Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
Microbiological evaluation of minimally processed foods. Genotypic and
phenotypic profile of Staphylococcus sp strains, regarding the production of
biofilm and enterotoxins
Avaliação microbiológica de alimentos minimamente processados. Perfil
genotípico e fenotípico de cepas de Staphylococcus sp, quanto à produção de
biofilme e enterotoxinas
DOI:10.34117/bjdv6n9-127Recebimento dos originais: 08/08/2020 Aceitação para publicação: 08/09/2020
Bruna Lourenço Crippa
Mestre em Biologia Geral e Aplicada
Instituição de atuação atual: Instituto de Biociências da Universidade Estadual Paulista – UNESP Campus de Botucatu
Endereço completo: Rua Dr. Plinio Pinto e Silva, S/N CEP: 18618-691. Distrito de Rubião Junior – Botucatu/SP
E-mail: lourencobruna@yahoo.com.br
Mirella Rossitto Zanutto Elgui
Doutora em Biotecnologia
Instituição de atuação atual: Instituto de Biociências da Universidade Estadual Paulista – UNESP Campus de Botucatu
Endereço completo: Rua Dr. Plinio Pinto e Silva, S/N CEP: 18618-691. Distrito de Rubião Junior – Botucatu/SP
E-mail: mirella.rzanutto@gmail.com
Paulo Eduardo Budri
Doutor em Microbiologia Clínica pelo Royal College of Surgeons in Ireland Instituição de atuação atual: Forensic Science Ireland
Endereço completo: 123 Stephens Green, Dublin 2, Dublin E-mail: paulinhosbudri@gmail.com
Ivana Giovannetti Castilho
Doutora em Biologia Geral e Aplicada
Instituição de atuação atual: Instituto de Biociências da Universidade Estadual Paulista – UNESP Campus de Botucatu
Endereço completo: Rua Dr. Plinio Pinto e Silva, S/N CEP: 18618-691. Distrito de Rubião Junior – Botucatu/SP
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
Nathália Cristina Cirone Silva
Doutora em Biologia Geral e Aplicada
Instituição de atuação atual: Faculdade de Engenharia de Alimentos da Universidade Estadual de Campinas - UNICAMP
Endereço completo: Rua Monteiro Lobato, 80. CEP: 13083-862. Campinas/SP E-mail: ncirone@unicamp.br
Vera Lúcia Mores Rall
Prof. Associado
Instituição de atuação atual: Departamento de Microbiologia e Imunologia, Instituto de Biociências da Universidade Estadual Paulista – UNESP Campus de Botucatu
Endereço completo: Rua Dr. Plinio Pinto e Silva, S/N CEP: 18618-691. Distrito de Rubião Junior – Botucatu/SP
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
ABSTRACT
Nowadays, consumers are increasingly looking for quality food, that is healthy, easy to prepare and easy to consume and, consequently, minimally processed foods have emerged as this alternative. The objective of this work was to evaluate the microbiological quality of 200 minimally processed fruits and vegetables samples. The samples were collected from products marketed in the state of São Paulo, Brazil, in order to determine the most probable number of thermotolerant coliforms (TC) and to detect the presence of Salmonella and Staphylococcus sp. For the Staphylococcus sp strains, the presence of genes related to the production of classical enterotoxins and biofilm was tested. Additionally, the production of enterotoxins and biofilm in vitro by the isolated Staphylococcus sp strains was evaluated. Among the 200 fruits and vegetables samples, 157 (78.5%) were out of the acceptable limits for thermotolerant coliforms. Furthermore, strains producing sed enterotoxin in
vitro and strains producing biofilm on stainless steel and glass surfaces were found. Therefore,
according to the obtained results, the analyzed products are improper for consumption, presenting contamination levels above law recommendation.
Keywords: biofilm, enterotoxin, coagulase-negative staphylococci, coagulase-positive
staphylococci.
RESUMO
Atualmente, os consumidores buscam cada vez mais alimentos de qualidade, saudáveis, fáceis de preparar e consumir e, consequentemente, alimentos minimamente processados têm surgido como essa alternativa. O objetivo deste trabalho foi avaliar a qualidade microbiológica de 200 amostras de frutas e hortaliças minimamente processadas. As amostras foram coletadas de produtos comercializados no estado de São Paulo, Brasil, para determinar o número mais provável de coliformes termotolerantes (CT), detectar a presença de Salmonella e Staphylococcus sp. Para as cepas de Staphylococcus sp, foi testada a presença de genes relacionados à produção de enterotoxinas clássicas e biofilme. Adicionalmente, foi avaliada a produção de enterotoxinas e biofilme in vitro pelas cepas isoladas de Staphylococcus sp. Entre as 200 amostras de frutas e vegetais avaliadas, 157 (78,5%) estavam fora dos limites aceitáveis para coliformes termotolerantes. Além disso, foram encontradas cepas produtoras de entotoxina sed in vitro e cepas produtoras de biofilme em superfícies de aço inoxidável e vidro. Portanto, de acordo com os resultados obtidos, os produtos analisados são impróprios para consumo, apresentando níveis de contaminação acima do recomendado pela legislação.
