Universidade de Trás-os-Montes e Alto Douro
Application of RNA interference in the silencing of genes
involved in Rheumatoid Arthritis inflammatory process in
primary human macrophages
Dissertação de Mestrado em Genética Molecular Comparativa e Tecnológica
Andreia Vilar Mendes
Orientadora: Doutora Patrícia Ribeiro Nogueira
Co-orientadora: Professora Doutora Maria Manuela do Outeiro Matos
Universidade de Trás-os-Montes e Alto Douro
Application of RNA interference in the silencing of genes
involved in Rheumatoid Arthritis inflammatory process in
primary human macrophages
Dissertação de Mestrado em Genética Molecular Comparativa e Tecnológica
Andreia Vilar Mendes
Orientadora: Doutora Patrícia Ribeiro Nogueira
Co-orientadora: Professora Doutora Maria Manuela do Outeiro Matos
II
Universidade de Trás-os-Montes e Alto Douro
Application of RNA interference in the silencing of genes
involved in Rheumatoid Arthritis inflammatory process in
primary human macrophages
Dissertação de Mestrado em Genética Molecular Comparativa e Tecnológica
Andreia Vilar Mendes
Orientadora: Doutora Patrícia Ribeiro Nogueira
Co-orientadora: Professora Doutora Maria Manuela do Outeiro Matos
Composição do Júri: ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________
III
“A verdadeira viagem de descobrimento não consiste em procurar novas paisagens mas em ter novos olhos”
IV Agradecimentos
Esta dissertação é o resultado de um ano lectivo de trabalho o qual não teria sido conseguido sem a contribuição e apoio de várias pessoas e instituições e, como tal não poderia deixar de expressar alguns agradecimentos sinceros:
Ao magnífico Reitor da Universidade de Trás-os-Montes e Alto Douro e à Universidade de Trás-os-Montes e Alto Douro.
Ao Instituto de Biologia Celular e Molecular (IBMC) por me ter acolhido durante este ano e permitir-me o desenvolvimento do trabalho.
Ao Prof. Doutor Alexandre do Carmo, obrigada pela oportunidade única que me concedeu, ao aceitar-me no seu grupo de investigação para desenvolver o meu trabalho.
À Doutora Patrícia Nogueira, agradeço por tudo. Por todos os ensinamentos que me transmitiu, pela disponibilidade constante, por toda ajuda, por todos os conselhos que me deu, e por toda a paciência durante este ano que passou.
Ao Doutor Jaime Freitas, por todos os ensinamentos, pela grande paciência, por todos os conselhos, por toda a exigência e por todas as “perguntas”.
À Prof. Doutora Manuela Matos, pela orientação, pela ajuda e pela constante disponibilidade que sempre demonstrou.
Ao projecto NANOFOL – “Folate-based nanobiodevices for integrated diagnosis/therapy targeting chronic inflammatory diseases” (NMP4-LA-2009-228827), pelo financiamento.
A todos os meninos e meninas dos grupos: cell activation and gene expression (CAGE), thymic epithelial cells (TECs) e gene regulation (GR), por todo o apoio, por toda a ajuda e companheirismo, que foram uma grande contribuição para que tenha sido possível a conclusão do meu trabalho com sucesso.
Aos “Vilarealenses, Lisboenses e Barcelonenses”, que desde há 5 anos traçaram o mesmo caminho que eu, obrigada por tudo, mas sobretudo pelo apoio e pela amizade para a vida. A todos os meus amigos em geral, pela amizade e principalmente pela compreensão de que o dever está sempre em primeiro lugar.
Ao meu irmão, Germano e à minha cunhada Marli, obrigada pela amizade, e pelo apoio. E por último, o mais importante de todos. Obrigada por tudo, Pai Germano e mãe Fátima, por todo o apoio em todas as decisões tomadas por mim ao longo de todo o meu precurso académico. Obrigada pelo carinho, compreensão e principalmente por todo o esforço que fizeram para que pudesse concluir o meu trabalho.
V Abstract
Rheumatoid arthritis (RA) is an autoimmune disease affecting the majority of the elderly population around the world, with a high social and economic impact. Despite decades of research, there is a lack of effective treatments capable of permanently eliminate the symptoms and recede the progress of the disease. Among the cells implicated in the RA etiology, macrophages play a central role by releasing several pro-inflammatory cytokines and chemokines, which activate and recruit other immune cells to the inflamed sites.
Depending on the stimuli in their environment, macrophages can acquire different surface markers associated with diverse functions. In the present work, different macrophage populations were characterized and the great plasticity of this cell type was confirmed. It was also shown that specific markers respond faster than others to changes in their microenvironment. It was also shown that pro-inflammatory macrophages (stimulated with com LPS-INFγ) express higher levels of MCL1 and IRF5, the selected RA-related genes.
Due to the potential use as a therapeutic tool in several diseases, the RNAi technology is quickly evolving to allow higher levels of specificity and gene silencing. Locked nucleic acids (siLNA) are a new class of RNAi molecules that show great specificity and stability. In the present work, RA-related genes were silenced in vitro using siLNAs in pro-inflammatory primary human macrophages. The selected target genes were the anti-apoptotic MCL1 and the transcription factor IRF5.
Whereas after an efficient IRF5 silencing no biological effect could be demonstrated, silencing of MCL1 led to a 6-fold increase in the number of apoptotic cells. The results reported here, although preliminary, are quite promising, as they demonstrate the potential use of MCL1 as target and LNA as a tool for future RNAi-based treatments in RA. The present study, besides indicating MCL1 as a serious RA target gene, also developed a methodology to efficiently transfect RNAi molecules in the hard-to-transfect primary macrophages, with a minimal cytotoxicity.
VI Resumo
A artrite reumatóide (AR) é uma doença autoimune que afecta a maioria da população idosa em todo o mundo, com um grande impacto a nível social e económico. Após décadas de investigação, ainda não são conhecidos tratamentos eficientes que sejam capazes de eliminar permanentemente os sintomas desta doença e a regressão do seu progresso. Entre outras células importantes na etiologia da AR, os macrófagos desempenham um papel central, devido à secreção de citocinas pro-inflamatórias e quimiocinas que activam e recrutam outras células imunitárias para os locais de inflamação.
Dependendo do estímulo, os macrófagos podem adquirir diferentes marcadores superficiais associados a diversas funções. Neste trabalho foram caracterizadas diferentes populações de macrófagos e a grande plasticidade destas células foi confirmada. Foi também mostrado que os macrófagos pro-inflamatórios (estimulados com LPS-INFγ) expressam altos níveis de MCL1 e IRF5, genes relacionados com AR.
Devido ao seu potencial uso como ferramenta terapêutica em algumas doenças, a tecnologia do RNAi tem evoluído no sentido de serem atingidos maiores níveis de especificidade e silenciamento génico. “Locked-nucleic acids” ou LNAs são uma nova classe de moléculas de RNAi caracterizadas por uma grande especificidade e estabilidade. Neste trabalho, genes relacionados com a AR foram silenciados in vitro usando LNAs em macrófagos primários pro-inflamatórios. Os genes alvo selecionados foram o anti-apoptótico
MCL1 e o fator de transcrição IRF5.
Enquanto que após o silenciamento do IRF5 não foram demonstrados efeitos biológicos, o silenciamento do MCL1 levou a um aumento de seis vezes no número de células apoptóticas. Os resultados aqui apresentados apesar de preliminares são promissores, uma vez que demonstram o potencial do uso de MCL1 como alvo e o LNA como ferramenta para futuros tratamentos da AR baseados no RNAi. Este estudo, para além de apontar o MCL1 como um sério candidato alvo no tratamento da AR, também resultou no desenvolvimento de uma metodologia para eficientemente transfetar moléculas de RNAi nos difíceis de transfetar macrófagos primários e com uma baixa citotoxicidade.
