Universidade de Aveiro Departamento de Química
2012
Lydie Ferreira dos Análise de Chá Preto por Cromatografia
Santos
Líquida Bidimensional
Analysis of Black Tea by Two-Dimensional
Liquid Chromatography
Universidade de Aveiro Departamento de Química
2012
Lydie Ferreira dos Análise de Chá Preto por Cromatografia
Santos
Líquida Bidimensional
Analysis of Black Tea by Two-Dimensional
Liquid Chromatography
Dissertação apresentada à Universidade de Aveiro para cumprimento dos requisitos necessários à obtenção do grau de Mestre em Química Analítica e Qualidade, realizada sob a orientação científica do Doutor Armando da Costa Duarte, Professor Catedrático do Departamento de Química da Universidade de Aveiro, e da Doutora Regina Maria Brandão de Oliveira Duarte, Investigadora Auxiliar do Centro de Estudos do Ambiente e do Mar (CESAM) da Universidade de Aveiro.
O júri
Presidente Prof. Doutor Artur Manuel Soares da Silva
Professor Catedrático do Departamento de Química da Universidade de Aveiro
Prof. Doutora Teresa Alexandra Peixoto da Rocha Santos
Professora Associada do Instituto Piaget de Viseu
Prof. Doutor Armando da Costa Duarte
Professor Catedrático do Departamento de Química da Universidade de Aveiro
Doutora Regina Maria Brandão de Oliveira Duarte
Investigadora Auxiliar do Centro de Estudos do Ambiente e do Mar (CESAM) da Universidade de Aveiro
Agradecimentos Agradeço aos meus pais, irmão e namorado por todo o apoio e carinho que me deram ao longo desta etapa da minha vida. Sem eles não teria conseguido terminar esta nova fase da minha vida!
Agradeço também aos meus orientadores por todo o apoio e dedicação que depositaram em mim, simpatia e paciência que tiveram comigo devido às minhas dificuldades ao longo deste percurso.
Agradeço aos meus colegas de mestrado e laboratório pelo apoio, mas em especial ao Mestre João Tiago Viana de Matos pela preciosa ajuda que me deu nas Secções 2.4.2 e 4.3 desta Dissertação. Obrigado pela atenção e tempo disponibilizado.
Palavras-chave Cromatografia Líquida Bidimensional, Cromatografia Líquida Bidimensional Compreensiva, Chá Preto, Coluna de Modo Misto de Fase Reversa/Troca Aniónica, Coluna de Fase Reversa, Interações de Troca Aniónica, Interações Hidrofílicas.
Resumo O chá (Camellia sinensis) é uma das bebidas mais consumidas no mundo, existindo vários processos de produção que originam os diversos tipos de chá comummente conhecidos: verde (não fermentado), oolong (semi - fermentado) e preto (fermentado). Actualmente, assiste-se a um amplo debate acerca das alegadas propriedades anti-oxidantes, anti-carcinogénicas e anti-inflamatórias de diversos tipos chás. É pois compreensível que a caracterização de chás, com vista ao conhecimento integral dos seus potenciais benefícios na saúde, seja um tópico de interesse não só do ponto de vista académico, mas também para os operadores da indústria do chá.
Este trabalho pretende mostrar a viabilidade e as vantagens da aplicação da técnica de cromatografia líquida bidimensional compreensiva para a separação dos compostos existentes em amostras de chá preto. Para o efeito, foram desenvolvidas duas metodologias de eluição utilizando, na primeira dimensão, uma coluna com fase estacionária de modo misto de fase reversa/troca aniónica (Acclaim Mixed-Mode WAX-1, denominação em inglês) e, na segunda dimensão, uma coluna de fase reversa convencional de octadecil sílica (C18). O sistema separativo bidimensional foi acoplado a um detector de fotodíodos e a um detector de fluorescência molecular, em série. A qualidade de separação cromatográfica dos compostos, assim como, a ortogonalidade do sistema foram avaliadas utilizando funções matemáticas previamente desenvolvidas.
Keywords Dimensional Liquid Chromatography, Comprehensive Two-Dimensional Liquid Chromatography, Black Tea, Mixed-Mode Reversed - Phase/Anionic Change Column, Reversed – Phase Column, Anionic Exchange Interactions, Hydrophilic Interactions.
Abstract Tea (Camellia sinensis) is one of the most widely consumed beverages in the world. Depending on the production process, there are three types of tea available: green (unfermented), oolong (semi - fermented) and black (fermented). Currently, there is a large discussion about the alleged anti-oxidant, anti-carcinogenic, and anti-inflammatory properties of various types teas. It is, therefore, understandable, that the characterization of tea has become a topic of interest not only from an academic point of view, but also for those working in tea industry.
Therefore, this work aims at showing the application of comprehensive two-dimensional liquid chromatography for the separation of compounds contained in black tea samples. For accomplishing this objective, two methodologies of elution have been developed comprising, in the first dimension, a mixed-mode reversed-phase/anionic change column (Acclaim Mixed-Mode WAX-1) and, in the second dimension, a conventional C18 reversed-phase column. The comprehensive two-dimensional chromatographic system was coupled to a photodiode array and fluorescence detectors, in series. The quality of chromatographic separation of the compounds, as well as the orthogonality of the system were evaluated by applying mathematical functions previously developed.
Table of contents
XV
Table of contents
List of figures ... XVII List of tables... XXIII List of abbreviations ... XXV
I. Aims and structure of dissertation ...1
1.1. Introduction...2
1.2. Aims of the dissertation...2
1.3. Structure of the dissertation ...3
II. Basics associated to tea and to comprehensive two-dimensional liquid chromatography ...5
2.1. Production of tea...6
2.2. Chemistry of the black tea ...6
2.3. Liquid chromatography for tea analysis ... 11
2.4. Two-dimensional liquid chromatography (2D-LC)... 12
2.4.1. Chromatographic response function (CRF2D) ... 14
2.4.2. The orthogonality of separation in 2D-LC ... 16
2.5. Application of 2D-LC in tea analysis ... 18
III. Experimental conditions for chromatographic analysis of black tea samples ... 21
3.1. Chemicals... 22
3.2. Preparation of black tea samples ... 22
3.3. Excitation-Emission matrix (EEM) fluorescence spectroscopy ... 23
3.4. Instrumentation ... 23
3.5. Chromatographic conditions ... 24
3.5.1. Development a chromatographic method for Mixed-Mode WAX-1 and RP-C18 columns .. 24
3.5.2. Chromatographic conditions used for LC x LC (Mixed-Mode WAX-1 x RP-C18) analysis of black tea ... 25
3.6. Software ... 26
IV. One- and two-dimensional chromatographic analysis of black tea samples ... 27
4.1. Characterization of the black tea samples by EEM fluorescence spectroscopy ... 28
4.2. One-dimensional chromatographic separation of black tea samples ... 29
4.2.1. Separation of the black tea samples in the Mixed-Mode WAX-1 column ... 30
4.2.2. Separation of the black tea sample in the RP-C18 column ... 45
4.2.3. Separation of black tea samples by the Mixed-Mode WAX-1 x RP-C18 method ... 57
4.3. Application of a CRF2D to assess the quality of separation ... 78
4.4. Evaluating of the orthogonality of the LC x LC analysis of the black tea ... 87
V. Conclusion ... 91
VI. References ... 93
VII. Annexes ... i
Annex A: Description of the black tea sample used in this work ...ii
Table of contents
XVI
Annex B: Repeatability in the Mixed-Mode WAX-1 column (continued)... iv Annex C: Chromatograms of the black tea samples prepared with ultra-pure and distilled water.
