Potentiometric multi-syringe flow injection system for determination
of exchangeable potassium in soils with in-line extraction
M. Inês G.S. Almeida
a, Marcela A. Segundo
b, José L.F.C. Lima
b, António O.S.S. Rangel
a,⁎
aEscola Superior de Biotecnologia, Universidade Católica Portuguesa, Rua Dr. António Bernardino de Almeida, 4200-072 Porto, Portugal bREQUIMTE, Serviço de Química-Física, Faculdade de Farmácia, Universidade do Porto, Rua Aníbal Cunha, 164, 4099-030 Porto, Portugal
Abstract
A multi-syringe flow injection system for the potentiometric determination of exchangeable potassium in soil samples is proposed. Firstly, a manifold was devised to allow determination in soil extracts prepared off-line. It was possible to analyze samples prepared in extractants with different composition (Mehlich or Morgan) without physical or chemical modification of the manifold. A linear dynamic concentration range of 6–391 mg L− 1was obtained, allowing the direct introduction of soil extract without dilution. A determination frequency of 50 h− 1was achieved, with good repeatability for 10 consecutive injections of soil extracts (RSDb3.0%). The in-line preparation of soil extract was implemented by automatic addition of extractant solution to a previously weighed portion of soil, followed by in-line filtration. Good repeatability was attained as the variance of the extraction procedure was not significantly different from the variance obtained in consecutive measurements of the same extract. Furthermore, results comparable to those obtained by off-line extraction and determination by flame emission spectrometry were attained for the two soil samples tested. Using this procedure, a determination frequency of 13 h− 1and a sampling rate of 4 h− 1were achieved.
Keywords: Multi-syringe flow injection analysis; Potentiometry; Exchangeable potassium; Soil; In-line extraction
Introduction
The determination of exchangeable potassium in soil samples is an important routine analysis, as the quantity of potassium in the soil solution and the readily available pool is assessed to ascertain if potassium fertilizer application is required or not [1]. The analytical procedure involves several steps prior to measurement of potassium, including sampling, drying, sieving, weighing, extraction, and filtration, which makes the determination complex, tedious and time-consuming. For these reasons, the automation of the preliminary operations should be regarded as important as the automation of the mea-surement of potassium.
In fact, this task can be accomplished using flow systems for automating the extraction and/or filtration steps. The direct introduction of solid soil samples or soil suspensions has been
described in flow manifolds incorporating filtration probes[2,3]
or micro-cartridges[4]. However, those approaches have never been applied to the determination of exchangeable potassium.
Therefore, the objective of the present work was the development of a robust flow analyser comprising the in-line extraction and measurement of exchangeable potassium in soils using potentiometric detection. Multi-syringe flow injection analysis (MSFIA) was chosen because this technique, intro-duced in 1999 [5], combines the multi-channel operation of flow injection analysis [6] to the flexibility of the multi-commutation concept[7]. Several works have already revealed the potentialities of MSFIA through analytical applications relying on different detection methods[8]. The potentiometric detectors based on ion-selective electrodes (ISEs) are not an exception, though only applied to the determination of chloride
[9,10]. These detectors are specially suited to flow measure-ments because of their low cost, few instrumental requiremeasure-ments, constant sensitivity over a wide range of linear response, rapid analysis time, high selectivity in most cases, and simple chemistry[11].
⁎ Corresponding author. Tel.: +351 225580064; fax: +351 225090351. E-mail address:aorangel@esb.ucp.pt(A.O.S.S. Rangel).
In this particular application, the features offered by MSFIA were exploited in order to perform the determination in soil extracts with different compositions, including pH and ionic strength (Mehlich and Morgan extractants). Furthermore, in-line preparation of soil extract, including a filtration step prior to measurement of potassium, was also addressed.
Experimental
Reagents and solutions
Deionised water was used to prepare all solutions and che-micals were analytical-reagent grade.
For extraction of potassium from soil samples, two extractants were prepared: the Mehlich solution was prepared by dilution of 4.0 mL of concentrated hydrochloric acid (d = 1.19; 37%) and 2.2 mL of sulfuric acid (6.0 mol L− 1) per liter of water, and the Morgan solution was prepared by dissolving 78.8 g of sodium acetate in about 900 mL of water. After addition of 30 mL of
glacial acetic acid (d = 1.05; 100%), the pH was adjusted to 4.8 and the volume was made up to 1 L.
