w w w . r b h h . o r g
Revista
Brasileira
de
Hematologia
e
Hemoterapia
Brazilian
Journal
of
Hematology
and
Hemotherapy
Review
article
The
role
of
magnetic
resonance
imaging
in
the
evaluation
of
transfusional
iron
overload
in
myelodysplastic
syndromes
Emmanouil
Petrou
a,∗,
Sophie
Mavrogeni
a,
Vasiliki
Karali
b,
Genovefa
Kolovou
a,
Marie-Christine
Kyrtsonis
b,
Petros
P.
Sfikakis
b,
Panayiotis
Panayiotidis
baOnassisCardiacSurgeryCenter,Athens,Greece
bAthensUniversityMedicalSchool,Athens,Greece
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received11November2014 Accepted31March2015 Availableonline19May2015
Keywords:
Myelodysplasticsyndromes Bloodtransfusion
Ironoverload Magnetic
a
b
s
t
r
a
c
t
Myelodysplasticsyndromesrepresentagroupofheterogeneoushematopoieticneoplasms derivedfromanabnormalmultipotentprogenitorcell,characterizedbyahyperproliferative bonemarrow,dysplasiaofthecellularhemopoieticelementsandineffectiveerythropoiesis. Anemiaisacommonfindinginmyelodysplasticsyndromepatients,andbloodtransfusions aretheonlytherapeuticoptioninapproximately40%ofcases.Themostserioussideeffect ofregularbloodtransfusionisironoverload.
Currently, cardiovascular magnetic resonanceusing T2is routinely used to identify patientswithmyocardialironoverloadandtoguidechelationtherapy,tailoredtoprevent irontoxicityintheheart.Thisisamajorvalidatednon-invasivemeasureofmyocardial ironoverloadingandissuperiortosurrogatessuchasserumferritin,liveriron,ventricular ejectionfractionandtissueDopplerparameters.
Theindicationforironchelationtherapyinmyelodysplasticsyndromepatientsis cur-rentlycontroversial.However,cardiovascularmagneticresonancemayofferanexcellent non-invasive,diagnostictoolforironoverloadassessmentinmyelodysplasticsyndromes. Furtherstudiesareneededtoestablishthepreciseindicationsofchelationtherapyandthe clinicalimplicationsofthistreatmentonsurvivalinmyelodysplasticsyndromes.
©2014Associac¸ãoBrasileiradeHematologia,HemoterapiaeTerapiaCelular.Published byElsevierEditoraLtda.Allrightsreserved.
Introduction
Myelodysplastic syndrome (MDS) comprises an acquired primitive stem cell disorder resulting in ineffective hematopoiesismanifestedbyvariabledegreesandnumbers
∗ Correspondingauthorat:DivisionofCardiology,OnassisCardiacSurgeryCenter,356SyggrouAve.,GR-17674,Athens,Greece.
E-mailaddress:emmgpetrou@hotmail.com(E.Petrou).
ofcytopenias,aswellasanincreasedriskoftransformation toacuteleukemia.MDSisrelativelycommonwithareported incidence of 3.5–4.9 per 100,000 people.1 The incidence increasesto28–36per100,000inover80-year-oldindividuals, making it ascommon asmyelomain thisage group.2 Red blood cell (RBC) transfusions comprise the most effective
http://dx.doi.org/10.1016/j.bjhh.2015.03.014
treatmentofanemiainMDSpatients,intheexpenseoforgan damagingironoverload.
Magneticresonanceimaging(MRI)hasbeensuccessfully usedfortheevaluationofmyocardialandliverironoverload. MRIistheonlytechniqueabletoprovidenon-invasive infor-mationaboutironoverload,aswellasmicrocirculationdefects andthedetectionofmyocardialscars.