Palavras-chave: biofilme, enterotoxina, estafilococos coagulase-negativas, estafilococos
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
1 INTRODUCTION
The search for minimally processed foods, especially fruits and vegetables, is growing because of practicality, and due to being ready for consumption (Nascimento et al., 2014).
Minimally processed foods may be obtained from fresh products through selection, washing, peeling, cutting, sanitization, rinse, drying and packing, in order to extend shelf life and retain the nutritional and sensorial properties (Cruz et al., 2006).
These steps might not be efficient to eliminate microbiological contamination of those foods. Furthermore, storage under refrigeration may favor the growing of psychotropic pathogens and deteriorating microorganisms (Gleeson & O’Beirne, 2005).
S. aureus is considered the only pathogenic species among the Staphylococcus, while
coagulase-negative staphylococci (CoNS) have been classified as contaminants (Kloos and Bannerman, 1994). Recently, several authors have suggested that this group of microorganisms should be better studied, in order to ascertain its real role on intoxication cases, since the genes responsible for the production of enterotoxins may be present, but without the occurrence of production (Veras et al., 2008; Rall et al., 2010a; Rall et al, 2010b). Staphylococcal enterotoxins (SE) are the main poisoning agents of bacterial origin in humans, being reported in several outbreaks of foodborne diseases (Lamaita et al., 2005), and may cause intoxications whose symptoms may include diarrhea, vomit, prostration, shiver and fever (Bergdoll, 1989).
Biofilms are microorganisms aggregates soaked in a polymeric matrix and adhered to a solid surface, forming a highly hydrated porous structure that contains exopolysaccharides and small canals, opened between the microcolonies. This type of organization is extremely advantageous to all microorganism species for providing protection against adversities, such as dehydration and bacteriophage colonization, and for providing resistance to antimicrobial agents (Melo, 2008). The biofilm composition is heterogeneous by the microorganism diversity, with different physiological and metabolic properties, as response to the pH and to nutritional requirements inside the polymeric matrix. Furthermore, the microorganism distribution inside the biofilm is usually not uniform, and interdependence between species may occur, which contributes to increase the resistance to antimicrobial agents (Marques, 2005).
Cell proliferation to adhere and form biofilm is mediated by the production of the polysaccharide intercellular adhesion (PIA) or poly-N-succinyl-β-1,6-glucosamine and its synthesis is codified by the genes icaABCD. The genes and products of the ica locus were proven to be fundamental to the formation of biofilm and virulence of microorganisms (O’ TOOLE et al., 2000; STANLEY & LAZAZZERA, 2004).
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
Bap (biofilm-associated protein) is another protein that also seems to be associated with biofilm production. The bap gene is contained inside a pathogenicity island (SaPlbov2) and codifies a protein with 2276 amino acids that acts at the first phase of the surface adherence (CUCARELLA et al., 2004). Bap promotes both the primary bonding to inert surfaces and the intracellular adherence, where PIA/PNAG seems to be involved only on the intracellular adherence. Interestingly, the bap gene is contained inside a mobile pathogenicity island (Ubeda et al., 2003).
Therefore, this work aims the hygienic-sanitary evaluation of minimally processed foods, by the determination of the most probable number of thermotolerant coliforms, the research of
Salmonella, the enumeration of Staphylococcus sp, and the research for the presence of the genes
related to the classical enterotoxins production and biofilm production, as well as the research for the production of these virulence factors in vitro.
2 MATERIALS AND METHODS
2.1 FOOD SAMPLES
A total of 200 samples of minimally processed fruits and vegetables were analyzed, which were acquired at stores in the Botucatu/SP municipality. Samples of green beans, carrots, beets, cabbage and kale were the most frequent among vegetables, while samples of guava, melon, pineapple and papaya were the most frequent among fruits. The samples were transported under refrigeration to the Food Microbiology Laboratory, in the Microbiology and Immunology Department of the Biosciences Institute, UNESP/Botucatu.
All the culture mediums used are from Oxoid brand, except when specified.
2.2 MICROBIOLOGICAL ANALYSIS
For the analysis, 25g were homogenized in 225 ml of buffered water in the Stomacher Lab Blender 400/30s. Starting from the 10-1 dilution, a series of decimal dilutions was prepared.
2.3 DETERMINATION OF THE MOST PROBABLE NUMBER (MPN) OF THERMOTOLERANT COLIFORMS
The research for thermotolerant coliforms was done using the multiple tubes technique. 1ml from each dilution was transferred to three tubes via dilution in Lauryl Sulfate broth with inverted Durham tube. After incubation at 35°C/48h, three portions from the positive tubes were transferred to the EC broth and incubated at 45°C/24h. Based on the positive tubes, the MPN/g was calculated (KORNACKI & JOHNSON, 2001).
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761 2.4 SALMONELLA PRESENCE DETECTION
In order to detect the presence of Salmonella, 25 grams were homogenized in 225ml of buffered peptone water and incubated at 35°C/24h. After this, 1ml was transferred to the tetrathionate broth (0.2ml of potassium iodate) and incubated at 35°C/24h. Another 0.1ml part was transferred to the Rappaporte-Vassiliadis broth and was incubated at 42°C/24h. After this period, each broth was inoculated in xylose lysine deoxycholate agar (XLD) and Salmonella-Shigella agar, incubated at 35°C/24h. The bacterial colonies were tested on TSI, phenylalanine and API-20E (Biomérieux), which were confirmed by the use of somatic polyvalent and flagellar serum (Probac) (ANDREWS et al, 2001).
