Contents lists available atScienceDirect
Journal of Neuroimmunology
journal homepage:www.elsevier.com/locate/jneuroim
Sex in
fl
uences in behavior and brain in
fl
ammatory and oxidative alterations
in mice submitted to lipopolysaccharide-induced in
fl
ammatory model of
depression
Bruna Stefânia Ferreira Mello
a, Adriano José Maia Chaves Filho
a, Charllyany Sabino Custódio
a,
Rafaela Carneiro Cordeiro
a, Fabio Miyajima
a, Francisca Cléa Florenço de Sousa
a,
Silvânia Maria Mendes Vasconcelos
a, David Freitas de Lucena
a, Danielle Macedo
a,b,⁎aNeuropharmacology Laboratory, Drug Research and Development Center, Department of Physiology and Pharmacology, Faculty of Medicine, Universidade Federal do
Ceará, Fortaleza, CE, Brazil
bNational Institute for Translational Medicine (INCT-TM, CNPq), Ribeirão Preto, Brazil
A R T I C L E I N F O
Keywords: Depression Lipopolysaccharide Inflammation Sex
Oxidative stress Lipid peroxidation
A B S T R A C T
Peripheral inflammation induced by lipopolysaccharide (LPS) causes a behavioral syndrome with translational relevance for depression. This mental disorder is twice more frequent among women. Despite this, the majority of experimental studies investigating the neurobiological effects of inflammatory models of depression have been performed in males. Here, we sought to determine sex influences in behavioral and oxidative changes in brain regions implicated in the pathophysiology of mood disorders (hypothalamus, hippocampus and prefrontal cortex - PFC) in adult mice 24 h post LPS challenge. Myeloperoxidase (MPO) activity and interleukin (IL)-1βlevels were measured as parameters of active inflammation, while reduced glutathione (GSH) and lipid peroxidation as parameters of oxidative imbalance. We observed that male mice presented behavioral despair, while females anxiety-like alterations. Both sexes were vulnerable to LPS-induced anhedonia. Both sexes presented increased MPO activity in the PFC, while male only in the hippocampus. IL-1βincreased in the PFC and hypothalamus of animals of both sexes, while in the hippocampus a relative increase of this cytokine in males compared to females was detected. GSH levels were decreased in all brain areas investigated in animals of both sexes, while increased lipid peroxidation was observed in the hypothalamus of females and in the hippocampus of males after LPS exposure. Therefore, the present study gives additional evidence of sex influence in LPS-induced behavioral alterations and, for thefirst time, in the oxidative changes in brain areas relevant for mood regulation.
1. Introduction
Depression is a common chronic-recurrent mental disorder. Recently, the World Health Organization reported that globally > 300 millions of people of all ages suffer from depression, highlighting de-pression as the leading cause of disability worldwide (WHO | Depression, 2015). Depression causes great social and economic costs. For example, in the United States a survey revealed that 8.3% of all years lived with disability (YLDs) were associated to depression, with an enormous economic burden in the order of 210.5 billions of dollars each year (Greenberg et al., 2015). Furthermore, there is a well-es-tablished overall gender difference in the prevalence of depression, with women outnumbering men 2:1. Additionally, the onset of de-pression in women occurs at younger ages with women also presenting
more recurrent episodes (Schuch et al., 2014).
In the last decades, compelling evidences revealed an important contribution of immune-inflammatory alterations to the pathophy-siology of depression (Miller et al., 2009;Rosenblat et al., 2014). In-deed, recent meta-analyses have confirmed that depressed patients present increased serum levels of inflammatory markers, such as in-terleukin-1β(IL-1β), IL-6, tumor necrosis factor (TNF)-alpha and IL-10, and markers of cell activation, such IL-2 receptors (sIL-2Rs) and neopterin (Farooq et al., 2016;Köhler et al., 2017). Furthermore, the systemic injection of cytokines or of the bacterial endotoxin lipopoly-saccharide (LPS) induce depressive symptoms in health humans and depressive-like behavior in rodents (Suarez et al., 2004; Vogelzangs et al., 2016).
In preclinical approaches, LPS-based models of depressive-like
https://doi.org/10.1016/j.jneuroim.2018.04.009
Received 21 November 2017; Received in revised form 12 April 2018; Accepted 12 April 2018
⁎Corresponding author at: Drug Research and Development Center, Department of Physiology and Pharmacology, Universidade Federal do Ceará, Rua Cel. Nunes de Melo 1000, Fortaleza 60431-270, CE, Brazil.
E-mail address:danielle.macedo@ufc.br(D. Macedo).
0165-5728/ © 2018 Elsevier B.V. All rights reserved.
behavior are accompanied by time-dependent alterations related to sickness behaviors (behavioral inhibition, anorexia, weight loss, fa-tigue, hyperalgesia, malaise symptoms, neurocognitive impairment and anxiety) (Dantzer et al., 2008;Maes et al., 2012) and depressive-like behavior. In this regard, some studies (Custódio et al., 2013;Dantzer et al., 2008;Frenois et al., 2007) have demonstrated that most beha-vioral alterations elicited by the acute (1.5–2 h) exposure to LPS com-pose the spectrum characteristic of sickness behavior, in which a marked pyrexia, anorexia and locomotor inhibition occurs. On the other hand, 24 h later, the emergence of depressive-like behavior characterized by despair-, anhedonia- and anxiety-like behaviors takes place. Twenty-four h after LPS exposure motor activity, food and drink consumption returns to normal (Dantzer et al., 2008).
The influence of sex in the behavioral responses to LPS is not fully understood. In this regard, there are studies pointing to a greater sen-sibility of female animals to LPS and cytokine effects in some behavioral aspects, such as sexual activity as well as sucrose and food consumption (Avitsur and Yirmiya, 1999;Merali et al., 2003). On the other hand, other studies reported better coping strategies of females in despair-like conditions, such as forced swimming test (Pitychoutis et al., 2009). Furthermore, thefindings in the literature about sex influences in LPS-induced behavioral effects changes markedly accordingly to the dose of LPS used, time of assessment and murine specie tested (Badalà et al., 2008;Cai et al., 2016).
It is well estabished that depression is accompanied by increased levels of reactive oxygen- (ROS) and nitrogen- (RNS) species, such as superoxide, peroxynitrite and hydrogen peroxide, and oxidative da-mage (Maes et al., 2011a). These pro-oxidative alterations are triggered in some patients by inflammation and mitochondrial metabolic pro-cesses. In clinical samples, some studies pointed that sex could be a determining factor for ROS and RNS levels in health subjects (Bellanti et al., 2013;Massafra et al., 2002), however little is known about the influence of sex in a depressive subset of patients, that is, those patients with depression induced by inflammatory alterations. Also, regarding animal models, several studies demonstrated the contribution of oxi-dative stress in the molecular signature of depressive-like alterations (Lucca et al., 2009;Zhang et al., 2009). However, most of them were conducted in male animals, thus not assessing the possible influence of sex in the oxidative response triggered by depressive-like conditions induced by inflammatory challenge. This issue regarding sex influences in depression deserves a better comprehension, since it will contribute to the development of personalized treatments against this mental disorder.