Detector: DAD... v Annex C: Chromatograms of the black tea samples prepared with ultra-pure and distilled water.
Detector: DAD (continued) ... vi Annex D: Chromatograms recorded with DAD at 320 and 370 nm in the RP-C18 column ... vii Annex E: Repeatability in the RP-C18 column ... viii
List of figures
XVII
List of figures
Figure 1 - Chemical structure of catechins found in tea. ... 7
Figure 2 - Chemical structure of theaflavins found in tea. ... 8
Figure 3 - Chemical structure of flavonols found in tea. ... 9
Figure 4 - Chemical structure of gallic acid found in tea. ... 10
Figure 5 - Chemical structure of hydroxycinnamic acids found in tea. ... 10
Figure 6 - Chemical structure of methylxanthines found in tea. ... 10
Figure 7 - Schematic representation of a LC x LC system. (1) first dimension pump; (2) first dimension column; (3) second dimension pump; (4) loop 1; (5) loop 2; (6) switching valve; (7) second dimension column; (8) waste; (9) detector. ... 14
Figure 8 - Degrees of orthogonality in a hypothetical 2D-LC system where each point represents an analyte, whose respective retention time is normalized for the column void volume: (A) orthogonal separation, (B) separation with partial correlation, and (C) highly correlated separation. R is the correlation coefficient. ... 17
Figure 9 - EEM fluorescence profiles of a black tea sample prepared with ultra – pure water (A) and distilled water (B). ... 28
Figure 10 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 200 mM CH3COONH4 (pH 4.6) and 83% (v/v) CH3CN; flow rate: 0.5 mL/min; fluorescence detector operating at λExc/λEm = 240/424 nm. ... 31
Figure 11 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 200 mM CH3COONH4 (pH 4.6) and 83% (v/v) CH3CN; flow rate: 0.5 mL/min; evaporative light scattering detector. ... 31
Figure 12 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 200 mM CH3COONH4 (pH 4.6) and 83% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 225 nm. ... 32
Figure 13 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 200 mM CH3COONH4 (pH 4.6) and 83% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 280 nm. ... 33
Figure 14 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 200 mM CH3COONH4 (pH 4.6) and 83% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 320 nm. ... 33
Figure 15 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 200 mM CH3COONH4 (pH 4.6) and 83% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 370 nm. ... 34
Figure 16 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; fluorescence detector at different λExc/λEm pairs. ... 36
Figure 17 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; evaporative light scattering detector. ... 36
Figure 18 - Chromatogram of a black tea sample in the Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 225 nm. ... 37
Figure 19 - Chromatogram of a black tea sample in the Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 280 nm. ... 38
List of figures
XVIII
Figure 20 - Chromatogram of a black tea sample in the Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array
detector operating at 320 nm. ... 38 Figure 21 - Chromatogram of a black tea sample in the Mixed-Mode WAX-1 column. Mobile phase: 20
mM CH3COONH4 (pH 6.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array
detector operating at 370 nm. ... 39 Figure 22 - Chromatogram of a black tea sample prepared with ultra-pure and distilled water in a
Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v)
CH3CN; flow rate: 0.5 mL/min; evaporative light scattering detector. ... 40
Figure 23 - Chromatogram of a black tea sample prepared with ultra-pure and distilled water in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v)
CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 225 nm. ... 40
Figure 24 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 5.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; fluorescence detector
operating at λExc/λEm = 300/425 nm. ... 41
Figure 25 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 5.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; evaporative light
scattering detector. ... 42 Figure 26 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM
CH3COONH4 (pH 5.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector
operating at 225 nm. ... 43 Figure 27 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM
CH3COONH4 (pH 5.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector
operating at 280 nm. ... 43 Figure 28 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM
CH3COONH4 (pH 5.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector
operating at 320 nm. ... 44 Figure 29 - Chromatogram of a black tea sample in a Mixed-Mode WAX-1 column. Mobile phase: 20 mM
CH3COONH4 (pH 5.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min; diode array detector
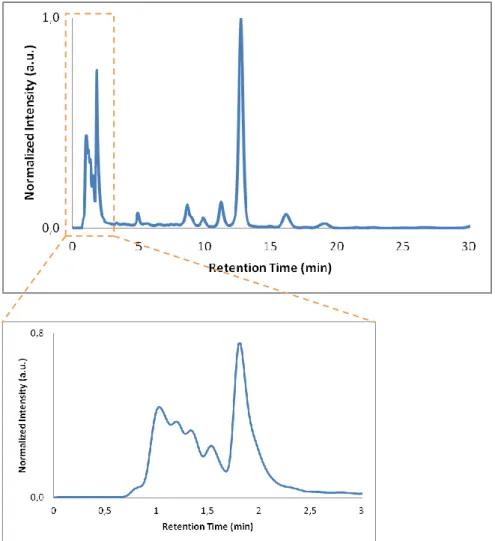
operating at 370 nm. ... 44 Figure 30 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v)
CH3COOH and 10% (v/v) CH3CN; flow rate: 2.5 mL/min; fluorescence detector at different
λExc/λEm pairs. ... 46
Figure 31 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 10% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
225 nm. ... 46 Figure 32 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v)
CH3COOH and 10% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
280 nm. ... 47 Figure 33 - Chromatograms of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v)
CH3COOH and 5% (v/v) CH3CN; flow rate: 2.5 mL/min; fluorescence detector at different
λExc/λEm pairs. ... 47
Figure 34 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 5% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
225 nm. ... 48 Figure 35 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v)
CH3COOH and 5% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
List of figures
XIX
Figure 36 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 12% (v/v) CH3CN; flow rate: 2.5 mL/min; fluorescence detector at different
λExc/λEm pairs. ... 49
Figure 37 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 12% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
225 nm. ... 50 Figure 38 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v)
CH3COOH and 12% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
280 nm. ... 50 Figure 39 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v)
CH3COOH and 14% (v/v) CH3CN; flow rate: 2.5 mL/min; fluorescence detector at different
λExc/λEm pairs. ... 51
Figure 40 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 14% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
225 nm. ... 51 Figure 41 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: 0.01% (v/v)
CH3COOH and 14% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
280 nm. ... 52 Figure 42 - Chromatogram of the eluent of the first dimension (CH3COONH4 20 mM (pH 6.0) and 40%
(v/v) CH3CN) in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 14% (v/v)
CH3CN; flow rate: 2.5 mL/min; fluorescence detector operating at λExc/λEm = 240/424 nm.. 53
Figure 43 - Chromatogram of the eluent of the first dimension (CH3COONH4 20 mM (pH 6.0) and 40%
(v/v) CH3CN) in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 14% (v/v)
CH3CN; flow rate: 2.5 mL/min; diode array detector operating at 225 nm. ... 53
Figure 44 - Chromatogram of the eluent of the first dimension (CH3COONH4 20 mM (pH 6.0) and 40%
(v/v) CH3CN) in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 14% (v/v)