Myelodysplastic
syndromes
MDSrepresentagroupofheterogeneoushematopoietic dis-ordersderivedfromanabnormalmultipotentprogenitorcell, characterizedbyahyperproliferative bonemarrow, dyspla-sia of the cellular hemopoietic elements, and ineffective hematopoiesis (Figure 1). Peripheral blood cytopenias and markedmorphologicdysplasiasare prominentand ineffec-tiveerythropoiesisresultsinsymptomaticanemia.3Cellular dysfunctionresultsinanincreasedriskofinfection,bleeding tendencyduetothrombocytopenia,andneedfortransfusions in most MDS patients.4 MDS can be classified as primary (idiopathic)or secondary (therapy-related), the latterbeing associatedwithpriorradiotherapy,chemotherapeuticagents, andimmunosuppressiontherapy.5OtherriskfactorsforMDS developmentincludebenzeneexposure,occupational chemi-cals,tobaccoexposure,excessivealcohol,viralinfections,and autoimmunedisorders,aswellaschronicinflammation.5 A usefulclassificationofMDSaccordingtotheirpathogenesis, cytological features and specific karyotypes, was proposed initially by the French-American-British (FAB) Cooperative StudyGroup.6Morerecently,theWorldHealthOrganization (WHO)workedoutanupdatedclassificationthatrepresents anextensionoftheFABproposal,withseveralmodifications.7 Alterations in many individual biological pathways have beenimplicatedinMDS pathophysiology.However,the pri-maryhypothesisinvolvesaninitialdeleteriousgeneticevent withinahematopoieticstem cell,subsequent development of excessive cytokines/inflammatory response leading to a proapoptotic/proliferative state, resulting in peripheral cytopeniasdespiteahypercellularbonemarrow.Furthermore,
the presence of detectable cytogenetic abnormalities in approximately40–70%ofpatientswithprimaryMDSandover 80%withsecondaryMDS,aswellasthevalidatedprognostic valueofspecificcytogeneticaberrationsinMDS,supportsthe theoryofanincidentalgeneticevent.8
Anemia,
transfusion
and
iron
overload
in
myelodysplastic
syndrome
patients
A limited number ofeffective treatmentoptions are avail-abletotreatanemiaandthushelptopreventironoverload and other transfusion-related sideeffects inMDS patients. A direct approach is to correct anemia by administering hematopoieticgrowthfactors,i.e.erythropoietinwithor with-out granulocyte-colony stimulating factor (G-CSF).9 Other drugs,suchaslenalidomide,cyclosporine-Aand antithymo-cyteglobulinactincertainsubgroupsofMDSpatientsandmay improve or correctanemia.10–12 Allogeneic stem cell trans-plantationistheonlycurativeapproach.13
RBC transfusions are considered in MDS patients when hemoglobin(Hb) <8g/dL,and may providetemporary relief fromthesymptomsofanemia,buttheyalsoaddextrairon tothebody.14And whilethereare therapies,asmentioned above,thatcanrestoretheproductionofRBCsothatpatients canbecometransfusionindependent,theyare noteffective inall MDSpatients. Infact, forapproximately40% ofMDS patients,transfusionsaretheonlyoptiontotreatthe symp-tomsofanemia.4
SupportivetherapywithregularRBCtransfusionscanlead to elevated levels of iron in the blood and other tissues. The actual prevalence of ironoverload in transfused MDS patientshasnotbeensystematicallydocumented.15Eachunit ofpackedRBCcontainsabout250mgofiron.Asageneralrule, ironoverloadoccursafterthetransfusionof20unitsofRBC.15 Thus,MDSpatientswhoreceivetransfusionsfortheiranemia areatriskforironoverload.Inadditiontoironoverloadasa resultofmultipletransfusions,MDSpatientswith sideroblas-ticanemiamaydevelopironoverloadsubsequenttoexcessive absorptionofironfromfood.16
MDS patients considered to be eligible for iron chela-tion therapy are transplant recipient candidates. In recent years,differentstudies highlighted ironoverloadasa neg-ative prognosticindicator inpatients undergoingstem cell transplantation.Inacohortof590patientswhounderwent myeloablativestemcelltransplantation,Armandetal.found astrongnegativeassociationbetweenhighserumferritin lev-elsandoverallanddisease-freesurvival.Thisassociationwas seeninpatientsaffectedeitherbyacutemyeloidleukemiaor MDS.17
Iron
overload
and
survival
in
myelodysplastic
syndrome
Transfusiondependency isassociatedwithshortened over-allsurvivalandleukemia-freesurvivalinMDS;however,itis notclearwhetherthiseffectismediatedbytransfusionaliron overloaditselforifneedforRBC transfusionisamarkerof diseaseseverity.18Thecontributionofanemiaitselftocardiac dysfunctioninapredominantlyolderpatientpopulationas wellaslackofconsiderationintheInternationalPrognostic ScoringSystem(IPSS)oftheseverityofanemiaare confound-ingfactorsthatmakethisevaluationdifficult.Furthermore, worseningofsurvivalwithincreasingserumferritin values hasbeenobservedinpatientswithrefractoryanemia, refrac-toryanemiawithringedsideroblasts,and5q-typesofMDS, but notinpatients withrefractorycytopenia and multilin-eagedysplasia,suggestingthatthelongevityorotherfactors inpatientswiththeaforementionedsubtypesmakethem sus-ceptibleto theadverse effectsofiron overload.19 However, itmustbenotedthatall thisevidenceisindirectand large prospectivestudies thatcorrelate accuratemarkers ofiron overload,such MRImeasurements oftissue ironand non-transferrin-bound iron (NTBI)/labile plasma iron (LPI) with survivalarenecessarytoconclusivelydeterminetheimpact ofironoverloadonsurvivalinMDS.