Therefore, taking into account the important and poorly understood influence of sex in the manifestation of depressive-like phenomenology by LPS immune challenge, in this study, we investigated the behavioral (despair-, anhedonia- and anxiety-like) alterations in male and female mice 24 h post-LPS administration, a time related to the emergence of depressive-like phenotype without locomotor bias (Custódio et al., 2013). Also, we evaluated the influence of sex in brain inflammatory alterations, namely myeloperoxidase (MPO) activity and IL-1β, and, to our knowledge for the first time, in the oxidative stress parameters reduced glutathione (GSH) and lipid peroxidation in relevant brain areas for mood regulation: prefrontal cortex (PFC), hippocampus and hypothalamus.
2. Materials and methods
2.1. Drugs
Lipopolysaccharide (LPS) fromEscherichia coli, strain 055:B5 (Sigma e Aldrich Corp., St Louis, (USA) was used. The drugs were made up freshly for the study. All other chemicals used were of analytical grade.
2.2. Animals
Male and female adult Swiss mice (weighing 25 to 30 g) obtained from the Animal house of the Federal University of Ceara were used. Animals were housed 10 per cage under standard polycarbonate cages (42 × 20.5 × 20 cm) and at normal environmental conditions (22 ± 1 °C, humidity of 60 ± 5%; reversed 12 h light cycle/darkness with lights on at 18:00) with access to food (FRILAB Mouse II, FRIRibe) and water ad libitum. All experimental procedures were performed between 8:00 AM and 02:00 PM and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH, 2011) and the Brazilian law for scientific use of animals from the Brazilian College of Animal Experimentation (COBEA). This research protocol was approved by the local ethics committee of the Federal University of Ceara.
2.3. Experimental protocol
The animals were randomly divided into four experimental groups: male LPS-challenged group, male control-group, female LPS-challenged group, female control-group (n = 24 mice/group). LPS-challenged groups received an intraperitoneal injection of 0.5 mg/kg LPS dissolved in 0.2 ml sterile endotoxin-free SF 0.9% - saline (as vehicle). Control animals received saline, 0.1 ml/10 g weight. Twenty-four hours after LPS or saline exposure, the behavioral tests and sample collection were performed. The behavioral tests were conducted in the order of the less stressful to the more stressful, i.e., openfield, elevated plus maze and forced swimming test. A different set of animals was used to perform sucrose preference test due the potential stressful conditions of food and water deprivation involved in this test. In all behavioral determina-tions, two raters blinded to the experimental treatment performed the tests. Two independent experiments were performed to ensure the re-producibility of the data. To avoid some potential confounding effect of the behavioral testing in the neurochemical parameters, a different set of animals was used to the removal of brain structures. The animals were killed by decapitation, and the brain areas were quickly dissected namely prefrontal cortex (PFC), hippocampus and hypothalamus. All samples were immediately stored−70 °C until assay. The dose of LPS used and the time of assessment was based on previous studies de-monstrating depressive-like behavior and neuroinflammatory altera-tions induced by LPS in mice (Custódio et al., 2013; Frenois et al., 2007).
2.4. Behavioral determinations
2.4.1. Forced swimming test (FST)
The animals were placed individually in an acrylic cylinder (25 cm of height × 10 cm of diameter) containing 8 cm of water at 24 °C. After a habituation period of 1 min, the immobility time (in seconds) of the animals was evaluated for 5 min. Immobility was defined as the absence of targeted escape behavior, such as swimming, jumping, lifting, smell or diving (Porsolt et al., 1977). Any mouse seeming to have trouble keeping its head out of the water was removed from the cylinder and excluded from the analysis. In this study, two experienced evaluators blinded to the treatment group independently assessed the mice beha-vior.
2.4.2. Sucrose preference test (SPT)
individual cages and had free access to two bottles containing 100 ml of sucrose solution (10% w/v) and 100 ml of water, respectively. After 1 h, the volumes of sucrose solution and water consumed were recorded and preferably sucrose consumption was calculated by the following for-mula:
= ×
+ Sucrose preference (sucrose consumption 100%)
/(water consumption sucrose consumption)
2.4.3. Openfield test (OFT)
The openfield arena was made of acrylic (30 × 30 × 15 cm) with transparent walls and a blackfloor, divided into nine squares of equal areas. The openfield test was used to evaluate the locomotor and ex-ploratory activity of the animal during 5 min (Archer, 1973). The parameters number of squares crossed by the animal (crossings number), number of rearings (vertical locomotion), number of self-cleaning grooming behavior, and total time in the center of arena were observed over 5 min and were registered in seconds.
2.4.4. Elevated plus maze test (EPM)
The elevated plus-maze consisted of two open (30 × 5 cm) and two darkened, closed arm (30 × 5 × 15 cm) emanating from a common central platform (5 × 5 cm), to form a plus shape (Pellow and File, 1986). The entire apparatus was raised 45 cm above the base, and the test was performed under dim red light (60 W × 2). The test started by placing a mouse on the central platform, facing an open arm. An ob-servation period of 5 min was used, during which the total arms entries, and the amount of time spent by animals in the open and closed arms of the maze was measured. These data were used to calculate the number of total entries, % of open entries (i.e., open entries/total entries × 100) and % of time in the open arms (time in open arms/total time of the test × 100).
2.5. Neurochemical determinations
2.5.1. Analysis of myeloperoxidase (MPO) activity
Myeloperoxidase is a highly oxidative enzyme. The extracellular activity of this enzyme gives an estimate of the oxidative stress in in-flammatory conditions (Pulli et al., 2013). Peroxidase activity with 3,30,5,50-Tetramethylbenzidine (TMB) was measured as described elsewhere (Suzuki et al., 1983). Absorbance was determined at 450 nm in two time-points, 0 and 3 min, to estimate MPO activity (U MPO/min/ mg tissue).
2.5.2. Immunoassay for IL-1β
The brain areas were homogenized in 8 volumes of PBS buffer with protease (EMD Biosciences) inhibitor and centrifuged (10.000 rpm, 5 min). The concentration of the cytokines in 50 ml samples was de-termined by the immunoenzymatic assay ELISA (R&D systems, Minneapolis, MN, USA) according to the manufacturer's protocol and expressed in pg/g tissue.
2.5.3. Determination of reduced glutathione (GSH) levels
The levels of GSH were evaluated to estimate endogenous defenses against oxidative stress. The method was based on Ellman's reagent (DTNB) reaction with free thiol groups (Sedlak and Lindsay, 1968). The reaction was read in the absorbance of 412 nm, and the product was expressed as ng of GSH/mg wet tissue.
2.5.4. Measurement of lipid peroxidation
Lipid peroxides formation was analyzed by measuring the thio-barbituric-acid reacting substances (TBARS) in the brain homogenates (Mihara and Uchiyama, 1978), as an index of reactive oxygen species (ROS) production. Lipid peroxidation was assessed by the absorbance at 532 nm and expressed asμmol of malonaldehyde (MDA)/mg of tissue.
2.6. Statistical analyses
Data are present in scatter dot plot with lines representing mean ± S.E.M. (standard errors of the mean) and were compared by regular two-way ANOVA with Tukey's as post hoc test. The factors used were“sex” (male and female) and“LPS treatment” (LPS and saline treatment). The significance level was set at P≤0.05. The statistical program used was GraphPad Prism 6.0 Version for Windows, San Diego, CA, USA.
