Use of intravenous magnesium sulfate for the
treatment of severe acute asthma in children in
emergency department
Uso do sulfato de magnésio venoso para tratamento da asma
aguda grave da criança no pronto-socorro
INTRODUCTION
Asthma is a chronic inlammatory disease involving the lower airways that is characterized by variable airlow obstruction and respiratory symptoms. his disease is clinically manifested by recurrent symptoms that often include wheezing, shortness of breath, chest tightness and coughing. In children under ive years of age, the symptoms are often variable and nonspeciic, making diagnosis diicult.(1)
Asthma is the most common disease in childhood and is a major cause of morbidity, as assessed by school absenteeism, emergency department visits and hospital admissions. In the United Kingdom, asthma afects 5.2 million people, 1.1 million of them children. In the UK, asthma is responsible for approximately 60,000 hospital
Tânia Mara Baraky Bittar1, Sérgio
Diniz Guerra1,2
1. Lato Sensu Postgraduate Program in Pediatric Emergencies, Faculdade de Ciências Médicas de Minas Gerais, Fundação Educacional Lucas Machado – Belo Horizonte (MG), Brazil.
2. Pediatric Intensive Care Unit, Hospital João XXIII, Fundação Hospitalar do Estado de Minas Gerais – Belo Horizonte (MG), Brazil.
ABSTRACT
Severe acute asthma is a medical emergency that must be quickly diagnosed and treated. Initial treatment includes a bronchodilator agent and systemic corticosteroids. In severe cases with poor response to the standard treatment, intravenous magnesium sulfate is a therapeutic option. his article aimed a literature review on the use of intravenous magnesium sulfate in the emergency room treatment of children with acute asthma. he treatment parameters of efectiveness, indication, dosage, adverse efects and contraindications were assessed.
A narrative review of the literature based on a search of the Medline and Lilacs databases and the Cochrane Database of Systematic Reviews for articles published between 2000 and 2010 was conducted. he keywords used included the following: asthma, children, emergency and magnesium sulfate. Eight controlled clinical trials, three meta-analyses, one retrospective study, eight review articles and one cross-sectional study were included.
A total of 21 articles were analyzed. Several authors reported that intravenous magnesium was efective in the treatment of acute asthma in children. Adverse efects were rare. he use of intravenous magnesium sulfate was indicated for patients with moderate to severe acute asthma not responding to initial treatment with a bronchodilator agent and systemic corticosteroids. Few contraindications were reported but included kidney failure and atrioventricular block. Reports of adverse drug interactions with magnesium were rare. Although reported as safe, intravenous magnesium is infrequently used in children with acute asthma. Most often it is used in severe, progressed cases to prevent respiratory failure and/ or admission to the intensive care unit. Intravenous magnesium was concluded to be efective and safe in children with severe acute asthma, although its use in the emergency room is still limited.
Keywords: Asthma/drug therapy; Children; Emergency; Magnesium sulfate/ therapeutic use
Conlicts of interest: None.
Submitted on July 13, 2011 Accepted on February 1, 2012
Corresponding author:
Tânia Mara Baraky Bittar
Rua Delim Moreira, 177/1601 - Centro Zip Code: 36010-570 - Juiz de Fora (MG), Brazil.