In addition to negatively impacting prognosis, anemia andtransfusiondependencehavesignificantimpactonthe quality oflifeofMDS patients. Despite the effects of ane-miaandtransfusion dependenceon diseaseoutcomes and patient quality of life,the clinical impact ofiron overload inMDS patients remainscontroversial.20 Studies of hered-itary hemoglobinopathies (e.g. -thalassemia) have shown causationforironoverloadandorgantoxicities.21Clinical con-sequences oftransfusion ironoverload innon-thalassemic adults have been previously reported by Schafer et al.22 These authors also reported that long-term deferoxamine ironchelationtherapywaseffectivenotonlyinretardingbut evenreversing organ damagecaused by parenchymal iron overload.23However,evidencelinkingorganiron accumula-tionwithmorbidityinMDSisindirect.Inastudyofrefractory anemiawithringedsideroblasts,itwasfoundthatmildiron overloadwascommonatpresentation,butclinical manifes-tationsoccurredonlyinpatientswhohadaregularneedfor RBC transfusions.Complications ofiron overloadwere the mostcommon causes ofdeath.24 More recently,the effect oftransfusiondependence andsecondary ironoverloadon survivalofMDS patients, classifiedaccording tothe World HealthOrganization(WHO)criteria,werealsostudied.Overall,
transfusiondependencewasfoundtosignificantlyworsenthe probability of survivaland toincrease the risk of progres-siontoleukemiainMDSpatients.Aninverserelationshipwas observedbetweentransfusionsrequirementandprobabilityof survival.Thenegativeimpactoftransfusiondependencywas morepronouncedinpatientswithrefractoryanemia, refrac-toryanemiawithringedsideroblastsandMDSwithisolated deletion5q.Overall,themostcommonnon-leukemiccause of deathwas heart failure. Transfusional ironoverload, as assessedbyserum ferritin,wasassociatedwithworse sur-vivalinpatientsreceivingregularRBCtransfusions.Theeffect ofironoverloadwasmainlynoticeableamongpatientswith refractoryanemia,whohaveamediansurvivalofmorethan fiveyearsandaremorepronetodeveloplong-termtoxicityof ironoverload.25Theseobservationsindicatethatthe develop-mentofsecondaryironoverloadperseworsensthesurvivalof subgroupsoftransfusiondependentpatientswithMDS. Find-ings ofaretrospective,singleinstitutionstudy suggestthat iron overloadsignificantlycontributes totreatment related mortalityinMDS.Finally,ironoverloadinMDSpatients under-goingallogeneicstemcelltransplantationmaybeassociated withadverseoutcomes.26
Small studies using cardiovascular magnetic resonance (CMR)techniqueshaveshownvariable andinfrequent inci-dence of cardiac iron accumulation. Regardless of organ damage,ironoverloadmayincreaseriskofinfectionby sup-plyingreadilyavailableirontosupportmicroorganismgrowth, whileseveralretrospectivestudieshavesuggestedthat trans-fusiondependenceinfluencessubsequentoverallsurvivaland evolution toleukemia.27 Additionalprospectivetrialsusing accurateironoverloadmarkersare requiredtoconclusively determinetheimpactofironoverloadonoverallsurvivalin patientswithMDS.
Management
of
transfusion-related
iron
overload
in
myelodysplastic
syndrome
patients
Iron overloadtherapeuticoptionscan decrease transfusion needs as well as improve quality of life in MDS patients. Attainment oftransfusion independencein the absenceof cytogenetic responses with disease-modifying agents has been associated with improved overall survival.28 Patients who receive hypomethylating agents have demonstrated improvements in quality of life measures. Iron overload remainsariskforpatientswhohavecontinuedtransfusion dependence evenwithhypomethylating therapy; therefore, ironchelationtherapymayberecommended.29
Byconsensus,thefollowinggroupsofMDSpatientsshould beregardedascandidatesforironchelatingtherapy:
(i) Patientswithfrankironoverload(e.g.stable/increasing serumferritin >1000ng/mLwithoutsigns of inflamma-tionorliverdisease),whoaretransfusiondependent(at anyfrequency)andhavealifeexpectancyofatleastone year.
with frankiron deficiency,e.g. chronicgastrointestinal tractbleeding).