3. Results
3.1. Male mice are more susceptible to despair-like behavior while both sexes present anhedonia without motor alterations 24 h post-LPS
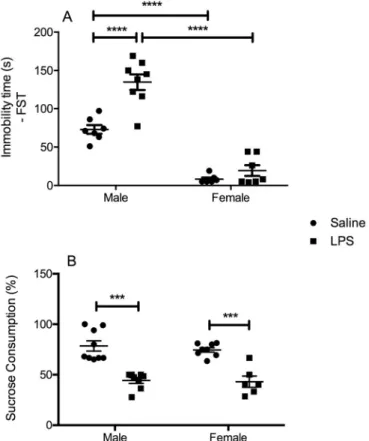
In the evaluation of despair-like behavior using the FST, two-way ANOVA revealed a significant interaction between the factors“sex”and “LPS challenge” [F(1, 27) = 4.989, P = 0.0340], with a significant main effect“sex”[F(1, 27) = 59.21, P < 0.0001] and“LPS challenge” [F(1, 27) = 8.867, P = 0.0061]. Post hoc test revealed a significant increase in the immobility time in LPS-challenged male mice when compared to male controls (P < 0.0001). No significant alterations were observed in female animals exposed to LPS. We also observed increased immobility time in male control animals in relation to female ones (P < 0.0001), being this sex-related increase in immobility time also observed in LPS-challenged males when compared to females (P < 0.0001) (Fig. 1A).
In the evaluation of anhedonia with the SPT (Fig. 1B), we detected a significant main effect of“LPS challenge” in the percent of sucrose consumption [F(1, 25) = 29.05, P < 0.0001], without significant in-teraction between factors. Post hoc analysis revealed a significant de-crease in the percent of sucrose consumption in LPS-challenged male (P < 0.001) and female (P < 0.001) mice in relation to sex-matched controls.
The number of crossings in the OFT was used as a parameter of horizontal locomotor activity. As shown inFig. 2A, LPS immune chal-lenge did not induced significant alterations in the number of crossings in both male and female animals when compared to their sex-matched controls, as well no significant difference was noted when comparing each treatment group in relation to sex. In the exploratory behavior, we did not observe significant differences in the number of rearings post-LPS challenge in animals of both sexes (Fig. 2B).
Regarding grooming behavior and the time spent in the center of the arena, both parameters related to anxiety-like behavior, we observed in grooming behavior (Fig. 2C) a significant main effect of“LPS challenge” [F(1, 27) = 5.984, P = 0.0212] without significant interaction between factors. In this behavioral parameter, we observed that LPS-challenged female mice presented increased grooming behavior in relation to fe-male controls. On the other hand, in the analysis of the time spent in the center of the arena, two-way ANOVA demonstrated no significant al-terations (Fig. 2D).
3.2. Female mice seem to be more susceptible to anxiety-like behavior in the EPM test 24 h post-LPS
In the EPM test, we did not detect significant differences in the total number of entries (Fig. 3A) as well as in the percent of time in the open arms (Fig. 3C). Nevertheless, in the percent of entries in the open arms, a significant main effect of “LPS challenge” [F(1, 21) = 7.863, P = 0.0106], without a significant interaction between factors was observed. In the post hoc test, a significant decrease in the percentage of open arms entries was noted in LPS-challenged female mice when compared to female controls (P < 0.05) (Fig. 3B).
3.3. Male and female mice present brain alterations in MPO activity, IL-1β levels and oxidative parameters 24 h post-LPS
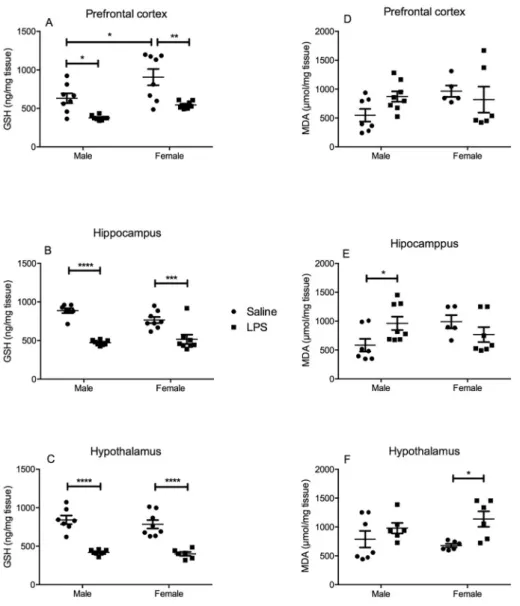
Regarding MPO activity in the PFC (Fig. 4A), two-way ANOVA demonstrated a significant main effect of each factor alone ([F(1, 26) = 23.26, P < 0.0001], for“sex”; [F(1, 26) = 36.48, P < 0.0001], for“LPS challenge”), without significant interaction between factors. Post hoc comparisons revealed a significant increase in MPO activity in the PFC of both male and female subjects challenged with LPS com-pared to their sex-matched controls (P < 0.0001 for males and fe-males). Furthermore, male control animals presented higher MPO ac-tivity when compared to female control ones (P < 0.05). In the hippocampus (Fig. 4B), ANOVA analysis revealed a significant inter-action between factors [F(1, 24) = 8.064, P = 0.0091], with a sig-nificant main effect of each one alone: [F(1, 24) = 8.900, P = 0.0065], for“sex”; [F(1, 24) = 8.523, P = 0.0075], for“LPS challenge”. In the post hoc comparisons, we evidenced a significant increase in MPO ac-tivity in the hippocampus of female mice after LPS challenge (P < 0.01). We also observed higher MPO activity in the hippocampus of male control mice when compared to female controls (P < 0.01). In the hypothalamus ANOVA analysis showed no significant interaction nor main effect of any of the factors analyzed (Fig. 4C).
in relation to sex-matched controls (P < 0.001 for males and P < 0.05 for females). Also, male control mice presented increased levels of IL-1β in relation to female ones (P < 0.05).
Regarding the levels of the main endogenous antioxidant, GSH, we observed in the PFC, a significant main effect of both factors alone: [F (1, 26) = 10.44, P = 0.0033], for “sex” and [F(1, 26) = 26.93, P < 0.0001], for“LPS challenge”, without significant interaction. In the post hoc test, we identified a significant decrease in GSH contents in the PFC of both male (P < 0.05) and female (P < 0.01) mice chal-lenged with LPS when compared to their sex-matched controls. Female control mice presented higher levels of GSH when compared to control male mice (P < 0.05) (Fig. 5A). In the hippocampus, two-way ANOVA revealed a significant main effect of “sex” [F(1, 27) = 1.626, P = 0.2131] and“LPS challenge”[F(1, 27) = 54.69, P < 0.0001], and a significant interaction between factors [F(1, 27) = 5.290, P = 0.0294]. In post hoc test, we detected a significant decrease in GSH levels in the hippocampus of animals of both sexes challenged with LPS
when compared to their same sex controls (P < 0.0001, for males; P < 0.001, for females) (Fig. 5B). In the hypothalamus, there was a significant main effect of “LPS challenge” [F(1, 25) = 88.34, P < 0.0001]. Post hoc comparisons demonstrated a significant de-crease in GSH levels in the hypothalamus of animals of both sexes challenged with LPS compared to sex-matched saline controls (P < 0.0001, for males; P < 0.0001, for females) (Fig. 5C).