2.5 ISOLATION AND IDENTIFICATION OF STAPHYLOCOCCUS SP
For S. aureus enumeration (Lancette & Bennett, 2001), serial dilutions of food homogenates were plated on Baird Parker agar (Oxoid) with 5% egg yolk tellurite emulsion (Oxoid) and incubated at 35 °C for 48 hours. After this period, characteristic colonies were counted and were transferred to tubes of nutrient agar. The colonies were tested for catalase and coagulase production, confirmed by using the Staphytect Plus Dry Spot Kit (Oxoid) and the Voges Proskauer test. Coagulase-negative staphylococci strains were identified using the API Staph (Biomérieux).
2.6 PCR
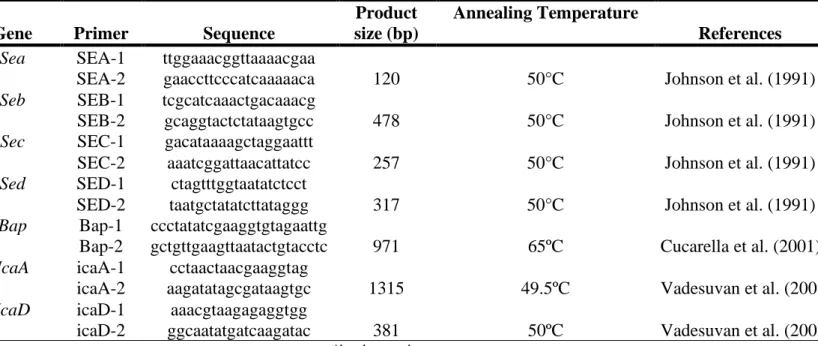
DNA was extracted using a commercial kit (Mini Spin Kit; GE Healthcare) following the supplier’s instructions. The primers used to detect the classical enterotoxins producing genes and the PCR characteristics were performed according to Johnson et al. (1991), and are described on the Table 01. The positive controls used were S. aureus ATCC 13565 (sea), ATCC 14458 (seb), ATCC 19095 (sec), FRI 361 (sed), and for biofilm genes ATCC 12228 (negative) e ATCC 25923 (positive).
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
TABLE 01. Oligonucleotides and its properties used on the detection of biofilm and enterotoxins producing genes, in the Staphylococcus sp strains.
Gene Primer Sequence
Product size (bp)
Annealing Temperature
References Sea SEA-1 ttggaaacggttaaaacgaa
SEA-2 gaaccttcccatcaaaaaca 120 50°C Johnson et al. (1991)
Seb SEB-1 tcgcatcaaactgacaaacg
SEB-2 gcaggtactctataagtgcc 478 50°C Johnson et al. (1991)
Sec SEC-1 gacataaaagctaggaattt
SEC-2 aaatcggattaacattatcc 257 50°C Johnson et al. (1991)
Sed SED-1 ctagtttggtaatatctcct
SED-2 taatgctatatcttataggg 317 50°C Johnson et al. (1991)
Bap Bap-1 ccctatatcgaaggtgtagaattg
Bap-2 gctgttgaagttaatactgtacctc 971 65ºC Cucarella et al. (2001)
IcaA icaA-1 cctaactaacgaaggtag
icaA-2 aagatatagcgataagtgc 1315 49.5ºC Vadesuvan et al. (2003)
IcaD icaD-1 aaacgtaagagaggtgg
icaD-2 ggcaatatgatcaagatac 381 50ºC Vadesuvan et al. (2003)
*bp: base pairs
2.7 BIOFILM FORMATION ON MICROPLATES
Each strain was inoculated in TSB (tryptic soy broth), incubated at 37°C/24h. After this, the culture was diluted at 108 CFU (0.5 on the MacFarland scale), using TSB. A 200µl portion was plated in quadruplicate, in a 96 wells microplate with flat bottom. The CoPS strains were incubated for 48 hours and the CoNS strains were incubated for 72 hours, without agitation in both cases. After the incubation period specific for each group, the plate was washed three times with buffered saline solution (PBS, pH 7.4), dried at ambient temperature and colored with crystal violet 1%/15 minutes. After three washes with distilled water, the dried plate was read in a ELISA reader (Babsystems, MultiSkan EX), with 570nm readings. Non inoculated TSB was used to correct the absorbance value, where the final value was the average of the four wells. The strains that presented corrected values higher than 0.1 were considered biofilm producers (MACK et al., 2000, VASUDEVAN et al., 2003; OLIVEIRA et al., 2007).
Positive and negative controllers were used (S. aureus ATCC 13.556 and S. epidermidis ATCC 12.228, respectively). The significance level employed was 5%.
