H. Uronic acid and metals control. In: International Symposium on Wood and Pulping Chemistry, 9., 1997, Montreal. Proceedings... Montreal: PAPTAC, Oral presentation (M5-1 - M5-4), 1997.
Vuorinen, T., Teleman, A., Fagerström, Pp., Buchert, J., Tenkanen, M. Selective hydrolysis of hexenuronic acid groups and its application in ECF and TCF bleaching of kraft pulps. In: International Pulp Bleaching Conference, 1996, Washingtonº. Proceedings... Washington, 1996. vol. 1, pp. 43-52.
República Federal do Brasil. Key Henricson. Tratamento ácido de polpa a alta temperatura em conexão com alvejamento. BR nº. PI 9611258-1 A, Oct. 13, 1996; July 13, 1999.
Ratnieks, E., Foelkel, C., Sacon, Vol., Sauer, M.J. Improved Eucalyptus pulp bleachability via high temperature acid treatment. In: International Emerging Technologies Conference and Exhibition, 1997, Orlando. Proceedings... Orlando, March 9-13, 1997.
Furtado, F.P., Evtuguin, D.Vol., Gomes, T.M. Effect of the acid stage in ECF bleaching on Eucalyptus globulus kraft pulp bleachability and strength. In: International Pulp Bleaching Conference, 2000, Halifax. Proceedings... Halifax: Poster Presentation, June 27-30, 2000. vol.1, pp. 111-114.
Technical Association of The Pulp and Paper Industry. Tappi Standard Methods. Atlanta: TAPPI, 2000.
International Organization for Standardization. International Standard. ISO,
PTS, Papiertechnishe Stiftung fur Furchung und Ausbildung in Papierzeugung und
Papierver-Arbeitung. Determination of the Total Halogenated Organics OX Content. 32 Pulp Testing, 1990.
German Standard Methods for Examination of Water, Waste Water and Sludge. Deustshe Norm Germany: DIN 38 414-S; 18, Nov. 1989.
Scandinavian Pulp, Paper and Board. Testing Committee. Stockholm: SCAN W9:1989.
Examination of Water And Wastewater (APHA/AWWA/WEF). Standard Methods. 20th ed., 1998.
Jiang, Z-H., Audet, A. Sullivan, J. Lierop, B.Vol. and Berry, R. Method for quantify Hexenuronic acid groups in chemical pulps. In: Intl. Symposium on Wood and Pulping Chem., Japan, Proc. pp. 384-389 (1999).
Teleman, A., Hausalo, T., Tenkanen, M., Vuorinen, T. Identification of the acidic degradation products of hexenuronic acid and characterization of hexenuronic acid-substituted xylooligosaccharides by NMR spectroscopy. Carbohydrate Research, vol. 280, pp. 197-208, 1996.
Jiang, Z., Van Lierop, B., Nolin, A., Berry, B. A new insight into the bleachability of kraft pulps. In: International Pulp Bleaching Conference, 2000, Halifax. Proceedings... Montreal: PAPTAC, 2000. pp. 163-168.
Gellerstedt, G. Li, J., Sevastyanova, O. The relationship between kappa number and oxidizable structures in bleached kraft pulps. In: International Pulp Bleaching Conference, 2000, Halifax. Proceedings... Halifax: Oral presentation, June 27-30, 2000. vol. 1, pp. 203-206.
Pascoal Neto, C., Daniel, A.I.D., Evtuguin, D.Vol., Silvestre, A.J.D. Influence of kappa number of unbleached pulp on ECF bleachability of Eucalyptus globulus kraft pulps. In: International Pulp Bleaching Conference, 2000, Halifax. Proceedings... Halifax: Poster presentation, June 27-30, 2000. vol. 1, pp. 107–110.
Freire, C.S.R., Silvestre, A.J.D., Pascoal Neto, C., Silva, A.M.S., Evtuguin, D.Vol., Cavaleiro A.S. Easily degradable chlorinated compounds derived from glucuronoxylan in filtrate from chlorine dioxide bleaching of eucalyptus globulus Kraft pulp. Holzforschung, vol. 57(1), pp. 81-87, 2003. Freire, C.S.R., Silvestre, A.J.D., Pascoal Neto, C.
Carbohydrate-derived chlorinated compounds in ECF bleaching of hardwood pulps: formation, degradation and contribution to AOX in bleached kraft pulp mill. Environ. Sci. Technol. Vol. 37, pp. 811-814, 2003.
chlorine bleaching. Proceedings...International Pulp Bleaching Conference, Tappi Press, vol. 2, pp. 417-420. (1996).
Elsander, A., Ek. M., and Gellerstedt, G., Oxalic acid formation during ECF and TCF bleaching of kraft pulp, Proceedings..., TAPPI Minimum Effluent Symposium, Tappi Press, pp. 63-66 (1997)
Krasowski, J.A., Marton, J., Formation of oxalic acid during bleaching of kraft pulp. Journal Wood Chemistry Technology, vol. 3, pp. 445-458, 1983. Vuorinen, T., Fagerström, Pp., Räsänen, E., Vikkula,
A., Henricson, K., Teleman, A. Selective hydrolysis of hexenuronic acid groups opens new possibilities for development of bleaching processes. In: International Symposium Wood and Pulping Chemistry (ISWPC), 9, 1997, Montreal, Canada. Proceedings... Montreal: Oral
Presentation (M4-1 M4-4), June 9-12, 1997. vol. 2, 901 pp.
Kempf A.W., Dence C.W. The reactions of hardwood lignin model compounds with alkaline hydrogen peroxide. Tappi Journal, vol. 58 (6) pp. 104–108, 1975.
Costa, M.M., Correia, F. M, Bissiat, J.I., Molinar, P. Estudos de solubilização química de incrustações no estágio D2 da seqüência ECF - D0EoD1D2. In:
Conferência Associação Brasileira Técnica de Celulose E Papel, 1999, São Paulo. Proceedings... São Paulo, 1999.