Phone: + 55 (32) 3211-0440 / + 55 (32) 8831-4044
admissions yearly.(2) Although most children in asthma crisis
respond well to initial treatment with inhaled bronchodilators and oral corticosteroids, asthma may still cause death in a number of cases. In the United Kingdom, approximately 25 children die from asthma yearly, and poor quality emergency care may be responsible for up to one-third of these deaths.(3)
Severe acute asthma is a medical emergency that must be quickly diagnosed and treated. Airlow obstructions during exacerbations can be severe, resulting in life-threatening respiratory failure. Initial, emergent therapy requires the administration of oxygen, ß2 agonists and systemic steroids. For patients who fail to respond to this standard therapy, magnesium sulfate may be a therapeutic option.(2)
Magnesium is an essential cofactor in several enzymatic reactions and helps maintain cell homeostasis. he role of magnesium in asthma is not yet fully understood, but some studies have helped to clarify its mode of action. Magnesium causes bronchodilation via modulation of calcium ion low, which inhibits the release of acetylcholine from nerve terminals. Magnesium stabilizes T cells, preventing their activation, and inhibits mastocyte degranulation, therefore limiting the production of inlammatory mediators. It also acts to stimulate nitric oxide and prostacyclin production, possibly reducing the severity of asthma.(4,5)
Most acute asthma patients can be stabilized in an emergency department or an equivalent care setting.(6) According to the
Global Initiative for Asthma (GINA) recommendations, during severe exacerbations all patients initially should be given oxygen, ß2 agonists, anticholinergics and corticosteroids. he onset of bronchodilation may occur within minutes, while corticoids may take hours to take efect. Magnesium sulfate may be a therapeutic option for patients who do not respond to these initial therapies.(2)
Although magnesium sulfate is potentially beneicial in severe acute asthma in children who respond poorly to initial therapy with ß2 agonists and systemic corticosteroids, its use remains limited in pediatric emergency settings.
In 2007, Barbosa et al. published a review article in he Brazilian Journal of Intensive Care on the use of magnesium sulfate for treating acute asthma patients in the emergency department. hey searched Medline for articles published from 2000 to 2006 and included both adult and pediatric patient populations. Considering the relevance of this subject, we performed our search with a speciic focus on initial care in the emergency department, broadened our search to include the Lilacs and Cochrane databases and considered articles published from 2000 to 2010. We restricted our search to studies in children that were published in Portuguese, English or Spanish.(7)
his review aimed to assess the efectiveness of intravenous
magnesium sulfate in treating severe acute asthma in the pediatric emergency setting, with particular consideration for the indications, dosages, adverse efects and contraindications of magnesium use in asthma crisis.
METHODS
he literature on the use of intravenous magnesium sulfate for acute asthma in children in the emergency department was searched via Medline, Lilacs and the Cochrane Database of Systematic Reviews. Articles published in these databases from 2000 to 2010, in Portuguese, English or Spanish, were reviewed. he following keywords were used: asthma, children, emergency and magnesium sulfate. Controlled trials, meta-analyses, guidelines, cohort studies, systematic reviews and classical articles published during the speciied timeframe and relevant secondary references were included.
his search yielded 25 articles. Eight controlled trials, three meta-analyses, one retrospective study, eight review articles and one cross-sectional study were included. One case report and three narrative reviews with redundant information were excluded.
CURRENT LITERATURE EVIDENCE
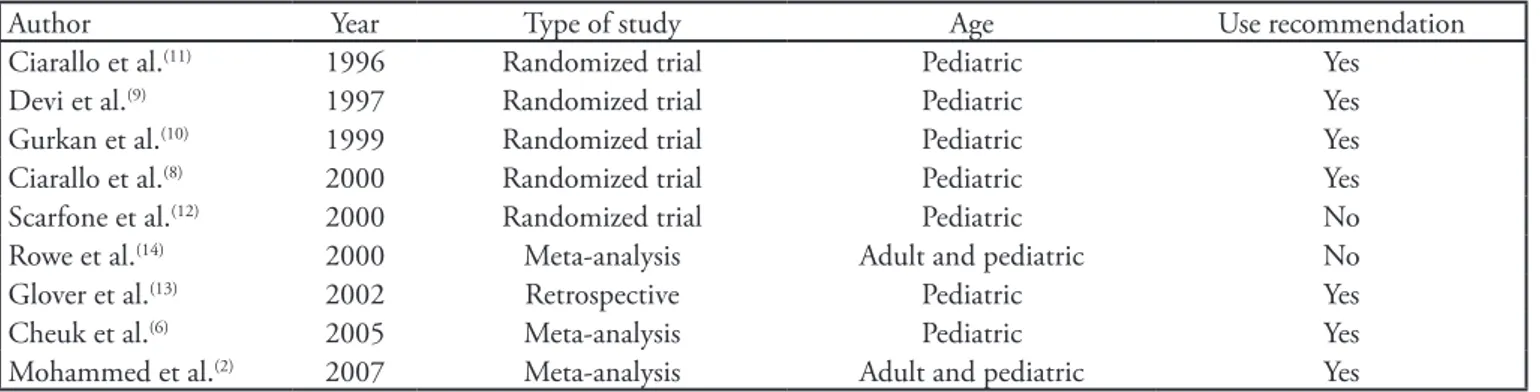
Table 1 shows the treatment recommendations from the culled Medline articles on intravenous magnesium sulfate administration in children with acute asthma. Dosages and therapy-related adverse events are shown in table 2.