A potassium stock solution of 1.00 mol L− 1was prepared by dissolving 7.456 g of potassium chloride (previously dried at 100 °C overnight) in 100 mL of water. Working standards from 1 × 10− 4to 1 × 10− 2mol L− 1were obtained by rigorous dilution of stock solution and made up with either Mehlich or Morgan solutions.
The total ionic strength adjustment buffer (TISAB) solution consisted of a mixture of 16.4 g of sodium acetate, 11.4 mL of glacial acetic acid, 100 mL of sodium chloride (2.0 mol L− 1) and 10 mL of potassium (1 × 10− 3mol L− 1), subsequently made up to 1 L with water.
Apparatus
A Crison (Allela, Spain) multi-syringe burette was used to propel all solutions through the flow network. This device is a multiple-channel piston pump, where all pistons are driven by a
MS
F N water S1/V1 St N S2/V2 S3/V3 TISAB MC F V5 HC S4/V4 V6 N F ISE RE w w P GE GEMS
S1/V1 St S2/V2 S3/V3 TISAB MC V5 HC S4/V4 V6 ISE RE w w P GE GE water V7 F N ME SS FD f MEMS
F N water S1/V1 St N S2/V2 S3/V3 TISAB MC F V5 HC S4/V4 V6 N F ISE RE w w P GE GEMS
S1/V1 St S2/V2 S3/V3 TISAB MC V5 HC S4/V4 V6 ISE RE w w P GE GE water V7 F N ME SS FD f ME(a)
(b)
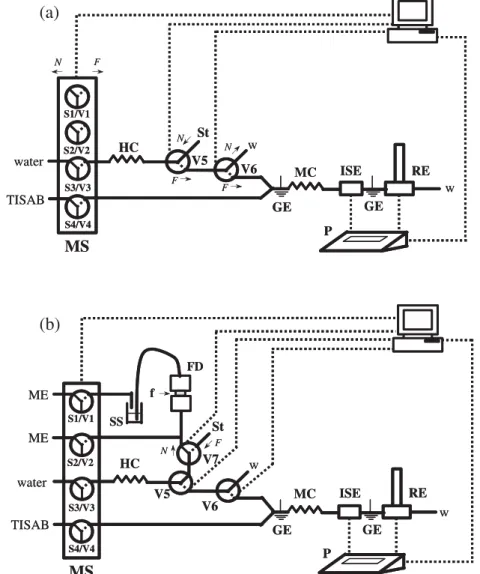
Fig. 1. MSFIA system for the determination of exchangeable potassium in soil extracts (a) or in soil samples (b), comprising in-line extraction. MS, multi-syringe; Si, syringe; Vi, solenoid valves; N, position‘on’ (solid line); F, position ‘off’ (dotted line); TISAB, total ionic strength adjustment buffer; St, standard or soil extract; HC, holding coil; MC, mixing coil; ISE, tubular potassium ion-selective electrode; RE, reference electrode; GE, ground electrode; P, millivoltmeter; w, waste; ME, Morgan extractant; SS, soil suspension; FD, filtration device; f, filter.
single motor, controlled by computer software through a serial port. A three-way commutation valve (NResearch, ref. 161T031) was connected to the head of each syringe.
At most, 4 syringes with different volume capacities can be applied simultaneously. In this application three syringes of 10 mL each were placed in positions 1, 3 and 4 and one syringe of 5 mL in position 2. Three extra commutation valves were also included in the manifold. For all valves, the exchange options were classified in on/off lines. The‘on’ line was assigned to the solution flasks and the ‘off’ line was reserved for the flow network.
A personal computer (Samsung SD 700), running lab-made software written in QuickBasic 4.5 (Microsoft), controlled the multi-syringe operation (number of steps, direction of piston displacement and position of all commutation valves). Data acquisition was performed through a PCL-818L interface card at 4 Hz, using the same software developed for controlling the flow system.
For the potentiometric measurements, a millivoltmeter (Crison, model 2002) and a double-junction reference electrode (Russell, model 90-0029) were used. As indicator electrode, a tubular shaped potassium ion-selective electrode without inner reference solution was used[12]. The tubular electrode consists of a sensing element made up by a polyvinylchloride (PVC) membrane coating a tube of conductive material which serves both as support for the PVC film and as electric contact. The sensor system includes valinomycin as ionophore, potassium tetrakis(4-chlorophenyl)borate as an additive, dioctyl sebacate as the mediator solvent, and PVC. This mixture is dissolved in tetrahydrofuran (THF) and it is applied dropwise down the cylindrical hole of the electrode, placed vertically, in order to cover its wall. A thin layer inside the tubular electrode is formed after evaporation of the THF. A response time lower than 2.5 s was achieved with this electrode.