2.8 STAINLESS STEEL AND GLASS
In the plates preparation, circles of stainless steel with 1cm diameter and glass chips with 1.3cm diameter were used. These materials were cleaned and sterilized. Then, each chip was laid on the bottom of a well of a 24 wells plate, sterile with lid. The Staphylococcus sp strains were
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
incubated in broth (BHI) at 35°C/24h. After, the culture was diluted to 108 CFU of bacteria, with aid of the Densichek (Biomeriéux). Portions of 300µl of this dilution were distributed, in triplicate, in the plate cavities and incubated at 35°C for 48h for the CoPS strains, and for 72h for the CoNS strains. After this, the chips were transferred to a new plate. This step aims to quantify the biofilm eventually formed on the plastic, surrounding the chips of different materials. Once in the new plate, the chips were washed three times with buffer solution (PBS, pH 7.4), for the removal of unfixed cells colored with crystal violet 1%, for 15 minutes. The dye was removed and the plate was washed again. Then, the biofilm was resuspended in 300µl of glacial acetic acid, for 15 minutes, which ensured the homogeneity of the colored material. 200µl were transferred to a 96 wells microplate, read in an ELISA reader (Babsystems, MultiSkan EX), at 570nm. After this, a measurement of the three wells was performed (VASUDEVAN et al., 2003). The results were interpreted according to Stepanovic et al (2000).
2.9 ENTEROTOXINS PRODUCTION BY STAPHYLOCOCCUS SP
The S. aureus strains were cultivated in BHI broth, at 37°C/24h. After, 0.1ml of these growths were spread on a sterilized cellophane, laid upon the agar BHI together with 1% of yeast extract, and incubated at 37°C/24h. Then, the bacterial growth was homogenized with 2.5ml of Na2HPO4 0.01M and the volume was centrifuged at 10,000g/10min/4°C and frozen (DONNELLY
et al., 1967). For the CoNS enterotoxin production, dialysis bags (Inlab) were used tied at the ends with 50ml of BHI broth on double concentration. The bags were put in Erlenmeyers with 36ml PBS buffer. The strains tested were inoculated in BHI broth and, after 24h at 37°C, one portion was inoculated in the PBS buffer, incubated at 37°C/24h, at 140g. After this, 1ml of the PBS buffer was centrifuged at 11,000g/15min, filtered (Milipore, 45µm) and frozen (ROBBINS, GOULD, & BERGDOLL, 1974).
The supernatants obtained by both methodologies were tested on the toxins A, B, C and D, by the RPLA method (Oxoid), according the manufacturer recommendations.
3 RESULTS AND DISCUSSION
3.1 HYGIENIC-SANITARY QUALITY OF FRUITS AND VEGETABLES
Among the 200 fruits and vegetables samples, 157 (78.5%) were out of the acceptable limits for thermotolerant coliforms, which is up to 5 x 102 NMP/g for fruits and up to 102 for vegetables,
with the absence of Salmonella in 25 grams of the product (BRASIL, 2001). According to the data collected in this work, among the 57 fruit samples analyzed, 43 (75.4%) presented themselves in
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
disagreement with current legislation, for the excess of thermotolerant coliforms. Regarding the minimally processed vegetables, among the 143 analyzed samples, 112 (78.3%) fell out of the microbiological standards by the same parameter. A lower contamination rate was observed by Pinheiro et al. (2005), who evaluated the microbiological quality of minimally processed fruits commercialized at supermarkets in Fortaleza (CE), and found that 43% of the samples were unsuitable for human consume, according to RDC nº 12. Adjrah et al (2011) analyzed 90 samples of minimally processed vegetables in Lomé, in Togo, and verified that 87% of the samples were out of the established standard for thermotolerant coliforms. In a study evaluating 144 minimally processed food samples from the cities of São Paulo, Lavras and Brasília, it was found the presence of coliforms in about 50% of the samples and the absence of E. coli and Salmonella sp. (TEIXEIRA et al., 2013). In Portugal, Graça et al. (2015) found coliforms in all samples of minimally processed apples. The results have shown that these foods were dangerous to the consumers, thus a higher hygiene control from production to the commercialization is needed, in addition to controlling the temperature during storage. This high percentage of foods unsuitable for consumption is due to the processing of low quality feedstock and/or bad practices in its manipulation (CHITARRA, 2005).
3.2 SALMONELLA
The presence of Salmonella was not detected in any of the analyzed samples. Similar results were observed by Aguila et al. (2006), whose analysis of minimally processed radishes did not find the presence of this pathogen in any sample. Sasaki et al. (2006) obtained the same results, verifying the absence of this microorganism in all the pumpkin samples analyzed. However Fröder et al. (2007), in São Paulo, observed that 3% of the analyzed samples were contaminated with Salmonella. Abadias et al. (2008), in Spain, analyzed the microbiological quality of 300 minimally processed fruits and vegetables samples and observed four (1.3%) samples positive for Salmonella sp. Studies in Italy, carried out by Caponigro et al. (2010), have not revealed any case of salmonellosis linked to the consume of minimally processed vegetables.