VII Index of Contents Agradecimentos IV Abstract V Resumo VI Index of Figures IX Index of Tables IX List of Abbreviations X I- INTRODUCTION 1
1. The Immune System 2
1.1. Cells of the immune system 3
1.2. Monocytes 4
1.3. Macrophages 5
2. Autoimmune diseases 7
2.1. Rheumatoid Arthritis 8
2.2. The role of macrophages and cytokines in RA 11
2.3. RA therapies 13
2.3.1. Gene Therapy and its application in RA 14
3. RNA interference 14
3.1. LNA-modified siRNA (siLNA) 16
3.2. Target Genes 17
3.2.1. MCL1 18
3.2.2. IRF5 19
4. Aims 20
II- MATERIALS AND METHODS 21
1. Cell Culture 22
1.1. Monocyte isolation 22
1.2. In vitro differentiation and activation of macrophages 22
2. Cell surface markers’ expression 23
3. Gene silencing using siLNAs 23
3.1. Optimization of siLNA transfection in primary macrophages 23
VIII
4. Determination of protein levels 25
4.1. Total protein extracts 25
4.2. SDS-PAGE and Western-Blotting 25
5. Gene expression 26
5.1. Total mRNA isolation 26
5.2. First strand cDNA synthesis 27
5.3. Real-Time Polymerase Chain Reaction (qPCR) 27
6. Apoptosis and cell viability analysis 28
7. Enzyme-linked immunosorbent assay (ELISA) 28
8. Statistical Analysis 29
III- RESULTS AND DISCUSSION 30 1. Macrophage can generate diverse populations in response to different
activation stimuli 31
2. IRF5 and MCL1 genes are highly expressed in macrophages activated
with LPS-IFNγ 34
3. Lipofectamine® RNAiMAX performs the highest transfection efficiency
in primary human macrophages with low toxicity 36 4. MCL1 knockdown with siLNAs promotes apoptosis in M1 macrophages 39 5. IRF5 knockdown with siLNAs did not lead to the regression of
inflammatory phenotype 42
IV- FINAL CONSIDERATIONS 46
IX Index of Figures
Figure 1. Monocyte development and heterogeneity 4 Figure 2. Schematic comparison between a normal joint (left) and a joint affected
by RA (right) 11
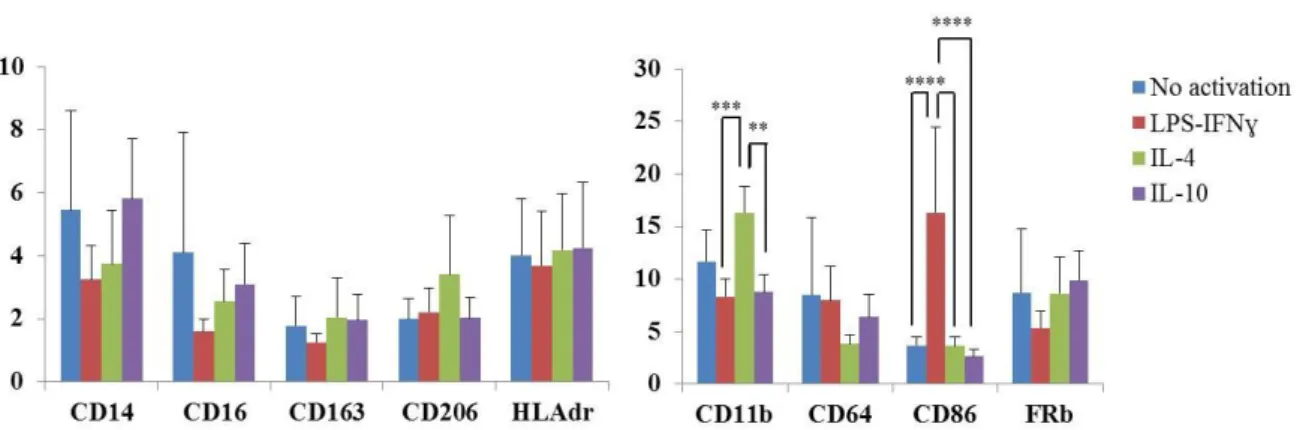
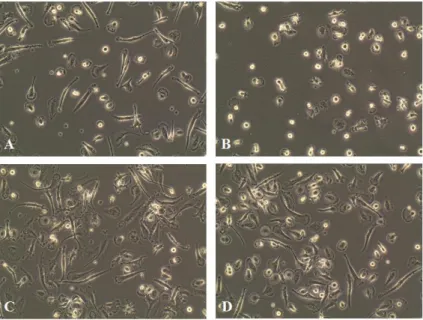
Figure 3. Cytokine signaling pathways involved in inflammatory arthritis 12 Figure 4. Schematic representations of chemical structures of siRNA and siLNA 17 Figure 5. Essential mechanisms to maintain macrophage viability 19 Figure 6. Morphology of macrophages activated with different stimuli for 48 hours 32 Figure 7. Cell surface expression of different markers on macrophages activated with
different stimuli for 48 hours 32
Figure 8. Morphology of macrophages activated with different stimuli for 24h 33 Figure 9. Cell surface expression of different markers on macrophages activated
withLPS-IFNγ for 24 hours 34
Figure 10. Relative gene expression of MCL1 and IRF5 in macrophages activated with
different stimuli for 48 hours 35
Figure 11. Relative gene expression of MCL1 and IRF5 in macrophages polarized
for 24 hours 36
Figure 12. Transfection of siRNA and siLNA in primary human macrophages 37 Figure 13. Optimization of siLNA transfection in primary human macrophages 38 Figure 14. Silencing of MCL1 gene in primary human macrophages 39 Figure 15. Apoptosis levels induced by MCL1 knockdown in primary macrophages 41 Figure 16. Transfection of negative control and IRF5 siLNAs in M1 macrophages 42 Figure 17. Silencing of IRF5 in primary human macrophages 43 Figure 18. Cytokine production in IRF5-silenced primary human macrophages 44
Index of Tables
Table 1. Concentration of cytokines used to induce macrophage activation 23 Table 2. Antibodies used for cell phenotyping of monocyte-derived macrophages,
together with the respective labeled fluorochrome 24
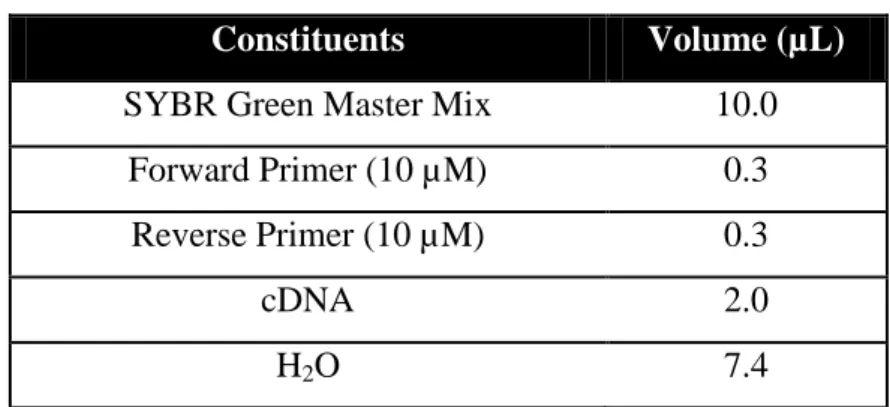
Table 3. Constituents of the qPCR reaction 28
X List of Abbreviations
BSA bovine serum albumin CD cluster of differentiation
cDNA complementary deoxyribonucleic acid CO2 carbon dioxide
ddH2O double-distilled water
dNTPs deoxyribonucleoside triphosphates EDTA ethylenediamine tetraacetic acid ELISA enzyme-linked immunosorbent assay FACS fluorescence activated cell sorting FAM carboxyfluorescein
FCS fetal calf serum
HLA human leucocyte antigen HSCs hematopoietic stem cells IFNγ interferon gamma IgG immunoglobulin G
IL interleukin
IRF5 interferon regulatory factor 5 LPS lipopolysaccharide
MCL1 myeloid cell leukemia 1
M-CSF macrophage-colony stimulating factor MFI mean fluorescence intensity
MHC major histocompatibility complex mRNA messenger ribonucleic acid NaCl sodium chloride
NP-40 nonidet P-40
PBMCs peripheral blood mononuclear cells PBS phosphate buffer saline
PI propidium iodide
PMSF phenylmethanesulfonylfluoride RA rheumatoid arthritis
RISC ribonucleic acid-induced silencing complex RNAi ribonucleic acid interference
ROS reactive oxygen species RPMI roswell park memorial institute SD standard deviation
SDS-PAGE sodium dodecyl sulfate polyacrylamide gel electrophoresis siLNA short-interfering locked nucleic acid
siRNA short-interfering ribonucleic acid TBS-T tris-buffered saline with tween 20 TNF-α tumor necrosis factor-alpha
1
2 1. The Immune System
The immune system’s first function is to defend the organism from the wide range of infectious agents relentlessly attacking the human body. A remarkable feature of this system is the ability to distinguish between self and non-self proteins, cells and tissues. This same remarkable feature can also be quite dangerous when due to external or internal occurrences stops to recognize its own tissues. This activates a network of strong immune responses against the organism it was built to protect, leading to the development of severe diseases that, in extreme cases, can result in death (Seeley, Stephens ans Tate, 2004; Abbas, Lichtman and Pillai, 2012 (a)).
