REVISTA
BRASILEIRA
DE
REUMATOLOGIA
ww w . r e u m a t o l o g i a . c o m . b r
Review
article
Polyunsaturated
omega-3
fatty
acids
and
systemic
lupus
erythematosus:
what
do
we
know?
夽
Mariane
Curado
Borges
a,∗,
Fabiana
de
Miranda
Moura
Santos
a,b,
Rosa
Weiss
Telles
c,
Maria
Isabel
Toulson
Davisson
Correia
d,
Cristina
Costa
Duarte
Lanna
eaProgramofSciencesAppliedtoAdultHealth,FacultyofMedicine,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil bHospitaldasClínicas,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil
cDepartmentofInternalMedicine,FacultyofMedicine,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil dDepartmentofSurgery,FacultyofMedicine,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil
eDepartmentofMusculoskeletalSystem,FacultyofMedicine,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16October2013 Accepted10December2013 Availableonline23October2014
Keywords:
Systemiclupuserythematous(SLE) Polyunsaturatedfattyacidsomega-3 Eicosapentanoicacid(EPA)
Docosahexanoicacid(DHA) Antioxidantsanddiet
a
b
s
t
r
a
c
t
Variousstudieshavedemonstratedtheimpactofomega-3fattyacidsontheconcentration ofCreactiveprotein(CRP),pro-inflammatoryeicosanoids,cytokines,chemokinesandother inflammatorymediators.Therefore,thesupplementationofthesetypesoflipidsmay rep-resentadditionaloptiontreatmentforchronicsystemicdiseases,suchasSystemicLupus Erythematousandother rheumaticdiseases. Theroleoftheselipidshasnot beenwell established,yet.However,itseemsthereisadirectrelationshipbetweenitsintakeand thedecreaseofthediseaseclinicalmanifestationsaswellasoftheinflammatorystatus ofthepatients.Thus,theaimofthismanuscriptistopresentathoroughreviewonthe effectsofomega-3fattyacidsinpatientswithSLE.BibliographicdatasetastheMedical Lit-eratureAnalysisandRetrievalSystemOnline(MEDLINE)andLiteraturaLatino-Americana edoCaribeemCiênciasdaSaúde(LILACS)weresearchedusingaskeywords:systemic lupuserythematous(SLE),polyunsaturatedfattyacidsomega-3,eicosapentanoicacid(EPA), docosahexanoicacid(DHA),antioxidantsanddiet.ManuscriptspublisheduptoSeptember 2013wereincluded.Therewere43articlesrelatedtothetopic,howeveronly15pertained humanstudies,withthreereviewarticlesand12clinicalstudies.
©2014ElsevierEditoraLtda.Allrightsreserved.
DOIoforiginalarticle:http://dx.doi.org/10.1016/j.rbr.2013.12.002. 夽
Institution:FromtheClinicofRheumatology,HospitaldasClínicas,UFMG/DepartmentofMusculoskeletalSystemandDepartmentof Surgery,FacultyofMedicine,UFMG,Post-graduationPrograminSciencesAppliedtoAdultHealth,FacultyofMedicine,UFM,majorarea inClinicalSciences.
∗ Correspondingauthor.
E-mail:maricurado@gmail.com(M.C.Borges). http://dx.doi.org/10.1016/j.rbre.2013.12.002
Ácidos
graxos
poli-insaturados
ômega-3
e
lúpus
eritematoso
sistêmico:
o
que
sabemos?
Palavras-chave:
Lúpuseritematososistêmico (LES)
Ácidosgraxospoli-insaturados ômega-3
Ácidoeicosapentaenoico(EPA) Ácidodocosahexaenoico(DHA) Antioxidantes
r
e
s
u
m
o
Diversosestudostêmdemonstradoa habilidadedosácidosgraxosômega-3emreduzir as concentrac¸ões de proteína C-reativa (PCR), eicosanoides pró-inflamatórios, citoci-nas, quimiocinas e de outrosbiomarcadores da inflamac¸ão. Poressas propriedades, a suplementac¸ãocomessaclassedelipídeospoderepresentarterapiaadicionalao trata-mentodedoenc¸asinflamatóriascrônicassistêmicas,comoolúpuseritematososistêmico (LES)eoutrasdoenc¸asreumáticas.OpapeldessaclassedelipídeosnoLESaindanãoestá bemestabelecido.Noentanto,parecehaverrelac¸ãoentreoconsumodestetipodegordura eadiminuic¸ãodasmanifestac¸õesedaatividadeinflamatóriadadoenc¸a.Sendoassim,este artigoapresentarevisãodaliteraturacientíficasobreosefeitosdosácidosgraxosômega-3 empacientescomLES.Realizou-selevantamentobibliográficojuntoaosbancosdedados MedicalLiteratureAnalysisandRetrievalSystemOnline(MEDLINE)eLiteratura Latino-AmericanaedoCaribeemCiênciasdaSaúde(LILACS),utizando-secomopalavras-chave: lúpuseritematososistêmico(LES), ácidosgraxospoli-insaturadosômega-3,ácido eicos-apentaenoico(EPA),ácidodocosahexaenoico(DHA),antioxidantesedieta.Foramincluídos artigospublicadosatésetembrode2013.Quarentaetrêsartigosrelacionadosaotemaforam encontrados.Apóslimitarabuscaapenasparaestudosrealizadosemsereshumanosforam encontrados15artigos,sendotrêsderevisãoe12ensaiosclínicos.
