0103 - 5053 $6.00+0.00
Article
* e-mail: pfardim@abo.fi
Present Address: Åbo Akademi University, Porthansgatan 3, FIN-20500, Turku/Åbo, Finland.
Effects of Kraft Pulping on the Interfacial Properties of
Eucalyptus
Pulp Fibres
Pedro Fardim*,a and Nelson Durán a,b
a
Instituto de Química, Universidade Estadual de Campinas, CP 6154, 13083-970 Campinas - SP, Brazil and bNCA-Universidade de Mogi das Cruzes, Mogi das Cruzes - SP, Brazil
Os efeitos da polpação kraft nas propriedades interfaciais da polpa de eucalipto brasileiro foram investigados usando titulação com polieletrólito (PT) e medidas de ângulo de contato. Mudanças na composição química da superfície foram avaliadas por espectrometria de reflexão total atenuada no infravermelho (FTIR-ATR), combinada com análise de componentes principais (PCA). O aumento do álcali ativo na polpação provocou a remoção de lignina da superfície, de acordo com FTIR-ATR e PCA. A carga superficial diminuiu enquanto a energia superficial e trabalho de adesão com a água (Wap) aumentaram. O componente ácido-base (Wap-AB) foi indentificado como o causador do aumento em Wap. Nossos resultados sugeriram que a remoção de lignina superficial combinada com a exposição de carbohidratos e mobilidade superficial de extrativos são as principais causas das modificações nas propriedades interfaciais geradas pela polpação kraft. A contribuição das propriedades superficiais no desenvolvimento de seqüências de branqueamento e novos produtos de polpa também foram discutidos.
The effects of kraft pulping on the interfacial properties of eucalypt pulp fibres were investigated using polyelectrolyte titration (PT) and contact angle measurements. Changes on surface composition were assessed by FTIR-ATR spectrometry combined with principal component analysis (PCA). Surface lignin was removed by increase in active alkali in pulping as indicated by FTIR-ATR and PCA. In parallel, the surface charge decreased while the surface energy and work of adhesion with water (Wap) increased. The acid-base component (Wap-AB) explained the increase of Wap. Our results suggested that the removal of surface lignin, exposure of carbohydrates and surface mobility of extractives are the main contributors to modifications of interfacial properties by kraft pulping. The role of interfacial properties on development of bleaching sequences and new pulp products was also briefly discussed.
Keywords: eucalyptus, cellulosic fibre, surface charge, surface energy, work of adhesion
Introduction
The interfacial properties of fibres are relevant in many interactions during paper manufacture, printing and converting. Reactions with hydrophobic compounds in paper sizing, coating with polymer for use in packaging and fibre-to-fibre bonding in paper are significantly affected by the surface charge.1 Adhesion between toner and paper surface in electrostatic printing (laser and photocopy) and mottle in ink-jet printing can also be evaluated in terms of surface energy and work of adhesion.2,3 However, it should be mentioned that the determination of interfacial properties in heterogeneous systems such as pulp fibres requires a critical approach.4
Surface energy and work of adhesion with water are thermodynamic properties usually estimated by measuring contact angle between the fibre surface and certain solvents. In fact, the measured contact angle is an average of local deformations and the energy minimization for the overall system, i.e. an apparent contact angle (θap). Thus, the interfacial properties estimated in this case are apparent properties, which can only be evaluated in relative terms. The interactions between fiber surface and liquids were investigated for hardwood and softwood unbeaten pulps using the Wilhelmy,5 inverse gas chromatography (IGC)6 and θap7 methods. Determinations of θ
J. Braz. Chem. Soc.
and still require improvements.12-14 As a consequence, a strict experimental set-up and certain assumptions are needed. The work of adhesion between water and a solid surface can also be estimated in terms of liquid surface tension and θap measurements.5 The apparent work of
adhesion (Wap) is assumed as a combination of two
components named Lifishitz-van der Waals (Wap-LW) and acid-base (Wap-AB).15 The W
ap-LW component is due to London or dispersion forces across the interface and also includes Keesom and Debye forces. The Wap-AB component is due to specific chemical interactions across solid-liquid interface, where hydrogen bonding is considered. Surface energy can be estimated by the empirical method of Zisman16,17 that involves contact angle measurements between the solid surface and specific liquids. This technique assumes that if there is no hydrogen bonding at the interface, a plot of cosθ versus the surface tension of liquid gives a straight line that extrapolated to unit yields an estimation of surface critical energy.
