Ar
ti
cle
J. Braz. Chem. Soc., Vol. 21, No. 10, 1807-1813, 2010.
Printed in Brazil - ©2010 Sociedade Brasileira de Quím ica 0103 - 5 05 3 $ 6 .00+ 0.00
* e-m ail: m aj ok ato@ iq .usp .b r
Antifungal Activity of Natural and Synthetic Amides from Piper species
Joaquim V. Marques,a Alberto de Oliveira,a,b Ludmila Raggi,c Maria C. M. Youngc and
Massuo J. K ato*,a
aInstituto de Química, Universidade de São Paulo, CP 260 7 7 , 0 5 5 1 3 - 9 7 0 São Paulo- SP, Brazil
bInstituto de Química, Universidade F ederal de Ub erlândia, CP 5 9 3 , 3 8 4 0 0 - 9 0 2 Ub erlândia- M G , Brazil
cSeção de F isiolog ia e Bioq uímica de Plantas, Instituto de Botânica, CP 4 0 0 5 , 1 0 0 5 1 São Paulo- SP, Brazil
E x trato de f olh as de Pip er scutif olium ap resentou ativ idade antif úng ica sig niicativ a contra
Cladosp orium cladosp orioides e C. sp haerosp ermum e seus p rincip ais com p onentes ativ os,
p ip erina, p ip erlong um inina e corcov adina f oram isolados p or m eio de p uriicação b iom onitorada, ap resentando lim ites de detecção de 1 µg . F oi realizado um estudo da relação estrutura-ativ idade
b aseado na síntese de doze análog os com v ariações no núm ero de insaturações, no p adrão de sub stituição no anel arom ático e no g rup o am ídico. A s am idas sem sub stituintes no anel arom ático e com ap enas um a lig ação dup la f oram as m ais ativ as e os deriv ados N ,N-dietil-sub stituídos ap resentam m aior dose-dep endência.
T h e antif ung al leav es ex tract f rom Pip er scutif olium w as sub m itted to b ioactiv ity -g uided
ch rom atog rap h ic sep aration ag ainst Cladosp orium cladosp orioides and C. sp haerosp ermum
y ielding p ip erine, p ip erlong um inine and corcov adine as th e activ e p rincip les w h ich disp lay ed a detection lim it of 1 µg . Structure-activ ity relationsh ip s w ere inv estig ated w ith th e p rep aration of tw elv e analog s h av ing dif f erences in th e num b er of unsaturations, arom atic ring sub stituents and in th e am ide m oiety . A nalog s h av ing a sing le doub le-b ond and no sub stituent in th e arom atic ring disp lay ed h ig h er activ ity , w h ile N ,N,-dieth y l analog s disp lay ed h ig h er dose-dep endent activ ity .
Keywords: Pip er, antif ung al, analog s, am ides
Introduction
Pip er ( Pip eraceae) are com m only h erb s, sh rub s or
inf req uently trees w ith ov er 1000 sp ecies describ ed so f ar
m ostly on trop ical reg ions.1 I n addition to th e h ig h econom ical v alue of b lack p ep p er (P. nig rum) as a sp ice, th ere are sev eral m edicinal uses describ ed f or dif f erent Pip er sp ecies, including anti-inf lam m atory , analg esic and treatm ent of snak e-b ite.2,3 Sev eral Pip er sp ecies h av e b een p h y toch em icaly inv estig ated4 -6 and a p leth ora of secondary m etab olites h as b een f ound including k av alactones,7,8 lig noids,9 -11 ch rom enes,12,13
terp enes,14 ,15 p reny lated b enzoic acids13,16 ,17 and also am ides as th e m ost ch aracteristic classes of com p ounds.18,19
T h e m ost p rom inent ex am p le of a Pip er am ide is
p ip erine, th e p ung ent p rincip le of b lack p ep p er (P. nig rum) . I t w as th e irst natural p roduct isolated f rom Pip er sp ecies b ack in 1819 . Pip erine and sev eral oth er am ides h ave sh ow n a v ariety of b iolog ical activ ity , e.g., antitum oral,20-22
ef lux -p um p inh ib itor,23-25 insecticidal19 ,26 -36 and antif ung al activ ity .37-39 T h e im p ortance of insecticidal activ ity of
Pip er am ides is considerab le and th us sev eral studies h av e b een addressed to inv estig ate p ossib le structu re-activ ity relationsh ip s. F or instance, isob uty lam ides h av e sh ow n h ig h activ ity ag ainst dif f erent insects including
M usca domestica,4 0 A edes aeg y p ti36 and A . tog oi4 1 and
com p ounds b earing m eth y lenediox y p h eny l sub stituents can act in som e cases as sy nerg ists f or oth er com p ounds b y
interf ering w ith cy toch rom e P4 5 0-m ediated detox iications of insecticides.34 ,4 2,4 3 I n sp ite of th e occurrence of sev eral antif ung al am ides in p lants,37,38,4 4 ,4 5 th e data concerning th e m ech anism of action f or such com p ounds is scarce and th us a com p reh ensiv e structure-activ ity relationsh ip studies
could not b e p erf orm ed so f ar.
I n th e course of b iop rosp ecting studies f or antif ung al com p ounds f rom Pip eraceae sp ecies,12,37,4 6 -4 8 leaf ex tracts
f rom P. scutif olium disp lay ed sig niicant activ ity ag ainst
th e p h y top ath og enic f ung i Cladosp orium cladosp orioides
A ntif ung al A ctiv ity of Natural and Sy nth etic A m ides f rom Pip er sp ecies J. Braz. Chem. Soc. 1808
a com b ined ch rom atog rap h ic and b ioautog rap h ic m eth od y ielded th e am ides p ip erine (4) , p ip erlong um inine (2e)
and corcov adine (5) ( F ig ure 1) as th e m aj or b ioactiv e
com p ounds. Since th e detection lim it of 1 µg w as com p arab le to th at of p ositiv e controls m iconazole and ny statin, sev eral analog s w ere sy nth esized in order to ev aluate p relim inary structure-activ ity relationsh ip s.