©2014ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Beneficialeffectsofomega-3polyunsaturatedfattyacidshave beenextensivelystudied,mostlythoseactingonthe cardio-vascularsystem.1–4Overthelastfewyears,theinterestinthe
roleofthisnutrientinreducinginflammationhasgrown.5
Thesefattyacidsareconsideredessentialandshouldbe providedbythedietorfromsupplements.Theycompetewith thearachidonicacid(AA),amemberoftheomega-6family throughthesameenzymaticpathwayandstimulateseries3 prostaglandinsandseries5leukotrienes,whichhavealower inflammatoryactionthanthoseAA-derivedeicosanoids.6
Several studies have demonstrated that omega-3 fatty acids can reduce the concentrations of C reactive protein (CRP),proinflammatoryeicosanoids,cytokines,chemokines, andotherinflammatorybiomarkers.7–10 Inaddition,
eicosa-pentaenoicacid(EPA)anddocosahexaenoicacid(DHA),both membersofomega-3family,areprecursorsofthelipid medi-atorsresolvinsandprotectins,whichhaveanti-inflammatory andimmunomodulatorycharacteristics.11–13
Inviewoftheseproperties,supplementswiththis class oflipids mightrepresentanadditionaltherapyofsystemic chronicinflammatorydiseases,suchassystemiclupus ery-thematosus (SLE) and other rheumatic conditions. Studies conducted insubjects with rheumatoid arthritis report an improvementingeneralphysicalevaluation,pain, morning stiffnessandreducedanti-inflammatoryagentusefollowing supplementationwithomega-3.14–16
TheroleofthesefattyacidsinSLEisnotfullyestablished yet.However,theintakeofthiskindoffatseemstoberelated tothereductionindiseasemanifestationsandinflammatory activity.17–25
Theauthorspresentaliteraturereviewontheeffectsof omega-3fattyacidsonpatientswithSLE.
Method
A literaturesearchon MEDLINEand LILACSdatabases was performed with the following keywords: systemic lupus erythematosus (SLE), omega-3 polyunsaturated fatty acids, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), antioxidants, and diet.Articles publishedup to September 2013wereincluded. Forty-threearticlesrelatedtothetopic havebeenidentified.Afterthesearchwaslimitedtostudies conductedinhumans,15articleswerefound,withthreeof thembeingreviewarticlesand12clinicaltrials.
SystemicLupusErythematosus
Systemic lupus erythematosus (SLE) is a multisystemic autoimmuneinflammatorydiseasewithalargespectrumof manifestationsthatisclinicallycharacterizedbyrelapsesand remissionperiodswithavaryingcourseandoutcome.The dis-easeseverityrangesfrommildtosevere.However,complete andprolongedremissionisrare.26
Although SLE etiology is not known, an interaction of genetic, hormonal and environmentalfactors are probably involvedinthediseasedevelopment,thusleadingtoalossof cellimmunoregulationbalance.27Thisimmunedisturbance
occursthroughlossoftolerancetonuclearantigens,poorly regulatedactivationofB-andTlymphocytesandsubsequent B-lymphocyte polyclonalactivation, with largenumbers of reactive self-antibodies being produced and immune
self-antibodies,areprimarilyresponsiblefortissuelesionand organdamage.28
This complex process involves interaction of various cytokines, chemokines, adhesion molecules, and pattern-recognitionreceptors(PRRs)triggeringcellactivation.29
TlymphocyteswithaTH1functionalresponseare stimu-latedbyinterleukin(IL)-12toproducetumornecrosisfactor alpha(TNF-),interferongamma(IFN-),andIL-2.Ontheother hand,TH2lymphocytes produceIL-4, IL-5,and IL-13when stimulated by IL-4. A significant cytokine elevation result-ing from the TH1 response is observed in the serum of patientswithSLE,thusindicatingadisturbanceinTH1/TH2 responsepattern is likely.28 The TH17functional response
and serum levels of other cytokines, such as IL-23, IL-18, IL-21,and IL-23arealsoelevated, but theirrole inthe dis-easepathogenesisisstillunderinvestigation.28Manyofthese
cytokinesareelevatedintheserumofpatientswithSLEand areassociatedwithahigherdiseaseactivityorcertainclinical manifestations.29–32
Fewstudiesanalyzedtherole ofnutritioninSLE patho-genesistothismoment.Omega-3polyunsaturatedfattyacids mighthavebeneficialclinicaleffectsonSLEbecauseoftheir anti-inflammatoryactions,whichwouldwarranttheiruseas anothertreatmentoptionforthedisease.13
Omega-3polyunsaturatedfattyacids
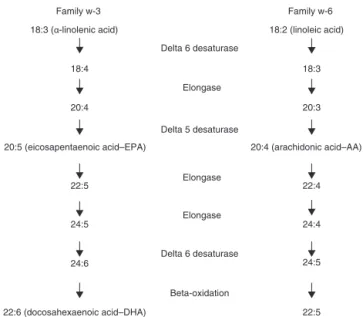