3.3 IDENTIFICATION OF STAPHYLOCOCCUS SP
Among the analyzed samples, 50 (25%) showed the presence of coagulase-negative staphylococci (CoNS) and 7 (3.5%) showed the presence of coagulase-positive staphylococci (CoPS), with the occurrence of more than one species in several samples. From seven CoPS positive samples, 10 strains were isolated and identified, where 6 (60%) were identified as Staphylococcus
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
aureus and 4 (40%) were identified as S. hyicus. Among the 50 CoNS positive samples, 62 strains
were identified, where 53 (85.5%) were identified as Staphylococcus xylosus, 6 (9.7%) were identified as S. epidermidis and 3 (4.8%) were identified as S. warneri. All the 57 (100%) contaminated samples have shown counts higher than 103 CFU/g, reaching up to 105 CFU/g. The ECP contamination rate obtained in this study was lower than the one found by Jung et al. (2005), in Korea, where 37% of the minimally processed lettuce samples were contaminated by S. aureus. In the state of Salvador/Brazil, when comparing microbiological aspects between minimally processed kale and in natura kale, Santos et al., (2015) found the presence of Staphylococcus aureus in all minimally processed kale samples and all in natura kale samples. The presence of this genus is also considered a hygienic indicator of the process, since many species of this genus colonize human hands and mucosa, as Staphylococcus aureus, S. epidermidis, S. warnery, S. xylosus, among others (FRANCO and LANDGRAF, 1996). Therefore, the samples contaminated with these pathogens indicate improper manipulation during their processing.
3.4 RESEARCH FOR THE GENES RESPONSIBLE BY ENTEROTOXINS PRODUCTION AND PRODUCTION IN VITRO
The PCR technique used evidenced the absence of the sea, seb and sec genes in the CoNS and CoPS isolated strains. Only the sed gene was observed in 10 CoPS strains, occurring in 4 (40%)
S. aureus strains and in 1 (10%) S. hyicus strains. Among the 62 CoNS strains, 12 (19.3%) S. xylosus strains and 1 (1.6%) S. epidermidis strains were also positive for the presence of this gene.
However, only one (7.6%) S. epidermidis strains among the 13 positive for this gene produced the enterotoxin D in vitro (4+). All CoPS strains with the presence of the sed gene produced this enterotoxin in vitro (4+). In Korea, Young-Ho et al. (2010) analyzed the enterotoxin production of 40 S. aureus strains isolated from minimally processed vegetables and observed that the gene was not found in any of the isolated strains, however sea was present in 23 (57.5%) strains followed by sed, present in 2 (5%). In 2008, Zell et al. analyzed the classical enterotoxin production capacity of 35 CoNS strains isolated from foods and starters, and observed that 51.4% of the strains were producers of at least one kind of enterotoxin, SED and SEH being the most frequent ones.
Staphylococci are capable of producing enterotoxins, the major causes of bacterial poisoning on humans (Hata et al., 2006). These enterotoxins are thermostable and capable of staying active on the environment for several days, in addition to being resistant to several proteolytic enzymes, which allow their passage through the intestinal tract without loss of activity (Cliver, 1994). Poisoning by
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
Salmonella, and with numbers similar to Clostridium perfringens. In France, in 1997, S. aureus was
identified as the etiologic agent in 569 of 1142 intoxication cases registered. The unexplained cases can be explained by the presence of other coagulase positive or negative staphylococci, or by the production of others enterotoxins, other than the classical ones (ROSEC & GIGAUD, 2002; MARTIN et al., 2004). In spite of the literature that demonstrates SEA as the most prevalent enterotoxin isolated from S. aureus (NORMMANNO et al., 2007), in this study SED was the only enterotoxin found. The D type staphylococcal enterotoxin (SED) is the second most common type associated to cases of food poisoning. The entD gene is the responsible for this enterotoxin, which is located at the PIB 485 plasmid (BAYLES and IANDOLO, 1989). Species as S. hyicus, S.
intermedius and several coagulase-negative staphylococci (CoNS) have been involved in cases of
food poisoning. Udo et al. (1999), isolated CoNS from food handlers at 50 restaurants in Kuwait city and 6% of the strains showed the presence of one or more of the classical enterotoxins, where SEB was the most frequent. In 1996, on a study carried in the south of France, 213 Staphylococcus
aureus and 51 CoNS strains were isolated from several foods, whereas 65 (30.5%) and 9 (17.6%)
were enterotoxin producers, respectively. In the research on the classical enterotoxins production, the distribution frequency found was 66% for SEC and 20.1%, 15.4% and 7.6% for SED, SEA and SEB, respectively (ROSEC et al., 1997).
3.5 BIOFILM
In this study, bap, icaA and icaD genes were not observed in any of the isolated CoPS strains. Regarding CoNS, none presented bap and icaA genes, however 5 (8%) S. xylosus strains were positive for the icaD gene.
Among the 62 CoNS strains and the 10 CoPS strains, none was capable of producing biofilm on polystyrene plates. These results were expected by the absence of the main genes involved in this production.