CH3CN; flow rate: 2.5 mL/min; diode array detector operating at 280 nm. ... 54
Figure 45 - Chromatogram of the eluent of the first dimension (CH3COONH4 20 mM (pH 6.0) and 40%
(v/v) CH3CN) in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 14% (v/v)
CH3CN; flow rate: 2.5 mL/min; diode array detector operating at 320 nm. ... 54
Figure 46 - Chromatogram of the eluent of the first dimension (CH3COONH4 20 mM (pH 6.0) and 40%
(v/v) CH3CN) in a RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 14% (v/v)
CH3CN; flow rate: 2.5 mL/min; diode array detector operating at 370 nm. ... 54
Figure 47 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: CH3COONH4 20
mM (pH 6.0) and 40% (v/v) CH3CN; flow rate: 2.0 mL/min; fluorescence detector at
different λExc/λEm pairs. ... 55
Figure 48 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: CH3COONH4 20
mM (pH 6.0) and 40% (v/v) CH3CN; flow rate: 2.0 mL/min; diode array detector operating
at 225 nm. ... 56 Figure 49 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: CH3COONH4 20
mM (pH 6.0) and 40% (v/v) CH3CN; flow rate: 2.0 mL/min; diode array detector operating
at 280 nm. ... 56 Figure 50 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: CH3COONH4 20
mM (pH 6.0) and 40% (v/v) CH3CN; flow rate: 2.0 mL/min; diode array detector operating
at 320 nm. ... 57 Figure 51 - Chromatogram of a black tea sample in a RP-C18 column. Mobile phase: CH3COONH4 20
mM (pH 6.0) and 40% (v/v) CH3CN; flow rate: 2.0 mL/min; diode array detector operating
List of figures
XX
Figure 52 - Contour plot obtained with fluorescence detection at λExc/λEm = 240/424 nm in the
Mixed-Mode WAX-1 and RP-C18 analysis of the black tea. Mobile phases: CH3COONH4 20 mM (pH
6.0) and 40% (v/v) CH3CN for the first dimension and 0.01% (v/v) CH3COOH and 14%
(v/v) CH3CN for the second dimension; flow rate: 0.050 mL/min in first dimension and 2.5
mL/min in second dimension... 58 Figure 53 - Complete DAD data set of the black tea sample in the Mixed-Mode WAX-1 x RP-C18 method.
Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN for the first dimension
and 0.01% (v/v) CH3COOH and 14% (v/v) CH3CN for the second dimension; flow rate:
0.050 mL/min in first dimension and 2.5 mL/min in second dimension... 59 Figure 54 - Contour plot obtained with UV detection at 215 nm (A), 235 nm (B) and 275 nm (C) in the
Mixed-Mode WAX-1 x RP-C18 analysis of the black tea. Mobile phases: CH3COONH4 20 mM
(pH 6.0) and 40% (v/v) CH3CN for the first dimension and 0.01% (v/v) CH3COOH and 14%
(v/v) CH3CN for the second dimension; flow rate: 0.050 mL/min in first dimension and 2.5
mL/min in second dimension. ... 60 Figure 55 - Contour plot obtained with fluorescence detection at λExc/λEm = 240/424 nm in the
Mixed-Mode WAX-1 x RP-C18 analysis of the black tea. Mobile phases: CH3COONH4 20 mM (pH
6.0) and 40% (v/v) CH3CN for the first dimension and 0.01% (v/v) CH3COOH and 14%
(v/v) CH3CN for the second dimension; flow rate: 0.033 mL/min in first dimension and 2.5
mL/min in second dimension. ... 61 Figure 56 - Contour plot obtained with UV detection at 215 nm (A), 235 nm (B) and 275 nm (C) in the
Mixed-Mode WAX-1 x RP-C18 analysis of the black tea. Mobile phases: CH3COONH4 20 mM
(pH 6.0) and 40% (v/v) CH3CN for the first dimension and 0.01% (v/v) CH3COOH and 14%
(v/v) CH3CN for the second dimension; flow rate: 0.033 mL/min in first dimension and 2.5
mL/min in second dimension... 62 Figure 57 - Complete DAD data set in the Mixed-Mode WAX-1 x RP-C18 method. Mobile phases:
CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN in both dimensions; flow rate: 0.031
mL/min in first dimension and 2.0 mL/min in second dimension. ... 64 Figure 58 - Contour plot obtained with fluorescence detection at λExc/λEm = 300/425 nm in the
Mixed-Mode WAX-1 x RP-C18 analysis of black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0)
and 40% (v/v) CH3CN in both dimensions; flow rate: 0.031 mL/min in first dimension and
2.0 mL/min in second dimension... 65 Figure 59 - Contour plot obtained with UV detection at 227 nm in the Mixed-Mode WAX-1 and RP-C18
analysis of black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN in
both dimensions; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension. ... 66 Figure 60 - Contour plot obtained with UV detection at 279 nm in the Mixed-Mode WAX-1 and RP-C18
analysis of black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN in
both dimensions; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension. ... 67 Figure 61 - Contour plot obtained with UV detection at 311 nm in the Mixed-Mode WAX-1 and RP-C18
analysis of black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN in
both dimensions; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension. ... 68 Figure 62 - Contour plot obtained with UV detection at 343 nm in the Mixed-Mode WAX-1 and RP-C18
analysis of black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN in
both dimensions; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension. ... 69 Figure 63 - Contour plot obtained with fluorescence detection at λExc/λEm = 300/425 nm in the
List of figures
XXI
and 40% (v/v) CH3CN in both dimensions; flow rate: 0.031 mL/min in first dimension and
2.0 mL/min in second dimension; (A) is the first replica and (B) is the replica. ... 70 Figure 64 - Contour plot obtained with UV detection at 227nm in the Mixed-Mode WAX-1 x RP-C18
analysis of black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN in
both dimensions; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension; (A) is the first replica and (B) is the replica. ... 71 Figure 65 - Elution program applied into the first and second dimensions... 72 Figure 66 - Contour plot obtained with fluorescence detection at λExc/λEm = 300/425 nm in the
Mixed-Mode WAX-1 x RP-C18 analysis of black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0)
and 40% (v/v) CH3CN for the first dimension and CH3COONH4 20 mM (pH 6.0) and 40%
(v/v) CH3CN until the 37 min and CH3COONH4 20 mM (pH 6.0) and 20% (v/v) CH3CN until
the 288 min for the second dimension; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension... 73 Figure 67 - Contour plot obtained with UV detection at 227 nm in the Mixed-Mode WAX-1 x RP-C18
analysis of black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN for
the first dimension and CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN until the 37
min and CH3COONH4 20 mM (pH 6.0) and 20% (v/v) CH3CN until the 288 min for the
second dimension; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension. ... 74 Figure 68 - Contour plot obtained with UV detection at 279 nm in the Mixed-Mode WAX-1 x RP-C18
analysis of the black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v)
CH3CN for the first dimension and CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN until
the 37 min and CH3COONH4 20 mM (pH 6.0) and 20% (v/v) CH3CN until the 288 min for
the second dimension; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension. ... 75 Figure 69 - Contour plot obtained with UV detection at 311 nm in the Mixed-Mode WAX-1 x RP-C18
analysis of the black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v)
CH3CN for the first dimension and CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN until
the 37 min and CH3COONH4 20 mM (pH 6.0) and 20% (v/v) CH3CN until the 288 min for
the second dimension; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension. ... 76 Figure 70 - Contour plot obtained with UV detection at 343 nm in the Mixed-Mode WAX-1 x RP-C18