Estimations of surface charge are presently limited to extrapolation of adsorption isotherms were fiber is used as substrate for adsorption of a high-molar mass polyelectrolyte.18 The dimensions of the polymers are supposed to limit the sorption to the external surfaces. However, screening of polyelectrolyte charges in solution, deviation of stoichiometry and presence of non-electrostatic interactions were reported as drawback for this method.19 In this work, we have used a direct titration of surface charges using a high-molar mass polyelectrolyte in deionized water and monitored the streaming potential using a particle charge detector. Surface energy and work of adhesion with water were estimated using apparent contact angle measurements.
Experimental
Pulps, kappa and viscosity
Nine Eucalyptus grandis kraft pulp samples were
obtained by digesting wood chips in a 20 L laboratory reactor using active alkali (AA) 15.5, 18.1, 19.4, 20.7, 22.0, 23.3, 25.9, 28.5 and 31.1% (m/v) as NaOH. The reactor conditions used in all experiments were: pulping temperature 165 ± 2°C, heating rate 2.8 ± 0.1°C min-1, sulfidity 27.2 ± 0.1% as NaOH, liquor-wood ratio 4:1, H-factor 400 ± 20 and 1000 g (o.d.) of chips. After pulping, the unbleached pulps were washed with tap water until the pH of the filtrate was proximate 6.8 to simulate industrial conditions. Kappa and viscosity were determined according to TAPPI methods T236:85 and T230:94, respectively. Pulp hand sheets were prepared in a Rapid
Köthen apparatus using deionised water and properly conditioned at the temperature of 23 ± 1°C and relative humidity of 50 ± 2% prior to FTIR spectrometry and contact angle measurements. Samples were identified according to the AA used in pulping as H15, H18, H19, H21, H22, H23, H26, H29 and H31.
FTIR spectrometry and principal component analysis
Attenuated total reflectance (ATR) spectra were obtained using a spectrometer Perkin Elmer 1000 equipped with a deuterated triglycine sulphate (DTGS) detector and coupled to a SpectraTech HATR accessory (ZnSe crystal). An incident angle of 45°, spectral resolution of 4 cm-1, number of scans of 256 and temperature of 23°C were the standard conditions used. The contact between samples and the ATR crystal was kept to a constant level in all measurements. All FTIR spectra were obtained on the wire side of the hand sheets. Principal component analysis (PCA) was used to identify the effects of active alkali in pulping on the FTIR spectra. PCA is a multivariate data analysis method where a matrix X with m rows and n
columns, with each spectrum of a sample being a row and each intensity at a given wavenumber being a column, is decomposed as the sum of outer products of vectors ti and p
i plus a residual matrix E. The ti vectors are known as scores and give information on how the spectra are related to each other. The p
i vectors are known as loadings and give information on how the spectral intensities are related to each other, i.e., gives a sub-spectrum of a data set. A
detailed description of PCA method can be found in the literature.20 The ATR spectra were initially pre-processed using a Savitsky-Golay smoothing21,22 and then normalized using the position at 1936 cm-1. A 9 x 601 data matrix was built containing 9 spectra and normalized absorbance at wavenumbers in a range of 800-2000 cm-1 with data points in 2 cm-1 interval. Then, the data matrix was mean centred. No matrix scaling was used and the PCA was performed using a computer program (MATLAB 6.1).