Fattyacidsmaybeclassifiedassaturated(nodoublebonds betweenindividualcarbonatoms)fattyacidsandmono-or polyunsaturatedfattyacidsaccordingtothenumberof dou-ble bondsinthe chain. Themost commonsaturated fatty acidsinhumanfoodare:lauric,myristic,palmitic,andstearic acids(rangingfrom12to18carbonatoms).Unsaturatedfatty acidsareclassifiedintotwomaincategories:polyunsaturated fattyacids,representedbyomega-6fats(withmain represent-ativesbeinglinoleicandarachidonicacids)andomega-3fats (withmainrepresentativesbeing-linolenic,eicosapentaenoic [EPA],anddocosahexaenoic[DHA]acids)ormonounsaturated fattyacids,representedbytheomega-9(omega-9–oleic)fats. Omega-3andomega-6fattyacidsareconsideredessential,as theyarenotsynthesizedbythehumanbody.Thelinoleicacid (18:2omega-3)istheprecursoroftheotherpolyunsaturated fattyacidsinomega-6fats,whichhassoy,corn,andsunflower vegetableoilsastheirmainfoodsources.Intheomega-3 fam-ily,the -linolenicacid(18:3 omega-3)isfoundinanumber ofvegetables,suchascanolaandlinseed,whereasEPA(20:5 omega-3)andDHA(22:6omega-3)arefoundindeepseacold waterfish(mackerel,sardines,salmon,herring).Ontheother hand,oleicacid(18:1omega-9)canbesynthesizedbythebody anditsmaindietsourcesareoliveoil,canolaoil,olives, avo-cadoandnuts(peanuts,chestnuts,walnuts,almonds).33
Mechanismsofactionofomega-3fattyacids
Thefirst datasuggestinga possibleanti-inflammatoryrole for omega-3 fatty acids are derived from an epidemiolog-ical study in Greenland Eskimos conducted by Kronmann and Green in 1980. The authors found that the preva-lenceofdiseaseswithaninflammatorycomponent,suchas acutemyocardiuminfarction,diabetesmellitus(DM),multiple
Family w-3
Delta 6 desaturase
Delta 5 desaturase
Delta 6 desaturase
Family w-6 18:3 (α-linolenic acid) 18:2 (linoleic acid)
20:4 (arachidonic acid–AA) 20:5 (eicosapentaenoic acid–EPA)
22:6 (docosahexaenoic acid–DHA) 18:4
Elongase
Elongase
Elongase
Beta-oxidation 20:4
22:5
18:3
20:3
22:4
24:4
24:5
22:5 24:5
24:6
Figure1–Omega-3andomega-6fatty-acidmetabolism AdaptedfromLeonardetal.,2004.37.
sclerosis,bronchialasthma,andthyrotoxicosis,waslowerin the studypopulationthan inWesterncountry populations. Eskimosdiethavealargeamountofseafood,whichisasource ofpolyunsaturatedfattyacids.34 Thereafter,several studies
wereconductedtofindtheroleoftheselipidsinmanychronic diseasesinwhichinflammationplaysamajorpathogenetic role.
Theomega-3andomega-6families,throughtheir mem-bersEPA,DHA,and arachidonicacid (AA),are theessential lipidclassesforeicosanoidsynthesis.Eicosanoidsareamong the major inflammation mediators and regulators. They includeprostaglandins(PG),leukotrienes(LT),thromboxanes, andotheroxidizedderivatives.Theyareproducedbyoxidative pathwaysoftheenzymescyclooxygenase(COX)and lipoxyge-nase(LOX).35
Due to their molecular similarity, linoleic acid and -linolenicacidcompeteforthesameenzymes tosynthesize derivatives with20 carbonatoms: AA(20:4omega-6), eico-sapentaenoicacid(EPA)(20:5omega-3)eeicosatrienoicacid (ETA) (20:3 omega-9). The -linolenic acid has a 22-carbon atomderivative,thedocosahexaenoicacid(DHA)(22:6
omega-3)33,36,37(Fig.1).
A high intake of linoleic acid favors an increased AA content in membrane phospholipids, thus increasing the productionof series2 and 4eicosanoids (prostaglandinE2 and leukotriene B4) through cyclooxygenaseand lipoxyge-nase enzymatic pathways, respectively. A high production ofeicosanoidsisrelated tothe occurrenceofimmune dis-orders, cardiovascular and inflammatory diseases. On the other hand,the intakeofomega-3family fatty acids,such as -linolenic acid, EPA or DHA, which compete with AA for the same enzymatic pathways, competitively inhibits thearachidonicacidoxidationbycyclooxygenase(COX)into prostaglandins and their conversioninto leukotrienes (LTs) via5-lipoxygenase(LOX)pathway.6,33Theomega-6fattyacid
Arachidonic Acid
EPA
DHA
Series-2 PGs Series-4 LTs Series-3 PGs Series-5 LTs Series-E resolvins Series-D resolvins and protectins
COX LOX COX LOX COX and LOX
COX and LOX
High proinflammatory potential Low proinflammatory potential Anti-inflammatory and resolution
of inflammation potentials
-
-Figure2–SynthesesandactionsoflipidmediatorsproducedbyAA,EPA,andDHA
AA,arachidonicacid;EPA,eicosapentaenoicacid;DHA,docoxahexaenoicacid;COX,cyclooxygenase;LOX,lipoxygenase; PG,prostaglandins;LT,leukotrienes
AdaptedfromCalderetal.,2010.13.