analysis of the black tea. Mobile phases: CH3COONH4 20 mM (pH 6.0) and 40% (v/v)
CH3CN for the first dimension and CH3COONH4 20 mM (pH 6.0) and 40% (v/v) CH3CN until
the 37 min and CH3COONH4 20 mM (pH 6.0) and 20% (v/v) CH3CN until the 288 min for
the second dimension; flow rate: 0.031 mL/min in first dimension and 2.0 mL/min in second dimension. ... 77 Figure 71 - Contour plot obtained with fluorescence detection at λExc/λEm = 300/425 nm in the
Mixed-Mode WAX-1 x RP-C18 analysis of the black tea by isocratic elution (additional details in Figure 57). ... 78 Figure 72 - Contour plot obtained with fluorescence detection at λExc/λEm = 300/425 nm in the
Mixed-Mode WAX-1 x RP-C18 analysis of the black tea by “segment-in-fraction” gradient elution (additional details in Figure 66). ... 80 Figure 73 - Contour plot obtained with UV detection at 227 nm in the Mixed-Mode WAX-1 x RP-C18
analysis of the black tea by isocratic elution (additional details in Figure 59). ... 81 Figure 74 - Contour plot obtained with UV detection at 279 nm in the Mixed-Mode WAX-1 x RP-C18
analysis of the black tea by isocratic elution (additional details in Figure 60). ... 83 Figure 75 - Contour plot obtained with UV detection at 311 nm in the Mixed-Mode WAX-1 x RP-C18
List of figures
XXII
Figure 76 - Contour plot obtained with UV detection at 343 nm in the Mixed-Mode WAX-1 x RP-C18 analysis of the black tea by isocratic elution (additional details in Figure 62). ... 85 Figure 77 - Representation of the regions to the different UV wavelengths. ... 87 Figure 78 - Normalized fluorescence plot for Mixed-Mode WAX-1 x RP-C18 using isocratic elution. ... 88 Figure 79 - Normalized fluorescence plot for Mixed-Mode WAX-1x RP-C18 using “segment-in-fraction”
gradient elution... 89 Figure 80 - Normalized UV plot at 227 nm for Mixed-Mode WAX-1x RP-C18 using isocratic elution. ... 89 Figure 1B - Replica of the chromatograms of the black tea samples in the Mixed-Mode WAX-1 column
and recorded with fluorescence detector at λExc/λEm = 240/424 nm (A), 260/340 nm (B),
300/425 nm (C) and 310/440 nm (D). Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40%
(v/v) CH3CN; flow rate: 0.5 mL/min……….iii
Figure 2B - Replica of the chromatograms of the black tea samples in the Mixed-Mode WAX-1 column and recorded with evaporative light scattering detector. Mobile phase: 20 mM CH3COONH4
(pH 6.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min………...…iii
Figure 3B - Replica of the chromatograms of the black tea samples in the Mixed-Mode WAX-1 column and recorded with DAD at 225 nm (A), 280 nm (B), 320 nm (C) and 370 nm (D). Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v) CH3CN; flow rate: 0.5 mL/min...…..iv
Figure 1C - Chromatogram of a black tea sample prepared with ultra – pure and distilled water in the Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v)
CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 280 nm………...…v
Figure 2C - Chromatogram of a black tea sample prepared with ultra – pure and distilled water in the Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v)
CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 320 nm………...…v
Figure 3C - Chromatogram of a black tea sample prepared with ultra – pure and distilled water in the Mixed-Mode WAX-1 column. Mobile phase: 20 mM CH3COONH4 (pH 6.0) and 40% (v/v)
CH3CN; flow rate: 0.5 mL/min; diode array detector operating at 370 nm………...…v
Figure 1D - Chromatogram of a black tea sample in the RP-C18 column. Mobile phase: 0.01% (v/v) CH3COOH and 10% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
320 nm……….………..….vi Figure 2D - Chromatogram of a black tea sample in the RP-C18 column. Mobile phase: 0.01% (v/v)
CH3COOH and 10% (v/v) CH3CN; flow rate: 2.5 mL/min; diode array detector operating at
370 nm………..…..vi Figure 1E - Replica of the chromatograms of the black tea samples in the RP-C18 column and recorded
with fluorescence detector at λExc/λEm = 240/424 nm (A), 260/340 nm (B), 300/425 nm (C),
310/440 nm (D). Mobile phase: 0.01% (v/v) CH3COOH and 14% (v/v) CH3CN; flow rate: 2.5
mL/min……….…vii Figure 2E - Replica of the chromatograms of the black tea samples in the RP-C18 column and recorded
with DAD at 225 nm (A), 280 nm (B), 320 nm (C), 370 nm (D). Mobile phase: 0.01% (v/v) CH3COOH and 14% (v/v) CH3CN; flow rate: 2.5 mL/min……….………….vii
List of tables
XXIII
List of tables
Table I - Mobile phase composition and flow rate used in the Mixed-Mode WAX-1 column. ... 25 Table II - Mobile phase composition and flow rate used in the RP-C18 column. ... 25 Table III - Chromatographic conditions used in the LC x LC (Mixed-Mode WAX-1 x RP-C18) analysis of
black tea samples. ... 26 Table IV - Fluorescence data derived from the EEM profiles of the four black tea samples... 29 Table V - Retention times of the peaks and their individual peak purity values using the fluorescence
data of Figure 71. ... 79 Table VI - Retention times of the peaks and their individual peak purity values using the fluorescence
data of Figure 72. ... 80 Table VII - Retention times of the peaks and their individual peak purity values using the UV data of
Figure 73. ... 82 Table VIII - Retention times of the peaks and their individual peak purity values using the UV data of
Figure 74. ... 83 Table IX - Retention times of the peaks and their individual peak purity value using the UV data of
Figure 75. ... 84 Table X - Retention times of the peaks and their individual peak purity value using UV data of Figure
76. ... 85 Table XI - Parameters calculated on the basis of the information entropy for the different LC x LC
List of abbreviations
XXV
List of abbreviations
AXC: anion exchange chromatography API: atmospheric pressure ionization
C: catechin ((2R,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol) CXC: cation exchange chromatography
CG: catechin gallate ([(2R,3S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3, 4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate)
DAD: diode array detector EEM: emission – excitation matrix
EC: epicatechin ((2S,3S)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol)
ECG: epicatechin gallate ([(2S,3S)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate)
EGC: epigallocatechin ((2S,3S)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol)
EGCG: epigallocatechin gallate ([(2S,3S)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate)
ELSD: evaporative light scattering detector ESI: electrospray ionization
FLD: fluorescence detector
GC: gallocatechin ((2R,3S)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol)
GCG: gallocatechin gallate ([(2S,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate)
GPC: gel permeation chromatographic
HILIC: hydrophilic interaction chromatography LC: liquid chromatography
LC x LC: comprehensive two-dimensional liquid chromatography LC-LC: heart-cutting two-dimensional liquid chromatography MS: mass spectrometry
Q-TOF: quadrupole – time-of-flight RP: reversed phase
SEC: size-exclusion chromatography UV: ultraviolet
1D-LC: one-dimensional liquid chromatography 2D-LC: two-dimensional liquid chromatography
Chapter I: Aims and structure of disseration
2
1.1. Introduction
Tea has been one of the beverages most analyzed, because it is consumed worldwide due to its aroma and flavor, and also provides many health benefits associated to the presence of polyphenols with consequent antioxidant capacity.