For the in-line extraction a lab-made metacrylate filtration device holding a MoBiTec filter (ref. M2210, 10μm of pore size) was used.
The reference method was carried out using a Corning 410 flame emission spectrometer.
MSFIA manifold andp rocedure
All connections in the manifolds described in Fig. 1 were made of polytetrafluoroethylene (PTFE) tubing (Omnifit) with Gilson end-fittings and connectors. The internal diameter of tubing was 0.8 mm, except for the sampling tubing connected to the filtration device (1.6 mm i.d.) and the tubing connecting the filtration device to the confluence point (1.0 mm i.d.) presented in
Fig. 1b. The holding coil (HC) and the mixing coil (MC) were 400 and 50 cm long, respectively, and the tube for direct introduction of soil extract or standard was 10 cm long. The sampling tube placed inside the extraction vessel, immersed in the soil suspension, was 17 cm long.
The automation of the determination of exchangeable potassium was performed in two stages. Firstly, the determination in soil extracts prepared off-line was implemented and evaluated, using the manifold presented inFig. 1a. Subsequently, the in-line
extraction was introduced after addition of other elements to the previous manifold, as depicted inFig. 1b.
The analytical cycle applied in the potentiometric determina-tion in soil extracts prepared off-line is referred in Table 1. It comprises three steps: in the step 1, 0.100 mL of sample (soil extract) were aspirated towards to the HC. Then, sample was merged with TISAB solution at a total flow rate of 6.0 mL min− 1and the potential was monitored (step 2). After filling the syringes up to 40% of their capacity the system was ready to begin another cycle (step 3). Before each analytical cycle it was neces-sary to fill the sampling tube with the new standard or soil extract solution. Hence, 0.250 mL of sample were aspirated towards the HC in order to fill the sampling tube and then the excess was sent through valve V6 (position‘on’) towards the waste (dispense of 0.750 mL). After that, the syringes were refilled and positioned at their initial position (pick up of 0.500 mL).
The protocol sequence for the in-line extraction and determination of potassium in soil is shown inTable 2. In the first step, 9 mL of extractant solution were dispensed through syringe 1 into the flask that contains a portion of soil previously weighed. Then, this mixture was shaken during 5 min with a magnetic stirrer and rested for about 1 min, whilst the syringes were filled (step 2). After that, the soil suspension was aspirated through the filtration device into the HC, filling the connecting tube with soil extract (step 3). After washing the HC with water (step 4), water and TISAB solution were sent towards the detector for establishment of the baseline (step 5). The next two steps were similar to the procedure described inTable 1. Hence, 0.100 mL of soil extract were aspirated towards to the HC (step 6) and merged with TISAB solution at a total flow rate of 6.0 mL min− 1and the potential was monitored (step 7). These two steps were repeated three times, providing three replicate measurements from the same extract. After filling the syringes up to 95% of their capacity (step 8), extractant was dispensed to wash the filter (step 9). Before beginning another cycle it was necessary to fill the syringes up to 90% of their capacity (step 10). In order to calibrate
Table 1
Protocol sequence for the determination of exchangeable potassium in soil extracts Step Volume (mL) Flow ratea (mL min− 1) Piston movement Position of solenoid valvesb Description V3 V4 V5 V6 1 0.100 5.0 Pick up F N N F Standard or soil extract is aspirated into the holding coil 2 2.000 3.0 Dispense F F F F The sample is
merged with TISAB solution and send through the mixing coil for detection and data acquisition 3 1.900 10 Pick up N N F F The syringes
are filled
a The indicated values for flow rate and volume refer to a 10 mL syringe. b
the system, standards were introduced through valve V7 (position ‘off’), and the analytical cycle was the same applied in the determination of soil extracts prepared off-line.
Reference method and sample preparation
The reference method was based on flame emission spectrometry (FES) [13] and it involved the extraction of potassium from soil, dilution of extract to fit the linear working concentration range of FES detector and measurement of emission.