Experimental
G eneral ex p erimental p rocedures
U V sp ectra w ere recorded in a U V/ Visib le Sh im adzu U V-16 01PC sp ectrop h otom eter using C H C l3 as solv ent. I R sp ectra w ere ob tained in a Perk in-E lm er m odel 175 0 sp ectrom eter. 1H NM R sp ectra w ere recorded at 200 and 300 M H z and 13C NM R at 5 0 and 75 M H z in Bruk er D PX -200 and D R X -300 sp ectrom eters, resp ectiv ely . C D C l3 ( A ldrich ) w as used as solv ent and T M S ( A ldrich ) as internal standard. C h em ical sh if ts are rep orted in d units
( p p m ) and coup ling constants (J) in H z. G C L R E I M S w ere
m easured in a Sh im adzu G C -17A ch rom atog rap h interf aced w ith a M S-QP-5 05 0A m ass sp ectrom eter. T em p erature p rog ram m ing w as p erf orm ed f rom 15 0 to 300 °C at
15 °C m in−1, th en isoth erm al at 300 °C f or 5 m in. T h e inj ector and detector tem p eratures w ere 15 0 and 320 °C , resp ectiv ely , and h elium w as used as a carrier g as. A naly tical H PL C w as p erf orm ed using a D ionex C 18 ( 15 0 × 5 m m i.d. × 3 µm ) colum n w ith U VD -D A D 34 0U as a detector. Silica g el ( M erck , 230-4 00 m esh ) and Sep h adex L H -20 ( A m ersh am Biosciences) w ere used f or colum n ch rom atog rap h ic
sep aration, w h ile silica g el 6 0 PF25 4 ( M erck ) w as used f or
analy tical ( 0.25 m m ) and p rep arativ e T L C ( 1.0 m m ) .
Plant material
P. scutif olium J ack . leav es w ere collected in U b atub a,
SP, Brazil, in Sep tem b er 2002 and identif ied b y
D r. E lsie F rank lin G uim arães ( I nstituto de Pesq uisas J ardim Botânico do R io de J aneiro) . Vouch er sp ecim en ( K ato-281)
w as dep osited at th e H erb arium of th e J ardim Botânico do R io de J aneiro, R J , Brazil. D ried f ruits of P. nig rum w ere
p urch ased in th e local m ark et.
E x traction and isolation
D ried and p ow dered leav es of P. scutif olium ( 10 g )
w ere ex h austiv ely ex tracted w ith C H2C l2. C rude ex tract
w as f ractionated th roug h successiv e ch rom atog rap h y as p rev iously describ ed to y ield p ip erlong um inine ( 4 m g , m p 15 7-16 0 °C ) and corcov adine ( 10 m g , m p 14 3-14 5 °C ) .4 7 T o ob tain p ip erine, dried f ruits of
P. nig rum ( 1 k g ) w ere crush ed, ex tracted w ith eth anol and y ielded a cry staline solid af ter concentration under
v acuum . Successiv e recry stallization in E tO H y ielded p ure p ip erine ( 200 m g , m p 129 -131 °C ) w h ich w as identiied b ased on com p arison of 1H and 13C NM R data w ith th ose rep orted.4 9
Sy nthetic p rocedures
C om p ounds 1 c, 1 d, 2c, 2d e 3 c w ere sy nth esized using
W adsw orth -E m m ons according to Strunz and F inlay ’s m eth od.5 0 T h e starting aldeh y des reacted w ith trieth y l-p h osl-p h onocrotonate y ielding eth y l 5 -l-p h eny ll-p entadienoates. T h e ester p roducts w ere th en h y droly sed w ith out f urth er p urif ication to th e corresp onding carb ox y lic acid, ( 2E,4E) -5 -p h eny lp enta-2,4 -dienoic acid (6) and ( 2E,4E)
-5 -( 3-m eth ox y p h eny l) p enta-2,4 -dienoic acid (7) . T h eir
corresp onding ch lorides ob tained b y reaction w ith ox aly l ch loride reacted w ith am ines y ielding th e desired am ides w ith an ov erall y ield of ca. 80% . C om p ounds 1 d and 2d
w ere sy nth esized according to de Paula and co-w ork ers,32
w ith p ip eric acid ob tained f rom p ip erine h y droly sis w h ich w as th en conv erted into th e desired am ides sim ilarly as
ab ov e describ ed. A m ides 1 a, 2a and 3 c w ere p rep ared
N H O O O N H O O O N O O O O O Piperine (4)
Piperlonguminine (2e) Corcovadine (5)
M arq ues et al. 1809 Vol. 21, No. 10, 2010
using com m ercial cinnam ic acid. A m ides 1 b and 3 b w ere
sy nth esized f rom com m ercial b enzo[ d] [ 1,3] diox ole-5 -carb aldeh y de (8) w h ich w as conv erted into (E)
-3-( b enzo[ d] [ 1,3] diox ol-5 -y l) acry lic acid -3-(9) , y ielding th e
am ides as ab ov e describ ed.5 1
Sp ectroscop ic data of 1 a, 1 e, 2a, 3 e, 6 and 9 w ere in accordance w ith th ose p ub lish ed.32,5 1-5 4 C om p ounds 1 d and
3 c w ere not p rev iously describ ed.
( E ) - 3 - ( Benzo[ d] [ 1 ,3 ] diox ol- 5 - y l) - N ,N - diethy lacry lamide (1 b)5 5
Y ield 34 %, colorless oil; I R νm ax/ cm−1 34 5 7, 29 32,
29 01, 16 4 6 , 15 9 8, 15 03, 14 9 1, 1038, 9 29 , 811, 5 23 (K Br) ;
1H NM R ( C D C l
3, 200 M H z) d 1.18 ( t, 3H, J 7.0 H z, H 2”a), 1.25 ( t, 3H, J 7.0 H z, H 2”b ), 3.4 0-3.5 0 ( m , 4 H, H 1”a
and H 1”b ), 5 .9 8 ( s, 2H, O C H2O ), 6 .6 5 ( d, 1H , J 15 .4 H z,
H 2), 6 .79 ( d, 1H , J 8.0 H z, H 6 ’), 7.00 ( d, 1H, J 8.0 H z,
H 5 ’), 7.03 ( s, 1H, H 2’) , 7.6 2 ( d, 1H, J 15 .4 H z, H 3) ;
13C NM R ( C D C l
3, 5 0 M H z) d 13.1 ( C 2”a), 14 .9 ( C 2”b ), 4 0.9 ( C 1”a), 4 2.0 ( C 1”b ), 101.3 ( O C H2O ), 106 .2 ( C 2’),
108.3 ( C 5 ’), 115 .6 ( C 2), 123.5 ( C 6 ’), 129 .7 ( C 1’), 14 1.8
( C 3), 14 8.0 ( C 4 ’), 14 8.7 ( C 3’) , 16 5 .6 ( C = O ) ; E I M S m/ z
24 7 ( M+, 24 % ) , 176 ( 18) , 175 ( 75 ) , 14 5 ( 5 6 ) , 117 ( 35 ) ,
89 ( 100) , 87 ( 11) , 72 ( 28) , 6 5 ( 14 ) , 6 3 ( 5 8) , 6 2 ( 22) , 4 4 ( 26 ) , 4 2 ( 36 ) , 39 ( 33) .