(iii) Inselectedcases,ironchelatingtherapycanalsobe con-sidered when life expectancy is less than two years. Examplesareplannedcurativetherapy(stemcell trans-plantation), massive iron overload with consecutive organopathy,ormassiveironoverload,judgedto signif-icantlyreducethequalityoflife.Additionalparameters thatmayinfluencethedecisiontotreatindividualMDS patients with iron chelating agents are age (geriatric aspects), social and mental features, and comorbidity (organopathy).30
Cardiac
iron
in
myelodysplastic
syndromes
Theorgandamageofmostconcern,giventheadvancedage andcomorbiditiesinmanyMDSpatientsiscardiac dysfunc-tionresultingfrommyocardialirondeposition.Cardiaciron has been observed at autopsy in patients who have died ofacute leukemiaor other transfusiondependentanemias and correlateswiththe number ofRBC transfusions; how-everthesedataareconfoundedbythecontributionofanemia itselftocardiacdysfunctionaswellasthefactthat transfu-siondependence isa featureofdisease severity inMDS.31 However,recent studies using the CMR T2technique have shownthat cardiacironaccumulationisquite variable but infrequentamongpatientswithMDS.Moreover,cardiaciron inMDSpatientsdoesnotcorrelatewithserumferritinor hep-aticiron,butshowscorrelationwiththechelatableironpoolas determinedbyurinaryironexcretion,asurrogateforLPI.32It remainstobedeterminedwhetherLPIdirectlycorrelateswith myocardialironinMDS.Mechanismsleadingtocardiaciron depositioninparticularpatientswithlowergradesofMDS, especiallyasitrelatestohepcidinlevel,ineffective erythro-poiesis,and elevated LPI,need furtherevaluationinlarger studies.
Prospectivestudies correlatingcardiac ironwithcardiac functionandsurvivalaswellasstudiesshowingimprovement incardiacfunction withchelationwillbenecessary before
avertingorreversingcardiacdysfunctioncanbeestablished asaprimarygoalofironchelationinMDS.
Role
of
magnetic
resonance
imaging
in
the
evaluation
of
iron
overload
MRI uses the magnetic properties of the human body to provide pictures of any tissue (Figure 2). Hydrogen nuclei areaprincipalconstituentofbodytissuesinwaterandlipid moleculesandproduceadipolemoment(magneticfield)that caninteractwithanexternalmagneticfield.MRImachines generate a strong, homogenous magnetic field by using a largemagnet,madebypassinganelectricfieldthrough super-conductingcoilsofwire.Hydrogennucleiinthebody,which normallyhaverandomlyorientedspins,whenexposedtothe magneticfield,aligninadirectionparalleltothemagnetic field.TheMRImachineappliesshortelectromagneticpulses ataspecificradiofrequency(RF).Thehydrogennucleiabsorb the RF energy and precess away from equilibrium. When theRFpulseisturnedoff,theprecessingnucleireleasethe absorbed energyand returntonormal.Thestrengthofthe signalvaries,dependingontheRFmagneticfieldapplied.The examinedtissuereturnstonormalinthelongitudinalplane overacharacteristicintervalcalledT1relaxationtime.Inthe transverseplane,thereturntonormaloccursovera character-isticintervalcalledT2relaxationtime.UsingMRI,tissueiron isdetectedindirectlybytheeffectsonrelaxationtimesof fer-ritinandhemosiderinironinteractingwithhydrogennuclei. Thepresenceofironinthe humanbodyresultsinmarked alterationsoftissuerelaxationtimes.33 WhileT1decreases onlymoderately,T2demonstratesasubstantial decrease.34 Myocardial T2, a parameter measured by spin echo tech-niques,hasbeenshowninexperimentalanimalstohavean inversecorrelationwithmyocardialironcontent.35Inastudy byourgroupthatcomparedmyocardialT2withironcontent inheartbiopsy,anagreementwasfoundbetweenmyocardial biopsy andtheMRIresults.36 Unfortunately,theMRIsignal isaffectedbymultipleacquisitionvariables.AlthoughT2is relativelyindependentoffieldstrength,thereisanexception
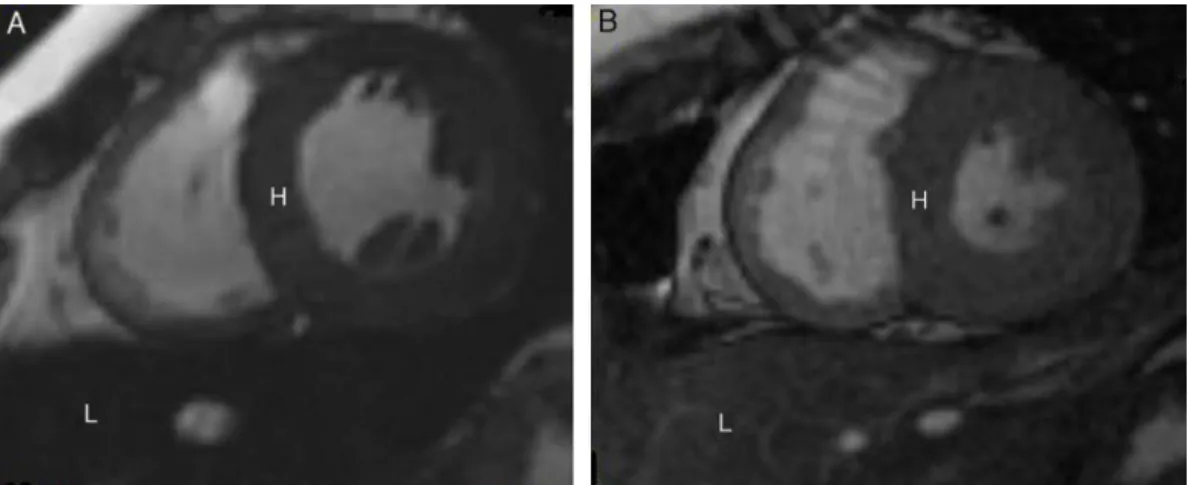
Figure2–CardiovascularmagneticresonanceT2*imagesshowingtheheart(H)andliver(L)fromtwodifferentpatientsat thesameechotime(10.70ms).(A)Darksignalindicatingseveremyocardialandliversiderosis.(B)Normalmyocardialand liversignalsuggestingmildirondeposition.