The extraction step was performed using either Mehlich[14]
or Morgan[15]methods. The procedure was similar for both extractants. Hence, 5 g of soil were weighed into a 50 mL flask, and 25 mL of extractant (Mehlich or Morgan) were added. This mixture was shaken for 5 min at 20 °C, and filtered through paper (Whatman No.1, 11 cm of diameter). The filtrate was then introduced directly into the MSFIA system and analysed by FES after dilution.
For the MSFIA system with in-line extraction, 1.8 g of soil was weighed into a 25 mL flask before determination.
Results and discussion
MSFIA system for determination of exchangeable potassium
Firstly, a MSFIA system for determination of exchangeable potassium in soil extracts prepared off-line was developed. Therefore, the manifold (Fig. 1a) was arranged to allow the introduction of a portion of soil extract into the flow network through a commutation valve (V5).
This was necessary because it is not feasible to place the sample inside one of the available syringes as it would take a long time of washing steps to avoid carry-over between conse-cutive samples. Moreover, another commutation valve (V6) was also added to perform sample exchange without disturbing the detector. A confluence point was also included for addition of an ionic strength adjustment (ISA) solution to the whole sample
plug prior to measurement of potential. Chemical and physical parameters were studied using aqueous standards of potassium and the lower limit of linear response (LLLR), the limit of detection (LD)[16]and the sensitivity were assessed.
The presence of low potassium content in the flowing stream through the electrode guarantees the baseline stabilization, as it provides a constant conditioning of the membrane. Nevertheless, its quantity should be strictly controlled in order to attain a low limit of detection. Therefore, the background concentration of potassium was varied between 1 × 10− 6 to 5 × 10− 5 mol L− 1, while the sample volume was 0.200 mL, and the mixing coil length was 50 cm. The ionic strength was adjusted by using a NaCl 0.2 mol L− 1solution (ISA solution) and the total flow rate was fixed at 6.0 mL min− 1. As expected, an increase in the concentration values did not influence the sensitivity and the stability of the baseline improved with increasing concentrations. The LLLR and LD values obtained for the different background concentration of potassium were very similar except for the values obtained for the highest concentration studied. For the highest concentration tested, the results obtained revealed a carry-over effect probably owing to adsorption of potassium ions to the membrane, and an increase of about three times in the LLLR and LD values was noticed. For these reasons, the chosen concen-tration for further studies was 1 × 10− 5mol L− 1.
The configuration and length of the mixing coil were also studied, using the conditions described above. Linear, coiled and knitted reactors were tested, but no significant difference was noticed. Therefore, the coiled configuration was selected to study the influence of coil length between 25 and 200 cm. As expected, neither sensitivity nor LLLR were affected. More-over, LD values were similar (about 2 × 10− 5mol L− 1). With the 25 cm reaction coil, an unstable baseline was noticed owing to insufficient mixing between carrier and ionic strength adjust-ment solution. As similar results were obtained for larger length values, the chosen length for the mixing coil was 50 cm to avoid unnecessary production of effluents and to attain a higher de-termination frequency.
Using the conditions described above, a sample volume of 0.200 mL, and NaCl 0.2 mol L− 1as ionic strength adjustment
Table 2
Protocol sequence for the in-line extraction, filtration and determination of exchangeable potassium in soil samples Step Volume (mL) Flow ratea (mL min− 1) Piston movement
Position of solenoid valvesb Description
V1 V2 V3 V4 V5 V6 V7
1 9.000 20 Dispense F N N N F F F Extractant is added to the soil sample previously weighed 2 7.200 1.2 Pick up N N N N F F F The mixture is shaken during 5 min and the syringes are filled 3 1.500 1.0 Pick up N N F N N F N The slurry is filtered through aspiration into the holding coil 4 2.500 10 Dispense N N F N F N F The holding coil is washed with water
5 0.500 3.0 Dispense N N F F F F F The baseline is set
6c 0.100 5.0 Pick up N N F N N F N Soil extract is aspirated into the holding coil
7c 2.000 3.0 Dispense N N F F F F F The sample is merged with TISAB solution and send through
the mixing coil for detection
8 9.500 10 Pick up N N N N F F F The syringes are filled
9 9.500 20 Dispense N F N N F F F The filter is washed with extractant
10 9.000 10 Pick up N N N N F F F The syringes are filled
a
The indicated values for flow rate and volume refer to a 10 mL syringe.
b
N and F represent position‘on’ and ‘off’, respectively.
c
solution, the total flow rate was varied between 3.0 and 12 mL min− 1. The value 6.0 mL min− 1was chosen as a compromise between sample throughput and stability of the baseline. More-over, no influence was verified on the sensitivity, LLLR or LD values.