For analyzing tea samples, liquid chromatography (LC) is the most preferred technique due its ability for separating the compounds of interest. The possibility of combining two or more columns brought to LC an even greater capacity of separation and higher resolution. However, despite this new multi-column or multi-dimensional technique showing many advantages over the one-column or one-dimensional system, only two works have been published where green and black teas were analyzed, but in an offline mode. Therefore, this work is the first where black tea analysis is performed by a two-dimensional liquid chromatography (2D-LC) technique in a comprehensive mode.
1.2. Aims of the dissertation
The main objective of this dissertation is the application of comprehensive two-dimensional liquid chromatography (LC x LC) for the separation of compounds contained in black tea samples. The separation system comprises in the first dimension a mixed-mode reversed-phase/anionic change (Mixed-Mode WAX-1) column and in second dimension a conventional C18 – reversed phase column; the separation system was further coupled to a diode array and fluorescence detectors in series. The chromatographic conditions were firstly optimized as one-dimensional liquid chromatography (1D-LC) with the same columns that were subsequently used in LC x LC. In addition, an algorithm was applied for evaluating the quality of the separation of compounds as well as the degree of orthogonality.
Chapter I: Aims and structure of disseration
3
1.3. Structure of the dissertation
This dissertation is divided into seven chapters. Chapter I contains a brief and general introduction on tea, followed by a description of the most widely used technique for analyzing this type of matrix, as well as the state of the art about the analysis of tea by 2D–LC. Finally, the various objectives of this work and its structure are presented in this same chapter.
Chapter II describes how leaves and leaf buds of the Cammelia sinensis are processed for obtaining the various types of teas. Subsequently, it is presented and discussed the chemical composition of tea and the results obtained by 1D-LC available in the literature for the separation and characterization of teas. Several concepts of 2D-LC and a detailed description of LC x LC are also presented in this second chapter. Finally, the only two works carried out with the 2D-LC technique for analysis of green and black teas are discussed in detail.
Chapter III describes the methodology used for the preparation of black tea infusion, the work using fluorescence spectroscopy to identify the excitation-emission wavelengths, and the conditions used for the analysis of samples by 1D-LC and LC x LC.
Chapter IV introduces and discusses the experimental results obtained along the work of chromatographic analysis of black tea samples.
Chapter V contains the main conclusions of the work developed in this dissertation. Finnaly, there is a list of references used along the work, followed by Annexes A to E containing experimental data used in the discussion throughout this dissertation.
II.
Basics associated to tea and to comprehensive
two-dimensional liquid chromatography
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
6
2.1. Production of tea
Camellia sinensis (L.), which belongs to the family of Theaceae, are the plants from which leaves and leaf buds are used for producing tea. This drink originated in China around 2700 BC, it was later introduced in Japan, Indonesia, India, and now, appears all over the world, being the most widely consumed beverage after water (Weisburger, 1997).
Depending on the manufacturing process, there may be three categories of the teas based in specific polyphenols and an enzymes, polyphenol oxidase and peroxidase, contained in the leaves of C. sinensis: unfermented (green tea), semi-fermented (oolong tea), and fully semi-fermented (black tea) (Sang et al., 2011). The green tea is based on the dehydration of the cut leaves, by exposing them firstly to heat or hot steam, and then dried at the sun or by a stream of hot air. This heat treatment inactivates the enzyme responsible for the oxidation and consequently the fermentation does not occur, as it happens in the other two categories of teas. The oolong tea consists of leaving the cut leaves to stand, allowing the enzyme to act moderately, and thus forming a semi-fermented product. Finally, the black tea is produced by fermentation (oxidation) of the leaves that are left to stand for a period of time, thus allowing the polyphenol oxidases and peroxidases to act upon the polyphenols. The fermentation produces complex condensed molecules, which provide aroma and color to the black tea (Weisburger, 1997; Wang et al., 2000; Wang and Ho, 2009).
2.2. Chemistry of the black tea
Tea is a complex chemical mixture containing several groups of compounds, from which the polyphenols is one of the most important. However, there are other groups of compounds, such as phenolic acids and methylxanthines. Sang et al. (2011) reviewed the chemistry of the major constituents in tea. The catechins [gallocatechin gallate (GCG), gallocatechin (GC), catechin gallate (CG), and catechin (C) (Figure 1-A)] and their respective isomers [epigallocatechin gallate (EGCG), epigallocatechin (EGC),
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
7
epicatechin gallate (ECG), and epicatechin (EC) (Figure 1-B)] are flavonoids that belong to the group of the flavan-3-ols or flavanols. During the manufacturing and brewing processes the catechins undergo many chemical modifications, such as oxidation, epimerization and polymerization, and their content in tea decreases by 85% (Balentine et al., 1997). The epimerization occurs when the catechins are subjected to high temperatures where the structure is in position cis, as demonstrated by Wang and Helliwell (2000). The polymerization occurs during the oxidation process, where the polyphenol oxidase and peroxidase act on the catechins producing complex condensed molecules, theaflavins, and thearubigins (Wang and Ho, 2009). The interest in the analysis of catechins in tea has been increasing in recent decades due to their highly beneficial properties to human health. Ananingsih et al. (2011) reviewed these properties highlighting that catechins have oxidative, carcinogenic, microbial, viral, inflammatory and anti-diabetic capacities. (A) Compound R1 R2 (1) Gallocatechin gallate OH G (2) Gallocatechin OH H (3) Catechin gallate H G (4) Catechin H H (B) = G (galloyl) Compound R1 R2 (5) Epigallocatechin gallate OH G (6) Epigallocatechin OH H (7) Epicatechin gallate H G (8) Epicatechin H H
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
8
The content of theaflavins in black tea varies between 0.3 and 2%, and these compounds are formed when di-hydroxylated B ring of catechin and the tri-hydroxylated B ring of another catechin molecule are condensated, resulting in four chemical structures: theaflavin, theaflavins 3-gallate, theaflavine 3’-gallate and theaflavin 3,3’-digallate (Figure 2) (Balentine et al., 1997; Scharbet et al., 2004).
Figure 2 - Chemical structure of theaflavins found in tea.
The thearubigins are the most abundant group of compounds found in black tea accounting for 10 – 20% of the dry matter in black tea (Wang et al., 2000). Although its structure and its mechanism of formation are still not entirely clear, it is suggested that they are the product of oxidation between theflavins and catechins (Wang and Ho, 2009). The presence of the theaflavins and the thearubigins are exclusive of black tea, contributing to its characteristic organoleptic properties, and responsible for the intense dark colour. The theaflavins are also responsible for the astringency, brightness, and briskness, whereas the thearubigins contribute for the thickness of the tea (Ngure et al., 2009).