Lipid peroxidation represents the damage of lipid membranes caused by oxidative imbalance (Ramos-Loyo et al., 2013). In this con-text, we observed no lipid peroxidation in the PFC of LPS-challenged mice when compared to controls (Fig. 5D). In the hippocampus, two-way ANOVA analysis demonstrated a significant interaction between “sex”and“LPS challenge”[F(1, 23) = 6.131, P = 0.0211]. In the post hoc test, we evidenced a significant increase in MDA levels in the hippocampus of LPS-challenged male mice in relation to their same sex controls (P < 0.05) (Fig. 5E). In the hypothalamus (Fig. 5F), a sig-nificant increase in MDA levels was evidenced in LPS-challenged female mice in relation to their same sex controls (P < 0.05) (two-way ANOVA: main effect of “LPS challenge” [F(1, 21) = 8.211, P = 0.0093].
4. Discussion
In this study, we demonstrated that at the time-point of 24 h post systemic challenge with LPS, male and female mice present a distinctive spectrum of behavioral accompanied by proinflammatory and oxidative alterations. Of note, male animals presented behavioral despair and anhedonia, while females presented anhedonia and anxiety-like al-terations. Both sexes presented brain proinflammatory and pro-oxida-tive alterations. The main strengths of the present study were de-termining sex influences in anxiety-like behavior as well as proinflammatory and pro-oxidative alterations in brain areas relevant to mood regulation in mice submitted to an inflammatory (LPS) model of depressive-like behavior.
Men and women differ in the occurrence and presentation of several psychiatric disorders. This is specifically relevant for major depressive disorder (MDD), which is twice as common in females than in males. Interestingly, prior to puberty, subjects of both genders are equally affected by depression (Marcus et al., 2005). Many biological, psycho-social, and sociological theories attempted to explain this dramatic in-crease in the prevalence of depression among women, but none of them is fully satisfactory. In this context, animal models despite their lim-itations, represent a useful tool for the investigation of sex differences in the neurobiology of depression and antidepressant response (Dalla et al., 2010).
In line with the limited knowledge regarding sex influences in de-pression, only few studies were conducted aiming to determine sex influences in the behavioral and neurochemical alterations induced by LPS challenge. In this context,Pitychoutis et al., 2009reported that male and female rats presented different vulnerability to sickness be-havior at the time-point of 2 h post-LPS administration. In their study, LPS-challenged female rats showed a better coping strategy, evidenced by the increased time of swimming, while males presented a more ro-bust locomotor suppression. The other behavioral parameters eval-uated, such as sucrose consumption, social exploration and food intake were equally affected in animals of both sexes (Pitychoutis et al., 2009). Additionally, a recent study focusing on the sex-specific behavioral effects of LPS at the time-points of 6 and 24 h post LPS (Sens et al., 2017) found that the immobility duration was equally affected in ani-mals of both sexes. The same results were obtained when mice were tested for anhedonia by sucrose consumption. Notably, they found that the grooming behavior in the splash test, an index of motivation and self-care behavior, was persistently impaired in female mice, while anorexic behavior showed to be more pronounced in male counterparts (Sens et al., 2017).
In our experimental conditions, male and female mice showed a Fig. 3.Effect of LPS (0.5 mg/kg, i.p.) in the number of total entries (A), in the %
distinct behavioral pattern in the forced swimming test 24 h post LPS challenge. Our results pointed to an increased vulnerability of LPS challenged male animals to the development of despair behavior in the FST. Of note, we also observed that male control animals presented increased immobility time in relation to female ones. Taken together, these results obtained in male animals corroborates previous findings showing the ability of female animals in the development of coping strategies in the stressful FST (Pitychoutis et al., 2009). It is important to highlight that this previous study was conducted in Sprague–Dawley (SD) rats with LPS serotype 026:B6, i.e., different from the serotype used in the present study, and that the enhanced coping abilities ob-served in females were detected at the time-point of 2 h post LPS (Pitychoutis et al., 2009). This sex influence in the immobility time in the FST observed in our results was not observed at the time-point of 24 h post LPS serotype 026:B6 (Sens et al., 2017), revealing, thus, that the effects of LPS are not only time, but also specie and serotype de-pendent (Felgner et al., 2017).
In addition, we observed that anhedonia assessed by SPT was equally established in both male and female animals. As expected, 24 h post-LPS administration, no locomotor impairment was observed in male and female mice. This result is in line with previous ones showing that both sexes are vulnerable to anhedonia (Sens et al., 2017).
A well-established concept is that anxiety and inflammation walk together. In this regard,Bassi et al., 2012. described the anxiogenic-like
effects of LPS in male rats in several doses and in several behavioral tests (elevated plus maze, black-white box, openfield, elevated T maze, emission of ultrasonic vocalizations) 3–4 h after administration. They found that LPS induced a dose-dependent increase in anxiety-like be-haviors, forming an inverted U curve which peaks in 200μg/kg dose (Bassi et al., 2012). Several other studies demonstrated the anxiogenic-like effects of LPS in the sickness spectrum of alterations (few hours after injection) as well as in depressive-like ones (24 h after) (Li et al., 2017;Salazar et al., 2012;Savignac et al., 2016). We also observed, in a previous study, that LPS 0.5 mg/kg caused anxiety-like behavior in male animals at the time-point of 1.5 h post LPS, while at the time-point of 24 h no alteration was observed (Custódio et al., 2013). Furthermore, the intravenous administration of Salmonella abortus equi endotoxin to 20 healthy male volunteers caused a transient significant increase in the levels of anxiety and depressive mood at the time-points of 1, 3 and 9 h post administration. To the best of our knowledge, no previous study evaluated sex influences in anxious-like behavior in LPS-challenged animals (Reichenberg et al., 2001).
anxiety-like behavior in basal situations (Rodgers and Cole, 1993; Võikar et al., 2001). However, when exposed to stressful-aversive sti-muli, remarkable anxiety-like alterations are observed.
It is worth mentioning that adrenocorticotropic hormone (ACTH) and corticosterone responses are greater in female subjects than in male ones during the exposure to a variety of acute stressors (Doremus-Fitzwater et al., 2009; Iwasaki-Sekino et al., 2009). More recently,Vieira et al., 2017, confirmed this proposition by demonstrating that female rodents showed increased anxiety-like behavior and plasma corticosterone levels when exposed to chronic unpredictable stress and chronic homotypic stress (repeated restraint stress) (Vieira et al., 2017). In this context, our results revealing enhanced vulnerability of females to anxiety-like phe-notype in LPS inflammatory model of depressive-like behavior are in accordance with the previousfindings of female susceptibility to anxiety-like behavior against environmental stressful situations. It is important to mention that there is a discussion in the literature about the possible relation of inflammatory models of depression with one specific subtype of depression, namely atypical depression (Remus and Dantzer, 2016). In fact, atypical depression is the most common form of depression, four times more prevalent in women presenting high comorbidity with an-xiety disorder (Singh and Williams, 2006).
It is consistently recognized that LPS activates toll-like receptor 4 (TLR4). The TLR4 pathway is coupled to the activation inflammasome signaling, in special nucleotide-binding domain leucine-rich repeat (NLRP) 3 (Scholz and Eder, 2017). NLRP3 mediates, in large part, the processing and maturation of IL-1β(Cullen et al., 2015;Jo et al., 2016).