The sample volume was varied between 0.050 and 0.300 mL, aiming the decrease of LD value. An increase in the injection volume (0.050, 0.100, 0.200 and 0.300 mL), decreased the LD value (62%, 55% and 50% of that obtained for 0.050 mL), but for larger volumes a positive drift of the baseline was noticed. Hence, the sample volume chosen was 0.100 mL.
In order to enable the introduction of extracts with different pH and composition using the same flow configuration, the ISA solution was replaced by a total ionic strength adjustment buffer (TISAB) solution. Two TISAB solutions (acetate buffer 0.2 mol L− 1, pH 4.7 and phosphate buffer 0.05 mol L− 1, pH 7.2) were tested (ionic strength of about 0.2 mol L− 1). The results were similar for all conditions tested (about 1 × 10− 4and 3 × 10− 5mol L− 1for LLLR and LD, respectively). To avoid the occurrence of side reactions between sample matrix and phosphate buffer (e.g., precipitation), acetate buffer was chosen. In order to assure the adjustment of the ionic strength, NaCl (0.2 mol L− 1) was also added to the acetate buffer, providing a total ionic strength of about 0.4 mol L− 1.
The linear dynamic concentration range (LDCR)[17], defined in this case as the concentration interval for which the intensity of the signal is directly proportional to logarithm of potassium concentration, was not the same for both extractants. For Mehlich extracts the LDCR was 4–391 mg L− 1and for Morgan extracts it
was 6–391 mg L− 1. The lowest concentration values of those ranges corresponded to the LLLR. Hence, the LLLR for Mehlich and Morgan extracts was 4 and 6 mg L− 1, respectively, and the minimum detectable value (LD) was 1.5 (Mehlich) and 2.4 (Morgan) mg L− 1. Considering these figures of merit it is possible to analyse directly the soil extracts, with no additional treatment. The determination of exchangeable potassium in soil extracts prepared off-line comprised the analytical cycle (Table 1) and the routine for sample exchange. Therefore, it took 23 s to perform the
sample exchange and 216 s to perform three consecutive deter-minations. Hence, a determination frequency of 50 h− 1 and a sampling rate of 15 h− 1were achieved.
Soil extracts prepared using Mehlich or Morgan extractants were analyzed using the present methodology and the FES reference method (Table 3). In order to assess the accuracy of the developed methodology, a linear relationship (CMSFIA= C0+
S × CFES) was established, where C0= 0.13 (± 0.63), S = 1.013
(± 0.047) and R = 0.998 for Mehlich extracts, and C0= 0.28
(± 0.71), S = 1.023 (± 0.054) and R = 0.998 for Morgan extracts. The values in parentheses are the limits of the 95% confidence intervals[18]. Considering these values, the estimated slope and intercept do not differ significantly from the values 1 and 0, respectively, for both extractant solutions. Therefore, there is no evidence for systematic differences between the two sets of results obtained by the proposed methodology and by the refe-rence method for both extractants.
The repeatability of the proposed system was assessed by the relative standard deviation from ten consecutive injections of three soil extracts for each extractant containing different concentrations of potassium. The results were: 1.7%, 3.0% and 1.8% for Mehlich extracts with concentrations of 33.8, 11.6 and 6.3 mg L− 1, respectively, and 1.5%, 1.5% and 2.1% for Morgan extracts with concentrations of 25.5, 14.6 and 7.3 mg L− 1, respectively.