( 2E ,4 E ) - N ,N - D iethy l- 5 - p heny lp enta- 2,4 - dienamide (1 c)5 6
Y ield 5 7%, colorless oil; I R νm ax/ cm−1334 9 , 29 76 , 1711,
16 34 , 14 5 0, 126 8, 1216 , 1127 ( K Br) ; 1H NM R ( C D C l
3, 300 M H z) d 1.17 ( t, 3H, J 6 .9 H z, H 2”a), 1.23 ( t, 3H, J 6 .9 H z, H 2”b ), 3.4 2 ( q , 2H , J 6 .9 H z, H 1”a), 3.4 7 ( q , 2H , J 6 .9 H z, H 1”b ), 6 .4 1 ( d, 1H , J 14 .5 H z, H 2), 6 .80-7.00 ( m,
2H, H 4 and H 4 ’) , 7.24 -7.5 6 ( m , 6 H, H 2’, H 8, H 5 ’, H 6 ’, H 5
and H 3) ; 13C NM R ( C D C l
3, 75 M H z) d 13.2( C 2”a), 15 .0 ( C 2”b ), 4 1.0 ( C 1”a), 4 2.2 ( C 1”b ), 121.3 ( C 2), 126 .3 ( C 4 ),
127.0 ( C 2’ and C 6 ’),128.6 ( C 4 ’), 128.7 ( C 3’ and C 5 ’),
136 .5 ( C 1’), 138.7 ( C 5 ), 14 2.4 ( C 3) , 16 5 .8 ( C 1) ; E I M S m/ z 15 7 ( 12% ) , 129 ( 16 ) , 128 ( 4 3) , 127 ( 22) , 102 ( 17) , 77
( 4 5 ) , 72 ( 32) , 5 6 ( 24 ) , 5 1 ( 36 ) , 4 2 ( 100) , 39 ( 23) .
( 2E ,4 E ) N ,N D iethy l 5 ( 3 methox y p heny l) p enta 2,4 -dienamide (1 d)
Y ield 72% , colorless oil; I R νm ax/ cm−134 05 , 29 74 , 29 37,
29 16 , 16 32, 16 05 , 14 84 , 14 6 2, 126 4 , 104 4 , 784 , 6 87 ( K Br) ;
1H NM R ( C D C l
3, 200 M H z) d 1.13 ( t, 3H, J 7.4 H z, H 2”a), 1.23 ( t, 3H, J 7.4 H z, H 2”b ), 3.4 0 ( t, 2H, J 7.4 H z, H 1”a),
3.5 2 ( t, 2H, J 7.4 H z, H 1”b ), 3.74 ( s, 3H , O C H3), 6 .4 1 ( d, 1H , J 15 .0 H z, H 2), 6 .6 9 -6 .84 ( m , 3H , H 4 ’, H 5 and H 4 ),
6 .89 ( s, 1H, H 2’), 6 .9 7 ( d, 1H , J 7.9 H z, H 6 ’), 7.18 ( t, 1H , J 7.9 H z, H 5 ’) , 7.38 ( dd, 1H , J 15 .0 H z and 9 .1 H z, H 3) ;
13C NM R ( C D C l
3, 5 0 M H z) d 13.2 ( C 2”a), 14 .9 ( C 2”b ),
4 1.0 ( C 1”a), 4 2.2 ( C 1”b ), 5 5 .2 ( O C H3), 111.9 ( C 2’), 114 .4
( C 4 ’), 119 .6 ( C 6 ’), 121.0 ( C 2), 127.2 ( C 5 ), 129 .7 ( C 5 ’),
137.8 ( C 1’), 138.7 ( C 4 ), 14 2.4 ( C 3), 15 9 .8 ( C 3’) , 16 5 .8
( C 1) ; E I M S m/ z 135 ( 5 % ) , 100 ( 10) , 72 ( 5 5 ) , 5 7 ( 30) , 5 5
( 18) , 4 4 ( 6 6 ) , 4 3 ( 100) , 4 2 ( 6 4 ) , 4 1 ( 72) .
( 2E ,4 E ) - N - Isob uty l- 5 - p heny lp enta- 2,4 - dienamide (2 c)5 7
Y ield 79 %, w h ite cry stals, m p 15 4 .2-15 4 .9 °C ;
I R νm ax/ cm−1 329 2, 29 6 1, 16 4 6 , 16 12, 15 4 6 , 14 4 6 , 134 6 ,
116 0, 9 89 , 5 08 ( K Br) ; 1H NM R ( C D C l
3, 300 M H z) d 0.9 4 ( d, 6 H , J 6 .8 H z, H 3”), 1.82 ( h ep t, 1H , J 6 .8 H z, H 2”),
3.19 ( t, 2H, J 6 .6 H z, H 1”), 5 .71 ( sl, 1H, NH ), 5 .9 8 ( d, 1H , J 14 .9 H z, H 2), 6 .83-6 .88 ( m, 2H, H 4 and H 4 ’), 7.24 -7.5 0
( m , 6 H, H 2’, H 3’, H 5 ’, H 6 ’, H 5 and H 3) ; 13C NM R ( C D C l
3, 75 M H z,) d 20.1 ( C 3”), 28.6 ( C 2”), 4 7.0 ( C 1”), 124 .1 ( C 2),
126 .3 ( C 4 ), 126 .9 ( C 2’ and C 6 ’), 128.6 ( C 4 ’), 128.7 ( C 3’
and C 5 ’), 136 .3 ( C 4 ’), 139 .0 ( C 5 ), 14 0.8 ( C 3), 16 6 .1 ( C 1) ;
E I M S m/ z 229 ( M+, 34 % ) , 172 ( 13) , 15 8 ( 12) , 15 7 ( 100) ,
129 ( 35 ) , 128 ( 9 8) , 9 6 ( 4 2) , 4 1 ( 24 ) .
( 2E ,4 E ) N Isob uty l 5 ( 3 methox y p heny l) p enta 2,4 -dienamide (2 d)5 8
Y ield 82%, w h ite cry stals, m p 9 9 .5 -101.0 °C, I R νm ax/ cm−1 3308, 29 5 0, 16 4 4 , 16 09 , 15 78, 14 32, 116 1,
105 2, 9 9 0, 86 3, 5 72 ( K Br) ; 1H NM R ( C D C l
3, 300 M H z) d 0.9 4 ( d, 6 H , J 6 .6, H 3”), 1.83 ( m , 1H , J 6 .6 H z, H 2”),
3.19 ( t, 2H, J 6 .6 H z, H 1”), 3.81 ( s, 3H, O C H3), 5 .9 9 ( d, 1H , J 14 .5 H z, H 2), 6 .75 -6 .9 0 ( m , 3H , H 4 ’, H 5 and H 4 ),
6 .9 5 ( t, 1H , J 2.0 H z, H 2’), 7.03 ( d, 1H , J 7.9 H z, H 6 ’),
7.24 ( t, 1H , J 7.9 H z, H 5 ’) , 7.5 3 ( m , 1H , H 3) ; 13C NM R
( C D C l3, 75 M H z) d 20.1 ( C 3”) , 28.6 ( C 2”) , 4 7.0 ( C 1”) , 5 5 .2 ( O C H3), 112.1 ( C 4 ’), 114 .3 ( C 2’), 119 .6 ( C 6 ’), 124 .2
( C 2), 126 .6 ( C 4 ), 129 .7 ( C 5 ’), 137.7 ( C 1’), 138.9 ( C 5 ),
14 0.7 ( C 3), 15 9 .8 ( C 3’) , 16 6 .0 ( C 1) ; E I M S m/ z 25 9 ( M+,
4 % ) , 187 ( 35 ) , 16 0 ( 11) , 15 8 ( 71) , 14 5 ( 12) , 14 4 ( 5 2) , 115 ( 88) , 77 ( 11) , 6 4 ( 10) , 4 3 ( 100) , 4 1 ( 9 0) , 39 ( 4 1) .