IL-1βacts as a pleotropic proinflammatory cytokine, inducing its own synthesis and the synthesis of other cytokines that potentiate its effect, such as IL-18 and IL-6 (Weber et al., 2010). Mitochondria ROS is both a major trigger and product of NLRP3 inflammasome activation (Han et al., 2015).
Oxidative stress and inflammation are closely related pathophysio-logical processes. Inflammatory cells release reactive species at the site of inflammation leading to exaggerated oxidative stress. On the other hand, a number of reactive species can initiate intracellular signaling cascades that enhances proinflammatory gene expression (Biswas, 2016). In line with this evidence, MPO is an important enzyme of innate immune system that is activated in phagocytic cells during in-flammatory conditions (Arnhold and Flemmig, 2010). MPO generates strong oxidant radicals, in special, hypochlorous acid (HOCl), that can covalently modify lipids and/or proteins causing local damage and amplification of inflammatory response (Arnhold and Flemmig, 2010; Pattison et al., 2012).
Relevant studies reported increased MPO expression and activity in serum samples of depressive patients. For example,Vaccarino et al., 2008, demonstrated in 178 monozygotic and dizygotic twins, a strong association between serum MPO levels and depression scores in stan-dard scales (Vaccarino et al., 2008). Additionally, it was found that MPO mRNA and protein levels are markedly enhanced in peripheral blood cells of patients with recurrent depression, and present a positive correlation with the impaired performance in several cognitive tasks (Talarowska et al., 2015).
In the present study, we observed a brain region-dependent increase in MPO activity in animals submitted to LPS immune challenge. Indeed, MPO activity was increased in the PFC of male and female mice, while in the hippocampus this increase was observed only in female animals. Animal submitted to chronic stress presented higher MPO activity in selected brain areas, namely hippocampus and cortex, which was ac-companied by additional proinflammatory alterations such as micro-glial activation and NO release (Kasımay Çakır et al., 2017;Lisowski et al., 2013). Currently, as far as we know there is no available data about brain MPO activity in rodents submitted to LPS-based depressive-like model. Therefore, our results demonstrated not only the effects of LPS as an activator of MPO activity in the brain, as well as advocate for a possible sex related brain region-dependent response.
Still regarding inflammatory alterations triggered by LPS challenge, in the present study we assessed the levels of IL-1βin relevant brain areas related to mood regulation. We found 24 h post LPS increased levels of IL-1βin the PFC and hypothalamus of male and female mice. We also observed higher levels of IL-1β in the hippocampus of LPS-challenged male mice when compared to female ones, although this increase was not significant in relation to male control mice. It is im-portant to highlight that despite being classified as a cytokine, IL-1βis also a neuroactive molecule that interferes with several mechanisms related to depression: i) decreases hippocampi cell proliferation, being an antineurogenic mediator (Koo and Duman, 2008) and ii) is a potent stimulant of the serotonin metabolism (Ramamoorthy et al., 1995). Considering sex influences in LPS-induced inflammatory alterations, some authors reported an increased inflammatory response in females 24 h post nasal LPS administration (Badalà et al., 2008), while others showed increased sickness-behavior and serum cytokine levels in male mice 10 h post intraperitoneal LPS injection (Cai et al., 2016).
Regarding oxidative alterations in depression, considerable evi-dence reported diminished levels of GSH and/or impaired activity of the enzymes involved in GSH synthesis and restoration in the serum and post-mortembrain of depressed patients (Gawryluk et al., 2011;Maes et al., 2011b). Among the endogenous antioxidant systems, GSH is the most abundant thiol antioxidant present in mammalian cells protecting cells against ROS generated by the mitochondrial respiratory chain (Dringen and Hirrlinger, 2003). Accordingly, several preclinical studies showed that rodents submitted to environmental or pharmacological models of stress presented a marked reduction in total GSH levels and glutathione peroxidase activity (GPX) in brain areas related to mood regulation (Silva et al., 2016;Tao et al., 2016;Todorovićand Filipović, 2017).
Lipid peroxidation is an autoxidation process promoted by radical species against phospholipids and unsaturated fatty acids of the cellular membranes. This process severely hampers the membrane functions, increasing membrane rigidity and allowing the leakage of calcium ions (Fuchs et al., 2014). Malondialdehyde (MDA) is a byproduct of poly-unsaturated fatty acid and arachidonic acid peroxidation consistently employed as a measure of lipid peroxidation (Gawełet al., 2004). There are numerous studies supporting the involvement of lipid peroxidation in depression, and a lot of them demonstrating increased MDA levels in biological samples of depressive patients [for a detailed Review, see (Maes et al., 2011a)].
In our study, we found that male and female mice exposed to LPS immune challenge equally presented impaired GSH levels in all brain areas investigated, and a sex- and brain region-dependent increase in lipid peroxidation. Notably, male mice showed increased levels of MDA in the hippocampus, while females showed increased levels of MDA only in the hypothalamus. Despite previous studies have demonstrated important oxidative damage (lipid peroxidation, increased nitrite and PGE2 levels) and impairment in antioxidant systems (reduced GSH le-vels) in the brain of mice submitted to LPS challenge (Custódio et al., 2013; Mello et al., 2013; Sayd et al., 2014), all of them investigated oxidative changes only in male rodents. Therefore, as far as we know,
our study gives thefirst evidence of sex influences in brain oxidative status in LPS-induced depressive-like model in mice.
It is an important to mention that the neural circuitries related to anxiety situations or reward-pleasure stimuli involves basolateral amygdala and bed nucleus of the stria terminalis (BNST) (Duval et al., 2015; Russo and Nestler, 2013). BNST recruits projections from the ventral hippocampus and hypothalamic nuclei, promoting the pitui-tary-adrenal response (Duval et al., 2015). In reward-promoting situa-tions, orbitofrontal cortex (OFT) projects reward information to ante-rior cingulate cortex, that, in turn, send projections to anteante-rior ventromedial PFC, dorsolateral PFC and ventral hippocampus (Russo and Nestler, 2013). These nuclei are key regulators of decision-making based on reward value and the reinforcement for future actions (Gorwood, 2008).
Taking this above mecioned cirtuitry into consideration, it is pos-sible to suggest, in our experimental condition, that the presence of a pronounced inflammatory-oxidative damage in hippocampal-hypotha-lamic areas of female mice is in accordance with the emergence of an increased anxiety-like phenotype, since these neurocircuits are involved in the regulation of anxiety and stress response (Duval et al., 2015). On the other hand, in male animals, the predominance of oxidative damage in the PFC and hippocampal regions, which are key areas for the reg-ulation of brain reward circuitry (Russo and Nestler, 2013), can be a plausible factor for the development of a robust despair-like/anhedonia behavior in these animals. Finally, the hippocampus, as a region in-trinsically involved in the regulation of both circuits, was impaired in animals of both sexes. On the other hand, in our experimental condition it seems that hippocampus was more affected in males than in females. This study presents some limitations. Firstly, we did not assess the influence of estrous cycle changes in the behavioral and neurochemical effects of LPS in females. This is an interesting point for future research, since ovarian hormones, in special estrogens, are known to modulate not only female behavior, as well as the activity of several cellular antioxidant systems (Bellanti et al., 2013). Additionally, we decided to measure the parameters of oxidative damage: MPO, GSH, and lipid peroxidation, and not others, for example, catalase and superoxide dismutase enzymes, that despite being relevant for redox status, present controversial evidence in depressive patients (Maes et al., 2011a).