The first line of defense is provided by the “innate immune system” (the so-called natural or native immunity), a vital set of defense mechanisms that are always turned on. Briefly, the ensemble of pre-existing defense mechanisms that comprise the innate immune system are designed to prevent infection by pathogens or to mount an immediate response against an infectious agent, responding essentially the same way to repeated infections (Wood, 2006; Abbas, Lichtman and Pillai, 2012 (b)). These innate defense mechanisms can be divided in two distinct categories. The first consists of a passive defense (Virella, 2001) composed by physical and chemical barriers to prevent the microorganisms from gaining access to the body or their physical removal (Roitt and Delves, 2001 (b); Seeley, Stephens and Tate, 2004).
In contrast to innate immunity, the “acquired immunity” or “adaptive immunity” is developed as a response to an infection and is capable to adapt. This form of immunity is characterized by very specific inflammatory responses, comprising complex sequences of events and involving many immune mediators that can be humoral or cell-mediated. These responses are stimulated by substances called antigens, which can be originated by infectious agents (Stewart, 2004). The body also produces these substances (self-antigens), which can either be beneficial or harmful, if the body recognizes them as foreign, leading to undesired immune responses (Seeley, Stephens and Tate, 2004).
The immune system faces numerous challenges to generate effective protective responses against infectious pathogens (Abbas, Lichtman and Pillai, 2012 (c)). The response to infection can be divided into different stages: first, the immune system must be aware of infection and respond immediately to it. Second, if the immediate response to infection is not effective in eliminating the pathogen, there is a delayed response to infection, in which new
3 cells and chemical factors are generated to deal with it. Finally, the effector mechanisms of adaptive immunity locate and destroy the pathogens or eliminate them from the body, and develop immunity against them, in order to prevent the illness in the organism in case of a second infection with the same pathogen (Wood, 2006; Abbas, Lichtman and Pillai, 2012 (b)). The generation of a response of either the innate or acquired immunity requires the interaction of specific molecules, cells and tissues (Cruse and Lewis, 2012).
1.1. Cells of the immune system
The cells of the innate and adaptive immune system act in an extremely organized fashion, and are usually present as circulating cells in the blood and lymph. The anatomic organization of these cells and their ability to circulate and migrate to specific tissues is of critical importance for the generation of an immune response (Abbas, Lichtman and Pillai, 2012 (a)).
All blood cell types have their origin in bone marrow through a process called hematopoiesis, which is essentially mediated by self-renewing hematopoietic stem cells (HSCs). Briefly, through the interaction of not well known mechanisms, some cells grow, divide and ultimately become mature blood cells, whereas others return to their daughter-like state. This ensures the maintenance of the process over the lifetime of the individual (Traver and Akashi, 2004; Sompayrac, 2012).
The process of cell specification involves complex molecular mechanisms, controlled primarily by transcription factors determining a regulatory network of gene activation and silencing (Iwasaki and Akashi, 2007) that depending on their timing and expression levels play crucial roles in activating lineage specific programs.
One of the cells involved in innate immunity are monocytes that give rise to macrophages, but also to neutrophils, basophils, eosinophils, mast cells, and NK cells. B, T and dendritic cells, are the ones involved in adaptive immunity (Abbas, Lichtman and Pillai, 2012 (a)).
4 1.2. Monocytes
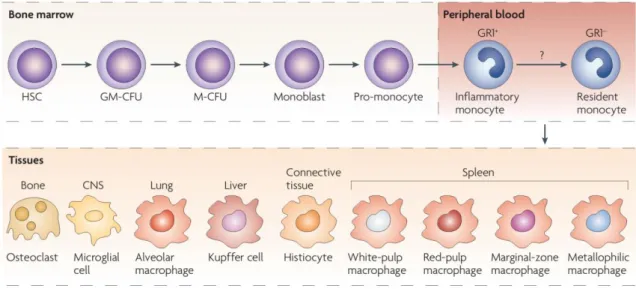
Monocytes are the biggest circulating leukocytes, representing approximately 10% of leukocytes in human blood (Auffray, Sieweke and Geissman, 2009). They circulate in peripheral blood together with other immune cells, with which they share a common progenitor in the bone marrow (Furth and Cohn, 1968). In the currently accepted model, first, HSCs undergo differentiation to the common myeloid progenitor and then to a monocyte lineage, which, in response to the growth factor macrophage-colony stimulating factor (M-CSF also known as Csf-1 and CD115), sequentially give rise to monoblasts, pro-monocytes and finally monocytes, that are released from the bone marrow into the bloodstream (Fig. 1) (Gordon and Taylor, 2005; Iwasaki and Akashi, 2007; Mosser and Edwards, 2008). This growth factor and its receptor are critical for monocyte differentiation from bone marrow progenitors (Auffray, Sieweke and Geissman, 2009).
Figure 1. Monocyte development and heterogeneity. Monocytes originate in bone marrow from a common
HSC. They suffer several differentiation steps before becoming monocytes, which then enter in the blood stream. Monocytes migrate to different tissues, where they differentiate into tissue-specififc macrophages (adapted from Mosser & Edwards, 2008 ).
When monocytes are released into the blood, they are exposed to a plethora of agents, including cytokines, chemokines, adrenergic and cholinergic agonists, fatty acids, hormones and immunoglobulins which are capable of impacting their functional and phenotypic characteristics (Stout and Suttles, 2004). The phenotype they acquire depends on the environment in which they accumulate and on an innumerous adhesion molecules,
5 chemokines and chemokine receptors. Monocytes thus exhibit morphological heterogeneity, including variations in granularity, nuclear morphology, cell size, nucleus-cytoplasm ratio and shape (Gordon and Taylor, 2005). Monocytes remain in the blood for several days, time during which they migrate to the capillaries until extravasating to tissues in a steady-state or in response to inflammation, and give rise to subsets of macrophage or dendritic cells populations (Fig.1) (Serbina et al., 2008; Sompayrac, 2012). In vitro, monocytes are impossible to maintain in culture as they quickly differentiate or die (Robbins and Swirski, 2010).
Although monocytes have long been considered as transitional cells whose main role is to repopulate body’s tissues with macrophages, today it is known that they are also important effectors of the innate immune system against pathogenic organisms, exerting a constant surveillance and immunoregulatory functions (Robbins and Swirski, 2010).
1.3. Macrophages
Macrophages are key players of the immune system and are, therefore, virtually present in all tissues of the body. They are divided in various types, performing different roles, and providing innate immune surveillance for every tissue in the body (Stout and Suttles, 2004). In organs like kidney, pancreas and others, macrophages are strategically located in big quantities, in order to monitor physiological processes (Hume et al., 2002).
The primary role of macrophages is related to their contribution to tissue homeostasis, by the clearance of senescent cells and other wreckage that can be a consequence of inflammation and tissue injury, or simply in order to keep tissue turnover (Kumar and Jack, 2006). Each day, they clear approximately 2 × 1011 erythrocytes, a vital metabolic process without which the host would not survive (Mosser and Edwards, 2008). During tissue remodeling, macrophages play an important role, because they efficiently clear the cells that undergo apoptosis (Kono and Rock, 2008).