inthecaseofironoverload.Inthesepatients, thereisthe lineardependenceofT2relaxivity(1/T2)onfieldstrength.37
Currently,CMRusingT2isroutinelyusedinmany coun-triestoidentifypatientswithmyocardialironoverloadand guidechelationtherapytailoredtotheheart.MyocardialT2is calibratedtothemyocardialironconcentration,andhasbeen shown toimprove withintensive ironchelationinparallel withtheejectionfraction.Moreover,itisthemajorvalidated non-invasivemeasureofmyocardialironoverloadandis supe-riortosurrogatessuchasserumferritin,liveriron,ventricular ejectionfractionandtissueDopplerparameters.38,39 Assess-ment of cardiac iron byMRI susceptometry hasalso been validated,althoughavailabilityofthetechniqueislimited.40In mostcases,chronicmyocardialsiderosisisbothpreventable and reversible with modern chelation regimes.41 Progress hasalsobeen madeinthe treatmentofacuteheartfailure andleftventricularsystolicdysfunction.42Asubstantial71% decreaseindeathshasbeenobservedintheUKthalassemia cohortsincetheintroductionofT2CMRandimprovediron chelation treatment.43 Other countries have also reported importantameliorationinmanagementofcardiacironusing T2CMR.44,45 Accordingtorecentdata,thereisasubstantial prevalenceof myocardial iron overload in a large interna-tionalsampleofthalassemia major(TM)patients,who are regularlytransfusedandtaking ironchelationtherapy. The use ofthethresholdofT2=10ms,belowwhich the riskof cardiac complications rises significantly, has been already confirmedandalternativeexplanationsforheartfailureapart from myocardialiron overloadseemunusual. Thefindings fromindependentcentersworldwideindicatethatmyocardial T2isarobustclinicaltoolwithpotentialforfurtherexpansion toguidechelationregimeswhicharetailoredtopreventthe developmentofheartfailureandprolongsurvival.46
Consequences
of
iron
deposition
in
myelodysplastic
syndromes
MRIhasalreadybeenusedfortheevaluationofmyocardial andliverironoverloadinMDS.Inastudybyourgroup,after comparisonofa population ofpatients withMDS and TM withoutevidenceofheartfailureusingMRI,weidentifiedthat MDSpatients,whoreceivedahigheramountofblood transfu-sionsforlongertimepresentedthesameMRIpatterninboth liverandheartasTMpatients.Onthecontrary,MDSpatients whoreceivedaloweramountofRBCtransfusionsforshorter timehadnoevidenceofmyocardialiron.However,theystill hadhepaticsiderosis,becauseliveristhefirstaffectedorgan inironoverload.Additionally,thesepatientshadhigher car-diacindices indicativeofhighoutput state,dueto chronic anemia.47
Similar findings were alsodocumented inother patient groupswithlessfrequenttransfusionscomparedtoTM,but similartopatientswiththalassemiaintermediaandsicklecell disease.48 Recentreportshavealsoassessedhepatic sidero-siswithoutevidenceofheartironoverloadinthesepatients. Additionally,ferritinreflectsthetotalbodyironandnotthe iron in individual organs, as has been already described inpreviousstudies.49Thesefindingsfurtheremphasizethe necessityofMRIintheevaluationofironload,particularly
afterrecentpublications provingthesignificanceofironin MDSprognosisandtreatment.
Clinical
implications
of
magnetic
resonance
imaging
findings
in
the
treatment
of
myelodysplastic
syndromes
Theironoverloadassessmentmayhaveseriousclinical impli-cationsinMDS.
Inafive-yearprospectiveregistrythatenrolled600lower risk MDSpatientswithtransfusional ironoverload,clinical outcomeswerecomparedbetweenchelatedandnon-chelated patients.Atbaseline,cardiovascularcomorbiditiesweremore common innon-chelatedpatients. At24months,chelation was associated with longer median overall survival (52.2 monthsvs.104.4months;p-value<0.0001)andatrendtoward longerleukemia-freesurvivalandfewercardiacevents.No dif-ferenceinsafetywasdocumentedbetweengroups.48Finally, a recent study showed that deferasirox was well tolerated andeffectiveinreducingS-ferritinandliveriron concentra-tion(LIC)levelintransfusionalironoverloadMDSpatientsor aplasticanemia.49Therefore,ironchelationtreatmentshould beconsideredintransfusedMDSpatientswhentransfusion relatedironoverloadisdocumented.