Surface charge, work of adhesion with water and surface energy
μS cm-1, respectively. Apparent work of adhesion with water (Wap) and its components acid-base (Wap-AB) and
Lifishitz-van der Waals (Wap-LW) were estimated from θap
determinations on hand sheet surfaces using water and di-iodomethane. Apparent surface energy (σap) was estimated
using diiodomethane, α-bromo-naphthalene and
benzaldehyde as probe liquids for θap measurements and a
Zisman plot method.17 Hand sheets were previously
conditioned at a temperature of 23 ± 1°C and relative humidity of 50 ± 2% for 6 hours. Liquids employed as probes were conditioned in the same way. The water was distilled twice in a quartz device and treated in a Nanopure deionization system with a subsequent purge of helium
for 20 min. Diiodomethane, α-bromo-naphthalene and
benzaldehyde were purified by vacuum distillation. The surface tensions of water (σ = 72.4 mJ m-2 at 23 °C), diiodomethane (σ = 72.4 mJ m-2 at 23 °C), α -bromo-naphthalene (σ = 44.5 mJ m-2 at 23 °C) and benzaldehyde (σ = 38.3 mJ m-2 at 23 °C) were checked using a ring tensiometer. The θap-DIM values were used for estimation of Wap-LW. The σL-LW assumed for water was 22.4 mJ m-2 at 23 °C.5 Measurements of θ
ap were made in a FIBRO DAT 1000 system. A drop of 3 μL of liquid probe was applied automatically by a liquid delivery system using a short stroke from an electromagnet. The parameters that control the stroke intensity are carefully optimized to avoid oscillation of the drop height immediately after application. The images of the drop in contact with the hand sheets were captured by a video camera at time intervals of 20 ms and shutter opening of 1 ms. The captured images were evaluated by an image analysis system and θap was calculated. A standard distance excluded from analysis was defined by determination of thickness using an electronic micrometer on 10 different hand sheet areas. The contact time used in all experiments was 0.1 s and a set of 10 determinations on each hand sheet was measured. Calibration and other details can be found in a standard method (TAPPI method T 588:97).
Results and Discussion
Effects of kraft pulping on fibre surface chemistry as analysed by FTIR and PCA
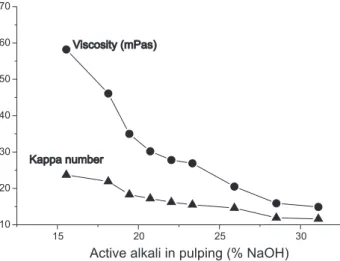
Kappa number and viscosity are routine parameters in the pulp and paper industry to monitor the extent of lignin removal and carbohydrate degradation by pulping, respectively. According to kappa number and viscosity data present in Figure 1, lignin was extensively removed and cellulose degradation increased according to the levels of AA used in pulping. Lignin was removed by cleavage
of α-and β-aril-ether bonds between phenyl propane units and subsequent dissolution in the pulping liquor, while cellulose was degraded by alkaline hydrolysis at high temperatures. It should be mentioned that the variation of AA aiming at changing the fibre chemistry was based on previous experiments using a factorial design and fast pulping cycles.24
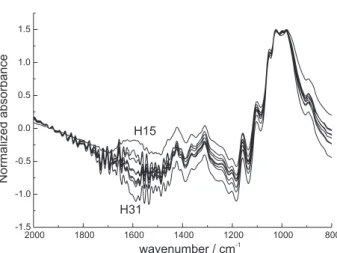
The effects of AA on the surface composition of pulps were assessed by FTIR-ATR combined with PCA. The average sampling depth of this technique is 1 μm, what corresponds to an external layer of 17% of the fibre wall thickness of eucalyptus kraft pulp.25 The ATR normalized spectra of the different pulps in the region of 800-2000 cm-1 are present in Figure 2. It was possible to observe significant changes on ATR spectra when different AA was used. The changes were assessed by PCA. The PCA score plot of the matrix containing spectral data showed that samples obtained with different AA in pulping were clearly separated by the PC1 (Figure 3). This PC described 94.1% of the variation in the data set and separated samples H15 and H31 in clear opposite positions located in different quadrants.