Ciarallo et al. conducted a randomized, double-blind, placebo-controlled trial involving 31 children (ages between six and 18 years) with moderate and severe acute asthma. Magnesium sulfate was used as intravenous 25 mg/kg (maximum 2 g) doses, within 20 minutes. he pulmonary function was improved and no adverse efect was reported.(8)
Devi et al. conducted a randomized, double-blind, placebo-controlled trial involving 47 children (between one and 12 years) with severe acute asthma. Magnesium sulfate was used as intravenous 100 mg/kg doses, within 35 minutes. he authors report improved signs and symptoms and expiratory peak low (EPF) above 70%. Only minor adverse efects were reported, such as heartburn, pain, tingling and numbing at the infusion site. No other signiicant adverse efects, such as hypotension or respiratory depression, were reported.(9)
treatment, with no adverse efects reported.(10)
Ciarallo et al. conducted a randomized, double-blind, placebo-controlled trial involving 30 children (ages between six and 18 years) with moderate to severe acute asthma. Again, magnesium sulfate was administered intravenously in 40 mg/ kg (maximum 2 g) doses over 20 minutes. EPF was improved above 80% of expected with marked improvement of pulmonary function, overall. No adverse efects were reported; patellar and brachial relexes and systolic blood pressures remained normal during the entire study time.(11)
Scarfone et al. conducted a randomized, double-blind, placebo-controlled trial involving 54 children (ages between one and 18 years). Magnesium sulfate was administered intravenously in 75 mg/kg (maximum 2.5 g) doses over 20 minutes. Analyzing the hospital admissions rate, the authors concluded that the routine use of intravenous magnesium sulfate in children with moderate or severe asthma was not efective. Some minor adverse efects were reported and included vomiting and lushing.(12)
In a retrospective study, Glover et al. identiied 40 patients aged between two months and 15 years who were admitted
to an intensive care unit (ICU) in asthma crisis in Florida. Fifteen of the 40 patients with severe acute asthma and who had not received magnesium sulfate required intubation. After magnesium administration, no patient required respiratory support. No adverse cardiovascular efects were reported.(13)
In a meta-analysis published in 2000, Rowe et al. evaluated the use of magnesium sulfate in conjunction with bronchodilators and systemic corticosteroids in seven studies (ive in adult and two in pediatric patient populations) involving a total of 668 patients. he authors found no evidence to support the routine use of magnesium sulfate in all cases of acute asthma. However, magnesium sulfate appeared to be safe and beneicial in the most severe cases.(14)
he efectiveness of intravenous magnesium sulfate in pediatric asthma crisis was assessed further in two subsequent meta-analyses,(2,6) with similar results. Five studies, with a total
of 182 patients aged between one and 18 years, were assessed. In four of these studies, 25 to 100 mg/kg of magnesium sulfate was administered in conjunction with the standard ß2 agonists and systemic corticosteroids. Magnesium sulfate was efective in treating moderate to severe acute asthma in children,
Table 2 - Dosage schedule and adverse efects reported
Reference Study group IV dosage (mg/kg/dose)
Adverse efects
Ciarallo et al. (11)
(1996)
31 ED children with moderate-severe acute asthma
25 mg/kg within 20 minutes (Max. 2 g)
None
Devi et al.(9)
(1997)
47 ED children with moderate-severe acute asthma
100 mg/kg within 35 minutes Heart burn, pain and numbing at the infusion site
Gurkan et al.(10)
(1999)
20 ED children with moderate-severe acute asthma
40 mg/kg within 20 minutes (Max. 2 g)
None
Ciarallo et al.(8)
(2000)
30 ED children with moderate-severe acute asthma
40 mg/kg within 20 minutes (Max. 2 g)
None
Scarfone et al.(12)
(2000)
54 ED children with moderate-severe acute asthma
75 mg/kg within 20 minutes (Max. 2.5 g)
Vomiting and lushing
IV - intravenous; ED - emergency department.