Among the 62 CoNS strains tested, 60 (96.7%) were considered non-producers and 2 (3.2%)
S. epidermidis strains were considered weak producers on stainless steel surfaces. Among the 10
CoPS strains tested, none was capable to produce biofilm on this surface. In the tests carried on glass surfaces, among the 62 CoNS strains, 59 (95.2%) were considered non-producers and 3 (4.8%)
S. xylosus strains were considered strong producers of biofilm on glass surfaces. Regarding the 10
CoPS strains tested, none was capable of producing biofilm on the same surface. The low percentage of biofilm producer strains on stainless steel and glass surfaces in this work can be explained by the absence of the genes responsible for this production. The biofilm formation process by
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
Staphylococcus that contain the ica operon occur by the production of transmembrane proteins
homologous to PIA trasferases by the icaA gene. The presence of the icaD gene will favor this production. The polymers formed by these two genes reach small sizes, whereas the new and bigger chains are synthesized due to the presence of the icaC gene (Gerke et al., 1998). After this phase, the icaB gene promotes the deacetylation of this molecule, forming the PIA (Gotz, 2002). In case there is no promotion of this last step, bacteria won’t have the capacity of forming biofilm via this gene, since they won’t have polysaccharides disposed for this function (Vuong et al., 2004). Biofilm formation is not dependant on the ica locus, which may be associated to a number of different factors, like other genes and proteins that can promote biofilm formation without necessarily have
ica interaction. One of the first studied mechanisms concerning the ica gene independence for the
biofilm formation was the verification of the Bap operon inexistence. The bap gene, in addition to being involved in the primary surface adhesion, has also an important factor on the intercellular relation after the bacterial agglomerate formation. This last process may also involve the PIA, as described by Cucarella et al. (2001), that the decrease or inactivation of the bap gene leads to a decrease of the PIA production. It is worth highlighting that regarding the dissociation step, considered the last step of the biofilm formation, the staphylococcal cells might have the capacity of dissociating from the biofilm matrix formed, in order to being able to pass to other tissues and surfaces. In this way, it is suggested that there is a way of non-expression of the genes that contribute for biofilm formation, as the dissociation process for the infection or contamination of new surfaces presuppose a negative phenotypic determination regarding the existence of proteins capable of the forming biofilm (NEVES, 2012).
By the obtained results, it is concluded that there is the need for more quality control in the minimally processed foods production, and that the presence of bacteria from the Staphylococcus genus with genes that codify for the enterotoxin production may cause intoxication on the population that consumes this kind of food.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
This work was funded by the São Paulo State Research Support Foundation – FAPESP (grant number 2011/05112-6).
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
REFERENCES
Abadias, M.; Usalla, J.; Angueraa, M.; Solsonaa, C.; Viñas, I. 2008. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments.
International Journal of Food Microbiology. 123, 121–129.
Adjrah, Y.; Karou, D.S., Djéri, B., Anani, K., Soncy, K., Ameyapoh, Y., Souza, C.; Gbeassor, M. 2011. Hygienic quality of commonly consumed vegetables, and perception about disinfecting agents in Lomé. International Food Research Journal, 18, 1499-1503.
Aguila, J.S.D.; Sasaki, F.F.; Heiffig, L.S.; Ongarelli, M.G.; Gallo, C.R. 2006. Determinação da microflora em rabanetes minimamente processados. Horticultura Brasileira, 24, 75 – 78.
Andrews, W.H.; Flowers, J.S.; Bailey, J.S. 2001. Salmonella. In: Compendium of methods for the microbiological examination of foods. downes, F.P.; Ito, K. American Public Health Association. Washington, 4, 357-380.
Bayles, K. W.; Iandolo, J. Genetic And Molecular Analyses Of The Gene Encoding Staphylococcal Enterotoxin D. 1989. Journal Of Bacteriology, 171, 4799-4806.
Bergdoll, M.S. Staphylococcus Aureus. 1989. In: Foodborne Bacterial Pathogens, 463-523.
Brasil. Resolução RDC n. 12 de 02 de janeiro de 2001 da Agência Nacional de Vigilância Sanitária. Dispões sobre o Regulamento técnico sobre padrões microbiológicos para alimentos. Diário Oficial da República Federativa do Brasil, Brasília, DF.
Caponigro, V., Ventura, M., Chiancone, I.; Amato, L., Parente, E.; Piro, F. 2010. Variation of microbial load and visual quality of ready-to-eat salads by vegetable type, season, processor and retailer. Food Microbiology, 27, 1071-1077.
Chitarra M. I. F, Chitarra, A. B. 2005. Pós-colheita de frutos e hortaliças: fisiologia e manuseio. 2, 185-190.
Cliver, D.O. 1994. Foodborne disease handbook: diseases caused by bacteria, 613.
Cruz A. G., Cenci A. S, Maia M. C.A. 2006. Pré-requisitos para implementação do sistema APPCC em uma linha de alface minimamente processada. Ciênc. Tecnol. Aliment. 26, 104-9.
Cucarella, C. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation, J.
Bacteriol. 183, 88–96.
Cucarella, C., Tormo, M. A., Ubeda, C., Trotonda, M. P., Monzon, M., Peris, C., Amorena, B., Lasa, I. & Penades, J. R. 2004. Role of biofilm-associated protein Bap in the pathogenesis of bovine Staphylococcus aureus. Infect Immun 72, 2177–2185.
Donnelly, C.B.; Leslie, J.E.; Black, L.A.; Lewis, K.H. 1967). Serological identification of enterotoxigenic estaphylococci from cheese. Appl. Environ., 15, 1382- 1387.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
Fröder, H., Martins, C. G. De Souza, K. L. Landgraf, M. Franco, B. D. Destro, M. T. 2007. Minimally processed vegetable salads: microbial quality evaluation. J. Food Prot., 70, 277- 1280.