Based on availabledata, the Expert Panel ofthe Italian SocietyofHematologyagreedthatironchelationwith defer-oxamineshouldbeconsideredasatherapyforMDSpatients, whohavepreviouslyreceivedmorethan50RBCunitsandfor whomalifespanlongerthansixmonthsisexpected.50The ExpertPaneloftheBritishSocietyofHematologyconcluded thatironchelationshouldbeconsideredonceapatienthas received25 RBCunits, butonlyinpatients forwhom long-term transfusion therapyislikely,suchasthose withpure sideroblasticanemiaorthe5q-syndromeanddeferoxamine 20–40mg/kgshouldbeadministeredby12hsubcutaneous(sc) infusion5–7daysperweek.51
Conclusion
Inconclusion,theMRIimagingpatternoftheliverandheartin MDSshowsthatironoverloadplaysacrucialrolein myocar-dialandhepaticpathophysiologyofthesepatientsandmay also influence MDS survival. However, further studies are neededinordertoidentifythethresholdfortransfusionsin respecttoprovokingmyocardialandhepaticirondeposition andtheroleofchelationinthelong-termprognosisofMDS patients.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. NeukirchenJ,SchoonenWM,StruppC,GattermannN,AulC, HaasR,etal.Incidenceandprevalenceofmyelodysplastic syndromes:datafromtheDüsseldorfMDS-registry.LeukRes. 2011;35(12):1591–6.
2. AulC,GiagounidisU,GermingU.Epidemiologicalfeaturesof myelodysplasticsyndrome:resultsfromregionalcancer surveysandhospital-basedstatistics.IntJHematol. 2001;73(4):405–10.
3. WarlickED,SmithBD.Myelodysplasticsyndromes:reviewof pathophysiologyandcurrentnoveltreatmentapproaches. CurrCancerDrugTargets.2007;7(6):541–58.
4. CazzolaM,DellaPortaMG,MalcovatiL.Clinicalrelevanceof anemiaandtransfusionironoverloadinmyelodysplastic syndromes.HematologyAmSocHematolEducProgram. 2008;(1):166–75.
5. DalamagaM,PetridouE,CookFE,TrichopoulosD.Riskfactors formyelodysplasticsyndromes:acase–controlstudyin Greece.CancerCausesControl.2002;13(7):603–8. 6. BennetJM,CatovskyD,DanielMT,FlandrinG,GaltonDA,
GralnickHR,etal.Proposalsfortheclassificationofthe myelosysplasticsyndromes.BrJHaematol.1982;51(2):189–99. 7. BrunningRD,OraziA,GermingU,LeBeauMM,PorwitA,
BaumannI,etal.Myelodysplasticsyndromes/neoplasms, overview.In:SwerdlowSH,CampoE,HarrisNL,JaffeES,Pileri SA,SteinH,etal.,editors.WorldHealthOrganization classificationoftumoursofhaematopoieticandlymphoid tissues.Lyon:IARCPress;2008.p.87–9.
8. OlneyHJ,LeBeauMM.Thecytogeneticsofmyelodysplastic syndromes.BestPractResClinHaematol.2001;14(3):479–95. 9. JäderstenM,MontgomerySM,DybedalI,Porwit-MacDonald A,Hellström-LindbergE.Long-termoutcomeoftreatmentof anemiainMDSwitherythropoietinandG-CSF.Blood. 2005;106(3):803–11.
10.ListA,KurtinS,RoeDJ,BureshA,MahadevanD,FuchsD, etal.Efficacyoflenalidomideinmyelodysplasticsyndromes. NEnglJMed.2005;352(6):549–57.
11.IssaJP,Garcia-ManeroG,GilesFJ,MannariR,ThomasD, FaderlS,etal.Phase1studyoflow-doseprolongedexposure schedulesofthehypomethylatingagent
5-aza-2′-deoxycytidine(decitabine)inhematopoietic
malignancies.Blood.2004;103(5):1635–40.
12.MolldremJJ,CaplesM,MavroudisD,PlanteM,YoungNS, BarretAJ.Antithymocyteglobulinforpatientswith
myelodysplasticsyndrome.BrJHaematol.1997;99(3):699–705.
13.CutlerC.Allogeneichematopoieticstem-celltransplantation formyelodysplasticsyndrome.HematologyAmSocHematol EducProgram.2010;2010(1):325–9.