(LTB4)].PGE2inducesanumber ofproinflammatoryeffects, suchasincreasedvascularpermeability,vasodilation, hyper-emia,andhiperalgesia.38–40TXA2promotesthesynthesisof
inflammatory cytokines, IL-1 and TNF-␣ by mononuclear phagocytes.39,41 On the other hand, LTB
4, in addition to
causingincreasedvascularpermeabilityandhyperemia,isa powerfulleukocytechemotacticagentinducingthe produc-tionoflysosomalenzymesandincreasingtheproductionof reactiveoxygenspeciesandcytokines,suchasTNF-,IL-1,and
IL-6(Fig.2).39,40
Thus,theomega-3fattyacidactsbyreducingthe forma-tionofeicosanoidswithinflammatorycharacteristics,since it competes with omega-6 fatty acids for the same enzy-maticpathway,leadingtotheinhibitionofTNF-,IL-1andIL-6 synthesisandreducingtheintercellularadhesionmolecule-1 (ICAM-1)expression.42Itisalsoasubstrateforthesynthesis
ofseries3and5eicosanoids,whichhavelessinflammatory characteristics(Fig.2).6
Studiesinculturedcellshavefurtherdemonstratedthat EPAandDHAcaninhibittheproductionofclassic inflamma-torycytokines,suchasTNF-,IL-1,IL-6,andothers.40,43–45In
healthysubjects,reducedproductionofTNF-,IL-1andIL6by monocytesandmononuclearcellshasalreadybeen demon-strated following fish oil supplementation,9,10,46 although
otherstudieshavenotconfirmedthosefindings.8,47,48These
differencesmighthaveoccurredbecauseofthedifferentdoses used,thedurationoftreatment,andnon-representative sam-ples.
T-cellproliferationinhibitionandIL-2productionwerealso foundinculturedcellsfollowingsupplementationwithEPA andDHA.49However,thesefindingsareinconsistentinhuman
studies.
Inaddition,EPAandDHAareprecursorsofthelipid medi-atorsresolvinsandprotectins.Thepathwayforproduction ofthesesmediatorsalsoinvolvestheenzymes cyclooxygen-ase and lipoxygenase (Fig. 2). In cultured-cell and animal studies,bothresolvinsandprotectinsdemonstratedtohave anti-inflammatory and immunomodulating actions.11–13,50
ResolvinsE1andD1andprotectinD1caninhibitneutrophil
transendothelialmigration,thuspreventingtheinfiltrationof immunecellsattheinflammationsite.ResolvinE1can fur-therinhibitIL-1productionandprotectinE1inhibitsIL-1and TNF-production.50Thus,theselipidmediatorscanplayarole
intheresolutionphaseofinflammationandinlimitingtissue damage.
Thus, the ratio between daily intake of omega-6 and omega-3 fatty-acid sources assumes great importance in humannutrition.36However,theWesterndietischaracterized
byahighomega-6polyunsaturatedfattyacidintakeandhigh omega-6/omega-3ratio,whichfavorspathogenesisina num-berofconditions,includingcardiovascular,inflammatory,and autoimmunediseases,aswellascancer.The omega-6/omega-3 ratio in Western diet is estimatedto be 15-20:1. On the otherhand,anincreasedomega-3intakeandareducedratio betweenthesefattyacidsasaconsequenceseemstoexerta suppressiveeffectondiseasepathogenesis.51
Omega-3andSystemicLupusErythematosus
Fewstudiesinvestigatingomega-3fattyacideffectson sub-jectswithSLEhavebeenconductedtothismoment.However, afewtrialssuggestthatsupplementationwiththisclassof lipids can represent an additionaltherapy to the standard pharmacologicaltreatmentofthisdiseaseduetotheir anti-inflammatoryproperties.17–25
SLEandahistoryofCVDhadhigherlevelsofplasma monoun-saturated fatty acids and higher levels of omega-6 family polyunsaturatedfattyacidsthanthosewithoutCVD.Patients takingprednisone had higher levels ofomega-3and lower AA/EPAratios comparedwiththose nottakingprednisone. However,thesedifferenceswerenotstatisticallysignificant.23
Low levels of omega-3 could contribute to worsen the inflammationalready present inthese patients, thus indi-catingthatthenutrientsupplementation,onitsturn,would contributetoimprovetheinflammatoryprofileand, conse-quently,thediseaseactivity,assubstantiatedbyElkanetal. (2012) in a study conducted in 114 patients with SLE and 122 subjects without the disease. The authors found that higherlevelsofEPAandDHAinfatcellsofpatientswithSLE were negativelyassociatedwithdisease activity (measured bySystemicLupus ErythematosusDisease Activity Index– SLEDAI)andthepresenceofatheroscleroticplaques.Onthe other hand, higher omega-6 levels were positively associ-atedwithahigherdamageindex(evaluatedbytheSystemic LupusInternationalCollaborationClinics/AmericanCollegeof RheumatologyDamageIndex–SLICC/ACR)andthepresence ofatheroscleroticplaques.Inthisstudy,foodintakeevaluation identifiedthat subjects withSLEdescribed a higher carbo-hydrateintakeand alower fiberand polyunsaturatedfatty acid(omega-6andomega-3) intakethan controls. Carbohy-drateintake was positively associatedwith the amount of omega-6fattyacidspresentinadiposetissue.Anassociation betweencarbohydrate,fiber,saturatedandmonounsaturated fattyacidintakeanddiseaseactivityordamageindexcould notbefound.24