Under inflammatory conditions, monocyte production in the bone marrow is increased and after the release into peripheral blood, they are rapidly recruited to infection sites where they are induced to differentiate into inflammatory macrophages. These can then contribute to the innate immune response by expression of inflammatory and effector activities, a process that is regulated by the microenvironment of the different tissues. The enormous plasticity of this system remains unclear; it is not known if macrophage fate is previously determined or if
6 it is constantly malleable, but it is thought that the exposure of macrophages to the multiple stimuli found in their surrounding environment, leads to a spectrum of different macrophage phenotypes (Gordon and Taylor, 2005).
Macrophages have been divided according to their roles and characteristics into two main groups: classically activated macrophages (also called M1 macrophages or CAM) and alternatively activated macrophages (M2 macrophages or AAM) (Gordon, 2003). This division is not rigid as there are characteristics that can be shared by more than one macrophage population, according to the different stimuli received for activation, which results in a spectrum of macrophage populations based on their function and activation stimulus. This supports the idea that there are many different shades of activation that have yet to be identified (Mosser and Edwards, 2008).
In general, M1 phenotype includes high levels of pro-inflammatory cytokines, like TNF-α, IL-1, IL-6, IL-12, IL-23, and low levels of IL-10. M1 macrophages are also effective producers of reactive oxygen species (ROS), and mediate the combat against pathogens and tumors (Mantovani, Sica and Locati, 2007; Martinez et al., 2008). Classical activation of macrophages can be induced in vitro by culturing macrophages with interferon-γ (IFN-γ) in combination with lipopolysaccharide (LPS), which is associated with high pro-inflammatory cytokine production, high microbicidal activity and cellular immunity (Gordon and Taylor, 2005; Martinez et al., 2008; Murray and Wynn, 2011).
There are many evidences that M2 encompasses cells with dramatic differences in their gene expression, biochemistry and physiology, which are linked to the stimulus received, and because of this they are further subdivided into three subtypes (Edwards et al., 2006; Murray and Wynn, 2011). Alternative activated macrophages can be observed after exposure to IL-4 or IL-13 (M2a), exposure to immune complexes in combination with IL-1β or LPS (M2b), or exposure to IL-10, TGF-β or glucocorticoids (M2c) (Gordon and Taylor, 2005; Martinez et al., 2008; Murray and Wynn, 2011). In general, M2 macrophages possess an anti-inflammatory phenotype, with high production levels of IL-10, IL-1 receptor antagonist, decoy IL-1RII, TGF-β and low levels of IL-12 and IL-23 (Mantovani, Sica and Locati, 2007).
Like monocytes, macrophages have a big plasticity in gene expression and are capable to adapt to the local environment and perform specialized functions. This ability allows them to efficiently respond to environmental alterations and signals, and to change their physiology by both innate and adaptive immune responses (Hume et al., 2002). Their phenotype changes
7 according to the type, concentration, and longevity of exposure to the stimulus received, involving different signaling cascades (Stout et al., 2005). A minimal disturbance of tissue normal environment, like infection, cell turnover or wounding, can cause rapid recruitment of macrophages and their activation, in order to rescue the normality (Hume et al., 2002).
In response to their tissue microenvironment, which can include cytokines produced by themselves, macrophages can change the patterns of gene expression and reverse their phenotype subsets (Stout et al., 2005). They can have opposite activities: pro-inflammatory versus anti-inflammatory, immunogenic versus tolerogenic, destructive versus tissue-restorative (Gordon, 2003; Stout and Suttles, 2004). After activation they remain plastic, in other words, polarization into one phenotype does not preclude re-polarization (Stout et al., 2005), for example, M1 macrophages can easily change to M2, and vice versa, if they suffer adequate stimulus (Murray and Wynn, 2011). This capacity has an important in vivo relevance particularly in the study of chronic diseases, in which there is the dominance of particular macrophage phenotypes with a significant role in disease pathology. Rheumatoid arthritis and other autoimmune diseases are examples of such phenomena.
2. Autoimmune diseases
Autoimmune diseases are a fascinating but a poorly understood group of diseases. Approximately 40 diseases of this nature are known, affecting about 5% of the world population (Roitt and Delves, 2001 (a)). The occurrence is more frequent in females than in males, probably because of the differences in hormonal patterns (Roitt and Delves, 2001 (a); Tsokos, Goust and Virella, 2001) and, in relation to age incidence, there are two peaks: one in puberty and the other one in the forties and fifties (Roitt and Delves, 2001 (a)).
Chronic inflammatory autoimmune diseases are the result of an imbalance between mediators that initiate and maintain inflammation and those that shut the process down, which leads to the inability to resolve acute inflammation, and consequently to chronic inflammatory states (Choy and Panayi, 2001; Kennedy et al., 2011). This is originated by the breakdown or failures in the immune system of normal tolerance to self-antigens, resulting in the development of an autoimmune response (Tsokos, Goust and Virella, 2001).
Autoimmune disorders are characterized by tissue lesions and fibrosis, reactions caused by the products of activated macrophages and other immune cells like lysosomic enzymes, ROS intermediaries, nitric oxide and pro-inflammatory cytokines (Abbas, Lichtman
8 and Pillai, 2012 (b)). These disorders can be organized as a spectrum, from organ specific to non-organ specific (Roitt and Delves, 2001 (a).
2.1. Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune, chronic inflammatory disease which involves inflammatory and degenerative lesions in joints, including shoulders, elbows, knees, fingers and ankles (Goust and Virella, 2001 (b); Abbas, Lichtman and Pillai, 2012 (a)). This disease cannot be cured yet and has substantial economic, social and personal costs (Choy and Panayi, 2001). It has a regular prevalence around the world, and it is estimated that it affects between 0.5 and 1% of worldwide population (Firenstein, 2005) .
Clinically, RA is characterized by synovial inflammation, which leads to the destruction of articular and bone cartilage. However, there can be a range of extra manifestations according with the stage of disease, due to abnormal immune responses (Firenstein, 2005; (Abbas, Lichtman and Pillai, 2012 (b)).
Despite all the research made, the etiology of this disease remains unclear, but it is clearly multifactorial with contributions from a blend of both genetic and environmental factors (Singal, Li and Zhu, 1999; Firenstein, 2005). Several genetic loci have been proposed as strictly related with the susceptibility and severity of this disease, including loci in major histocompatibility complex (MHC), cytokines, citrullinating enzymes and cytokine-receptor loci, among others (reviewed in (van der Helm, Wesoly and Huizinga, 2005) and in (Firenstein, 2005). However, other factors known to have an association with the predisposition to this disease and its severity were also described in distinct populations. One of them is the gender; this disease has a higher predominance in women, with a significant ratio female-to-male, but the specific mechanism responsible for this increased susceptibility to RA in women is uncertain.
Some environmental factors are also involved in the development and magnitude of this pathology. It is proved that smoking is an important risk factor for the development of the disease in certain populations. Smoking promotes the citrullination of self-proteins, so this can provide a stimulus for generation of anticitrullinated peptide antibodies, constantly activating the innate immunity. It is thought that this constant activation in genetically susceptible individuals, can potentially contribute to auto reactivity and initiation of the pathology (Klareskog, Padyukov and Alfredsson, 2007). The major function of those
9 molecules is to present antigenic peptides to CD4+ T cells, which strongly suggests that this disease is caused by an unidentified arthritogenic antigen (Gregersen, Silver and Winchester, 1987).
During many years, rheumatoid factors (antibodies specific for IgG) were the only known auto antigens, but some other candidates have been identified, including citrullinated proteins, human cartilage glycoprotein 39 and heavy-chain binding proteins (Bläss, Engel and Burmester, 2001). This state of auto reactivity precedes the clinical manifestations of disease and sometimes can last for years (Firenstein, 2003). However, the mechanisms by which this happens are poorly understood.