MSFIA system with in-line preparation of soil extract
After defining the working conditions for the potentiometric determination of potassium using extracts prepared off-line, the implementation of the in-line extraction was aimed. Hence, the previous manifold was modified in order to allow the addition of extractant solution to a portion of soil, and the filtration of the soil suspension (Fig. 1b). Therefore, a filtration device was included in the manifold, and a confluence point was also added to allow washing of the filtration device. Moreover, extractant solution was placed in the two available syringes whilst an extra commutation valve (V7) was included in the manifold to allow the introduction of standard solutions for calibration purposes. Initially, the connection between the filtration device and the confluence point was made by a piece of 0.8 mm i.d. PTFE tubing that was subsequently changed to 1.0 mm i.d. to avoid the for-mation of air bubbles during the filtration step. For this reason, the aspiration flow rate was also kept as low as possible (1.0 mL
Table 3
Results obtained by the proposed MSFIA methodology (CMSFIA) and by the
reference method (CFES) for the determination of exchangeable potassium in
Mehlich and Morgan soil extracts Soil extract samples Mehlich Morgan CMSFIA (mg L− 1) CFES (mg L− 1) RDa (%) CMSFIA (mg L− 1) CFES (mg L− 1) RDa (%) 1 13.4 ± 0.2 13.6 ± 0.1 −1.5 11.4 ± 0.3 11.6 ± 1.1 −1.7 2 14.1 ± 0.2 13.6 ± 0.2 + 3.7 14.5 ± 0.2 13.9 ± 0.4 + 4.3 3 6.91 ± 0.10 6.97 ± 2.65 −0.9 7.02 ± 0.10 7.46 ± 1.13 −5.9 4 4.61 ± 0.06 4.85 ± 0.09 −5.0 6.05 ± 0.00 5.81 ± 0.06 + 4.1 5 15.8 ± 0.2 15.2 ± 0.1 + 3.9 17.2 ± 0.4 17.8 ± 0.7 −3.4 6 6.70 ± 0.09 6.03 ± 0.07 + 11.1 7.68 ± 0.11 7.69 ± 0.22 −0.1 7 4.54 ± 0.06 4.60 ± 0.59 −1.3 6.16 ± 0.10 6.41 ± 0.87 −3.9 8 31.4 ± 0.8 31.1 ± 0.1 + 1.0 24.8 ± 0.3 24.5 ± 0.4 + 1.2 9 4.99 ± 0.07 4.93 ± 0.06 + 1.2 7.39 ± 0.10 7.84 ± 0.79 −5.7 10 9.87 ± 0.60 10.8 ± 0.3 −8.6 13.8 ± 0.2 13.8 ± 0.5 0.0 11 11.3 ± 0.0 11.8 ± 0.2 −4.2 15.9 ± 0.6 15.2 ± 0.6 + 4.6 a Relative deviation. Table 4
Results obtained by the proposed MSFIA methodology (CMSFIA) and by the
reference method (CFES) for the determination of exchangeable potassium with
in-line preparation of soil extract Soil sample CMSFIA
a (mg kg− 1) CFES b (mg kg− 1) RDc(%) tcalculated tcritical A 74.6 ± 5.8 75.8 ± 2.3 −1.6 0.73 2.09d B 52.1 ± 5.1 55.1 ± 2.6 −5.4 1.98 2.07e a n = 15. b n = 12. c Relative deviation. d Degrees of freedom = 20. e Degrees of freedom = 22.
min− 1). Furthermore, the sampling tubing immersed in the soil suspension has 1.6 mm i.d. to avoid blockage by soil particles.
Using this configuration, the volume of solution used in steps 3–5 was studied in order to keep it as low as possible while performing the operations required. Therefore, the values chosen were 1.500, 2.500 and 0.500, respectively. The condi-tions for measurement of potassium (steps 6–7) were the same applied in the determination in extracts prepared off-line. After 3 consecutive measurements, the filter was washed with ex-tractant solution. It was possible to analyze at least 10 soil samples before replacing the filter.
Considering the values of LDCR and LD presented in the previous section and the mass of soil (1.8 g) and the volume of extractant (9.00 mL) used in the present system, the LDCR is 30–1.9×103
mg kg− 1 and LD is 12 mg kg− 1for the metho-dology comprising the in-line extraction.
After defining the analytical cycle described inTable 2, it took 843 s to perform the extraction, filtration and three consecutive determinations. Hence, a determination frequency of 13 h− 1and a sampling rate of 4 h− 1were achieved.
Two soil samples were analyzed by the proposed system using Morgan extractant. The results obtained for 15 determina-tions obtained from 3 consecutive measurements on 5 aliquots of soil sample are summarized inTable 4. To assess the existence of significant differences between the extracts obtained from the 5 portions of soil, one-way ANOVA analysis was applied. When compared to the critical F value (F4,10= 3.48) for a level of
confidence of 95%, the calculated F values for samples A and B were 2.33 and 0.96. This indicates that the variance between the 5 portions of soil is not significantly different from the variance obtained within the 3 consecutive measurements performed in each aliquot of sample, accounting for the repeatability of the in-line extraction operation.