Thefirststudyevaluatingtheeffectofomega-3fattyacids on patients with SLE taking fish oil supplementation was reported in 1989 by Clark et al. Theauthors analyzed the effectsofomega-3(EPAandDHA)on12subjectswithSLEand nephritisandconcludedthatdietsupplementationwithfish oilcanaffectthemechanismsinvolvedininflammationand atherosclerosis.Thepatientswere givenasupplementation overfiveweekswithdailydosesof6goffishoil,followedby aperiodoffiveweekswithoutthesupplementationandthen fivemoreweekswith18goffishoildaily.AriseinEPAandDHA levelsinmembranephospholipidsandreducedAA incorpora-tionwerefound.Thesechangeswereassociatedwithreduced plateletaggregationandbloodviscosityandwithanincrease inredcellflexibility.LTB4productionbyneutrophilswasalso significantlyreduced.Thehigher fishoildose induced38% reductionintriglyceridelevelsanda39%reductionin VLDL-cholesterol,associatedwitha28%increaseinHDL-cholesterol levels.Theeffectsontheanti-DNAantibodiestitleand albu-minuriawasnotverified.17
Ontheotherhand,inanothertrialconductedbythesame group of investigators, different results were found. In 21 patientswithastablelupusnephritiswhoweregivenadaily doseof15gfishoilorplacebocontainingoliveoiloverone year,animprovementinkidney function orinthe disease activitywasnotfoundinthefishoilgroupcomparedwiththe placebogroup.Asforbloodlipids,onlyVLDL-cholesteroland triglycerideshadasignificantreductionfollowingthe treat-mentwithfishoil,whereasLDLandHDLfractionsremained unchanged.19
Westbergetal.(1990)evaluatedtheeffectsofomega-3fatty acids supplementationwith capsulescontaining fish oilor capsulesofoliveoilasaplacebo in60patientswith mod-eratelyactiveSLE.Overthefirstthreemonths,thepatients receivingomega-3supplementationhadsignificant improve-mentinclinicalandlaboratoryparameters.However,aftera six-monthsupplementation,nodifferencesbetweengroups couldbedetected,whichsuggests,accordingtotheauthors, thesupplementeffectsareshort-lived.18
In 2004, Duffy et al. published another trial evaluating theeffectofomega-3supplementsand/orcopperinpatients with SLE. Fifty-two patients were divided into four treat-ment groups. One of the groups was given 3 g of fish oil in capsules (EPA 540 mg+DHA 360 mg/capsule)and 3mg of copper; the second groupwas given 3 gof fish oil and copperasaplacebo;thethirdgroupreceivedasupplement with3mgofcopperand fish oilplacebo; the fourthgroup receivedplaceboinsteadofbothnutrients.Afterasix-month supplementation,areduceddiseaseactivityfrom6.12to4.69 (P < 0.05), measured by the Systemic Lupus Activity Mea-sure(SLAM)score,wasfound.Theintegument,neuromotor, and laboratory domains ofSLAM-R were most affected by supplementation.20
Omega-3 beneficial effects on reducing lupus nephritis activitywereevaluatedbyNakamuraetal.(2005).Theauthors examined theeffectofdailysupplementationwith1.8gof purifiedEPAinsixpatients withlupusnephritisdiagnosed bykidneybiopsy.Aftera3-monthsupplementation,reduced AA levels and increasedlevels ofEPA inplasma phospho-lipidsweredetected,inadditiontoreducedurinarylevelsof 8-isoprostane (from550113pg/mg CRto23549pg/mg CR, P=0.02).Intheauthors’opinion,thesefindingsindicatethat EPAcanpromotedecreasedoxidativestress,withconsequent benefits forlupusnephritis treatment. On the other hand, whenimmuneparameterswereevaluated,nosignificant dif-ferences were found in anti-DNA and serum complement valuesafteromega-3treatment.21
Wrightetal.(2008)comparedtheeffectsofdailyintakeof3 gofomega-3(1.200mgofDHA+1.800mgofEPA)withplacebo ofolive oilin 60 patientswith SLEover a 24-weekperiod. They noted that the omega-3 supplementationin patients withSLEcouldnotonlyhaveatherapeuticeffectonthe dis-easeactivitymeasuredthroughtheSystemicLupusActivity Measure(SLAM-R)andBritishIslesLupusAssessmentGroup (BILAG)indexes,butalsopromoteimprovementinendothelial functionandreducedoxidativestress,thusconferring cardio-vascularbenefits.22
Recently,Belloetal.(2013)havepublishedadouble-blind, placebo-controlledtrialinwhich85patientswithSLEwere randomizedtoreceiveeither3gofomega-3supplementsor placebo.Aftera12-weeksupplementation,nosignificant dif-ferencesbetweenthegroupswerefoundfordiseaseactivity, serumlevelsofinflammatorymarkers(ICAM-1,VCAM-1,and IL-6),andendothelialfunction.Asforthelipidprofile,patients receivingomega-3hadasignificantincreaseinLDLcholesterol levels,whereasthoseintheplacebogrouphadasignificant reductionofthislipoprotein.25
Table1–Interventionalstudieswithomega-3fattyacidsinpatientswithsystemiclupuserythematosus(SLE).