Numerous infectious organisms have also been associated with the development of RA, including some viruses, retroviruses, Mycoplasma and Mycobacteria, but it is not established yet a precise etiologic link between pathogens and the disease. The pathogen can potentially initiate the disease by a variety of mechanisms, like direct infection of synovium or by activating innate immunity through the receptors that recognize common molecules produced by them. These mechanisms, in a genetically susceptible individual, can contribute to the breakdown of tolerance and autoimmune reactions (Firenstein, 2005).
The accumulation of these auto reactivity reactions, together with environmental and genetic factors that challenge the normal regulatory mechanisms of the immune system can lead to a regulatory catastrophe, and initiate the shift from a healthy to a chronic inflammatory state (Bläss, Engel and Burmester, 2001).
As other autoimmune diseases, RA is initiated by CD4+ T cells, but the immune response is amplified through the stimulus given to other cells, like synovial fibroblasts, macrophages, chondrocytes and osteoclasts. Cytokines play an important role in this pathogenesis, being implicated in each stage of the disease and acting like mediators of the inflammatory process, connected via intricate networks (Choy and Panayi, 2001).
In the two first stages, damage is reversible, until the involvement of cartilage and bones. In state 3, clinical presentation involves joint pain, malaise, swelling and stiffness of joints, often associated with pain in the sole of the foot. With the worsen of the disease, in stages 4 and 5, the inflammation progresses from distal to the proximal joints, like ankles, knees and elbows, and there is an increase of gravity of symptoms, which may lead to deformities (Goust and Virella, 2001 (b)). These terrible consequences are due to infiltrations by immune cells and complex cell-cell interactions, affecting primarily the synovial tissue of
10 the joints, which leads to chronic inflammations and progressive joint destruction (Ma and Pope, 2005).
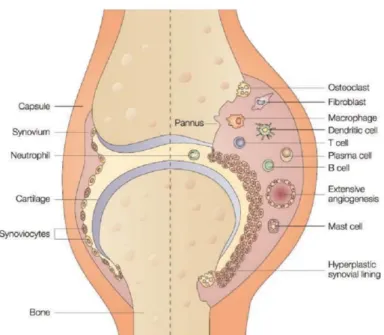
In healthy conditions, normal synovial fluid has an acellular environment, with many capillaries. This acellular stroma has a thin membrane, 1 to 3 cells in thickness, composed of two types of synoviocytes: type A, macrophage-like, and type B, fibroblast-like cells (Feldmann, Brennan and Maini, 1996; Goust and Virella, 2001 (a)). The relative number of type A and B cells is usually similar in normal synovium but, in contrast to this, in a chronic stage of RA, there is a big increase in the number of cells, especially in the macrophage-like cells (Firenstein, 2005). Synovial membrane is then greatly thickened to 6 to 8 cells and adopts a villous appearance, as a result of proliferation of synovial fibroblasts, which tend to accumulate in the more superficial regions of the lining layer (Fig. 2). There is also a massive infiltration of blood cells, like neutrophils, macrophages, lymphocytes and dendritic cells, together with the formation of blood vessels, forming what is known as pannus (Fig. 2) (Feldmann, Brennan and Maini, 1996; Goust and Virella, 2001 (b); Firenstein, 2005).
These cells produce abundant pro-inflammatory cytokines, chemokines and destructive proteases, such as matrix metalloproteinases (MMP), which promote cartilage destruction. The pannus has a tumor-like behavior, continuing to grow during months and years, filling the synovial fluid and increasing inflammation. With time, there is cell migration over the underlying cartilage and into the subchondral bone and tendons, which together with invader’s cells products causes the subsequent erosion of these tissues, leading to pain, movement limitations, flexion contractures and mechanical deformities (Allard et al., 1987).
In located disease, the first pathological alterations are seen in the microvasculature, whose permeability increases and allows the enrichment of synovial fluid with blood cells. At the time of initial symptoms, polymorphonuclear leukocytes like macrophages and monocytes predominate, but several weeks later, there can also be detected lymphocytes B and T, plasmocytes, and other types of inflammatory cells (Feldmann, Brennan and Maini, 1996; Goust and Virella, 2001 (a); Abbas, Lichtman and Pillai, 2012 (a)). In systemic disease, the most frequent symptoms are the formation of rheumatoid nodules in joint, constituted by necrotic cells and vasculitis, which is associated with granuloma formation indicating that cell-mediated immune processes are also involved. These symptoms have a clinical relevance, because they are associated with a poor prognosis (Goust and Virella, 2001 (b)).
11
Figure 2. Schematic comparison between a normal joint (left) and a joint affected by RA (right). In the left
side, the joint has a normal appearance without inflammation, whereas in the right side the joint is under an inflammatory state characterized by the thickness of the synovial lining, the accumulation of immune cells and the morphological swelling (adapted from Smolen & Steiner, 2003). (Smolen and Steiner, 2003)).
2.2. The role of macrophages and cytokines in RA
Macrophages have important functions in the process of synovial inflammation and in the development of RA, being already demonstrated that there is a correlation between the number of macrophages in the synovial lining and disease progression in RA patients (Tak et al., 1997). Cytokines are no less important in the development of this disease, since they are inflammatory mediators that have high expression and activity in synovial tissues, the focus of inflammation in RA. Cytokines are mainly produced via activation of T cells and local macrophages, varying their levels and roles with the patient and the stage of the disease (Fig. 3) (Feldmann, Brennan and Maini, 1996; Choy and Panayi, 2001; McInnes and Schett, 2007; Abbas, Lichtman and Pillai, 2012 (b)). The genetic predisposition to RA can determine the precise patterns of cytokine production, however the exact pathways and flux in synovium are still unclear. It is only known that these pathways are not autonomous, they act like paracrine and autocrine signals, and have a great contribution to inflammation (Firenstein, 2005).
In a healthy environment, macrophages are in a steady-state, but in inflammation they become activated, and regulate secretion of pro-inflammatory cytokines and enzymes involved in the inflammatory response and joint destruction (Firenstein and Zvaifler, 2002; Kennedy et al., 2011). The lining layer of the joint and the area of the pannus are very rich in
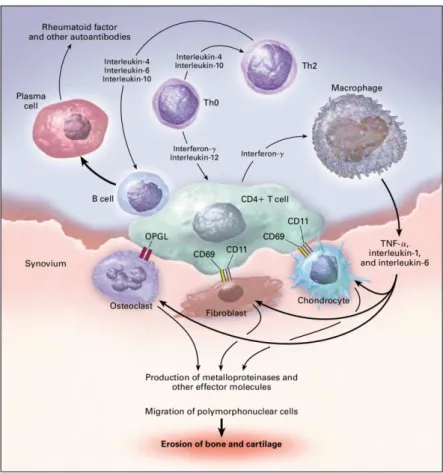
12 activated macrophages (30-40% of the cellular content in pannus), which occupy strategic sites within rheumatoid synovium (Feldmann, Brennan and Maini, 1996; Burmester et al., 1997). Macrophage activation is first triggered by cytokines produced by antigen-activated CD4+ T cells. They can be classically activated by the exposure to IFN-γ, produced by activated T helper 1 (Th1) cells, abundant in RA synovium. Once activated, macrophages play a central role by producing high levels of cytokines and chemokines that enter in complex networks responsible for the promotion of inflammation, until chronic stages (Fig. 3) (Dalton et al., 1993).
Figure 3. Cytokine signaling pathways involved in inflammatory arthritis. Macrophages and T cells, the
major cellular mediators of inflammation in a RA joint are shown together with the main molecular mechanisms that trigger the immune deregulation and development of the disease (adapted from Choy & Panayi, 2001).
The cytokines produced in larger amounts are pro-inflammatory like TNFα, IL-1 and IL-6 (Ma and Pope, 2005). The first two, together with others like IL-15, IL-18 and IL-32 also produced by synovial macrophages, can stimulate proliferation of fibroblasts and secretion of more IL-6, GM-CSF, chemokines and other effectors (Fig. 3). GM-CSF, produced either by macrophages or fibroblasts, can induce the production of IL-1, leading to a positive feedback.