N - Penty lcinnamamide (3 a)5 9
Y ield 9 5 %, w h ite cry stals, m p 87.6 -88.2 °C ;
I R νm ax/ cm−1 3276 , 29 5 5 , 29 33, 16 5 3, 16 07, 15 5 3, 14 75 ,
76 4 , 4 89 ( K Br) ; 1H NM R ( C D C l
3, 200 M H z) d 0.86 ( b rm , 3H, H 5 ”), 1.29 ( b rm , 4 H, H 3” and H 4 ”), 1.5 9 ( b rm , 2H,
H 2”), 3.38 ( q , 2H, J 6 .6 H z, H 1”), 6 .6 8 ( d, 1H , J 15 .8 H z,
H 2), 7.20-7.35 ( m , 4 H , H 3’, H 4 ’, H 5 ’ and NH ), 7.4 1-7.4 6
( m , 2H, H 2’ and H 6 ’), 7.6 4 ( d, 1H , J 15 .8 H z, H 3) ;
13C NM R ( C D C l
3, 5 0 M H z) d 13.7 ( C 5 ”), 22.1 ( C 4 ”), 28.9 ( C 3”), 29 .0 ( C 2”), 39 .6 ( C 1”), 121.3 ( C 2), 127.4 ( C 2’ and
C 4 ’), 128.4 ( C 3’ and C 5 ’), 129 .1 ( C 4 ’), 134 .7 ( C 1’), 139 .9
( C 3) , 16 6 .3 ( C = O ) ; E I M S m/ z 217 ( M+, 4 % ) , 16 0 ( 13) , 14 6
A ntif ung al A ctiv ity of Natural and Sy nth etic A m ides f rom Pip er sp ecies J. Braz. Chem. Soc. 1810
( E ) - 3 - ( Benzo[ d] [ 1 ,3 ] diox ol- 5 - y l) - N - p enty lacry lamide (3 b)5 5
Y ield 4 7% , orang e cry stals, m p 9 9 .8-100.2 °C ; I R νm ax/ cm−1 329 8, 29 34 , 16 5 5 , 16 21, 15 04 , 14 9 1, 9 7, 9 27
( K Br) ; 1H NM R ( C D C l
3, 200 M H z) d 0.79 ( b rm , 3H, H 5 ”), 1.24 ( b rm , 4 H, H 3” and H 4 ”), 1.4 8 ( b rm , 2H, H 2”), 3.28
( q , 2H, J 6 .7 H z, H 1”), 5 .86 ( s, 2H, O C H2O ), 6 .25 ( d, 1H , J 15 .4 H z, H 2), 6 .36 ( sl, 1H, NH ), 6 .6 6 ( d, 1H, J 8.0 H z,
H 6 ’), 6 .85 ( d, 1H, J 8.0 H z, H 5 ’), 6 .88 ( s, 1H, H 2’) , 7.4 3
( d, 1H, J 15 .4 H z, H 3) ; 13C NM R ( C D C l
3, 5 0 M H z) d 13.8 ( C 5 ”), 22.3 ( C 4 ”), 29 .0 ( C 3”), 29 .2 ( C 2”), 39 .7 ( C 1”),
101.2 ( O C H2O ), 106 .2 ( C 2’), 108.3 ( C 5 ’), 119 .1 ( C 2),
123.6 ( C 6 ’), 129 .2 ( C 1’), 14 0.1 ( C 3), 14 8.0 ( C 4 ’), 14 8.8
( C 3’) , 16 6 .2 ( C = O ) ; E I M S m/ z 26 1 ( M+, 19 % ) , 19 0 ( 5 3) ,
176 ( 35 ) , 175 ( 89 ) , 14 5 ( 5 6 ) , 135 ( 4 0) , 117 ( 29 ) , 89 ( 100) , 4 1 ( 5 8) , 39 ( 4 3) .
( 2E ,4 E ) - N - Penty l- 5 - p heny lp enta- 2,4 - dienamide (3 c)
Y ield 80%, y ellow ish cry stals, m p 103.9 -104 .3 °C ;
I Rνm ax/ cm−1 3287, 29 5 8, 16 4 4 , 16 15 , 15 4 7, 14 4 3, 114 6 ,
9 9 8 ( K Br) ; 1H NM R ( 200 M H z, C D C l
3) d 0.9 0 ( t, 3H,
J 6 .6 H z, H 5 ”), 1.25 -1.36 ( m , 4 H, H 3” and H 4 ”), 1.5 5 ( t,
2H, J 6 .6 H z, H 2”), 3.29 ( q , 2H, J 6 .6 H z, H 1”), 5 .77 ( sl,
1H, NH ), 5 .9 8 ( d, 1H , J 14 .9 H z, H 2), 6 .80-6 .89 ( m, 2H,
H 4 and H 5 ), 7.24 -7.5 0 ( m , 6 H, H 3, H 2’, H 3’, H 4 ’, H 5 ’ and
H 6 ’) ; 13C NM R ( C D C l
3, 5 0 M H z) d 13.9 ( C 5 ”), 22.3 ( C 4 ”), 29 .0 ( C 3”), 29 .2 ( C 2”), 39 .6 ( C 1”), 124 .3 ( C 4 ), 126 .3 ( C 2),
126 .9 ( C 2’ and C 6 ’),128.5 ( C 4 ’), 128.6 ( C 3’ and C 3’’),
136 .2 ( C 1’), 138.8 ( C 5 ), 14 0.4 ( C 3), 16 6 .1 ( C 1) ; E I M S m/ z 24 3 ( M+, 28% ) , 186 ( 17) , 15 7 ( 9 0) , 130 ( 23) , 129 ( 5 8) ,
128 ( 100) , 127 ( 4 5 ) , 115 ( 16 ) , 9 6 ( 4 1) , 9 1 ( 11) , 77 ( 22) , 6 4 ( 22) , 5 1 ( 17) , 4 3 ( 20) , 4 1 ( 35 ) , 39 ( 18) .