Study n Studydesign Results
Clarketal.,198917 12subjectswithlupus
nephritis
6gofomega-3supplements/day overfiveweeks,followedbyfive weekswithnosupplementsand additionalfiveweekswith18gof omega-3/day
RiseinEPAandDHAlevelsin membranephospholipidsand decreaseinAAlevels
Decreasedplateletaggregationand bloodviscosity
Increasedred-cellflexibility ReducedLTB4productionby neutrophils
38%reductionintriglyceridelevels and39%reductionofVLDL-Clevels 28%increaseinHDL-Clevels Noeffectsonanti-DNAand albuminurialevels Westbergetal.,199018 60subjectswith
moderatelyactiveSLE
Omega-3supplementsvs.placebo Supplementsforsixmonths
Improvementinclinicaland laboratoryparametersoverthefirst threemonthswithsupplements Unchangedaftersixmonthswith supplements
Clarketal.,199319 21subjectswithstable
lupusnephritis
Supplementswith15goffishoil vs.oliveoilplaceboforoneyear
Unchangedkidneyfunctionand diseaseactivity
ReducedVLDL-Candtriglyceride serumlevels
UnchangedLDL-CandHDL-Clevels Duffyetal.,200420 52subjectswithSLE Fourgroups:(1)3gofomega-3+3
mgofcopper;(2)3gof
omega-3+copperplacebo;(3)3mg ofcopper+omega-3placebo;(4) omega-3andcopperplacebo Supplementsforsixmonths
Reduceddiseaseactivityingroups receivingomega-3
Nakamuraetal.,200521 Sixsubjectswithlupus
nephritis
Supplementswithdailydosesof 1.8gofpurifiedEPA
Supplementsforthreemonths
ReducedAAlevelsandincreased EPAlevelsinmembrane phospholipids
Reducedurinelevelsof8 isoprostane
Unchangedanti-DNAandserum complementvalues
Wrightetal.,200822 60subjectswithSLE Supplementswith3g/dayof
omega-3vs.oliveoilplacebo Supplementsforsixmonths
Reduceddiseaseactivity Improvementofendothelial function
Reducedoxidativestress Aghdassietal.,201123 32subjectswithSLEand20
controlswithoutthe disease
Fattyacidcontentsinredcellsand plasmafrom:womenwithand withoutSLE;betweenSLEpatients withandwithoutahistoryofCVD; betweenpatientstaking
corticosteroidsornotwere compared
Loweromega-3levelsinred-cell membraneofsubjectswithSLE thaninthosewithoutthedisease Higherplasmaomega-6fatty-acid levelsinpatientswithSLEanda historyofCVD
Higheromega-3levelsinpatients takingcorticosteroids
Differences,however,werenot statisticallysignificant Elkanetal.,201224 114SLEpatientsand122
subjectswithoutthe disease
Omega-3andomega-6contentsin adiposetissuecellsofpatients withSLEwereevaluated
Negativeassociationbetween higherEPAandDHAlevelsin adiposetissuecellsanddisease activityandpresenceof atheroscleroticplaques
Positiveassociationbetweenhigher omega-6levelsinadiposetissue cells,damageindex,andpresence ofatheroscleroticplaques Belloetal.,201325 85subjectswithSLE Supplementswith3gof
omega-3/dayvs.placebo Supplementsfor12weeks
Unchangedendothelialfunction anddiseaseactivity
IncreasedLDL-Clevelsinsubjects receivingomega-3
Unchangedlevelsofinflammatory markers(IL-6,ICAM-1,and VCAM-1)
Finalremarks
Clinicalstudiessuggestthatsupplementationwithomega-3 polyunsaturatedfattyacidscanrepresentanadditionaloption toSLEstandardpharmacologicaltreatmentbecauseoftheir anti-inflammatoryproperties.17–25 Thislipidclassisrelated
totheproductionofeicosanoidswithalowerinflammatory actionthanthose producedbyfatty acidspertainingtothe omega-6family,additionallyreducingserumlevelsof inflam-matorycytokinesandTlymphocyteactivation.
Clinical trialsconducted usedvery different doses, ran-gingfrom 1.8 gto18 goffish oil, and differenttreatment lengths,rangingfromfiveweeksto12months.Whether pos-itive effectsare causedby EPAintake, DHA intakeor by a combinedactionofbothfattyacidsisquestionable.In addi-tion,moststudieshavesmallnumbersofsubjectsincluded, thereforeleadingtolimitedconclusions.
Thus, further studies with a larger number ofsubjects areneededtoevaluatetherealeffectofthesefattyacidsin patientswithSLE,theeffectivedose,andthedurationof treat-ment.
Funding
This research project received funding from Fundac¸ão de AmparoàPesquisadoEstadodeMinasGerais(Fapemig).
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. GruppoItalianoperloStudiodellaSopravvivenzanell’Infarto miocardico.Dietarysupplementationwithn−3
polyunsaturatedfattyacidsandvitaminEaftermyocardial infarction:resultsoftheGISSI-Prevenzionetrial.Lancet. 1999;354:447–55.
2. WangC,HarrisWS,ChungM,LichtensteinAH,BalkEM, KupelnickB,JordanHS,LauJ.N−3Fattyacidsfromfishor
fish-oilsupplements,butnotalpha-linolenicacid,benefit cardiovasculardiseaseoutcomesinprimary-and
secondary-preventionstudies:asystematicreview.AmJClin Nutr.2006;84:5–17.
3. KromhoutD,GiltayEJ,GeleijnseJM.N−3fattyacidsand
cardiovasculareventsaftermyocardialinfarction.NEnglJ Med.2010;363:2015–26.
4. Lorente-CebriánS,CostaAG,Navas-CarreteroS,ZabalaM, MartínezJA,Moreno-AliagaMJ.Roleofomega-3fattyacidsin obesity,metabolicsyndrome,andcardiovasculardiseases:a reviewoftheevidence.JPhysiolBiochem.2013;69:633–51. 5. CalvielloG,SuHM,WeylandtKH,FasanoE,SeriniS,Cittadini
A.Experimentalevidenceof-3polyunsaturatedfattyacid modulationofinflammatorycytokinesandbioactivelipid mediators:theirpotentialroleininflammatory,
neurodegenerative,andneoplasticdiseases.BiomedResInt. 2013;2013:743171.