The loads plot using the PC1 versus the variable number converted to wavenumber gave a sub-spectrum of the data set (Figure 4). This sub-spectrum gave information on what wavenumbers most contributed to the separation of pulp samples according the PC1 value. Characteristic peaks of lignin (C=C of aromatic at 1595 cm-1, 1550 cm-1 and 1500 cm-1; C-O-C of aryl-ether at 1225 cm-1 and 1263 cm-1, C-O-C of allyl-ether at 1137 cm-1 and 1188 cm-1, C-H of aromatic at 820 cm-1, 850 cm-1 and 930 cm-1) dominated the sub-spectrum. The PCA sub-spectrum clearly showed that surface lignin was removed when AA was increased. Interestingly, no lignin deposition seemed to occur. Other
Figure 1. Viscosity and kappa number of pulps obtained using
J. Braz. Chem. Soc.
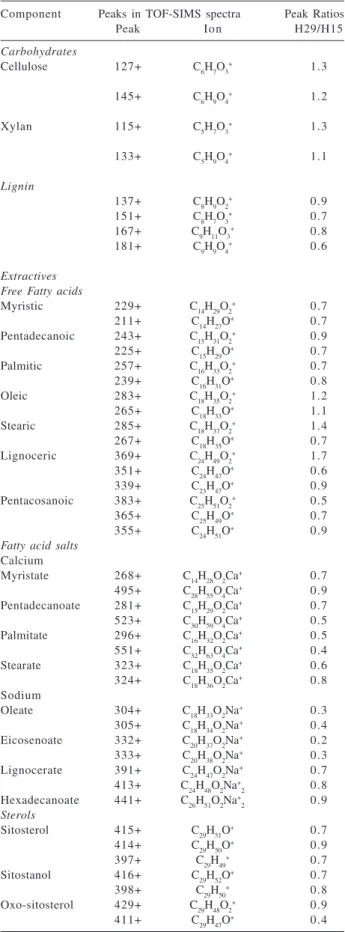
interesting observation was the effect on carbohydrates. Peaks of cellulose and hemicelluloses (C-O of primary alcohol at 1038 cm-1, C-O of secondary alcohol at 1088 cm-1) seemed to be unaffected by AA indicating a exposure of carbohydrates in parallel to lignin removal. The peaks of C-H at 1388 cm-1 and 1463 cm-1 also indicated extractive removal, but as the extractives content in eucalyptus is very low the influence of detection limit of FTIR-ATR cannot be discarded.26 The FTIR-ATR findings could be compared with a previous investigation reported for these samples using time-of-flight secondary ion mass spectrometry (ToF-SIMS) and X-ray photoelectron spectroscopy (XPS) (Table 1a and 1b).27 The removal of lignin and exposure of carbohydrates was clear as indicated by surface coverage using XPS and the ToF-SIMS peak ratio. Evidently, there are differences in the surface depth probed by these techniques. Thus, our results showed that the effects of kraft pulping on lignin and carbohydrates are apparently even in a layer of 1 nm to 1 μm.
Effects of kraft pulping on interfacial properties
The increase in AA reduced the surface charge in pulps, probably due to removal of lignin and uronic acid side groups in xylan (Figure 5). Other explanation may be the sorption of relocated xylan onto fibre surfaces after removal of its side groups by reactions in the pulping liquor.24 Additionally, the surface charge results indicated that the formation of metasaccharinic acids in cellulose29 appeared to occur in inner regions of the fibre wall. This was reasonable considering that the penetration of cooking liquor is believed to be through the fibre lumen.30
The θap measured for the pulp samples and different probe liquids is present in Table 2. The coefficient of variation (CV) was also calculated. No effects of pulping were observed on the θap-DIM, which was lower than θap-WA. This was evidence that the eventual variations in hand sheet roughness did not disturb the θap measurement significantly. Hand sheets are isotropic and no influence
Figure 2. Normalized FTIR-ATR spectra of hand sheets prepared with pulps obtained by using different levels of active alkali in pulping.
Figure 3. Scores plot of FTIR-ATR spectra of different pulp samples. Spectra were previously normalized and the data matrix was only mean centred. The circle showed that no outlier was present.
Figure 4. Loads plot for a data matrix containing FTIR-ATR spectra of different pulp samples.
of fibre orientation was present. The θap-DIM values were very similar as previously obtained for hardwoods using single fibers and the Wilhelmy method.5,31 The W
ap and its components Wap-AB and Wap-LW were estimated and presented in Figure 6. The Wap was dependent on the AA used in pulping. The major contributor for Wap was the Wap-LW component, however, the Wap-AB gradually increased with AA until equalizing the Wap-LW. This was a clear indication that sites for hydrogen bonding were made accessible by pulping. Interestingly, the dispersion forces represented by Wapp-LW did not change.