( 2E ,4 E ) - 5 - ( 3 - M ethox y p heny l) p enta- 2,4 - dienoic acid (7)60
Y ield 88%, colorless needles, m p 135 .9 -136 .6 °C ; I R νm ax/ cm−1 3025 , 3007, 16 87, 16 12, 15 79 , 14 26 , 115 8,
104 4 , 9 9 5 , 873 ( K Br) ; 1H NM R ( C D C l
3, 300 M H z) d 3.84 ( s, 3H, O C H3), 5 .9 9 ( d, 1H , J 15 .2 H z, H 2), 6 .85 -6 .9 5 ( m ,
2H , H 4 ’ and H 4 ), 6 .9 3 ( d, 1H , J 15 .7 H z, H 5 ), 6 .9 9 ( t, 1H , J 2.5 H z, H 2’), 7.07 ( d, 1H , J 7. 8 H z, H 6 ’), 7.28 ( t, 1H , J 7.8 H z, H 5 ’) , 7.5 3 ( ddd, J 15 .2, 7.5 and 2.3 H z, 1H , H 3) ;
13C NM R ( C D C l
3, 75 M H z) d 5 5 .3 ( O C H3), 112.3 ( C 4 ’), 115 .1 ( C 2’), 120.1 ( C 6 ’), 120.4 ( C 2), 126 .2 ( C 4 ), 129 .8
( C 5 ’), 137.2 ( C 1’), 14 1.5 4 ( C 5 ), 14 6 .9 ( C 3), 15 9 .9 ( C 3’) ,
172.3 ( C 1) ; E I M S m/ z 204 ( M+, 6 5 % ) , 15 9 ( 9 8) , 14 4 ( 100) ,
127 ( 4 0) , 115 ( 75 ) , 89 ( 12) , 77 ( 14 ) , 6 3 ( 20) , 5 1 (17) , 39 ( 19 ) .
A ntif ung al assay
T h e m icroorg anism s used in th e antif ung al assay ,
Cladosp orium cladosp orioides ( F resen) de Vries SPC 14 0
and C. sp haerosp ermum ( Perzig ) SPC 4 9 1, h av e b een m aintained at th e I nstituto de Botânica, São Paulo, SP,
Brazil. T h e assay w as carried out f or all am ides and th eir activ ities determ ined as p rev iously describ ed ( T ab le 3) .4 7 Ny statin and m iconazole w ere used as p ositiv e controls w h ereas am p icillin and ch loram p h enicol w ere used as neg ativ e controls.6 1
R esults and D iscussion
Natural am ides w ere isolated th roug h successiv e ch rom atog rap h ic p rocedures as p rev iously describ ed.4 7 A nalog s of ( 2E, 4E) -5 -p h eny lp enta-2,4 -dienam ides and
(E) -cinnam am ides w ere sy nth esized ( F ig ure 2) aim ing at determ ination of ov erall ef f ect of arom atic ring sub stitution
and nitrog en sub stituent in antif ung al activ ity .6 2,6 3 T h e
im p ortance of th e am ide m oiety f or th e antif ung al activ ity w as inv estig ated b y rep lacing th e natural p ip eridy l, isob uty l
or its acety lated deriv ativ e b y N ,N -dieth y l or N -p enty l analog s.
T h e rep lacem ent of isob uty l or p ip eridy l m oieties b y dieth y l g roup s in ( 2E, 4E) -5 -p h eny lp enta-2,4 -dienam ides
resulted in a noticeab le increase in th e dose-resp onse activ ity , as ob serv ed f or am ides 1 a, 1 b, 1 c, 1 d and 1 e ag ainst C. cladosp orioides and C. sp haerosp ermum.
Som e selectiv ity w as detected b etw een th e tw o strains in w h ich C. sp haerosp ermum w as m ore sensib le to 1 e th an
C. cladosp orioides ( T ab le 1) .
A naly sis of arom atic sub stitution p attern indicated
th at am ides h av ing m eth y lenediox y or m eth ox y l g roup s disp lay ed low er antif ung al p otency w h en com p ared to th ose h av ing no sub stituents. T h e am ides 2a, 2c, 3 a and
3 c disp lay ed h ig h er activ ities at 1 µg ag ainst b oth strains w h ile 1 d, 3 b and 3 e w ere th e least activ e am ong all am ides assay ed ag ainst C. cladosp orioides. A t low er concentrations
N -isob uty l and N-p enty l deriv ativ es (2a-2e, 3 a-3 c and 3 e)
sh ow ed h ig h er activ ity . I n th is case, th ere is an ap p arent p ositiv e correlation w ith th e lip op h y licity and th e am ides h av ing a α,β-conj ug ated carb ony l w ere m ore activ e th an th ose h av ing an ex tended α,β,γ,d-conj ug ated sy stem .
C onclusions
I nv estig ation of natural and sy nth etic am ides as antif ung al com p ounds h av e sh ow n p relim inary structure-activ ity relationsh ip in w h ich N ,N-dieth y l sh ow ed h ig h er dose dep endent activ ity w h ile N-p enty l and N -isob uty l
M arq ues et al. 1811 Vol. 21, No. 10, 2010
NR3R4
O
R1
R2
n
1a: n = 1; R1 = R2 = H; R3 = R4 = Et 1b: n = 1; R1+R2 = O
2CH2; R3 = R4 = Et
1c: n = 2; R1 = R2 = H; R3 = R4 = Et 1d: n = 2; R1 = OMe; R2 = H;R3 = R4 = Et 1e: n = 2; R1 + R2 = O
2CH2; R3 = R4 = Et
2a: n = 1; R1 = R2 = H; R3 = i-Bu; R4 = H 2c: n = 2; R1 = R2 = H; R3 = i-Bu; R4 = H 2d: n = 2; R1 = OMe; R2 = H; R3 = i-Bu; R4 = H 2e: n = 2; R1 + R2 = O
2CH2; R3 = i-Bu; R4 = H
3a: n = 1; R1 = R2 = H; R3 = pentyl; R4 = H 3b: n = 1; R1 + R2 = O
2CH2; R3 = pentyl; R4 = H
3c: n = 2; R1 = R2 = H; R3 = pentyl; R4 = H 3e: n = 2; R1 + R2 = O
2CH2; R3 = pentyl; R4 = H
4: n = 2; R1 + R2 = OCH
2O; R3 + R4 = piperidyl
5: n = 2; R1 + R2 = O
2CH2; R3 = CH2C(OCOCH3)(CH3)2; R4 = H
F igure 2. Sy nth etic and natural am ides ev aluated as antif ung al.
O
N
R3 R2
R1
Single double bond slightly increases activity
Diethyl amide activity is dose dependent while isobutyl shows higher activity at lower concentrations
Non substituted aromatic rings show higher activity
F igure 3. Prelim inary structure-activ ity relationsh ip f or antif ung al am ide.