Table 1 - Main results on the use of intravenous magnesium sulfate in acute asthma pediatric patients
Author Year Type of study Age Use recommendation Ciarallo et al.(11) 1996 Randomized trial Pediatric Yes
Devi et al.(9) 1997 Randomized trial Pediatric Yes
Gurkan et al.(10) 1999 Randomized trial Pediatric Yes
Ciarallo et al.(8) 2000 Randomized trial Pediatric Yes
Scarfone et al.(12) 2000 Randomized trial Pediatric No
Rowe et al.(14) 2000 Meta-analysis Adult and pediatric No
Glover et al.(13) 2002 Retrospective Pediatric Yes
Cheuk et al.(6) 2005 Meta-analysis Pediatric Yes
resulting in signiicantly improved respiratory function and a 30% decrease an average in hospital admissions.
An online cross-sectional study was conducted in collaboration with two large pediatric emergency medicine associations, one Canadian and one American, to assess for widespread usage of intravenous magnesium sulfate in severe cases of pediatric asthma crisis. In Canada, 124 out of 180 (69%) emergency medicine physicians responded, and 75 out of 108 (69%) eligible emergency departments in the United States responded. Although 88% of respondents overall were aware of magnesium’s efectiveness, only 14 of the 199 (7%) departments reported using it to prevent hospital admissions; however 142 of 199 (71%) reported administering magnesium in asthma crisis to prevent ICU admissions. A total of 38% departments reported using magnesium in less than 5% of stable acute episodes of pediatric asthma, while 79% reported its use in 50% or more of patients who show signs of imminent respiratory failure or are otherwise likely candidates for ICU admission. Among 79% of the respondents, fewer than 5% of patients who received intravenous magnesium were discharged to home from the emergency department. No fewer than 24% of respondents reported adverse events of severe hypotension requiring treatment, and 2% observed apnea in patients receiving intravenous magnesium.(15)
Interestingly, magnesium deiciency has been implicated as a risk factor in asthma exacerbations. One study found that intracellular magnesium levels, as measured in erythrocytes, were signiicantly lower in children with acute asthma when compared with levels found in the control group.(16)
Magnesium sulfate was shown to be beneicial in the treatment of moderate to severe asthma in children, and its bronchodilatory and anti-inlammatory efects may recommend its use as an adjuvant therapy in children in asthma crisis who fail to respond to conventional therapy.(4)
Intravenous administration of magnesium sulfate is safe overall. he most commonly reported side efects are rare and minor, such as lushing, pain and numbness at the infusion site, dry mouth and malaise.(12) Signiicant adverse efects were not
reported at therapeutic doses. Hypotension was documented for fast (less than 20 minutes) infusions. Magnesium toxicity can result in abnormal atrioventricular (AV) conduction leading to total AV blockade, loss of deep tendon relexes, muscle weakness, respiratory depression and cardiopulmonary arrest. However, serum magnesium levels generally have to exceed 12 mg/dL to have these efects, and these serum levels are only achieved with doses above 150 mg/kg, well above the therapeutic dose.(17)
In healthy children, serum magnesium levels range between 1.60 and 2.55 mg/dL. he bronchodilatory efects of magnesium only manifest once serum levels reach 4 mg/dL.