The apparent surface energy (σap) was estimated by a Zisman plot using the cosθap of different liquid probes. The σap also increased with AA in pulping (Figure 7), probably due to removal of lignin and exposure of carbohydrates. The σap for samples with low kappa number was similar as reported by using IGC for other eucalypts samples at the same kappa level.6 It is conceivable that the adhesion of two components with high surface energy such as cellulose (55 mJ m-2)32 and lignin (47 mJ m-2)33 in the fibre wall tends to lower considerably the free energy of the system. However, during pulping lignin is extensively removed and exposure of carbohydrates generates sites with relative high surface energy. At the same time, molecules with low surface energy, e.g. wood
extractives, tend to segregate to the surface and minimize the total surface free energy. The fact that extractives such as fatty acids have a hydrophobic tail and a hydrophilic head group should also be considered. Thus, a migration of extractives to sites of high surface energy and surface mobility according to the character of the interface involved are due to occur. Evidence of extractive migration and surface mobility can be observed when Wap, Wap-AB, σap and surface charge are taken into account and consequently a hypothesis is put forward: during pulping lignin and uronic acids are removed, the surface charge is reduced and carbohydrate sites of relative high surface energy are formed. Then, high energy sites in cellulose and hemicelluloses are occupied by extractive molecules that migrate from different regions inside the fibre wall or from external surfaces. This is thermodynamically favoured because the total surface
Table 1a. ToF-SIMS and XPSresults reported by Fardim and Durán.27
The peak ratios in ToF-SIMS were calculated after normalization by the spectrum total counts.