T ab le 1 . A ntif ung al activ ity of natural and sy nth etic com p ounds
NR3R4
O
R1
R2
n
n R1 R2 R3 R4 Cladosporium cladosporioides# Cladosporium sphaerospermum#
100 50 25 10 5 1 100 50 25 10 5 1
1 a 1 H H E t E t * * * * * * * * * * * * * * * * * * * * * * * * * * *
-1 b 1 O2C H2 E t E t * * * * * * * * * * * * * * * * * * * * * * * * * * * * *
1 c 2 H H E t E t * * * * * * * * * * * * * * * * * * * * * * * * * * *
-1 d 2 O M e H E t E t * * * * * * * * * * - * * * * * * * * * *
-1 e 2 O2C H2 E t E t * * * * * * * * - * * * * * * * * * * *
2a 1 H H iBu H * * * * * * * * * * * * * * * * * * * * * * *
2c 2 H H iBu H * * * * * * * * * * * * * * * * * * * * * * *
2d 2 O M e H iBu H * * * * * * * * * * * * * * * * * * * *
2 e 2 O2C H2 iBu H * * * * * * * * * * * * * * * * * * * * * *
3 a 1 H H p enty l H * * * * * * * * * * * * * * * * * * * * * * * *
3 b 1 O2C H2 p enty l H * * * * * * * * * * * * * * *
3 c 2 H H p enty l H * * * * * * * * * * * * * * * * * * * * * * * *
3 e 2 O2C H2 p enty l H * * * * * - * * * * * *
4 2 O2C H2 p ip eridy l * * * * * * * * * * * * * * * * * * * * * *
5 2 O2C H2 C H2C ( O C O C H3) ( C H3)2 H * * * * * * * * * * * * * * * * * * * * * *
A ntif ung al A ctiv ity of Natural and Sy nth etic A m ides f rom Pip er sp ecies J. Braz. Chem. Soc. 1812
F urth er inv estig ations including q uantitativ e b iolog ical
activ ity assessm ent and determ ination of p h y sicoch em ical descrip tors are req uired f or a th oroug h understanding of th e ob serv ed antif ung al activ ities and in th e dev elop m ent of ef f ectiv e antif ung al ag ents.
Supplementary Information
T h e sp ectral data are av ailab le f ree of ch arg e at h ttp : / / j b cs.sb q .org .b r, as p df ile.
Ack nowledgments
A uth ors th ank F A PE SP, C NPq and C A PE S f or inancial sup p ort.
R eferences
1. J aram illo, M . A .; M anos, P. S.; A m. J. Bot. 20 0 1, 8 8, 706 .
2. D i Stasi, L . C .; O liv eira, G . P.; C arv alh aes, M . A .; Queiroz J r, M .; T ien, O . S.; K ak inam i, S. H .; R eis, M . S.; F itoterap ia 20 0 2,
7 3, 6 9 .
3. G iorg etti, M .; Neg ri, G .; R odrig ues, E .; J. E thnop harmacol.
20 0 7, 1 0 9, 338.
4 . K ato, M . J .; F urlan, M .; Pure A p p l. Chem. 20 0 7, 7 9, 5 29 .
5 . Parm ar, V. S.; J ain, S. C .; G up ta, S.; T alw ar, S.; R aj w ansh i, V. K .; K um ar, R .; A zim , A .; M alh otra, S.; K um ar, N.; J ain, R .; Sh arm a,
N. K .; T y ag i, O . D .; L aw rie, S. J .; E rring ton, W .; H ow arth , O . W .; O lsen, C . E .; Sing h , S. K .; W eng el, J .; Phy tochemistry 1 9 9 8,
4 9, 106 9 .
6 . Parm ar, V. S.; J ain, S. C .; Bish t, K . S.; J ain, R .; T anej a, P.; J h a, A .; T y ag i, O . D .; Prasad, A . K .; W eng el, J .; O lsen, C . E .; Boll, P. M .; Phy tochemistry 1 9 9 7, 4 6, 5 9 7.
7. X uan, T . D .; F uk uta, M .; W ei, A . C .; E lzaaw ely , A . A .; K h anh , T . D .; T aw ata, S.; J. N at. M ed. 20 0 8, 62, 188.
8. W h itton, P. A .; L au, A .; Salisb ury , A .; W h iteh ouse, J .; E v ans, C . S.; Phy tochemistry 20 0 3, 64, 6 73.
9 . C h en, Y .-C .; L iao, C .-H .; C h en, I .-S.; Phy tochemistry 20 0 7, 68, 2101.
10. Bodiw ala, H . S.; Sing h , G .; Sing h , R .; D ey , C . S.; Sh arm a, S. S.; Bh utani, K . K .; Sing h , I . P.; J. N at. M ed. 20 0 7, 61, 4 18.
11. M artins, R . C .; L atorre, L . R .; Sartorelli, P.; Kato, M . J .; Phy tochemistry 20 0 0, 5 5, 84 3.
12. M orandim , A . A .; Berg am o, D . C . B.; K ato, M . J .; C av alh eiro, A . J .; Bolzani, V. S.; F urlan, M .; Phy tochem. A nal. 20 0 5, 1 6,
282.
13. Baldoq ui, D . C .; K ato, M . J .; C av alh eiro, A . J .; Bolzani, V. S.; Y oung , M . C . M .; F urlan, M .; Phy tochemistry 1 9 9 9, 5 1, 89 9 .
14 . Baldoq ui, D . C .; Bolzani, V. S.; F urlan, Mo, M.; K at. J .; M arq ues, M . O . M .; Quim. N ova 20 0 9, 3 2, 1107.
15 . Péres, V. F .; M oura, D . J .; Sp erotto, A . R . M .; D am asceno, F . C .; C aram ão, E . B.; Z ini, C . A .; Saf i, J .; F ood Chem. T ox icol.
20 0 9, 4 7, 2389 .
16 . L ag o, J . H . G .; C h en, A .; Y oung , M . C . M .; G uim arães, E . F .; O liv eira, A .; K ato, M . J .; Phy tochem. L ett. 20 0 9, 2, 9 6 .
17. R am os, C . S.; K ato, M . J .; J. Braz. Chem. Soc. 20 0 9, 20, 5 6 0.
18. C oting uib a, F .; R eg asini, L . O .; Bolzani, V. S.; D eb onsi, H . M .; Passerini, G . D .; C icarelli, R . M . B.; K ato, M . J .; F urlan, M .; M ed. Chem. R es. 20 0 9, 1 8, 703.
19 . Sriniv asan, K .; Crit. R ev. F ood Sci. N utr. 20 0 7, 4 7, 735 .