6. FerrucciL,CherubiniA,BandinelliS,BartaliB,CorsiA, LauretaniF,MartinA,Aandres-LacuevaC,SeninU,Guralnik
JM.Relationshipofplasmapolyunsaturatedfattyacidsto circulatinginflammatorymarkers.JCEM.2006;91:439–46. 7.SchwabJM,SerhanCN.Lipoxinsandnewlipidmediatorsin
theresolutionofinflammation.CurrOpinPharmacol. 2006;6:414–20.
8.ThiesF,MilesEA,Nebe-von-CaronG,PowellJR,HurstTL, NewsholmeEA,CalderPC.Influenceofdietary
supplementationwithlong-chainn-3orn-6polyunsaturated fattyacidsonbloodinflammatorycellpopulationsand functionsandonplasmasolubleadhesionmoleculesin healthadults.Lipids.2001;36:1183–93.
9.TrebbleT,ArdenNK,StroudMA,WoottonSA,BurdgeGC, MilesEA,BallingerAB,ThompsonRL,CalderPC.Inhibitionof tumournecrosisfactor-␣andinterleukin-6productionby mononuclearcellsfollowingdietaryfish-oilsupplementation inhealthymenandresponsetoantioxidant
co-supplementation.BritJNutr.2003;90:405–12.
10.WallaceFA,MilesEA,CalderPC.Comparisonoftheeffectsof linseedoilanddifferentdosesoffishoilonmononuclearcell functioninhealthyhumansubjects.BritJNutr.
2003;89:679–89.
11.SerhanCN,HongS,GronertK,ColganSP,DevchandPR,Mirick G,MoussignacRL.Resolvins:afamilyofbioactiveproductsof omega-3fattyacidtransformationcircuitsinitiatedbyaspirin treatmentthatcounterproinflammatorysignals.JExpMed. 2002;196:1025–37.
12.HongS,GronertK,DevchandP,MoussignacRL,SerhanCN. Noveldocosatrienesand17S-resolvinsgeneratedfrom docosahexaenoicacidinmurinebrain,humanbloodandglial cells:autocoidsinanti-inflammation.JBiolChem.
2003;278:14677–87.
13.CalderPC.Omega-3fattyacidsandinflammatoryprocesses. Nutrients.2010;2:355–74.
14.LauCS,MorleyKD,BelchJJ.Effectsoffishoilsupplementation onnon-steroidalanti-inflammatorydrugrequirementin patientswithmildrheumatoidarthritis-adouble-blind placebocontrolledstudy.BrJRheumatol.1993;32: 982–9.
15.BerbertAA,KondoCR,AlmendraCL,MatsuoT,DichiI. Supplementationoffishoilandoliveoilinpatientswith rheumatoidarthritis.Nutrition.2005;21:131–6.
16.LeeYH,BaeSC,SongGG.Omega-3polyunsaturatedfatty acidsandthetreatmentofrheumatoidarthritis:a meta-analysis.ArchMedRes.2012;43:356–62.
17.ClarkWF,ParbtaniA,HuffMW,ReidB,HolubBJ,FalardeauP. Omega-3fattyaciddietarysupplementationinsystemic lupuserythematosus.KidneyInt.1989;36:653–60.
18.WestbergG,TarkowskiA.EffectofMaxEPAinpatientswith SLE.Adouble-blind,crossoverstudy.ScandJRheumatol. 1990;19:137–43.
19.ClarkWF,ParbtaniA,NaylorCD,LevintonCM,MuirheadN, SpannerE,HuffMW,PhilbrickDJ,HolubBJ.Fishoilinlupus nephritis:clinicalfindingsandmethodologicalimplications. KidneyInt.1993;44:75–86.
20.DuffyEM,MeenaghGK,McMillanSA,StrainJJ,HanniganBM, BellAL.Theclinicaleffectofdietarysupplementationwith omega-3fishoilsandcopperinsystemiclupus
erythematosus.JRheumatol.2004;31:1551–6.
21.NakamuraN,KumasakaR,OsawaH,YamabeH,ShiratoK, FujitaT,MurakamiR,ShimadaM,NakamuraM,OkumuraK, HamazakiK,HamazakiT.Effectsofeicosapentaenoicacids onoxidativestressandplasmafattyacidcompositionin patientswithlupusnephritis.Invivo.2005;19:879–82. 22.WrightSA,O ´PreyFM,McHenryMT,LeaheyWJ,DevineAB,
23.AghdassiE,L.MaDW,MorrisonS,HillyerLM,ClarkeS, GladmanDD,UrowitzMB,FortinPR.Alterationsincirculating fattyacidcompositioninpatientswithsystemiclupus erythematosus:apilotstudy.JPEN.2011;35:
198–208.
24.ElkanAC,AnaniaC,GustafssonT,JogestrandT,HafstrõmI, FrostegardJ.Dietandfattyacidpatternamongpatientswith SLE:associationswithdiseaseactivity,bloodlipidsand atherosclerosis.Lupus.2012;21:1405–11.
25.BelloKJ,FangH,FazeliP,BoladW,CorrettiM,MagderLS,Petri M.Omega-3inSLE:adouble-blind,placebo-controlled randomizedclinicaltrialofendothelialdysfunctionand diseaseactivityinsystemiclupuserythematosus.Rheumatol Int.2013.Epubaheadofprint.
26.VasudevanA,GinzlerE.Clinicalfeaturesofsystemiclupus erythematosus.In:HochbergM,SilmanA,SmolenJ, WeinblattM,WeismanM,editors.Rheumatology. Philadelphia:MosbyElsevier;2011.