Gerke, C., Kraft, A., Sussmuth, R., Schweitzer, O., Gotz, F. 1998. Characterization oftheN-acetylglucosaminyl transferase activity involved in the biosynthesis oftheStaphylococcus epidermidispolysaccharide intercellular adhesin. J. Biol.Chem. 273, 18586–18593.
Gleeson, E., & O’beirne, D. 2005. Effects of process severity on survival and growth of Escherichia coli and Listeria innocua on minimally processed vegetables. Food Control, 16(8), 677- 685.
Götz, F. 2002. Staphylococcus and biofilms. Molecular Microbiology, 43, 1367–1378.
Graça, A., Santo, D., Esteves, E., Nunes, C., Abadias, M., Quintas, C. 2015 Evaluation of microbial quality and yeast diversity in fresh-cut apple. Food Microbiology.51, 179 – 185.
Hata, E.; Katsuda, K.; Kobayashi, H.; Ogawai, T.; Endo, T.; Eguchi, M. 2006. Characteristics and epidemiologic genotyping of Staphylococcus aureus isolates from bovine mastitis milk in Hokkaido, Japan. J. Vet. Med. Scie, 68, 165–170.
Johnson, W.M.; Tyler, S.D.; Ewan, F.E. Ashton, F. E. Pollard, D. R. and Rozee, K. R. 1991. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in
Staphylococcus aureus by the polymerase chain reaction. J. Clin. Microbiol., 29, 426-430.
Jung, H.J., Cho, J.I. Park, S.H. Ha S.D. Lee, K.H. Kim, C.H. Song, H.S. Chung, D.H. Kim, M.G. Kim, K.Y. Kim, K.S. 2005. Genotypic and phenotypic characteristics of Staphylococcus aureus isolates from lettuces and raw milk. Korean J. Food Sci. Technol. 37: 134-141.
KLOOS, W. E and BANNERMAN T. L. 1994. Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol, 7:117–140.
Kornacki, J. L and Johnson, J. L. 2001. Enterobacteriaceae, coliforms, and Escherichia coli as quality and safety indicators. In:Compendium of methods for the microbiological examination of foods. DOWNES, F.P.; ITO, K. American Public Health Association, 4, 69-82.
Lamaita, H.C., Cerqueira, M. M. O. P. Carmo, L. S. Santos, D. A. Penna, C. F. A. M. Souz, M. R. 2005. Staphylococcus sp. counting and detection of staphylococcal enterotoxins and toxic shock toxin syndrome from cooled raw milk. Arq. Bras. Med. Vet. Zootec., v.57, p.702-709.
Lancette, G. A and Bennett, R. W. 2001. Stapylococcus aureus and Staphylococcal enterotoxins. In: Downes, F. P., Ito, K. Compendium of methods for the microbiological examination of foods. 4 th ed. Washington: American Public Health Association, 39, 387-400.
Mack, D., Rohde, H. Dobinsky, S. Riedewald, J., Nedelmann, M. Knobloch, J. K. Elsner, H. and Feucht, H. 2000. Identification of three essential regulatory gene loci governing expression of Staphylococcus epidermidis polysaccharide intercellular adhesin and biofilm formation. Infection
and Immunity, 68, 3799- 3807.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
Marques, P. C. 2005. Formação de Biofilmes por Staphylococcus aureus na superfície de aço inoxidável e vidro e sua resistência a sanificantes químicos. Dissertação (Mestrado em Ciência e
Tecnologia de Alimentos). UFLA (Universidade Federal de Lavras).
Martin, M. C., Fueyo, J. M. Gonzalez-Hevia, M. A. and Mendoza, M. C. 2004. Genetic procedures for identification of enterotoxigenic strains of Staphylococcus aureus from three food poisoning outbreaks. Int J Food Microbiol, 94, 279– 286.
Melo, L. S. 2008. Estudo fenotípico e genotípico da produção de biofilmes por estirpes de
Staphylococcus aureus isoladas dos casos de mastite subclínica bovina. Dissertação (Mestrado em Medicina Veterinária Preventiva) - Faculdade de Ciências Agrárias e Veterinárias do Campus de
Jaboticabal–UNESP.
Nascimento, K. D. O., Augusta, I. M. Da Rocha Rodrigues, N. Pires, T. Batista, E. Júnior, J. L. B. Barbosa, M. I. M. J. 2014. Alimentos Minimamente Processados: Uma tendência de mercado.
Acta Tecnol. v. 9, n.1, p. 48-61.
Neves, T. C. C. C. 2012. Caracterização e avaliação da capacidade produtora de biofilmes em estafilococos coagulase negativos isolados de superfícies do ambiente fabril. Dissertação (Mestrado
em segurança alimentar). Faculdade de Medicina Veterinária - Universidade Técnica De Lisboa.
Normanno, G., La Salandra, G. Dambrosio, A. Quaglia, N. C. Corrente, M. Parisi, A. Santagada, G. Firinu, A. Crisetti, E. Celano, G. V. 2007. Occurrence, characterization and antimicrobial resistance of enterotoxigenic Staphylococcus aureus isolated from meat and dairy products. Int J Food
Microbiol, 115(3): p. 290-6.