Samples A and B were also analyzed using FES batch method (Table 4). Relative deviations were b5.4% and no evidence for significant difference was found between the results provided by the two methods at a level of confidence of 95% as tcalculatedbtcriticalfor both samples.
Conclusions
The proposed MSFIA systems allowed the determination of exchangeable potassium in soil extracts prepared off-line or in soil samples with automatic preparation of soil extract. The deter-mination in extractants with different composition (including pH) was accomplished without any physical or chemical modification in the manifold. Moreover, the application of potentiometric detection provided a large working concentration interval which allowed the direct introduction of soil extracts, eliminating the dilution step necessary to fit the working range in the FES refe-rence method.
The in-line preparation of the soil extract was successfully accomplished, providing a significant advantage over the flow system described previously for the same determination that required off-line extraction [19,20]. Moreover, MSFIA was a suitable tool for automation of preliminary operations as extraction and filtration, decreasing reagent and sample con-sumption, reducing sample manipulation and increasing sample throughput. This was possible through the combination of precise volume delivery enabled by piston pumps to the fle-xibility achieved from the use of commutation valves for flow management.
Acknowledgements
Financial support from IFADAP through project AGRO 273 is acknowledged. M.I.G.S. Almeida thanks FCT and FSE (III Quadro Comunitário de Apoio) for the grant SFRH/BD/8541/ 2002.
References
[1] A.E. Johnston, Understanding Potassium and its Use in Agriculture, European Fertilizer Manufacturers Association, Brussels, 2003, pp. 28–30. [2] E. Ballesteros, A. Ríos, M. Valcárcel, Analyst 122 (1997) 309. [3] L. Arce, A. Ríos, M. Valcárcel, Fresenius J. Anal. Chem. 360 (1998) 697. [4] M. Jimoh, W. Frenzel, V. Muller, H. Stephanowitz, E. Hoffmann, Anal.
Chem. 76 (2004) 1197.
[5] V. Cerdà, J.M. Estela, R. Forteza, A. Cladera, E. Becerra, P. Altimira, P. Sitjar, Talanta 50 (1999) 695.
[6] J. Ruzicka, E.H. Hansen, Flow Injection Analysis, 2nd ed., John Wiley and Sons, New York, 1988.
[7] B.F. Reis, M.F. Giné, E.A.G. Zagatto, J.L.F.C. Lima, R.A. Lapa, Anal. Chim. Acta 293 (1994) 129.
[8] M.A. Segundo, L.M. Magalhães, Anal. Sci. 22 (2006) 3.
[9] A. Andrade-Eiroa, J.A. Erustes, R. Forteza, V. Cerdà, J.L.F.C. Lima, Anal. Chim. Acta 467 (2002) 25.
[10] M.A. Segundo, H.M. Oliveira, J.L.F.C. Lima, M.I.G.S. Almeida, A.O.S.S. Rangel, Anal. Chim. Acta 537 (2005) 207.
[11] I.M.P.L.O. Ferreira, J.L.F.C. Lima, J. Flow Inject. Anal. 10 (1993) 17. [12] S. Alegret, J. Alonso, J. Bartrolí, J.M. Paulís, Anal. Chim. Acta 164 (1984)
147.
[13] Soil and Plant Analysis Council, Handbook on Reference Methods for Soil Analysis, Soil and Plant Analysis Council, Athens, GA, 1992, pp. 91–111. [14] A. Mehlich, Determination of P, Ca, Mg, K, Na and NH4, North Carolina
Soil Test Division, Raleigh, NC, 1953.
[15] M.F. Morgan, Conn., Agric. Exp. Sta., New Haven, Bull. 450 (1941). [16] R.P. Buck, E. Lindner, Pure Appl. Chem. 66 (1994) 2527.
[17] K. Tóth, K. Stulík, W. Kutner, Z. Fehér, E. Lindner, Pure Appl. Chem. 76 (2004) 1119.
[18] J.N. Miller, J.C. Miller, Statistics and Chemometrics for Analytical Chemistry, 5th ed., Pearson Education, Harlow (UK), 2005.
[19] J. Ruzicka, E.H. Hansen, E.A.G. Zagatto, Anal. Chim. Acta 88 (1977) 1. [20] R.M. Liu, D.J. Liu, A.L. Sun, Analyst 117 (1992) 1335.