14.BarziA,SekeresMA.Myelodysplasticsyndromes:apractical approachtodiagnosisandtreatment.CleveClinJMed. 2010;77(1):37–44.
15.ListAF.Ironoverloadinmyelodysplasticsyndromes: diagnosisandmanagement.CancerControl.2010;17 Suppl.:2–8.
16.CuijpersML,RaymakersRA,MackenzieMA,deWitteTJ, SwinkelsDW.Recentadvancesintheunderstandingofiron overloadinsideroblasticmyelodysplasticsyndrome.BrJ Haematol.2010;149(3):322–33.
17.ArmandP,KimHT,CutlerCS,HoVT,KorethJ,AlyeaEP,etal. Prognosticimpactofelevatedpretransplantationserum ferritininpatientsundergoingmyeloablativestemcell transplantation.Blood.2007;109(10):4586–8.
18.MalcovatiL,PortaMG,PascuttoC,InvernizziR,BoniM, TravaglinoE,etal.Prognosticfactorsandlifeexpectancyin myelodysplasticsyndromesclassifiedaccordingtoWHO criteria:abasisforclinicaldecisionmaking.JClinOncol. 2005;23(30):7594–603.
19.MalcovatiL.Impactoftransfusiondependencyand secondaryironoverloadonthesurvivalofpatientswith myelodysplasticsyndromes.LeukRes.2007;31Suppl.3:S2–6. 20.ShahJ,KurtinSE,ArnoldL,Lindroos-KolqvistP,TinsleyS.
Managementoftransfusion-relatedironoverloadinpatients withmyelodysplasticsyndromes.ClinJOncolNurs.2012;16 Suppl:37–46.
21.LiuP,HenkelmanM,JoshiJ,HardyP,ButanyJ,IwanochkoM, etal.Quantificationofcardiacandtissueironbynuclear magneticresonancerelaxometryinanovelmurine thalassemia-cardiacironoverloadmodel.CanJCardiol. 1996;12(2):155–64.
22.SchaferAI,CheronRG,DluhyR,CooperB,GleasonRE, SoeldnerJS,etal.Clinicalconsequencesofacquired transfusionalironoverloadinadults.NEnglJMed. 1981;304(6):319–24.
23.SchaferAI,RabinoweS,LeBoffMS,BridgesK,CheronRG, DluhyR.Long-termefficacyofdeferoxamineironchelation therapyinadultswithacquiredtransfusionalironoverload. AdvInternMed.1985;145(7):1217–21.
24.RoyNB,MyersonS,SchuhAH,BignellP,PatelR,WainscoatJS, etal.Cardiacironoverloadintransfusion-dependentpatients withmyelodysplasticsyndromes.BrJHaematol.
2011;154(4):521–4.
25.PullarkatV.Objectivesofironchelationtherapyin
myelodysplasticsyndromes:morethanmeetstheeye?Blood. 2009;114(26):5251–5.
26.AlessandrinoEP,DellaPortaMG,BacigalupoA,MalcovatiL, AngelucciE,VanLintMT,etal.Prognosticimpactof pre-transplantationtransfusionhistoryandsecondaryiron overloadinpatientswithmyelodysplasticsyndrome undergoingallogeneicstemcelltransplantation:aGITMO study.Haematologica.2010;95(3):476–84.
27.HoenB.Ironandinfection:clinicalexperience.AmJKidney Dis.1999;34Suppl2:30–4.
28.LeitchHA,VickarsLM.Supportivecareandchelationtherapy inMDS:arewesavinglivesorjustloweringiron?Hematology AmSocHematolEducProgram.2009:664–72.
29.SantiniV.Noveltherapeuticstrategies:hypomethylating agentsandbeyond.HematologyAmSocHematolEduc Program.2012;2012:65–73.
30.BennetJM.Consensusstatementonironoverloadin myelodysplasticsyndromes.AmJHematol. 2008;83(11):858–61.
32.WoodJC.Cardiacironacrossdifferenttransfusion-dependent diseases.BloodRev.2008;22Suppl2:14–21.
33.GomoriJM,GrossmanRI,DrottHR.MRrelaxationtimesand ironcontentofthalassemicspleens:aninvitrostudy.AmJ Roentgenol.1988;150(3):567–9.
34.MavrogeniSI,GotsisED,MarkussisV,TsekosN,PolitisC, VretouE,etal.T2relaxationtimestudyofironoverloadin b-thalassemia.MAGMA.1998;6(1):7–12.
35.AndersonLJ,HoldenS,DavisB,PrescottE,CharrierCC,Bunce NH,etal.CardiovascularT2-star(T2*)magneticresonancefor theearlydiagnosisofmyocardialironoverload.EurHeartJ. 2001;22(23):2171–9.