After parenteral administration, magnesium is mostly excreted in the urine, and only 1% to 2% is recovered from the stools. Its action is observed within minutes after infusion and lasts for approximately two hours.(17)
In clinical trials assessing the eicacy of magnesium administration in pediatric asthma crisis, administered magnesium doses ranged between 25 and 100 mg/kg (maximum 2 g). According to the British Guideline on the Management of Asthma (2011), magnesium should be given as no less than 40 mg/kg, not exceeding 2 g/dose.(18)
Pabon et al. recommend dosing at 50 mg/kg, titrating to a serum magnesium level of 4 mg/dL.(19) his dose was also
mentioned by Santana et al.(20) for the management of severe
asthma in children and in a study published in the Indian Journal of Pediatrics in 2010.(21) Magnesium should be diluted
in saline solution and infused over 30 minutes. he dose may be repeated once or twice after four- to six-hour intervals. Alternately, it can be given as a continuous infusion at 10 to 20 mg/kg/hour. Early signs of toxicity are seen with serum levels above 8 mg/dL. herefore, serum magnesium should be monitored, aiming to keep serum concentrations between 4 and 6 mg/dL.(17,21)
he concurrent use of magnesium and drugs that reduce its urinary excretion, such as glucagon and potassium-sparing diuretics, may increase serum magnesium levels. Patients taking these medications should be monitored for drug-drug interactions, and medications should be held temporarily when possible.(5) Magnesium is contraindicated in patients
with renal failure (creatinine clearance less than 30 mL/ minute), myasthenia gravis, AV block, as well as in patients with myocardial conditions.(17)
CLOSING REMARKS
Intravenous magnesium sulfate was shown to be efective in children with moderate to severe acute asthma who fail to respond to the standard initial therapy. he recommended dose is 50 mg/kg/dose (maximum 2 g/dose).
Literature-reported adverse efects were rare. Contraindications for magnesium administration are renal failure, myasthenia gravis, AV block and myocardial conditions.
Additional studies are required to further assess the safety of magnesium co-administration with drugs that are frequently used in pediatrics.
RESUMO
broncodilatadores e corticosteróides sistêmicos. Em casos graves, com fraca resposta ao tratamento padrão, o sulfato de magné-sio venoso surge como opção terapêutica. O objetivo deste artigo foi revisar a literatura sobre o uso do sulfato de magnésio venoso na asma aguda em crianças no pronto-socorro no que se refere a eicácia, indicação, dosagem, efeitos adversos e contraindicações. Realizada revisão narrativa por meio das Bases de dados Medli-ne, Lilacs e Cochrane Databaseof Systmatic Reviews, entre 2000 e 2010. Utilizados os descritores: asthma, children, emergency, magnesium sulfate. Incluídos oito ensaios clínicos controlados, três meta-análises, um estudo retrospectivo, oito artigos de revisão e um estudo transversal. A eicácia do magnésio venoso em crian-ças foi observada por vários autores, com raros efeitos adversos. Seu uso foi indicado para os pacientes com asma aguda moderada
e grave que não responderam ao tratamento inicial com bronco-dilatador e corticosteróide. As contraindicações em pediatria são poucas. Entre elas estão insuiciência renal e bloqueio atrioventri-cular. Existem poucos relatos da interação do magnésio com dro-gas de uso pediátrico. Apesar da segurança, na prática, o magnésio venoso é pouco usado na asma aguda em pediatria. Na maioria das vezes, é indicado tardiamente para impedir falência respira-tória e internação na unidade de cuidados intensivos. Os estudos demonstram que o magnésio venoso é uma droga eicaz e segura na criança com asma aguda grave, porém o seu uso no pronto--socorro ainda é limitado.
Descritores: Asma/quimioterapia; Criança; Emergência; Sulfato de magnésio/uso terapêutico
REFERENCES
1. Pedersen SE, Hurd SS, Lemanske RF Jr, Becker A, Zar HJ, Sly PD, Soto-Quiroz M, Wong G, Bateman ED; Global Initiative for Asthma. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1-17.