Proanthocyanidins are another group of polymeric compounds found in black tea that are based in catechins units. This group also can be divided in two sub-groups: procyanidins that are based on the monomer units of C and EC, and prodelphinidins based on GC and EC (Fraser et al., 2012).
Compound R1 R2
(9) Theaflavin H H
(10) Theaflavin 3-gallate G H
(11) Theaflavin 3’-gallate H G (12) Theaflavin 3,3’-digallate G G
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
9
Quercetin, kaempferol, myricitin are flavonols found in black tea, which may be non-glycosylated (aglycones), and glycosylated (Figure 3), where the sugar moieties presented in flavonols can be glucose, rhamnose, galactose, arabinose and fructose, which in turn can be mono-, di-, or tri-glycoside. The flavonols glycosylated are more abundant than the aglycones in tea, and this group represents a small fraction in tea of about 1%, expressed as dry weigth solids (Balentine et al., 1997; Wang et al., 2000). The flavonols also show several benefits for health human due to their anti-oxidative, anti-carcinogenic, anti-inflammatory, and anti-diabetic capacities, as well as, due to their role in prevention of cardiovascular disease and reduction of cholesterol (Dufresne and Farnworth, 2001).
´
Figure 3 - Chemical structure of flavonols found in tea.
Others compounds that can be found in tea are the phenolic acids, where their content can be up to 5% of dry weight, depending on the fermentation process. In this group there are two types of structures: hydroxybenzoic and hydroxycinnamic. The only compound from the first type of structures found in tea is the gallic acid (Figure 4), and its amount is significantly increased when de-esterification of the 3-galloyl substituted catechins occurs, either by native esterase or by oxidative degallation. The hydroxycinnamic structure (Figure 5) is represented in tea mainly by coumaric and caffeic acids. However, the chlorogenic acid can also be found in tea, which is constituted by caffeic acid esterified with quinic acid, that is, the quinic acid ester (Balentine et al., 1997; Stalikas, 2007; Sang et al., 2011).
Compound R1 R2
(13) Quercetin OH H
(14) Kaempferol H H
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography 10 O OH HO HO OH (16)
Figure 4 - Chemical structure of gallic acid found in tea.
Figure 5 - Chemical structure of hydroxycinnamic acids found in tea.
Besides polyphenols, there have been also other compounds identified in tea, such as methylxanthines, which play an important role on the quality of tea. In this group it can be included caffeine, theobromine, and theophylline (Figure 6), where caffeine is the compound present in highest amounts (about 1.5 - 4%) due to its stability during the fermentation process, while the others are present in such small amounts (0.2 – 0.4% for theobromine and 0.02% for theophylline) that become sometimes undetectable.
Figure 6 - Chemical structure of methylxanthines found in tea.
Compounds R1 R2 R3 R4 (17) o-Coumaric acid OH H H H (18) m-Coumaric acid H OH H H (19) p-Coumaric acid H H OH H (20) Caffeic acid H OH OH H Compound R1 R2 (21) Caffeine CH3 CH3 (22) Theobromine H CH3 (23) Theophylline CH3 H
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
11
2.3. Liquid chromatography for tea analysis
Liquid chromatography (LC) is among the most used analytical methods for the analysis of tea. LC is a physico-chemical method that allows the separation of analytes according to their distribution in two different phases: the stationary and the mobile phases. The stationary phase is usually in a column and consists of particles packed or a liquid coated on solid support, while the mobile phase is a solution pumped through the column. Various methods have been proposed for the determination of several compounds existing in teas using LC as a separation technique. Lin et al. (1998) found catechins, gallic acid, and methylxanthines in tea water extracts. The separation of compounds was achieved in a C18 column using two modes of elution: an isocratic elution with methanol/doubly distilled water/formic acid (19.5:80.2:0.3, v/v/v); and an gradient elution using as solvent A methanol/formic acid/water (20:0.3:79.7, v/v/v) and as solvent B methanol/formic acid (99.7:0.3, v/v). In both types of elution, the flow rate was 1.0 mL/min and an UV detector was used at 280 nm. Under these operational conditions, Lin et al. (1998) found very low levels of catechins, but very high levels of gallic acid and theobromine in black tea. They also studied the effect of fermentation on the levels of compounds and verified that all catechins, theophylline and theobromine decreased with the degree of fermentation. However, ECG, C, GCG, and theophylline were not observed in black tea due its high degree of fermentation, whereas the levels of gallic acid and caffeine increased due to this same process. Zuo et al. (2002) also used a C18 column for the determination of catechins, caffeine and gallic acids in several tea extracts obtained with 80% methanol. The mobile phase consisted of solvent A, water-acetic acid, 97:3 (v/v) and solvent B, methanol, in gradient elution at a flow rate of 1.0 mL/min. A diode array detector (DAD) was used in the range of 200-400 nm at two selected wavelengths of 280 and 360 nm. Under these operational conditions, Zuo et al. (2002) found that the levels of catechins in black tea are very low, whereas the levels of gallic acids are high. The contents of these compounds were related to the degree of fermentation, such as already found by Lin et al. (1998). Nishitani and Sagesaka (2004) also determined the levels of catechins, caffeine and phenolics
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
12
compounds in samples of tea prepared by extraction with water-acetonitrile (1:1, v/v) for 40 min. The LC system encompassed a C18 column operated at 40ºC and the composition of the mobile phase used was: solvent A, water/methanol/phosphoric acid (85:15:0.1) and solvent B, water/methanol/ethyl acetate/phosphoric acid (85:15:1:0.1), operated in gradient mode. An UV detector at 280 nm was shown to be the most appropriate for the detection of catechins and caffeine. The derivates of caffeoylquinic acid and coumaroylquinic acid were detected in major amounts in black tea. Horžić et al. (2009) also used LC in a study for testing the effect of different temperatures (60, 80 and 100ºC) in a 3 min infusion on the contents of polyphenols and methylxanthines in teas. The separation was carried out using a C18 column and gradient elution with the mobile phase composed by 3% formic acid as solvent A and methanol as solvent B, at a flow rate of 1.0 mL/min. For this study, DAD was used for detecting the compounds between 200 and 400 nm. The authors concluded that catequins, phenolics acids and methylxanthines contents attained maximum values for a water temperature of 100ºC.
2.4. Two-dimensional liquid chromatography (2D-LC)
Since tea is a complex mixture of compounds, it becomes necessary to use techniques of separation with a high resolving power, such as two-dimensional liquid chromatography (2D-LC). 2D-LC consists of two separation processes performed in two different columns, that is, in two different dimensions of chromatography. There are two different approaches for transferring the effluent from the first into the second column: offline and online. In offline 2D-LC, the effluent from the first column is collected in vials and re-injected for further separation into the second column. In the online 2D-LC, the columns are connected via an interface and it may be operated in two modes: heart-cutting and comprehensive. The major difference between these two online 2D-LC operational modes is the amount of effluent transferred from the first into the second column. In heart-cutting 2D-LC (LC-LC), only portions of interest of the effluent from the first dimension are collected in loops of predefined volume, and then reinjected into a second dimension, therefore increasing the chromatographic resolution of the first dimension. In LC x LC, constant volume
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
13
fractions of the effluent from the first dimension are transferred into the second dimension by means of a switching valve that acts as the interface. According to Horst and Schoenmakers (2003), the online approach shows several advantages: acquisition of maximum information on minimal amounts of material, allowing rigorous quantitative interpretation of the results, and total fractionation of the whole sample in both dimensions. However, in 2D-LC there are several issues to be faced in order to aim at a separation fit for purpose: interactions between stationary and mobile phases, isocratic vs. gradient elution, and column temperature. Furthermore, within the LC x LC mode, it is also necessary to take into account the compatibility of mobile phases, the interdependence of flow rates in both dimensions, and the volume of the fractions transferred into the second dimension.