Component Peaks in TOF-SIMS spectra Peak Ratios
Peak Ion H29/H15
Carbohydrates
Cellulose 127+ C6H7O3+ 1.3
145+ C6H9O4+ 1.2
Xylan 115+ C5H7O3+ 1.3
133+ C5H9O4+ 1.1
Lignin
137+ C8H9O2+ 0.9
151+ C8H7O3+ 0.7
167+ C9H11O3+ 0.8
181+ C9H9O4+ 0.6
Extractives Free Fatty acids
Myristic 229+ C14H29O2+ 0.7
211+ C14H27O+ 0.7
Pentadecanoic 243+ C15H31O2+ 0.9
225+ C15H29O+ 0.7
Palmitic 257+ C16H33O2+ 0.7
239+ C16H31O+ 0.8
Oleic 283+ C18H35O2+ 1.2
265+ C18H33O+ 1.1
Stearic 285+ C18H37O2+ 1.4
267+ C18H35O+ 0.7
Lignoceric 369+ C24H49O2+ 1.7
351+ C24H47O+ 0.6
339+ C23H47O+ 0.9
Pentacosanoic 383+ C25H51O2+ 0.5
365+ C25H49O+ 0.7
355+ C24H51O+ 0.9
Fatty acid salts Calcium
Myristate 268+ C14H28O2Ca+ 0.7
495+ C28H55O4Ca+ 0.9
Pentadecanoate 281+ C15H29O2Ca+ 0.7
523+ C30H59O4Ca+ 0.5
Palmitate 296+ C16H32O2Ca+ 0.5
551+ C32H63O4Ca+ 0.4
Stearate 323+ C18H35O2Ca+ 0.6
324+ C18H36O2Ca+ 0.8
Sodium
Oleate 304+ C18H33O2Na+ 0.3
305+ C18H34O2Na+ 0.4
Eicosenoate 332+ C20H37O2Na+ 0.2
333+ C20H38O2Na+ 0.3
Lignocerate 391+ C24H47O2Na+ 0.7
413+ C24H48O2Na+
2 0.8
Hexadecanoate 441+ C26H51O2Na+
2 0.9
Sterols
Sitosterol 415+ C29H51O+ 0.7
414+ C29H50O+ 0.9
397+ C29H49+ 0.7
Sitostanol 416+ C29H52O+ 0.7
398+ C29H50+ 0.8
Oxo-sitosterol 429+ C29H48O2+ 0.9
411+ C29H47O+ 0.4
Table 1b. Surface coverage (%-area) of carbohydrates (φcarb),
lig-nin (φlig) and extractives (φext) were estimated using XPS28
Surface coverage by XPS
H15 H18 H23 H29
φcarb 5 6 7 0 7 2 8 4
φlig 4 4 3 0 2 8 1 6
J. Braz. Chem. Soc.
energy of the system is minimized. In a fiber-water interface, there is surface mobility and the hydrophilic head of fatty acids will be oriented towards the water phase. As a consequence Wap and Wap-AB increase due to a combination of lignin removal, exposure of carbohydrates and relocation of extractives. In a fiber-solvent interface, there is also surface mobility, but the hydrophobic chains are oriented towards the solvent, as a result the σap is lower than the expected for solvent extracted eucalyptus pulps, i.e., nearly 50-60 mJ m-2.6,34
Influences of interfacial properties on bleaching sequences and pulp mechanical properties
Bleaching is usually done by addition of chemicals dissolved in water to a pulp suspension. During bleaching an interface fibre-water is present; however, the role of interfacial properties in bleaching is practically unexplored. Bleaching has been treated as a liquid phase reaction and consumption of bleaching chemicals as dependent on contents of residual lignin and hexenuronic
acid in pulp. Carbohydrates are degraded in bleaching and negative effects on mechanical and optical properties were extensively reported. Some attractive alternatives to be investigated are the utilization of surface active agents to protect the carbohydrate sites and the modification of interfacial properties to improve the adhesion between reagents and fibre phases. There is a great demand for development of fast bleaching sequences for high brightness pulps. At the same time, bleached pulps with special properties such as improved wettability and mechanical properties can be designed in bleaching. The impact of interfacial properties on reaction kinetics, consumption of chemicals and pulp bleachability is a suggestion for future work.
Mechanical properties of pulps are affected by fibre-to-fibre bonding and consequently by changes in interfacial properties during pulping, bleaching and refining. The utilization of fast pulping cycles, low AA and thin eucalyptus chips was suggested to reduce the xylan relocation, improving fibre wall swelling and flexibility and thus contributing to fibre-to-fibre bonding.24 More recently, it was also suggested that surface extractives can be detrimental to mechanical properties due to reduction of fibre-to-fibre bonding.35 Hence, according to the hypothesis presented here, any strategy to be developed for fibre modification or even optimization of pulping, bleaching and refining conditions should consider the migration and relocation of extractives onto fibre interfaces. Otherwise, no improvement in mechanical properties will be properly achieved.
Conclusions
We investigated the effects of kraft pulping on surface charge, work of adhesion with water and surface energy
Figure 6. Effects of AA in pulping on Wap, Wap-LW and Wap-AB as estimated by contact angle. The increase in Wap was a clear contribu-tion of Wap-AB.
Figure 7. Effects of AA in pulping on σap as estimated by the Zisman
method.
Table 2. Apparent contact angles (θap) of different probe liquids
measured on hand sheet surfaces. Results are average of 10 mea-surements and coefficient of variation is given in parentheses. Water (WA), diiodomethane (DIM), a-bromo naphthalene (BR) and ben-zaldehyde (BZ)
Sample θap-WA θap-DIM θap-BR θap-BZ
of pulp fibres. The increase in active alkali in pulping reduced the surface charge, increased of work of adhesion with water and surface energy, and improved the removal of surface lignin. The acid-base component was the main contributor for increasing in the work of adhesion with water. A hypothesis to clarify the modifications of interfacial properties by kraft pulping was put forward based on a combination of lignin removal, exposure of carbohydrates and surface mobility of extractives. The role of interfacial properties in bleaching sequences, improvement of mechanical properties and development of new pulp products was briefly discussed.
Acknowledgements
Support from Suzano Papel e Celulose and Åbo Akademi Process Chemistry Centre is acknowledged.
References
1. Barzyk, D.; Page, D. H.; J. Pulp Pap. Sci.1997, 23, 59. 2. Etzler, F. M.; Conners J. J. In Surface Analysis of Paper;
Conners, T.E.; Banerjee, S., eds.; CRC: Boca Raton, 1995, p. 90-108.