In conclusion, this study provides additional evidence of a sex-specific pattern of behavior in adult mice submitted to LPS-induced inflammatory model of depression. Notably, in our results, male ani-mals presented a greater vulnerability to despair-like behavior, while females developed anxiety-related alterations. Both sexes were equally vulnerable to LPS-induced anhedonia. The behavioral alterations in females were followed by increased oxidative changes in all brain areas investigated here, but especially in the hypothalamus, a brain area as-sociated to stress response and anxiety. Male animals presented a pre-ponderance for the involvement of oxidative damage in the PFC and hippocampal regions, which are key areas for the regulation of reward. Therefore, this study advances the knowledge about sex influence in inflammatory-based model of depression by presenting evidences of sex-specific pattern of behavioral alterations and, for thefirst time, of brain oxidative changes.
Disclosure
The authors declare no conflict of interests.
Acknowledgements
References
Archer, J., 1973. Tests for emotionality in rats and mice: a review. Anim. Behav. 21, 205–235.
Arnhold, J., Flemmig, J., 2010. Human myeloperoxidase in innate and acquired im-munity. Arch. Biochem. Biophys. 500, 92–106.http://dx.doi.org/10.1016/j.abb. 2010.04.008.
Avitsur, R., Yirmiya, R., 1999. The immunobiology of sexual behavior: gender differences in the suppression of sexual activity during illness. Pharmacol. Biochem. Behav. 64, 787–796.
Badalà, F., Nouri-mahdavi, K., Raoof, D.A., 2008. Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain leo-nardo. Neuropsychopharmacology 144, 724–732.http://dx.doi.org/10.1038/jid. 2014.371.
Bassi, G.S., Kanashiro, A., Santin, F.M., de Souza, G.E.P., Nobre, M.J., Coimbra, N.C., 2012. Lipopolysaccharide-induced sickness behaviour evaluated in different models of anxiety and innate fear in rats. Basic Clin. Pharmacol. Toxicol. 110, 359–369. http://dx.doi.org/10.1111/j.1742-7843.2011.00824.x.
Biswas, S.K., 2016. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxidative Med. Cell. Longev. 2016, 17–19.http:// dx.doi.org/10.1155/2016/5698931.
Bellanti, F., Matteo, M., Rollo, T., De Rosario, F., Greco, P., Vendemiale, G., Serviddio, G., 2013. Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol. 1, 340–346.http://dx.doi.org/10.1016/j.redox.2013.05.003. Cai, K.C., van Mil, S., Murray, E., Mallet, J.-F., Matar, C., Ismail, N., 2016. Age and sex
differences in immune response following LPS treatment in mice. Brain Behav. Immun. 58, 327–337.http://dx.doi.org/10.1016/j.bbi.2016.08.002.
Cullen, S.P., Kearney, C.J., Clancy, D.M., Martin, S.J., 2015. Diverse activators of the NLRP3 inflammasome promote IL-1beta secretion by triggering necrosis. Cell Rep. 11, 1535–1548.http://dx.doi.org/10.1016/j.celrep.2015.05.003.
Custódio, C.S., Mello, B.S.F., Cordeiro, R.C., de Araújo, F.Y.R., Chaves, J.H., Vasconcelos, S.M.M., Nobre Júnior, H.V., de Sousa, F.C.F., Vale, M.L., Carvalho, A.F., Macêdo, D.S., 2013. Time course of the effects of lipopolysaccharide on prepulse inhibition and brain nitrite content in mice. Eur. J. Pharmacol. 713, 31–38.http://dx.doi.org/ 10.1016/j.ejphar.2013.04.040.
Dalla, C., Pitychoutis, P.M., Kokras, N., Papadopoulou-Daifoti, Z., 2010. Sex differences in animal models of depression and antidepressant response. Basic Clin. Pharmacol. Toxicol. 106, 226–233.http://dx.doi.org/10.1111/j.1742-7843.2009.00516.x. Dantzer, R., O'Connor, J.C., Freund, G.G., Johnson, R.W., Kelley, K.W., 2008. From
in-flammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56.http://dx.doi.org/10.1038/nrn2297. Doremus-Fitzwater, T.L., Varlinskaya, E.I., Spear, L.P., 2009. Social and non-social
an-xiety in adolescent and adult rats after repeated restraint. Physiol. Behav. 97, 484–494.http://dx.doi.org/10.1016/j.physbeh.2009.03.025.
Dringen, R., Hirrlinger, J., 2003. Glutathione pathways in the brain. Biol. Chem. 384, 505–516.http://dx.doi.org/10.1515/BC.2003.059.
Duval, E.R., Javanbakht, A., Liberzon, I., 2015. Neural circuits in anxiety and stress disorders: a focused review. Ther. Clin. Risk Manag. 11, 115–126.http://dx.doi.org/ 10.2147/TCRM.S48528.
Farooq, R., Asghar, K., Kanwal, S., Zulqernain, A., 2016. Role of inflammatory cytokines in depression: focus on interleukin-1β(review). Biomed. Rep.http://dx.doi.org/10. 3892/br.2016.807.
Felgner, J., Jain, A., Nakajima, R., Liang, L., Jasinskas, A., Gotuzzo, E., Vinetz, J.M., Miyajima, F., Pirmohamed, M., Hassan-Hanga, F., Umoru, D., Jibir, B.W., Gambo, S., Olateju, K., Felgner, P.L., Obaro, S., Davies, D.H., 2017. Development of ELISAs for diagnosis of acute typhoid fever in Nigerian children. PLoS Negl. Trop. Dis. 11. http://dx.doi.org/10.1371/journal.pntd.0005679.
Frenois, F., Moreau, M., O'Connor, J., Lawson, M., Micon, C., Lestage, J., Kelley, K.W., Dantzer, R., Castanon, N., 2007. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypotha-lamus, that parallel the expression of depressive-like behavior.
Psychoneuroendocrinology 32, 516–531.http://dx.doi.org/10.1016/j.psyneuen. 2007.03.005.
Fuchs, P., Perez-Pinzon, M.A., Dave, K.R., 2014. Chapter 2–Cerebral ischemia in dia-betics and oxidative stress. In: Diabetes: Oxidative Stress and Dietary Antioxidants, pp. 15–23.http://dx.doi.org/10.1016/B978-0-12-405885-9.00002-4.
Gaweł, S., Wardas, M., Niedworok, E., Wardas, P., 2004. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 57, 453–455.
Gawryluk, J.W., Wang, J.-F., Andreazza, A.C., Shao, L., Young, L.T., 2011. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 14, 123–130. http://dx.doi.org/10.1017/S1461145710000805.
Gorwood, P., 2008. Neurobiological mechanisms of anhedonia. Dialogues Clin. Neurosci. 10, 291–299.
Greenberg, P.E., Fournier, A.-A., Sisitsky, T., Pike, C.T., Kessler, R.C., 2015. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 76, 155–162.http://dx.doi.org/10.4088/JCP.14m09298. Han, S., Cai, W., Yang, X., Jia, Y., Zheng, Z., Wang, H., Li, J., Li, Y., Gao, J., Fan, L., Hu, D.,
2015. ROS-mediated NLRP3 inflammasome activity is essential for burn-induced acute lung injury. Mediat. Inflamm. 2015.http://dx.doi.org/10.1155/2015/720457. Iwasaki-Sekino, A., Mano-Otagiri, A., Ohata, H., Yamauchi, N., Shibasaki, T., 2009.
Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or
psychological. Psychoneuroendocrinology 34, 226–237.http://dx.doi.org/10.1016/ j.psyneuen.2008.09.003.
Jo, E.-K., Kim, J.K., Shin, D.-M., Sasakawa, C., 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell. Mol. Immunol. 13, 148–159.http://dx.doi. org/10.1038/cmi.2015.95.
Kasımay Çakır, Ö., Ellek, N., Salehin, N., Hamamcı, R., Keleş, H., Kayalı, D.G., Akakın, D., Yüksel, M., Özbeyli, D., 2017. Protective effect of low dose caffeine on psychological stress and cognitive function. Physiol. Behav. 168, 1–10.http://dx.doi.org/10.1016/ j.physbeh.2016.10.010.
Köhler, C.A., Freitas, T.H., Maes, M., de Andrade, N.Q., Liu, C.S., Fernandes, B.S., Stubbs, B., Solmi, M., Veronese, N., Herrmann, N., Raison, C.L., Miller, B.J., Lanctôt, K.L., Carvalho, A.F., 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 135, 373–387.http://dx.doi.org/ 10.1111/acps.12698.
Koo, J.W., Duman, R.S., 2008. IL-1 is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. 105, 751–756.http://dx.doi.org/10. 1073/pnas.0708092105.
Li, C., Li, M., Yu, H., Shen, X., Wang, J., Sun, X., Wang, Q., Wang, C., 2017. Neuropeptide VGF C-terminal peptide TLQP-62 alleviates lipopolysaccharide-induced memory deficits and anxiety-like and depression-like behaviors in mice: the role of BDNF/ TrkB signaling. ACS Chem. Neurosci. 8, 2005–2018. (acschemneuro.7b00154). https://doi.org/10.1021/acschemneuro.7b00154.
Lisowski, P., Wieczorek, M., Goscik, J., Juszczak, G.R., Stankiewicz, A.M., Zwierzchowski, L., Swiergiel, A.H., 2013. Effects of chronic stress on prefrontal cortex transcriptome in mice displaying different genetic backgrounds. J. Mol. Neurosci. 50, 33–57.http:// dx.doi.org/10.1007/s12031-012-9850-1.
Lucca, G., Comim, C.M., Valvassori, S.S., Réus, G.Z., Vuolo, F., Petronilho, F., Dal-Pizzol, F., Gavioli, E.C., Quevedo, J., 2009. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem. Int. 54, 358–362.http://dx.doi.org/10. 1016/j.neuint.2009.01.001.
Maes, M., Galecki, P., Chang, Y.S., Berk, M., 2011a. A review on the oxidative and ni-trosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 35, 676–692.http://dx.doi.org/10.1016/j.pnpbp.2010.05.004. Maes, M., Mihaylova, I., Kubera, M., Uytterhoeven, M., Vrydags, N., Bosmans, E., 2011b.
Lower whole blood glutathione peroxidase (GPX) activity in depression, but not in myalgic encephalomyelitis/chronic fatigue syndrome: another pathway that may be associated with coronary artery disease and neuroprogression in depression. Neuro Endocrinol. Lett. 32, 133–140.
Maes, M., Berk, M., Goehler, L., Song, C., Anderson, G., Ga, P., 2012. Depression and Sickness Behavior Are Janus-Faced Responses to Shared Inflammatory Pathways 1–19.http://dx.doi.org/10.1186/1741-7015-10-66.
Mao, Q.-Q., Huang, Z., Zhong, X.-M., Xian, Y.-F., Ip, S.-P., 2014. Brain-derived neuro-trophic factor signalling mediates the antidepressant-like effect of piperine in chronically stressed mice. Behav. Brain Res. 261, 140–145.http://dx.doi.org/10. 1016/j.bbr.2013.12.020.
Marcus, S.M., Young, E.A., Kerber, K.B., Kornstein, S., Farabaugh, A.H., Mitchell, J., Wisniewski, S.R., Balasubramani, G.K., Trivedi, M.H., Rush, A.J., 2005. Gender dif-ferences in depression:findings from the STAR*D study. J. Affect. Disord. 87, 141–150.http://dx.doi.org/10.1016/j.jad.2004.09.008.
Massafra, C., Gioia, D., De Felice, C., Muscettola, M., Longini, M., Buonocore, G., 2002. Gender-related differences in erythrocyte glutathione peroxidase activity in healthy subjects. Clin. Endocrinol. 57, 663–667.
McLean, C.P., Asnaani, A., Litz, B.T., Hofmann, S.G., 2011. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J. Psychiatr. Res. 45, 1027–1035.http://dx.doi.org/10.1016/j.jpsychires.2011.03.006. Merali, Z., Brennan, K., Brau, P., Anisman, H., 2003. Dissociating anorexia and anhedonia elicited by interleukin-1beta: antidepressant and gender effects on responding for "free chow" and "earned" sucrose intake. Psychopharmacology 165, 413–418.http:// dx.doi.org/10.1007/s00213-002-1273-1.
Mihara, M., Uchiyama, M., 1978. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 86, 271–278.
Miller, A.H., Maletic, V., Raison, C.L., 2009. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. http://dx.doi.org/10.1016/j.biopsych.2008.11.029.
Mello, B.S.F., Monte, A.S., McIntyre, R.S., Soczynska, J.K., Custódio, C.S., Cordeiro, R.C., Chaves, J.H., Vasconcelos, S.M.M., Nobre, H.V., Florenço de Sousa, F.C., Hyphantis, T.N., Carvalho, A.F., Macêdo, D.S., 2013. Effects of doxycycline on depressive-like behavior in mice after lipopolysaccharide (LPS) administration. J. Psychiatr. Res. 47, 1521–1529.http://dx.doi.org/10.1016/j.jpsychires.2013.06.008.
NIH, N.R.C. (US) C. for the U. of the G. for the C. and U. of L, 2011. Guide for the Care and Use of Laboratory Animals. National Academies Press, Washington, D.C..http://dx. doi.org/10.17226/12910.
Pattison, D.I., Davies, M.J., Hawkins, C.L., 2012. Reactions and reactivity of myeloper-oxidase-derived oxidants: differential biological effects of hypochlorous and hy-pothiocyanous acids. Free Radic. Res. 46, 975–995.http://dx.doi.org/10.3109/ 10715762.2012.667566.
Pitychoutis, P.M., Nakamura, K., Tsonis, P.A., Papadopoulou-Daifoti, Z., 2009. Neurochemical and behavioral alterations in an inflammatory model of depression: sex differences exposed. Neuroscience 159, 1216–1232.http://dx.doi.org/10.1016/j. neuroscience.2009.01.072.
Porsolt, R.D., Le Pichon, M., Jalfre, M., 1977. Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732.
Pulli, B., Ali, M., Forghani, R., Schob, S., Hsieh, K.L.C., Wojtkiewicz, G., Linnoila, J.J., Chen, J.W., 2013. Measuring myeloperoxidase activity in biological samples. PLoS One 8, e67976.http://dx.doi.org/10.1371/journal.pone.0067976.
Ramamoorthy, S., Ramamoorthy, J.D., Prasad, P.D., Bhat, G.K., Mahesh, V.B., Leibach, F.H., Ganapathy, V., 1995. Regulation of the human serotonin transporter by inter-leukin-1 beta. Biochem. Biophys. Res. Commun.http://dx.doi.org/10.1006/bbrc. 1995.2659.