13 Together with TNFα, GM-CSF can increase HLA-DR expression on macrophages. The chemokines produced by macrophages and fibroblasts help in the recruitment of other specific cells into the synovium, like neutrophils, more macrophages and certain populations of B and T cells (Firenstein, 2005). TNF-α and IL-1 are also potent stimulators of mesenchymal cells, like osteoclasts, synovial fibroblasts, and chondrocytes, all producers of metalloproteinases. They also inhibit the production of tissue inhibitors of metalloproteinases by synovial fibroblasts (Shingu et al., 1993), leading to bone degradation (Firenstein, 2005).
Activated macrophages, together with other cells and their products, can also stimulate angiogenesis, which can justify the increased vascularity in RA synovium (Choy and Panayi, 2001).
2.3. RA therapies
RA is still considered a chronic disease because there is not a cure yet for it, despite all the research made. The existent therapies are focused in the control of symptoms and resolution of the acute inflammation, which allow the control of the disease, but the treatment still constitutes a major challenge (Colmegna, Ohata and Menard, 2012).
It is known that an early diagnosis is the basis for the success of treatments, but there are many questions to be answered before starting the treatment. At drugs level, the current medications used to treat RA in different stages of the disease are divided into three subsets: nonsteroidal anti-inflammatory drugs, corticosteroids and, the most used, disease-modifying antirheumatic drugs, all of them potent suppressors of the inflammatory response (O'Dell, 2004). For the majority of patients, a combination of different medications is required to obtain a small slowdown of disease progression, which frequently fails to achieve satisfactory results (Pap et al., 1999).
The majority of therapies used to treat this disease share important features. The mechanisms of function of the drugs used are based on the binding to proteins and alteration of their function (Juliano, Astriab-Fisher and Falke, 2001). This treatments are often marked by failures in pre-clinical test due to their disadvantages: they are extremely expensive, have a limited efficacy, have a significant toxic effect for the patients, increase the risk to a wide spectrum of infections, between a range of other side effects (Hurko, 2006). Furthermore, they do not improve the long-term prognosis of the disease (Scott et al., 1987).
14 In the last years, a significant progress has been made in the research for RA treatment. Until now, having into account the importance of cytokines in the RA pathogenesis, antagonizing their effects has been a priority in drug development (McInnes and Schett, 2007). However, the recent advances contributed to a better understanding of molecular mechanisms behind this pathology, and allowed the development of more effective therapies, directed to more specific molecular therapeutic targets (Adriaansen, Vervoordeldonk and Tak, 2006; Colmegna, Ohata and Menard, 2012).
2.3.1. Gene Therapy and its application in RA
Gene therapy is gaining growing importance and strength among the strategies to combat diseases. This type of therapy may be particularly useful in the case of RA, to overcome the current problems in drug delivery and efficacy in joint tissues (Bessis et al., 2002). There is a big interest in the progress of the in vitro studies and in experimental models, however a lot of work needs to be done in order to achieve high levels of stability, specificity and efficacy (França et al., 2010).
There are many different gene therapy approaches, being one of them by the delivery of gain-of-function genes, which give rise to therapeutic proteins that will interfere in signaling pathways responsible for the development and progression of the disease. Nevertheless, this approach has lost potential with the emergence of RNA potentialities to regulate gene expression (Pap et al., 1999). Nowadays, the best characterized and most promising RNA-mediated gene regulation is the post-transcriptional mechanism known as RNA interference (RNAi) (Caplen, 2004).
3. RNA interference
RNAi is a well-conserved mechanism that occurs spontaneously in vivo and that, in theory, allows the inhibition of the expression of any gene. It was first discovered in plants (Napoli, Lemieux and Jorgensen, 1990), but it is also known in animals (Tijsterman, Ketting and Plasterk, 2002). In vivo, RNAi-mediated silencing occurs with defined objectives in the regulation of basic biological processes, like the transition between developmental states (Pasquinelli and Ruvkun, 2002), or the defense of the organism against invasive foreign genetic material, introduced by mobile elements, such as viruses and transposons. When this
15 phenomenon was discovered in mammals, the prospect for harnessing this biological pathway for biomedical research was raised, and in the past years lots of efforts have been made to understand how RNAi could regulate gene expression and how this could be applied to the development of therapies for diseases (Dykxhoorn and Lieberman, 2005).
Due to its specificity and potency, RNAi is one of the most powerful current technologies to knockdown specific genes, because it allows differential silencing between similar genes that differ from each other in just a few nucleotides. This specificity permits a reduction in the number of off-target genes and directs the new therapies to more promising and specific genes in a quickest way than traditional drugs (Stevenson, 2004; Leung and Whittaker, 2005; França et al., 2010). In the field of autoimmune diseases, RNAi-mediated gene silencing offers an alternative therapeutic strategy to combat inflammation. It allows the target of specific genes in a level upstream in comparison to traditional drugs, inhibiting the expression of proteins before translation, instead of repressing their effects (Corey, 2007).
RNAi-mediated gene silencing can be induced by several mechanisms, depending on the structure of the initiating RNA, the proteins recruited and the target sequence. Silencing can occur by chromatin remodelation, translational blockade, or cleavage of target transcript (Caplen, 2004).
In vivo, the effector molecules that guide mRNAs for degradation are small (21-23)
double-strand (ds)RNA molecules, called small interfering RNAs (siRNAs), which are a result of the degradation of long dsRNAs (Zamore et al., 2000) by proteins of the highly conserved DICER family (Bernstein et al., 2001). The siRNAs are incorporated in a multisubunit ribonucleoprotein complex called RISC, which facilitates the search through the genome for RNA sequences complementary to one of the strands of the RNA duplexes, in other words, helps to find the target (Dykxhoorn, Novina and Sharp, 2003). This mechanism is still poorly understood. It was thought that one of the siRNA strands was then lost from the complex (sense strand), and the other (antisense strand) were matched with its complementary RNA and guides the endonuclease activity of RISC to that site, resulting in mRNA cleavage and thus preventing the synthesis of the protein, lowering its levels inside the cell (Dykxhoorn, Novina and Sharp, 2003). However, it is now known that both strand of the siRNA complex can find their target and are equally eligible to bind the target mRNA (Wei et al., 2009).
The mechanism of RNAi can also be initiated by the introduction of chemically synthesized siRNAs into cells (Dykxhoorn, Novina and Sharp, 2003). This technology applies
16 an evolutionary conserved biological pathway transversal to many organisms, because all cells in theory have the machinery required for the process and are potential targets, so in medicine the applications are infinite (Dykxhoorn and Lieberman, 2005).
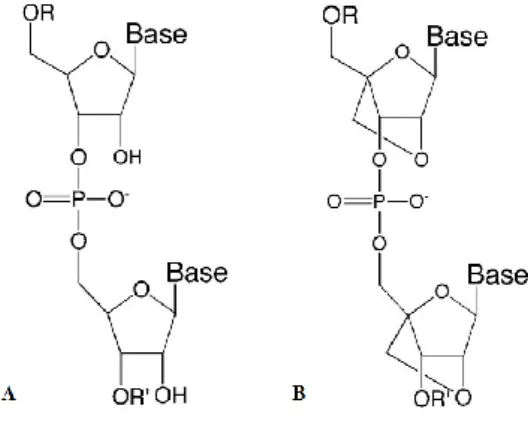
Synthetic siRNAs (Fig. 4) usually comprises small RNA duplexes, in which one of the strands is waived and the other is combined with cellular proteins to form the RISC structure (Dorsett and Tuschl, 2004). They are potent candidate molecules for RNA targeting with high biostability (Braasch et al., 2003), but are known to have a limited specificity and to cause several off-target effects. The studies will need to address the specificity and efficacy of any effect induced by the molecules triggering this mechanism (Akhtar and Benter, 2007).
Although siRNAs have been referred as a new generation of therapeutics, the main challenge in their use is to deliver them to the specific cells in vivo. One of the first barriers in the delivery is the stability of RNAs, when in the presence of the nuclease activity in plasma and tissues (Juliano et al., 2009). Once inside the cell, the siRNA (Fig. 4A) primarily act within cytoplasm, a place easier to access than nucleus, however it is very difficult to obtain an efficient uptake and long-term stability (Caplen, 2004).