27.CooperGS,DooleyMA,TreadwellEL,StClairEW,GilkesonGS. Riskfactorsfordevelopmentofsystemiclupus
erythematosus:allergies,infections,andfamilyhistory.JClin Epidemiol.2002;55:982–9.
28.YuSL,KuanWP,WongCK,LiEK,TamLS.Immunopathological rolesofcytokines,chemokines,signalingmolecules,and pattern-recognitionreceptorsinsystemiclupus erythematosus.ClinDevImmunol.2012;2012:715190. 29.HeinlenLD,McClainMT,MerrillJ,AkbaraliYW,EdgertonCC,
HarleyJB,JAJ.Clinicalcriteriaforsystemiclupus erythematosusprecedediagnosis,andassociated autoantibodiesarepresentbeforeclinicalsymptoms. ArthritisRheum.2007;56:2344–51.
30.JacobN,StohlW.Cytokinedisturbancesinsystemiclupus erythematosus.ArthritisRes.2011;13:228.
31.OhlK,TenbrockK.Inflammatorycytokinesinsystemiclupus erythematosus.JBiomedBiotechnol.2011;2011:432595. 32.SuDL,LuZM,ShenMN,LiX,SunLY.Rolesofproand
anti-inflammatorycytokinesinthepathogenesisofSLE.J BiomedBiotechnol.2012;2012:34714.
33.BhangleS,KolasinskiSL.Fishoilinrheumaticdiseases. RheumDisClinNAm.2011;37:77–84.
34.KronmannN,GreenA.Epidemiologicalstudiesinthe Upernavikdistrict,Greenland:incidenceofsomechronic diseases1950-1974.ActaMedScand.1980;208:401–6. 35.TilleySL,CoffmanTM,KollerBH.Mixedmessages:
modulationofinflammationandimmuneresponsesby prostaglandinsandthromboxanes.JClinInvest. 2001;108:15–23.
36.BistrianBR.Clinicalaspectsofessentialfattyacid
metabolism:JonathanRhoadslecture.JPEN.2003;27:168–75. 37.LeonardAE,PereiraSL,SprecherH,HuangHS.Elogationof
long-chainfattyacids.ProgLipidRes.2004;43:36–54.
38.SamuelssonB.Leukotrienes:mediatorsofimmediate hyersensitivityreactionsandinflammation.Science. 1983;220:568–75.
39.SalmmonJA,HiggsGA.Prostaglandinsandleukotrienesas inflammatorymediators.BrMedBull.1987;43:285–96. 40.MilesEA,CalderPC.Influenceofmarinen-3polyunsaturated
fattyacidsonimmunefunctionandsystematicreviewof theireffectsonclinicaloutcomesinrheumatoidarthritis.BrJ Nutr.2012;107:S171–84.
41.CaugheyGE,PouliotM,ClelandLG,JamesMJ.Regulationof tumornecrosisfactor-alphaandIL-1betasynthesisby thromboxaneA2innonadherenthumanmonocytes.J Immunol.1997;158:351–8.
42.HuguesDA,PinderAC.n-3polynsaturatedfattyacidsinhibit theantigen-presentingfunctionofhumanmonocytes.AmJ ClinNutr.2000;71:357S–60S.
43.DeCaterinaR,CybulskyMI,ClintonSK,GimbroneMA,Libby P.Theomega-3fattyaciddocosahexaenoatereduces cytokine-inducedexpressionofproatherogenican proinflammatoryproteinsinhumanendothelialcells. ArteriosclerThromb.1994;14:1829–36.
44.KhalfounB,ThibaultF,WatierH,BardosP,LebranchuY. Docosahexaenoicandeicosapentaenoicacidsinhibitinbitro humanendothelialcellproductionofinterleukin-6.AdvExp BiolMed.1997;400:589–97.
45.ZhaoY,Joshi-BarveS,BarveS,ChenLH,BarveS,ChenLH. EicosapentaenoicacidpreventsLSP-inducedTNF-alpha expressionbypreventingNF-kappaBactivation.JAmColl Nutr.2004;23:71–8.
46.MeydaniSN,EndresS,WoodsMM,GoldinBR,SooC, Morrill-LabrodeA,DinarelloCA,GorbachSL.Oral(n-3)fatty acidsupplementationsuppressescytokineproductionand lymphocyteproliferation:comparisonbetweenyoungand olderwoman.JNutr.1991;121:547–55.
47.KewS,BanerjeeT,MinihaneAM,FinneganYE,MuggliR, AlbersR,WilliamsCM,CalderPC.Lackofeffectoffoods enrichedwithplan-ormarine-derivedn-3fattyacidson humanimmunefunction.AmJClinNutr.2003;77:1287–95. 48.MilesEA,BanerjeeT,DooperMM,M’RabetL,GrausYM,
CalderPC.Theinfluenceofdifferentcombinationsof gamma-linolenicacid,stearidonicacidandEPAonimmune functioninhealthyyoungmalesubjects.BritJNutr. 2004;91:893–903.
49.CalderPC,NewsholmeEA.Polyunsaturatedfattyacids suppresshumanperipheralbloodlymphocyteproliferation andinterleukin-2production.ClinSci.1992;82:695–700. 50.SerhanCN,ChiangN,VanDykeTE.Resolvinginflammation:
dualanti-inflammatoryandpro-resolutionlipidmediators. NatureRevImmunol.2008;8:349–61.