Ramos-Loyo, J., Medina-Hernández, V., Estarrón-Espinosa, M., Canales-Aguirre, A., Gómez-Pinedo, U., Cerdán-Sánchez, L.F., 2013. Sex differences in lipid peroxidation and fatty acid levels in recent onset schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 44, 154–161.http://dx.doi.org/10.1016/j.pnpbp.2013.02.007. Reichenberg, A., Yirmiya, R., Schuld, A., Kraus, T., Haack, M., Morag, A., Pollmächer, T.,
2001. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58, 445.http://dx.doi.org/10.1001/archpsyc.58.5.445.
Remus, J.L., Dantzer, R., 2016. Inflammation models of depression in rodents: relevance to psychotropic drug discovery. Int. J. Neuropsychopharmacol.http://dx.doi.org/10. 1093/ijnp/pyw028.
Rodgers, R.J., Cole, J.C., 1993. Influence of social isolation, gender, strain, and prior novelty on plus-maze behaviour in mice. Physiol. Behav. 54, 729–736.
Rosenblat, J.D., Cha, D.S., Mansur, R.B., McIntyre, R.S., 2014. Inflamed moods: a review of the interactions between inflammation and mood disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 53, 23–34.http://dx.doi.org/10.1016/j.pnpbp. 2014.01.013.
Russo, S.J., Nestler, E.J., 2013. The brain reward circuitry in mood disorders. Nat. Rev. Neurosci. 14, 609–625.http://dx.doi.org/10.1038/nrn3381.
Savignac, H.M., Couch, Y., Stratford, M., Bannerman, D.M., Tzortzis, G., Anthony, D.C., Burnet, P.W.J., 2016. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-βlevels in male mice. Brain Behav. Immun. 52, 120–131.http://dx.doi.org/10.1016/j.bbi.2015.10.007. Sayd, A., Antón, M., Alén, F., Caso, J.R., Pavón, J., Leza, J.C., Rodríguez de Fonseca, F.,
García-Bueno, B., Orio, L., 2014. Systemic administration of oleoylethanolamide protects from neuroinflammation and anhedonia induced by LPS in rats. Int. J. Neuropsychopharmacol. 18.http://dx.doi.org/10.1093/ijnp/pyu111. Salazar, A., Gonzalez-Rivera, B.L., Redus, L., Parrott, J.M., O'Connor, J.C., 2012.
Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm. Behav. 62, 202–209. http://dx.doi.org/10.1016/j.yhbeh.2012.03.010.
Scholz, H., Eder, C., 2017. Lysophosphatidylcholine activates caspase-1 in microglia via a novel pathway involving two inflammasomes. J. Neuroimmunol. 310, 107–110. http://dx.doi.org/10.1016/j.jneuroim.2017.07.004.
Schuch, J.J.J., Roest, A.M., Nolen, W.A., Penninx, B.W.J.H., de Jonge, P., 2014. Gender differences in major depressive disorder: results from the Netherlands study of de-pression and anxiety. J. Affect. Disord. 156, 156–163.http://dx.doi.org/10.1016/j. jad.2013.12.011.
Sedlak, J., Lindsay, R.H., 1968. Estimation of total, protein-bound, and nonprotein sulf-hydryl groups in tissue with Ellman's reagent. Anal. Biochem. 25, 192–205. Sens, J., Schneider, E., Mauch, J., Schaffstein, A., Mohamed, S., Fasoli, K., Saurine, J.,
Britzolaki, A., Thelen, C., Pitychoutis, P.M., 2017. Lipopolysaccharide administration
induces sex-dependent behavioural and serotonergic neurochemical signatures in mice. Pharmacol. Biochem. Behav. 153, 168–181.http://dx.doi.org/10.1016/j.pbb. 2016.12.016.
Singh, T., Williams, K., 2006. Atypical depression. Psychiatry (Edgmont). 3, 33–39. Silva, M.C.C., de Sousa, C.N.S., Gomes, P.X.L., de Oliveira, G.V., Araújo, F.Y.R., Ximenes,
N.C., da Silva, J.C., Vasconcelos, G.S., Leal, L.K.A.M., Macêdo, D., Vasconcelos, S.M.M., 2016. Evidence for protective effect of lipoic acid and desvenlafaxine on oxidative stress in a model depression in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 64, 142–148.http://dx.doi.org/10.1016/j.pnpbp.2015.08.002. Suarez, E.C., Lewis, J.G., Krishnan, R.R., Young, K.H., 2004. Enhanced expression of
cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide sti-mulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology 29, 1119–1128.http://dx.doi.org/10.1016/j. psyneuen.2004.01.002.
Suzuki, K., Ota, H., Sasagawa, S., Sakatani, T., Fujikura, T., 1983. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal. Biochem. 132, 345–352.http://dx.doi.org/10.1016/0003-2697(83)90019-2.
Tao, W., Dong, Y., Su, Q., Wang, H., Chen, Y., Xue, W., Chen, C., Xia, B., Duan, J., Chen, G., 2016. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav. Brain Res. 308, 177–186.http://dx.doi.org/10.1016/j.bbr.2016.04. 039.
Talarowska, M., Szemraj, J., Gałecki, P., 2015. Myeloperoxidase gene expression and cognitive functions in depression. Adv. Med. Sci. 60, 1–5.http://dx.doi.org/10.1016/ j.advms.2014.06.001.
Todorović, N., Filipović, D., 2017. Prefrontal cortical glutathione-dependent defense and proinflammatory mediators in chronically isolated rats: modulation byfluoxetine or clozapine. Neuroscience 355, 49–60.http://dx.doi.org/10.1016/j.neuroscience. 2017.04.044.
Vaccarino, V., Brennan, M.-L., Miller, A.H., Bremner, J.D., Ritchie, J.C., Lindau, F., Veledar, E., Su, S., Murrah, N.V., Jones, L., Jawed, F., Dai, J., Goldberg, J., Hazen, S.L., 2008. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol. Psychiatry 64, 476–483.http://dx. doi.org/10.1016/j.biopsych.2008.04.023.
Vieira, J.O., Duarte, J.O., Costa-Ferreira, W., Morais-Silva, G., Marin, M.T., Crestani, C.C., 2017. Sex differences in cardiovascular, neuroendocrine and behavioral changes evoked by chronic stressors in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. http://dx.doi.org/10.1016/j.pnpbp.2017.08.014.
Vogelzangs, N., de Jonge, P., Smit, J.H., Bahn, S., Penninx, B.W., 2016. Cytokine pro-duction capacity in depression and anxiety. Transl. Psychiatry 6, e825.http://dx.doi. org/10.1038/tp.2016.92.
Võikar, V., Kõks, S., Vasar, E., Rauvala, H., 2001. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol. Behav. 72, 271–281.
Weber, A., Wasiliew, P., Kracht, M., 2010. Interleukin-1 (IL-1) pathway. Sci. Signal. 3 (cm1-cm1). https://doi.org/10.1126/scisignal.3105cm1.
WHO | Depression [WWW Document] (Ed.), 2015. Depress, Fact sheet [Internet]. Zhang, D., Wen, X., Wang, X., Shi, M., Zhao, Y., 2009. Antidepressant effect of