20. Bezerra, D . P.; M oura, D . J .; R osa, R . M .; Vasconcellos, M . C .; Silv a, A . C . R .; M oraes, M . O .; Silv eira, E . R .; L im a, M . A . S.; H enriq ues, J . A . P.; C osta-L otuf o, L . V.; Saf i, J .; M utat. R es./
G enet. T ox icol. E nviron. M utag en. 20 0 8, 65 2, 16 4 .
21. Bezerra, D . P.; Pessoa, C .; M oraes, M . O .; A lencar, N. M . N.;
M esq uita, R . O .; L im a, M . W .; A lv es, A . P. N.; Pessoa, O . D . L .; C h av es, J . H .; Silv eira, E . R .; C osta-L otuf o, L . V.; J. A p p l.
T ox icol. 20 0 8, 28, 5 9 9 .
22. L iu, D .; M eng , Y .; Z h ao, J .; C h en, L .; Chem. R es. Chin. Univ.
20 0 8, 24, 4 2.
23. M ich alet, S.; C artier, G .; D av id, B.; M ariotte, A . M .; D ij oux F ranca, M . G .; K aatz, G . W .; Stav ri, M .; G ib b ons, S.; Bioorg . M ed. Chem. L ett. 20 0 7, 1 7, 175 5 .
24 . Sang w an, P. L .; K oul, J . L .; K oul, S.; R eddy , M . V.; T h ota, N.; K h an, I . A .; K um ar, A .; K alia, N. P.; Qazi, G . N.; Bioorg . M ed.
Chem. 20 0 8, 1 6, 9 84 7.
25 . T h ota, N.; K oul, S.; R eddy , M . V.; Sang w an, P. L .; K h an, I . A .; K um ar, A .; R aj a, A . F .; A ndotra, S. S.; Qazi, G . N.; Bioorg .
M ed. Chem. 20 0 8, 1 6, 6 5 35 .
26 . Bezerra, D . P.; C astro, F . O .; A lv es, A . P. N.; Pessoa, C .; M oraes,
M . O .; Silv eira, E . R .; L im a, M . A . S.; E lm iro, F . J . M .; C osta-L otuf o, osta-L . V.; Braz. J. M ed. Biol. R es. 20 0 6, 3 9, 801.
27. Bina, S. S.; T ah sin, G .; Sab ira, B.; F arh ana, A .; F ouzia, A . S.; N at. Prod. R es. 20 0 5, 1 9, 14 3.
28. C h ristodoulop oulou, L .; T souk atou, M .; T ziv elek a, L . A .; Vag ias, C .; Petrak is, P. V.; R oussis, V.; J. A g ric. F ood Chem.
20 0 5, 5 3, 14 35 .
29 . K h an, I . A .; M irza, Z . M .; K um ar, A .; Verm a, V.; Qazi, G . N.; A ntimicrob . A g ents Chemother. 20 0 6, 5 0, 810.
30. L ee, S. A .; H ong , S. S.; H an, X . H .; H w ang , J . S.; O h , G . J .; L ee, K . S.; L ee, M . K .; H w ang , B. Y .; R o, J . S.; Chem. Pharm.
Bull. 20 0 5, 5 3, 832.
31. Nav ick iene, H . M . D .; Bolzani, V. S.; K ato, M . J .; Pereira, A . M . S.; Bertoni, B. W .; F rança, S. C .; F urlan, M .; Phy tochem.
A nal. 20 0 3, 1 4, 281.
32. Paula, V. F .; Barb osa, L . C . A .; D em uner, A . J .; Piló-Veloso, D .; Picanço, M . C .; Pest M anag e. Sci. 20 0 0, 5 6, 16 8.
33. R ib eiro, T . S.; L im a, L . F .; Prev iato, J . O .; Prev iato, L . M .; H eise, N.; L im a, M . E . F .; Bioorg . M ed. Chem. L ett. 20 0 4, 1 4, 35 5 5 .
M arq ues et al. 1813 Vol. 21, No. 10, 2010
35 . Brow n, D .; Z h ang , L .; W en, Z .; Scott, J . G .; A rch. Insect Biochem. Phy siol. 20 0 3, 5 4, 212.
36 . Sim as, N. K .; L im a, E . C .; K uster, R . M .; L ag e, C . L . S.; O liv eira F ilh o, A . M .; R ev. Soc. Bras. M ed. T rop . 20 0 7, 4 0, 4 05 .
37. A lecio, A . C .; Bolzani, V. S.; Y oung , M . C . M .; K ato, M . J .; F urlan, M .; J. N at. Prod. 1 9 9 8, 61, 6 37.
38. C unico, M . M .; M ig uel, O . G .; M ig uel, M . D .; C arv alh o, J . L . S.; Peitz, C .; A uer, C . G .; G rig oletti J r., A .; V isão A cadêmica
20 0 3, 4, 77.
39 . Silv a, R . V.; Nav ick iene, H . M . D .; K ato, M . J .; Bolzani, V. S.; M eda, C . I .; Y oung , M . C . M .; F urlan, M .; Phy tochemistry
20 0 2, 5 9, 4 79 .
4 0. E lliott, M .; F arnh am , A . W .; J anes, N. F .; J oh nson, D . M .; Pulm an, D . A .; Saw ick i, R . M .; A g ric. Biol. Chem. 1 9 8 6, 5 0, 134 7.
4 1. Park , I . K .; L ee, S. G .; Sh in, S. C .; Park , J . D.; A h n, Y . J .; J. A g ric. F ood Chem. 20 0 2, 5 0, 186 6 .
4 2. Pring , B. G .; J. Chem. Soc., Perk in T rans.1 1 9 8 2, 14 9 3. 4 3. Scott, I . M .; Puniani, E .; D urst, T .; Ph elp s, D .; M erali, S.;
A ssab g ui, R . A .; Sanch ez-Vindas, P.; Pov eda, L .; Ph ilog ène, B. J . R .; A rnason, J . T .; A g ric. F orest E ntomol. 20 0 2, 4, 137.
4 4 . Nav ick iene, H . M . D .; A lécio, A . C .; K ato, M . J.; Bolzani, V. S.; Y oung , M . C . M .; C av alh eiro, A . J .; F urlan, M .; Phy tochemistry
20 0 0, 5 5, 6 21.
4 5 . Silv a, R . V.; Nav ick iene, H . M . D .; K ato, M . J .; Bolzani, V. S.; M eda, C . I .; Y oung , M . C . M .; F urlan, M .; Phy tochemistry
20 0 2, 5 9, 5 21.
4 6 . L ag o, J . G . L .; R am os, C . S.; C asanov a, C . C . D .; M orandim , A . A .; Berg am o, C . D .; C av alh eiro, A . J .; Bolzani, V. S.; F urlan,
M .; G uim arães, E . F .; Y oung , M . C . M .; K ato, M . J .; J. N at. Prod. 20 0 4, 67, 1783.