2. Mohammed S, Goodacre S. Intravenous and nebulised magnesium sulphate for acute asthma: systematic review and meta-analysis. Emerg Med J. 2007;24(12):823-30. Review. 3. Carroll W, Lenney W. Drug therapy in the management
of acute asthma. Arch Dis Child Educ Pract Ed. 2007;92(3):ep82-6.
4. Bichara MD, Goldman RD. Magnesium for treatment of asthma in children. Can Fam Physician. 2009;55(9):887-9. 5. Guerrera MP, Volpe SL, Mao JJ. herapeutic uses of
magnesium. Am Fam Physician. 2009;80(2):157-62. Review. 6. Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous
magnesium sulphate for treating acute asthma. Arch Dis Child. 2005;90(1):74-7.
7. Barbosa FT, Barbosa LT, Cunha RM, Gonçalves GP, Souza DA. Uso do sulfato de magnésio por via venosa e nebulização para o tratamento da asma aguda na emergência. Rev Bras Ter Intensiva. 2007;19(3):369-73.
8. Ciarallo L, Brousseau D, Reinert S. Higher-dose intravenous magnesium therapy for children with moderate to severe acute asthma. Arch Pediatr Adolesc Med. 2000;154(10):979-83. 9. Devi PR, Kumar L, Singhi SC, Prasad R, Singh M. Intravenous
magnesium sulfate in acute severe asthma not responding to conventional therapy. Indian Pediatr. 1997;34(5):389-97. 10. Gürkan F, Haspolat K, Bosnak M, Dikici B, derman O, Ece
A. Intravenous magnesium sulphate in the management of moderate to severe acute asthmatic children nonresponding to conventional therapy. Eur J Emerg Med. 1999;6(3):201-5. 11. Ciarallo L, Sauer AH, Shannon MW. Intravenous
magnesium therapy for moderate to severe pediatric asthma: results of a randomized placebo controlled Trial. J Pediatr.
1996;129(6):809-14.
12. Scarfone RJ, Loiselle JM, Jofe MD, Mull CC, Stiller S, hompson K, Gracely EJ. A randomized trial of magnesium in the emergency department treatment of children with asthma. Ann Emerg Med. 2000;36(6):572-8.
13. Glover ML, Machado C, Totapally BR. Magnesium sulfate administered via continuous intravenous infusion in pediatric patients with refractory wheezing. J Crit Care. 2002;17(4):255-8.
14. Rowe BH, Breatzlaf JA, Bourdon C, Bota GW, Camargo CA Jr. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cocharane Database Syst Rev. 2000;(2):CD001490. Review.
15. Schuh S, Macias C, Freedman SB, Plint AC, Zorc JJ, Bajaj L, et al. North American practice patterns of intravenous magnesium therapy in severe acute asthma in children. Acad Emerg Med. 2010;17(11):1189-96.
16. Sedighi M, Poupark Z, Bavarian B, Safaralizadeh R, Zare A, Moin M. Low magnesium concentration in erithrocytes of children with acute asthma. Iran J Allergy Asthma Immunol. 2006;5(4):183-6.
17. Monem GF, Kissoon N, DeNicola L. Use of magnesium sulfate in asthma in childhood. Pediatr Ann. 1996;25(3):136, 139-44.
18. British Guideline on the Management of Asthma. England: British horacic Society; 2008. [upddate June 2009, cited 2011 Mar 22] . Available from: http://www.brit-thoracic.org. uk/guidelines/asthma-guidelines.aspx
19. Pabon H, Monen G, Kissoon N. Safety and eicacy of magnesium sulfate infusions in children with status asthmaticus. Pediatr Emerg Care. 1994;10(4):200-3.
20. Santana JC, Barreto SSM, Piva JP, Garcia PCR. Controlled study on intravenous magnesium sulfate or salbutamol in early treatment of severe acute asthma attack in children. J Pediatr (Rio J). 2001;77(4):279-87.