3. Duke, C. B.; Noolandi, J.; Thieret, T.; Surf. Sci. 2002, 500, 1005.
4. Adamson, A. W.; Physical Chemistry of Surfaces, 5th ed., Wiley:
New York, 1990.
5. Berg, J.C.; Nord. Pulp Pap. Res. J.1993, 8, 75.
6. Shen, W.; Parker, I.H.; Sheng, Y.J.; J. Adhesion Sci. Technol. 1998, 12, 161.
7. Carlsson, G.; Ström,G.; Nord. Pulp Pap. Res. J.1995, 10, 17. 8. Mejier, M.; Haemers, S.; Cobben, W.; Militz, H.; Langmuir
2000,16, 9352.
9. Shen, W.; Filonanko, Y.; Truong, I. H.; Parker, N.; Brack, P.; Pigram, P.; Liesegang, J.; Colloid Surface A2000, 173, 117. 10. Kwok, D.; Neuman, W.; J. Phys. Chem. B2000, 104, 741. 11. Gerdes, S.; Cazabat, A.; Ström,G.; Langmuir1997, 13, 7258. 12. Della Volpe, C.; Penati, A.; Peruzzi, R.; Siboni, S.; Toniolo, L.;
Colombo, C.; J. Adhes. Sci. Technol.2000, 14, 273.
13. Neimark, A.; J. Adhes. Sci. Technol.2000, 13, 1137. 14. McHale, G.; Rowan, S.M.; Newton, M.I.; Käb, N.A.; J. Adhes.
Sci. Technol. 2000, 13, 1457.
15. Fowkes, F.M.; McCarthy, D.C.; Mostafa, M.A.; J. Colloid Interface Sci 1980, 78, 200.
16. Fox, H.W.; Zisman, W.A.; J. Colloid Interface Sci.1952, 7, 109.
17. Jacob, P.; Berg, J.; Tappi J.1993, 76, 105.
18. Wågberg, L.; Ödberg, L.; Glad.Normark, G.; Nord. Pulp Pap. Res. J.1989, 4, 71.
19. Rojas, O. J.; Ernstsson, M.; Neuman, R. D.; Claesson, P. M.; J. Phys. Chem. B2000, 104, 10032.
20. Wold, S.; Esbensen, K.; Geladi, P.; Chemometr. Intell. Lab. Syst.1987, 2, 37.
21. Savitzky, A.; Golay, M.J.E.; Anal. Chem.1964, 36, 1627. 22. Madden, H. H.; Anal. Chem.1978, 50, 1383.
23. Laine, J.; Buchert, J.; Viikari, L.; Stenius, P.; Holzforschung 1996, 50, 208.
24. Fardim, P.; Durán, N.; J. Braz. Chem. Soc.2004, 15, 514. 25. Fardim, P.; PhD Thesis, Universidade Estadual de Campinas,
Brazil, 1999.
26. Kokkonen, P.; Fardim, P.; Holmbom, B.; Nord. Pulp Pap. Res. J.2004, 19, 318.
27. Fardim, P.; Durán, N.; Colloid Surf. A 2003, 223, 263. 28. Ström, G.; Carlsson, G.; J. Adhes. Sci. Technol.1992, 6, 745. 29. Buchert, J.; Tenkanen, M.; Tamminen, T.; Tappi J.2001, 84,
70.
30. Maloney, T.C.; Paulapuro, H.; J. Pulp Pap. Sci. 1999, 25, 430.
31. Jacob, P.; Berg, J.; Tappi J.1993, 76, 133.
32. van Oss, C.J.; Interfacial Forces in Aqueous Media, Marcel Dekker: New York, 1994.
33. Belgacem, M. N.; Blayo, A.; Gandini, A.; J. Colloid Interface Sci.1996, 182, 431.
34. Shen, W.; Parker, I. H.; Cellulose1999, 6, 41.
35. Fardim, P.; Durán, N.; J. Braz. Chem. Soc.2005, 16, 163.
Received: September 24, 2004
Published on the web: July 14, 2005