Since naked siRNAs are not efficient for direct in vivo administration, because of their instability and rapid degradation, it is thought that improved designs should avoid the off-target effects through an increased stability, selectivity and reduced toxicity (Juliano et al., 2009). Each chemical modification in the sense and antisense strands may provide different properties to the siRNAs, improving the silencing activity, stability, resistance to nucleases, and cellular uptake, and reducing the off-target and side effects (Corey, 2007).
3.1. Short-interfering locked nucleic acid (siLNA)
Short interfering locked nucleic acids (siLNAs) are a class of synthetic RNA analogues, with a special conformation (Fig. 4B). They adopt an RNA structure mimicking a N-type sugar conformation, by the introduction of a methylene linkage between the 2’-oxygen and the 4’-, constraining the ribose ring (Fig. 4B). The monomers are then linked by the phosphate backbone common to DNA or RNA. siLNAs have a high affinity to complementary DNA and RNA sequences (Braasch and Corey, 2001).
LNA monomers are tolerated by the RNAi machinery and provide thermal stability towards complementary RNA, with a high degree of protection from nucleolytic degradation, and a significant reduction of off-target effects, in comparison to normal siRNAs (Braasch et
17 al., 2003; Kauppinen, Vester and Wengel, 2005). LNAs show also low levels of toxicity for the organism and the capacity of being synthesized by standard methods. These characteristics suggest that LNAs can be potential agents for in vivo gene silencing (Braasch and Corey, 2001).
Figure 4. Schematic representations of chemical structures of siRNA (A) and siLNA (B) (adapted from
Corey, 2007).
3.2. Target Genes
The technology of RNAi has been widely applied in vitro in the study of RA (Liu et al., 2005; Courties et al., 2009). There are many possible therapeutic genes potentially useful for gene therapy of arthritis, and this number of candidate molecules is still growing. It is possible to target several molecules simultaneously, and there is also the possibility of combining gene therapy with other types of therapy (Adriaansen, Vervoordeldonk and Tak, 2006).
The criteria should be based in the importance of the targets in the development and maintenance of the pathology and also on the biological effects of the application of RNAi therapies on those targets (Bessis et al., 2002). Before using these methodologies, it is very important to have a wide knowledge of the molecular mechanisms behind pathology of the disease in order to achieve a more specific therapeutics (França et al., 2010).
There are several cytokines, chemokines, transcription factors and other molecules expressed by immune cells, which silencing could be helpful in the resolution of inflammation in joints (França et al., 2010). Among them are the anti-apoptotic protein MCL1 and the transcription factor IRF5.
18 3.2.1. MCL1
In RA synovium, the mechanisms that lead to a persistent chronic inflammatory state are not well characterized, but it is thought that an excessive cell accumulation or insufficient cell death can contribute to the excess of cells in the joints and to chronic inflammation (Pope, 2002; Firenstein, 2005). There are studies revealing that this cell accumulation is not originated in cell proliferation, because the majority of cells are derived from peripheral blood infiltrations (Pope, 2002). Other studies concluded that there are low apoptosis levels in RA synovium (Sugiyama et al., 1996) and, in addition, apoptotic bodies in macrophages have not been identified in patients (Perlman et al., 2001 (b)).
Apoptosis is a process of programmed cell death, so damaged or mutated cells can be safely eliminated from the body, playing thus an important role in maintaining body homeostasis. Disorders in this process contribute to the development of a variety of diseases including cancer, neurodegenerative and autoimmune diseases. Its importance in RA is related with the elimination of activated T cells and the termination of inflammatory responses by rapidly removing neutrophils and macrophages. The exact mechanisms that mediate the resistance to apoptosis in RA synovial macrophages remain little elucidated, but theories describe complex and variable mechanisms (Ma and Pope, 2005). A better understanding of these mechanisms may provide some knowledge for the development of new approaches for the RA therapy (Pope, 2002). However, the forced induction of apoptosis in these cells seems a candidate for the treatment of this inflammatory disease (Liu and Pope, 2003).
Thereby, it is important to target genes that have important roles in the signaling pathways responsible for mediating cell survival or apoptosis. Myeloid Cell Leukemia 1 (MCL1) gene may be an important target for silencing, because it has been shown to be upregulated in RA synovium and to be critical for the survival of macrophages in the joints of patients with RA (Liu et al., 2006). The forced underexpression of this gene through the use of RNAi technology may induce apoptosis in macrophages in RA synovium and thus lead to a decrease in the inflammatory process (Liu et al., 2001; Perlman et al., 2001 (a)).
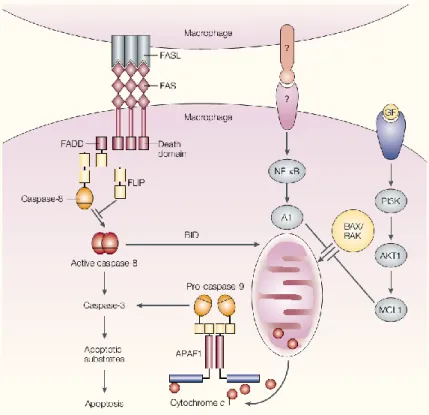
The MCL1 protein is an anti-apoptotic protein that belongs to the Bcl-2 family (Kozopas et al., 1993). In normal macrophages, MCL1 intervenes in the PI3K-AKT1 pathway, which is constitutively activated, contributing to protect macrophage survival. The suppression of MCL1 leads to the inhibition of either PI3K or AKT1 inducing macrophage
19 apoptosis (Fig. 5). This is initiated by the loss of mitochondrial transmembrane potential and is independent of the death-receptor ligation or caspase activation (Liu et al., 2001).
Figure 5. Essential mechanisms to maintain macrophage viability. When FAS and FAS ligand (FASL) are in
contact, apoptosis is prevented by the presence of FLIP. A second mechanism involves the constitutively activated NF-κB and A1. In the third pathway PI3K–AKT1, which is also constitutively activated in normal macrophages, keeps the levels of MCL1 high enough to prevent apoptosis (adapted from Pope, 2002).
3.2.2. IRF5
Transcription factors involved in the expression of pro-inflammatory cytokines are also potential targets to silence in chronic inflammatory diseases like RA. The family of transcription factors termed IFN regulatory factors (IRFs), bearing a helix-turn-helix DNA-binding motif, represents the most responsible regulators of the activation genes coding inflammatory cytokines. IRF5 gene, member of this group, is a transcription factor that is activated by Toll-like receptors (TLR) 7, 8 and 9 (Takaoka et al., 2005), and it has been implicated in driving the classically activated phenotype in macrophages, the most inflammatory, and in the inhibition of the alternative activation (Krausgruber et al., 2011). IRF5 is responsible for the activation of genes that encode inflammatory cytokines, and that are involved in RA and other autoimmune diseases pathologies such as IL-6, IL-12, TNFα,
20 and IFN α/β (Baccala, Kono and Theofilopoulos, 2005; Takaoka et al., 2005). Furthermore, IRF5 is also responsible for the repression of anti-inflammatory cytokines like IL-10 (Krausgruber et al., 2011).
The expression of IRF5 is known to be affected by alternative splicing, responsible for functional modifications in IRF5 messenger RNA, and consequently in its expression. Many polymorphisms were also described, as well as their functional relevance in its regulation, which lead to the expression of IRF5 variants with different cell-type specific expression, cellular localization and functions (Mancl et al., 2005; Sigurdsson et al., 2005). Some IRF5 polymorphisms have been found to be strongly associated with the susceptibility to the development of autoimmune diseases like RA (Dieguez-Gonzalez et al., 2008) and disease Systemic Lupus Erythematosus (Sigurdsson et al., 2005).