Dixon et al. (2006), in their review about the application of comprehensive multi-dimensional LC to biomedical and pharmaceutical analysis, concluded that the development of LC x LC started in 1972, when in the Oak Ridge National Laboratory, Scott et al. (1972) connected two columns by a six-port valve to analyze body fluids. They used anion exchange chromatography (AXC) in the first dimension and cation exchange chromatography (CXC) in the second dimension; the columns were operated in series and later in parallel.
In 1978, Erni and Frei (1978) verified that the resolution power of 1D-LC was not enough for resolving complex plant extract and then used a switching valve for designing the first LC x LC system. Erni and Frei (1978) used the column filled with gel permeation chromatographic (GPC) material in the first dimension and reversed phase (RP) in the second dimension. Although there have been several developments, such as the availability of sophisticated software and ten-port switching interfacing valves, the LC x LC has not evolved much. As shown in Figure 8, a typical LC x LC system consists of two pumps, two columns, one injector, one interface, and one or several detectors at the end of the chromatographic system.
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
14
Figure 7 - Schematic representation of a LC x LC system. (1) first dimension pump; (2) first dimension column; (3) second dimension pump; (4) loop 1; (5) loop 2; (6) switching valve; (7) second dimension column; (8) waste; (9) detector.
2.4.1. Chromatographic response function (CRF2D)
In LC x LC, it is possible to estimate an index of quality of separation through a mathematical function that is based on the peak purity, number of 2D peaks appearing in the chromatogram, and the time of analysis, namely the two-dimensional chromatographic response function (CRF2D) (Duarte et al., 2012). The
CRF2D can be calculated by following a three step procedure: a) detection of 2D peaks;
b) evaluation of each peak purity (Pi); and c) incorporation of all the parameters in
the final calculation of CRF2D (Duarte et al., 2012).
Before the final evaluation of the quality of separation, it is necessary to perform a data pre-treatment in order to reduce certain unwanted variations. This preliminary step starts by the normalization of the chromatographic data followed by spline interpolation of the data points in the second dimension; then the chromatogram is divided into slices and, finally, each slice is subtracted from its minimum value, in order to eliminate the background noise in the baseline (Duarte et al., 2012).
After the above mentioned corrections, then the chromatogram is ready for assessing the value of the CRF2D. The first step consists in plotting the sum of the two
partial second-derivates of the original chromatogram, and in the second step, the results are divided in different clusters and subsequently fitted to the following Gaussian type model as suggested by Matos (2011):
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
15
Eq. (1) where h0 is the maximum peak height, σ1D and σ2D are the standard deviations along
the first and second dimension, respectively, and s2D is a parameter related to the
symmetry of the peak and its coordinates (tR,1D and tR,2D). An estimate of the volume of
a 2D peak is then possible through the sum of all the values of the chromatogram and multiplying this result by the grid spacings in both dimensions. After the estimate of the volume of the peaks, the next step entails the calculation of the volume of the overlapped region as follows (Duarte et al., 2012):
Eq. (2) where pi is the peak under study, and pi+1 are the remaining overlapped peaks.
Subsequently, it is possible to calculate the value of Pi (Duarte et al., 2012):
Eq. (3) where is the volume of the overlapped region and Vi is the total volume of the
peak.
Finally, the value of CRF2D can be evaluated by the following equation, as
suggested by Duarte et al. (2012):
Eq. (4)
where ∑Pi is the sum of all peak purities, N is the number of 2D peaks, and f(t) is a
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
16
Eq. (5) where and are the retention times of the last eluted peaks in the first and second dimensions, respectively, and and are the elution times corresponding to the void volumes of the columns of the first and second dimensions, respectively.
2.4.2. The orthogonality of separation in 2D-LC
In 2D-LC there is another important concept, the orthogonality, which characterizes the capacity of various separation mechanisms to be independent from each other, and where the compounds show different retention profiles due to different selectivity derived from different combinations of stationary and mobile phases. Hence, the separation is considered to be orthogonal when the retention times of solutes in both dimensions are completely independent from each other. The concept of orthogonality when applied to 2D-LC is depicted in Figure 8, where the values of retention times were normalized to a range between 0 to 1 in both dimensions, as suggested by Slonecker et al. (1996). The retention times were normalized by dividing the corrected retention time of the compounds and the corrected total retention time; the above mentioned correction factor is the retention time corresponding to the column void volume. As shown in Figure 8-A, when the combination of chromatographic phases is orthogonal the separation area is randomly and fully filled, because the two-dimensional separation system is uncorrelated (R ≈ 0); in Figure 8-B, when the combination has some degree of correlation (0 < R < 1), the separation area is not fully filled; and finally, in Figure 8-C, the separation is extremely correlated (R ≈ 1), and the orthogonality is either zero or close to zero.
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
17
Figure 8 - Degrees of orthogonality in a hypothetical 2D-LC system where each point represents an analyte, whose respective retention time is normalized for the column void volume: (A) orthogonal separation, (B) separation with partial correlation, and (C) highly correlated separation. R is the correlation coefficient.
Recently, Pourhaghighi et al. (2011) proposed a different approach for accessing the orthogonality of separation based on the conditional entropy. Firstly, the values of retention times are normalized in a range from 0 to 1 in both dimensions, as suggested by Slonecker et al. (1996). The retention times are then subsequently standardized by dividing the corrected retention time of the peak by the corrected total retention time, and finally, the separation space is divided into a number of bins where peaks are allocated. The information entropy, H(X), of the first dimension is calculated as follows (in units of bits):
Eq. (6) where p(x) is the number of the peaks in the first dimension at specific retention time. The information entropy of the second dimension, H(Y), is calculated using an equation similar to that of Equation 6.
The information entropy of the entire separation space in LC x LC, considered the distribution of peaks, is calculated as follows:
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
18
where the variable X is the first dimension and the variable Y is the second dimension, and p(x, y) is the number of the peaks in each bin.
The conditional entropy, that is, the entropy of the second dimension conditioned on the first dimension, is calculated as follows:
Eq. (8) The orthogonality of the system (O %) can then be finally quantified as:
Eq. (9) where H(Y) is the information entropy of the second dimension.
The percentage of orthogonality obtained by this approach range between 0% for non-orthogonal systems, and 100% for orthogonal systems.
2.5. Application of 2D-LC in tea analysis
In order to obtain more information about the chemistry of tea, the 2D-LC is more appropriate due its higher power of resolution when applied to complex samples. However, only two studies (Kalili et al. 2010; Scoparo et al., 2012) have been carried out with this technique for analyzing green and black teas.