4 7. M arq ues, J . V.; K itam ura, R . O . S.; L ag o, J . H. G .; Y oung , M . C . M .; G uim arães, E . F .; K ato, M . J .; J. N at. Prod. 20 0 7, 7 0, 2036 .
4 8. Sartorelli, P.; Y oung , M . C . M .; K ato, M . J .; Phy tochemistry
1 9 9 8, 4 7, 1003.
4 9 . C h en, Z .; W u, J . B.; Z h ang , J .; L i, X . J .; Sh en, M . H .; Sep . Sci. T echnol. 20 0 9, 4 4, 1884 .
5 0. Strunz, G . M .; F inlay , H .; T etrahedron 1 9 9 4, 5 0, 11113. 5 1. M itra, A . K .; D e, A .; K arch audh uri, N.; Sy nth. Commun. 1 9 9 9,
29, 5 73.
5 2. C oncellón, J . M .; Pérez-A ndrés, J . A .; R odríg uez-Solla, H .; Chem.- - E ur. J. 20 0 1, 7, 306 2.
5 3. H uang , Z . Z .; W en, L . W .; H uang , X .; Sy nth. Commun. 1 9 9 0, 20, 25 79
5 4 . Sch auer, D . J .; H elq uist, P.; Sy nthesis 20 0 6, 36 5 4 .
5 5 . Z h ang , X . H .; L i, R . L .; C ai, M . S.; Beij ing M ed. Colleg e T rans.
1 9 8 0, 1 2, 83. ( C A 9 3: 14 26 76 ) .
5 6 . Step anov a, O . S.; M azurenk o, G . A .; T h o, N. V.; D erk ach , N.; F iziolog ichesk i A k tivny e V eshchestva 1 9 7 6, 8, 86 . ( C A 86 : 189 4 22) .
5 7. X iao, W . J .; Sh i, L . L .; C h en, Z . Q.; H uang , Y . Z .; L ang , S. A .; H eteroat. Chem. 1 9 9 0, 1, 24 5 .
5 8. Vig , B.; K anw ar, R .; Sing h , V.; Indian J. Chem., Sect. B: O rg . Chem. Incl. M ed. Chem. 1 9 7 7, 1 5 B, 104 8.
5 9 . D elaney , A . D .; C urrie, D . J .; H olm es, H . L .; Can. J. Chem.
1 9 6 9, 4 7, 3273.
6 0. K h an, A . M .; Proctor, G . R .; R ees, L .; J. Chem. Soc., C 1 9 6 6, 9 9 0.
6 1. H om ans, A . L .; F uch s, A .; J. Chromatog r., A 1 9 7 0, 5 1, 327. 6 2. H arm ath a, J .; Naw rot, J .; E ntomol. E x p . A p p l. 20 0 2, 1 0 4, 5 1.
6 3. M iy azaw a, M .; Y osh io, K .; I sh ik aw a, Y .; K am eok a, H .; J. A g ric. F ood Chem. 1 9 9 8, 4 6, 19 14 .
Sub mitted: F eb ruary 26, 20 0 9
Pub lished online: June 1 1 , 20 1 0
Su
pp
le
m
enta
ry
Inf
or
m
ati
on
J. Braz. Chem. Soc., Vol. 21, No. 10, S1-S10, 2010.
Printed in Brazil - ©2010 Sociedade Brasileira de Química 0103 - 5053 $6.00+0.00
*e-mail: majokato@iq.usp.br
Antifungal Activity of Natural and Synthetic Amides from Piper species
Joaquim V. Marques,a Alberto de Oliveira,a,b Ludmila Raggi,c Maria C. M. Youngc and
Massuo J. K atoa*
aInstituto de Química, Universidade de São Paulo, CP 260 7 7 , 0 5 5 1 3 - 9 7 0 São Paulo- SP, Brazil
bInstituto de Química, Universidade F ederal de Ub erlândia, CP 5 9 3 , 3 8 4 0 0 - 9 0 2 Ub erlândia- M G , Brazil
cSeção de F isiolog ia e Bioq uímica de Plantas, Instituto de Botânica, CP 4 0 0 5 , 1 0 0 5 1 São Paulo- SP, Brazil
Figure S1. 1H NMR (CDCl
Antifungal Activity of Natural and Synthetic Amides from Pip er species J. Braz. Chem. Soc. S2
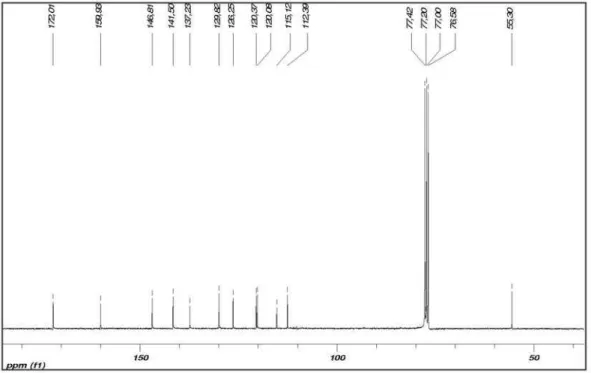
Figure S2. 13C NMR (CDCl
3, 50 MHz) of 1b.
Figure S3. 1H NMR (CDCl
Marques et al. S3 Vol. 21, No. 10, 2010
Figure S4. 13C NMR (CDCl
3, 50 MHz) of 1c.
Figure S5. 1H NMR (CDCl
Antifungal Activity of Natural and Synthetic Amides from Pip er species J. Braz. Chem. Soc. S4
Figure S6. 13C NMR (CDCl
3, 50 MHz) of 1d.
Figure S7. 1H NMR (CDCl
Marques et al. S5 Vol. 21, No. 10, 2010
Figure S8. 13C NMR (CDCl
3, 50 MHz) of 2c.
Figure S9. 1H NMR (CDCl
Antifungal Activity of Natural and Synthetic Amides from Pip er species J. Braz. Chem. Soc. S6
Figure S10. 13C NMR (CDCl
3, 50 MHz) of 2d.
Figure S11. 1H NMR (CDCl
Marques et al. S7 Vol. 21, No. 10, 2010
Figure S12. 13C NMR (CDCl
3,50 MHz) of 3a.
Figure S13. 1H NMR (CDCl
Antifungal Activity of Natural and Synthetic Amides from Pip er species J. Braz. Chem. Soc. S8
Figure S14. 13C NMR (CDCl
3, 50 MHz) of 3b.
Figure S15. 1H NMR (CDCl
Marques et al. S9 Vol. 21, No. 10, 2010
Figure S16. 13C NMR (CDCl
3, 50 MHz) of 3c.
Figure S17. 1H NMR (CDCl
Antifungal Activity of Natural and Synthetic Amides from Pip er species J. Braz. Chem. Soc. S10
Figure S18. 13C NMR (CDCl