4. Aims
This thesis is an approach of the recent iRNA technologies and their innumerous potential applications in the fields of medicine and biotechnology, like the treatment of inflammatory diseases. With the improvements of molecular biology they have been discovered some disease-related genes which may be potential targets for these new therapies. In the particular case of RA, the object of study in this work, they were chosen two potential target genes which knockdown would promote a decay in inflammation, IRF5 and MCL1, in primary human macrophages, cells with an important role in RA inflammatory pathways. In order to perform the best efficiency of silencing and analyze the biological effect of the knockdown in the cells, they were established the following objectives:
Selection of the best macrophage population for gene silencing in primary human macrophages.
Optimization of the transfection of primary macrophages with RNA interference against MCL1 and IRF5 genes.
Evaluation of gene silencing efficiency through analysis of mRNA and protein levels using RT-PCR and Western Blot.
Analysis of gene silencing effect in activated macrophages by the determination of cytokine production and/or induction of apoptosis.
21
22 1. Cell Culture
1.1. Monocyte isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from human peripheral blood of volunteer healthy donors from Hospital de São João (Porto, Portugal). The separation process was made by density gradient centrifugation using Lymphoprep (Axis- -Shield), following an optimized protocol. PBMCs were submitted to an erythrocyte lysis step, using a red blood cell lysis buffer (0.01 M Tris, 0.15 M NH4Cl, pH 7.2) followed by three washing steps with phosphate buffer saline (PBS, Invitrogen).
Monocytes were obtained from freshly isolated PBMCs by CD14+ positive selection, using CD14+ Microbeads (Miltenyi), according to manufacturer’s directions. The use of these beads allows the separation of human cells based on the surface expression of the CD14 antigen, which is highly expressed in monocytes and macrophages and little expressed in granulocytes.
1.2. In vitro differentiation and activation of macrophages
After isolation, CD14+ monocytes were plated in 6-well plates at a density of 0.5 x 106 cells/mL in RPMI-1640 medium (Sigma-Aldrich) supplemented with 10% FCS heat- -inactivated (Invitrogen), 2 mM L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin (Invitrogen). Macrophage-colony stimulating factor (M-CSF) (Peprotech) at 50 ng/mL (previously optimized) was also added to the culture medium for the differentiation of monocytes into macrophages. This cytokine is the primary regulator of macrophage survival, proliferation and differentiation. Cells were then maintained 6 or 7 days in culture (37º C, 5% CO2). On the third or fourth day of culture, cells were fed with fresh complete medium supplemented with 50 ng/mL M-CSF.
Monocyte-derived macrophages were then activated with different stimuli. Briefly, culture medium was removed and replaced by RPMI-1640 medium supplemented with 2% FCS and the desired agents to induce macrophage activation, which concentration was previously optimized (Table 1). Cells were incubated at 37º C, 5% CO2 for 24 or 48h, before analysis.
23
Table 1. Concentration of cytokines used to induce macrophage activation
Macrophage population Activator Concentration (ng/mL) M1 LPS 100 IFNγ 25 M2a IL-4 20 M2c IL-10 10
2. Cell surface markers’ expression
After activation, cells were analyzed by flow cytometry to assess the expression of different surface markers. Briefly, cells were detached from culture plates with Accutase™ (PAA Laboratories) and washed with PBS. Accutase™ allow an efficient dissociation of cells from surfaces, with very low levels of cell damage and, because it does not contain any mammalian or bacterially derived products, its use decreases the risk of contamination of cells by adventitious agents.
Macrophage fragment crystallizable (Fc) receptors were blocked using FcR Blocking Reagent (Miltenyi) diluted in FACS buffer (0.2% BSA, 0.1% azide) (6/100 dilution), for 10 min in the fridge, in order to block unspecific bindings. After blocking, cells were stained with different antibodies (Table 2), diluted in FACS buffer for 20 min on ice and dark. Appropriate isotype controls were also included. After two washing steps with FACS buffer cells were resuspended in PBS and analyzed on a FACSCanto II flow cytometer using BD FACSDivaTM software (BD Biosciences). Data were analyzed using FlowJo™ software (Tree Star).
3. Gene silencing using siLNAs
3.1. Optimization of siLNA transfection in primary human macrophages
The optimization of siLNA transfection in macrophages was performed in 48-well plates using siLNA Universal Negative Control A conjugated with FAM (Exiqon). This molecule acts a scramble, its nucleic acid sequence does not allow the binding of the molecule to any
24 specific target, and its presence is detected by flow cytometry in cells due to FAM fluorescence.
They were not used invasive transfection methodologies, to avoid high levels of dead cells after transfection. Different transfection reagents were tested, in order to evaluate which one produced the best transfection efficiency, with the lowest rate of cytotoxicity in macrophages:
GenMute™ siRNA Transfection Reagent for Primary Macrophages (SignaGen® Laboratories).
X-tremeGENE siRNA Transfection Reagent (Roche Applied Science). Lipofectamine ® RNAiMAX Reagent (Invitrogen).
INTERFERin® in vitro transfection reagent (Polyplus Transfection™).
jetPRIME® in vitro DNA & siRNA transfection reagent (Polyplus Transfection™). Transfections were performed following the manufacturer’s indications. The efficiency was measured 24h after transfection. Briefly, macrophages were detached with Accutase (PAA laboratories) and washed. The FAM fluorescence of the LNA Negative Control was analyzed on a FACSCanto II flow cytometer using BD FACSDivaTM software (BD Biosciences) and data were analyzed using FlowJo™ software (Tree Star). The percentage of dead cells was also determined in the flow cytometer after propidium iodide (PI, Sigma-Aldrich) incubation (1 μg/mL).
Table 2. Antibodies used for cell phenotyping of monocyte-derived macrophages, together with the respective
labeled fluorochrome.
Antibody Clone Fluorochrome Company
Anti - HLAdr MEM-12
Fluorescein isothiocyanate (FITC)
Exbio
Anti - CD14 MEM-15
Anti - CD206 DCN228 Miltenyi Biotec
Anti – FRβ 36b
Phycoerythrin (PE)
Exbio
Anti - CD163 GHI/61 BD Biosciences
Anti - CD1a HI149
Exbio
Anti - CD16 LNK16 Peridinin chlorophyll protein
(PerCP)
Anti - CD80 MEM-233
Anti - CD64 10.1
Allophycocyanin (APC)
Anti - CD86 BU63
25 3.2. Silencing of IRF5 and MCL1 genes in primary human macrophages
Lipofectamine® RNAiMAX Reagent (Invitrogen) was used in the following silencing experiments. Monocytes were plated in 6-well plates and differentiated and activated as described in section 1.2 of Material and Methods. According to the recommended protocol, culture medium was replaced by fresh RPMI-1640 complete medium, 30 to 60 min before transfection. siLNAs against IRF5 and MCL1 (Exiqon) as well as the Negative Control LNA (Exiqon), and the reagent were separately diluted in Opti-MEM® (Invitrogen). Diluted LNA and reagent were mixed and incubated for 15 min at room temperature to form liposomic complexes before being added to cells. The concentration of siLNA ranged according to the experiment, while the transfection reagent was always used in a proportion of 4 µL per million of cells to transfect, unless otherwise stated. Four to five hours after transfection, 1 mL of RPMI-1640 complete medium per million of cells was added, to ensure the nutritional requirements of cells. The transfection efficiency was measured 24 or 48h after transfection as described in the previous section. Protein expression (Section 4.), mRNA (Section 5.) as well as the percentage of apoptotic and dead cells (Section 6.) and cytokine secretion (Section 7.) levels were also analyzed.
4. Determination of protein levels
4.1. Total protein extracts
Macrophages were detached from plates using Accutase™ (PAA Laboratories), washed twice with PBS and lysed on ice for 30 min in lysis buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 1 mM PMSF and 1X protease inhibitor). The lysate was then centrifuged at maximum speed at 4º C to remove nuclei.
Total protein concentration in lysates was quantified using the Bradford Reagent (AppliChem), following the manufacturer’s instructions. The principle is based on the formation of a complex between the dye and proteins. The absorption of this complex can be measured by spectrophotometry at 595nm and it is proportional to the concentration of protein present in the solution. Bovine serum albumin (BSA) standards with known concentrations were used to construct a standard curve with absorbance versus concentration, to allow the determination of protein concentration in the samples.