Kalili et al. (2010) used offline 2D-LC for the separation at 50º C of green tea phenolics with a hydrophilic interaction chromatography (HILIC) for the first dimension, and a RP-C18 column for the second dimension, operated in a gradient elution mode in both dimensions. The mobile phases used were acetonitrile and acetic acid (99:1, v/v) for solvent A, and methanol/water/acetic acid (94.05:4.95:1, %v/v/v) for solvent B, at 0.050 mL/min in the first dimension; and 0.1% formic acid in water (v/v) for solvent A and acetonitrile for solvent B, eluted at 0.8 mL/min in the second dimension. The effluent from the HILIC column was automatically collected
Chapter II: Basics associated to tea and to comprehensive two-dimensional liquid chromatography
19
and transferred to vials. After that, all fractions were analyzed into RP-C18 column within two days of collection. The detectors coupled to the separation system were the DAD and the FLD. The UV spectra were recorded between 200 and 500 nm with selected wavelengths monitoring at 210 nm, 280 nm, 320 nm, and 370 nm. The FLD signals were obtained at an excitation wavelength of 276 nm and an emission wavelength of 316 nm. The off-line instrumental analysis was constituted by liquid chromatography-mass spectrometry (LC-MS) and LC-MS2, and the operational
conditions used were negative mode for electrospray ionization (ESI) coupled to a quadrupole-time-of-flight (Q-TOF). The tea extracts were obtained in a solution of 70% acetone in water (v/v), then freeze dried, and subsequently redissolved in 80% acetonitrile and 20% methanol just before further analysis. This 2D-LC methodology was proven to be suitable for the separation of phenolic compounds which could not be separated by 1D-LC. Isomers that were co-eluted on HILIC as well as flavones glycosides could now be separated in a two-dimensional system. This system also allowed to distinguish between flavonols glycosides and flavonols that are retained on HILIC according to the number of sugars, while in RP the separation depends on the nature of the flavonols non-glycosides and the glycoside moiety. Scoparo et al. (2012) used an offline 2D-LC method for analyzing hydroalcoholic extracts of teas. They used size-exclusion chromatography (SEC) with a mobile phase composed of water for solvent A and acetonitrile for solvent B in the first dimension; and in the second dimension it was used an ultra-fast RP (BEH-C18 column) with a mobile phase consisting of formic acid at 0.1% for solvent A and methanol for solvent B. In both dimensions a gradient elution was used at a flow rate of 1.0 ml/min. Analytical signal detection was provided by ELSD, DAD operating between 200-400 nm, and MS with positive and negative modes at atmospheric pressure ionization (API) with a triple quadrupole. Under these conditions, Scoparo et al. (2012) concluded that the combination of SEC and ultra-fast RP provided the orthogonality required for a 2D system of analysis of complex matrices. Furthermore, another important aspect highlighted by the authors was the capacity to identify some compounds by this technique that were not previously reported in the literature, such as saponin isomers and other flavonols glycosides containing gallic acid esters.
III.
Experimental conditions for chromatographic
analysis of black tea samples
Chapter III: Experimental conditions for chromatographic analysis of black tea samples
22
3.1. Chemicals
The mobile phases for 1D-LC and LC x LC were prepared with chromatographic grade acetonitrile (CH3CN), acetic acid (CH3COOH) and ammonium
acetate (CH3COONH4). All solutions were prepared with ultra - pure water (18 M Ω
cm) and filtered prior to their use through membrane filters (PVDF, Gelman Sciences) of 0.22 µm pore size and degassed in an ultrasonic bath (Branson, 2510).
3.2. Preparation of black tea samples
A pack of ten black tea bags (1.5 g of tea per bag) was purchased from a local supermarket (Annex A). Each black tea sample was prepared following the instructions included in the pack of bags. Accordingly, either 200 mL of boiling distilled water or hot (70ºC) ultra-pure water was poured over each tea bag, and left to stand for 3 minutes. This process was prepared in duplicate for each type of water. The tea bags were removed from the infusions after 3 minutes, and left to cool at room temperature. In a first stage, and for the 1D-LC analyses, the infusions were immediately placed in a freezer at 4ºC until analysis. However, for the LC x LC analyses, the freshly prepared infusions with boiling distilled water were filtered through membrane filters (PVDF, Gelman Sciences) of 0.22 µm pore size, in order to ensure that particles in suspension do not alter the composition of the black tea samples during storage at 4ºC. Before analysis, all tea samples were diluted 20% for 1D-LC analysis, except in the first analytical experiment for checking the signal intensity and perform a preliminary assessment of the separation. For LC x LC analysis, the samples were diluted 1% with the mobile phase of the first dimension (more details are given in Section 3.5.1). Before injection, all samples were filtered through HPLC Certified Syringe Filters (hydrophilic regenerated cellulose, SPARTAN, Whatman GmbH, Germany) of 0.20 µm pore size.
Chapter III: Experimental conditions for chromatographic analysis of black tea samples
23
3.3. Excitation-Emission matrix (EEM) fluorescence spectroscopy
Since one of the detectors employed in the LC x LC chromatographic system is a fluorescence detector (FLD), it becomes fundamental to identify the specific excitation/emission wavelengths (λExc/λEm) to be employed in the subsequent LC x LC
analysis of black tea. With this objective in mind, excitation-emission matrix (EEM) fluorescence spectra of four infusions, two of ultra-pure water and two of distilled water, were obtained in order to assess the general spectroscopic characteristics of the tea samples. The fluorescence spectra were recorded on a spectrophotometer JASCO, model FP-6500. Before analysis, the tea samples were subjected to 25-fold dilution, either with ultra-pure or distilled water. The EEM fluorescence spectroscopy involved scanning and recording of 18 individual emission spectra (230-500 nm) at sequential increments of 10 nm of excitation wavelength between 220 and 400 nm. The spectra were recorded at a scan speed of 100 nm min-1 using excitation and
emission slit bandwidths of 5 nm. The peaks due to water Raman scatter were eliminated from all spectra by subtracting either the ultra-pure or distilled water blank spectra.
3.4. Instrumentation
The first dimension consisted of a JASCO semi-micro HPLC pump (model PU-2085 Plus), a Rheodyne injection valve (model 7725i) equipped with a 20 µL loop, and an Acclaim Mixed-Mode WAX-1 column (Dionex, Sunnyvale, CA, USA; diameter 2.1 mm; length 150 mm; comprised of 5 µm high-purity, porous, spherical silica particles with 120 Å diameter pores bonded with alkyl amine functional groups). In the second dimension, a JASCO quaternary low pressure gradient pump (model PU-2089 Plus) and a RP Kromasil® 100-5-C18 column (Eka Chemicals AB–Separation
Products, Bohus, Sweden; diameter 4.6 mm; length 150 mm; particle size 5 µm; pore diameter 100˚A) were applied. The temperature of the analytical columns in both dimensions was maintained at 40ºC in a JASCO column oven (model CO-2065 Plus).
The outlet of the second dimension column was connected to three detectors in series: a diode array detector (DAD) (JASCO, model MD 2010 Plus) operating in the