O’toole, G., Kaplan, H. B. Kolter, R. 2000. Biofilm formation as microbial development. Annual
Review Microbiology., 54, 49–79.
Oliveira, M., Nunes, S. F. Carneiro, C. Bexiga, R. Bernardo, F. Vilela, C.L. 2007. Time course of biolfilm formation by Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet.
Microbiol.,124, 187–191.
Pinheiro, N. M. S. De Figueiredo, E. A. T. De Figueiredo, R. W. Maia, G. A. De Souza, P. H. M. 2005. Avaliação da qualidade microbiológica de frutos minimamente processados comercializados em supermercados de Fortaleza. Rev. Bras. Frut., Jaboticabal - SP, 27, 153-156.
Rall, V. L. M., Sforcin, J. M. Augustini, V. C. M. Watanabe, M. T. Fernandes, A. J. Sousa, D. C. Camargo, C. H. Gordinho, N. C. Galindo, L. A. Soares, T. C. S. Araújo, J. P. J. 2010. Polymerase Chain Reaction Detection of EnterotoxinsGenes in Coagulase-Negative StaphylococciIsolated from Brazilian Minas Cheese. Foodborne Pathogens and, 7, n 9.
Robbins, R., Gould, S. Bergdoll, M. 1974. Detection the enterotoxigenicity of Staphylococcus
aureus strains. Appl. Microbiol., 28, 946-50.
Rosec, J. O., Gigaud, O. 2002. Staphylococcal enterotoxin genes of classical and new types detected by PCR in France. J Food Microbiol. 77:61-7.
Braz. J. of Develop.,Curitiba, v. 6, n. 9, p.65789-65804 ,sep. 2020. ISSN 2525-8761
Rosec, J. P., Gigaud, O. Dalet, C. Richard, N. 1997. Enterotoxin production by staphylococci from foods in France. Inter. J. Food Microbiol. v. 35, p. 213-221.
Santos, K. R. S.B., Teixeira, C. N. S. Júnior, N. M. V. Santana, R. F. Miranda, A. S. Coutinho, R. G. 2015. Estudo comparativo da couve minimamente processada e in natura, segundo aspectos de qualidade microbiológica. Demetra; 10(2):279-287.
Sasaki, F. F., Aguila, J. S. D. Gallo, C. R. Ortega, E. M. M., Jacomino, A. P. Kluge, R. A. 2006. Alterações fisiológicas, qualitativas e microbiológicas durante o armazenamento de abóbora minimamente processada em diferentes tipos de corte. Horticultura Brasileira, 24, 170 - 174.
Stanley, N. R., Lazazzera, B. A. 2004. Environmental signals and regulatory pathways that influence biofilm formation. Molecular Microbiology., 52, n.4, p.917-924.
Stepanovic, S., Vukovic, D. Dakic, I. Savic, B. Vlahovic, M. S. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. Journal of Microbiology Methods., 40, 175-179.
Teixeira, L. E. B., Dos Santos, J. E. F. Dos Santos Moreira, I. De Sousa, F. C. Nunes, J. S. 2013. Qualidade microbiológica de frutas e hortaliças comercializadas na cidade de Juazeiro do Norte – CE. Rev. Verde Agroecol. Desenvol. Sust. v.8, n.3, 23 – 26.
Ubeda, C., Tormo, M. A. Cucarella, C. Trotonda, P. Foster, T. J. Lasa, I. and Penadés, J. R. 2003. Sip, na integrase protein with excision, circularization and integration activities, defines a new family of mobile Staphylococcus aureus pathogenicity islands. Molecular. Microbiology, 49, 193-210.
Udo, E. E. 1999. Enterotoxin production by coagulase-negative staphylococci in restaurant workers from Kuawait City may be a potential cause of food poisoning. J Med Microbiol. 48:819-23.
Vasudevan, P., Nair, M. K. M. Annamalai, T. Venkitanarayanan, K. S. 2003. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol., 92, 179–185.
Veras, J. F., Carmo, L. S. Tong, L. C. Shupp, J. W. Cummings, C. Santos, D. A. Cerqueira, M. M. O. P. Cantini, A. Nicoli, J. R. Jett, M. 2008. A study of the enterotoxigenicity of coagulase negative and coagulase positive staphylococcal isolates from food poisoning outbreaks in Minas Gerais. Brazil. J Infect Dis, 12:410–415.
Vuong, C., Kocianova, S. Voyich, J. M. Yao, Y. Fischer, E. R. Deleo, F. R. Otto, M. 2004. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence. J Biol Chem, 279, 881–886.
Young-Ho, S., Ji-Hyun, J. Kwang-Deog, M. 2010. Microbial evaluation of minimally processed vegetables and sprouts produced in Seoul, Korea. Food Science and Biotechnology. 19, 1283-1288.
Zell, C., Resch, M. Rosenstein, R. Albrecht, T. Hertel, C. and Götz, F. 2008. Characterization of toxin production of coagulase-negative staphylococci isolated from food and starter cultures. Int J