36.MavrogeniSI,MarkussisV,KaklamanisL,TsiaprasD, ParaskevaidisI,KaravoliasG,etal.Acomparisonofmagnetic resonanceimagingandcardiacbiopsyintheevaluationof heartironoverloadinpatientswithbeta-thalassemiamajor. EurJHaematol.2005;75(3):241–7.
37.CarpenterJP,HeT,KirkP,RoughtonM,AndersonLJ,de NoronhaSV,etal.OnT2*magneticresonanceandcardiac iron.Circulation.2011;123(14):1519–28.
38.TelferPT,PrestcottE,HoldenS,WalkerM,HoffbrandAV, WonkeB.Hepaticironconcentrationcombinedwith long-termmonitoringofserumferritintopredict complicationsofironoverloadinthalassaemiamajor.BrJ Haematol.2000;110(4):971–7.
39.LeonardiB,MargossianR,ColanSD,PowellAJ.Relationshipof magneticresonanceimagingestimationofmyocardialironto leftventricularsystolicanddiastolicfunctioninthalassemia. JACCCardiovascImaging.2008;1(5):572–8.
40.WangZJ,FischerR,ChuZ,MahoneyDHJr,MuellerBU, MuthupillaiR,etal.AssessmentofcardiacironbyMRI susceptometryandR2*inpatientswiththalassemia.Magn ResonImaging.2010;28(3):363–71.
41.AndersonLJ,WestwoodMA,HoldenS,DavisB,PrescottE, WonkeB,etal.Myocardialironclearanceduringreversalof sideroticcardiomyopathywithintravenousdesferrioxamine: aprospectivestudyusingT2*cardiovascularmagnetic resonance.BrJHaematol.2004;127(3):348–55.
42.KolnagouA,EconomidesC,EracleousE,KontoghiorghesGJ. Longtermcomparativestudiesinthalassemiapatients treatedwithdeferoxamineoradeferoxamine/deferiprone combinationIdentificationofeffectivechelationtherapy protocols.Hemoglobin.2008;32(1–2):41–7.
43.ModellB,KhanM,DarlisonM,WestwoodMA,IngramD, PennellDJ.Improvedsurvivalofthalassaemiamajorinthe
UKandrelationtoT2*cardiovascularmagneticresonance.J CardiovascMagnReson.2008;10:42.
44.PathareA,TaherA,DaarS.Deferasirox(Exjade)significantly improvescardiacT2*inheavilyiron-overloadedpatientswith beta-thalassemiamajor.AnnHematol.2010;89(4):
405–9.
45.LadisV,ChouliarasG,BerdoukasV,ChatziliamiA,
FragodimitriC,KarabatsosF,etal.Survivalinalargecohortof Greekpatientswithtransfusion-dependentbeta
thalassaemiaandmortalityratioscomparedtothegeneral population.EurJHaematol.2011;86(4):332–8.
46.CarpenterJP,RoughtonM,PennellDJ.Internationalsurveyof T2*cardiovascularmagneticresonancein-thalassemia major.Haematologica.2013;98(9):1368–74.
47.MavrogeniS,GotsisE,LadisV,BerdousisE,VerganelakisD, ToulasP,etal.Magneticresonanceevaluationofliverand myocardialirondepositioninthalassemiaintermediaand b-thalassemiamajor.IntJCardiovascImaging.
2008;24(8):849–54.
48.LyonsRM,MarekBJ,PaleyC,EspositoJ,GarboL,DiBellaN, etal.Comparisonof24-monthoutcomesinchelatedand non-chelatedlower-riskpatientswithmyelodysplastic syndromesinaprospectiveregistry.LeukRes. 2014;38(2):149–54.
49.CheongJW,KimHJ,LeeKH,YoonSS,LeeJH,ParkHS,etal. Deferasiroximproveshematologicandhepaticfunctionwith effectivereductionofserumferritinandliveriron
concentrationintransfusionalironoverloadpatientswith myelodysplasticsyndromeoraplasticanemia.Transfusion. 2013;54(6):1542–51.
50.AlessandrinoEP,AmadoriS,BarosiG,CazzolaM,GrossiA, LiberatoLN,etal.ItalianSocietyofHematology Evidence-andconsensus-basedpracticeguidelinesforthetherapyof primarymyelodysplasticsyndromes.Astatementfromthe ItalianSocietyofHematology.Haematologica.
2002;87(12):1286–306.
51.BowenD,CulliganD,JowittS,KelseyS,MuftiG,OscierD,etal. UKMDSGuidelinesGroupGuidelinesforthediagnosisand therapyofadultmyelodysplasticsyndromes.BrJHaematol. 2003;120(2):187–200.
52.CermakJ,JonasovaA,VondrakovaJ,CervinekL,Belohlavkova P,NeuwirtovaR.Acomparativestudyofdeferasiroxand deferiproneinthetreatmentofironoverloadinpatientswith myelodysplasticsyndromes.LeukRes.2013;37(12):