ww w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Omega-3
fatty
acids,
inflammatory
status
and
biochemical
markers
of
patients
with
systemic
lupus
erythematosus:
a
pilot
study
夽
Mariane
Curado
Borges
a,
Fabiana
de
Miranda
Moura
dos
Santos
b,
Rosa
Weiss
Telles
c,
Marcus
Vinícius
Melo
de
Andrade
c,
Maria
Isabel
Toulson
Davisson
Correia
d,
Cristina
Costa
Duarte
Lanna
b,∗aSecretariadeEstadodeSaúdedoDistritoFederal,Coordenac¸ãoGeraldeSaúdedaCrianc¸aeAleitamentoMaterno(CGSCAM),
Brasilia,DF,Brazil
bUniversidadeFederaldeMinasGerais(UFMG),FaculdadedeMedicina,DepartamentodoAparelhoLocomotor,BeloHorizonte,MG,
Brazil
cUniversidadeFederaldeMinasGerais(UFMG),FaculdadedeMedicina,DepartamentodeClínicaMédica,BeloHorizonte,MG,Brazil
dUniversidadeFederaldeMinasGerais(UFMG),FaculdadedeMedicina,DepartamentodeCirurgia,BeloHorizonte,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received1April2016 Accepted30August2016 Availableonline22October2016
Keywords: Omega3 Cytokines Adipokines Creactiveprotein
Systemiclupuserythematosus
a
b
s
t
r
a
c
t
Background:Studieshave shownthatomega-3 fattyacids reduce theconcentrations of eicosanoids,cytokines,chemokines,C-reactiveprotein(CRP)andotherinflammatory medi-ators.
Objective:Toinvestigatetheeffectsofomega-3fattyacidsoncirculatinglevelsof inflam-matorymediatorsandbiochemicalmarkersinwomenwithsystemiclupuserythematosus (SLE).
Methods:Experimental clinical study (clinical trial: NCT02524795); 49 women with SLE (ACR1982/1997) were randomized: 22 to the omega-3 group (daily intake of 1080mg EPA+200mg DHA,for12 weeks)and27 tothe controlgroup.The inflammatory medi-atorsand biochemicalmarkers atT0 and T1in omega-3 group werecompared using Wilcoxontest.U-Mann–Whitneytestwasusedtocomparevariationsofmeasuredvariables [V=pre-treatment(T0)−post-treatment(T1)concentrations]betweengroups.p<0.05was consideredsignificant.
Results:Themedian(interquartilerange–IQR)ofagewas37(29–48)yearsold,ofdisease durationwas7(4–13)years,andofSLEDAI-2Kwas1(0–2).Themedian(IQR)ofvariationin CRPlevelsbetweenthetwogroupsshowedadecreaseinomega-3groupwhiletherewasan increaseincontrolgroup(p=0.008).TheserumconcentrationsofIL-6andIL-10,leptinand adiponectindidnotchangeaftera12weektreatment.
Conclusions:Supplementationwithomega-3hadnoimpactonserumconcentrationsofIL-6, IL-10,leptinandadiponectininwomenwithSLE andlowdiseaseactivity.Therewasa
夽
StudyconductedatUnidadedeReumatologia,HospitaldasClínicas;FaculdadedeMedicina,DepartamentosdeSistemaLocomotor, CirurgiaeMedicinaInterna,UniversidadeFederaldeMinasGerais,BeloHorizonte,MG,Brazil.
∗ Correspondingauthor.
E-mail:duartelanna@gmail.com(C.C.Lanna). http://dx.doi.org/10.1016/j.rbre.2016.09.014
significantdecreaseofCRPlevelsaswellasevidencethatomega-3mayimpacttotaland LDL-cholesterol.
©2016PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCC BY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Ácidos
graxos
ômega-3,
estado
inflamatório
e
marcadores
bioquímicos
de
pacientes
com
lúpus
eritematoso
sistêmico:
estudo
piloto
Palavras-chave: Ômega-3 Citocinas Adipocinas ProteínaC-reativa
Lúpuseritematososistêmico
r
e
s
u
m
o
Introduc¸ão: Estudostêmmostradoqueosácidosgraxosômega-3reduzemasconcentrac¸ões séricasdeeicosanoides,citocinas,quimiocinas, proteínaC-reativa (PCR)eoutros medi-adoresinflamatórios.
Objetivo: Investigarosefeitosdosácidosgraxosômega-3sobreosníveiscirculantesde mediadoresinflamatóriosemarcadoresbioquímicosemmulherescomlúpuseritematoso sistêmico(LES).
Métodos: Ensaioclínicorandomizado(ensaioclínico:NCT02524795);foramrandomizadas 49mulherescomLES(ACR1982/1997):22paraogrupoômega-3(dosediáriade1.080mg deEPA+200mgdeDHAdurante12semanas)e27paraogrupocontrole.Osmediadores inflamatóriosemarcadoresbioquímicosemT0eT1nogrupoômega-3foramcomparados pelotestedeWilcoxon.OtesteUdeMann–Whitneyfoiusadoparacompararvariac¸ões dasvariáveismensuradas[V=concentrac¸õespré-tratamento(T0)−concentrac¸ões pós-tratamento(T1)]entreosgrupos.Ump<0,05foiconsideradosignificativo.
Resultados:Amediana(intervalointerquartil–IIQ)daidadefoide37anos(29-48),adurac¸ão dadoenc¸afoideseteanos(4-13)anoseoSystemicLupusDiseaseActivityIndex(SLEDAI-2K)foi de1(0-2).Amediana(IIQ)davariac¸ãonosníveisdePCRentreosdoisgruposmostrouum decréscimonogrupoômega-3,enquantohouveumaumentonogrupocontrole(p=0,008). Asconcentrac¸õesséricasdeIL-6eIL-10,leptinaeadiponectinanãosealteraramapósum tratamentode12semanas.
Conclusões:Asuplementac¸ãodeômega-3nãoteveimpactosobreasconcentrac¸õesséricasde IL-6,IL-10,leptinaeadiponectinaemmulherescomLESebaixaatividadedadoenc¸a.Houve umadiminuic¸ãosignificativanosníveisdePCR,bemcomoevidênciasdequeoômega-3 podeimpactarsobreocolesteroltotaleLDL.
©2016PublicadoporElsevierEditoraLtda.Este ´eumartigoOpenAccesssobuma licenc¸aCCBY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Omega-3 fatty acids have been considered antiinflamma-tory lipids based on epidemiological studies of Greenland Eskimos, whose dietis rich inpolyunsaturated fatty acids from fish. The prevalence of diseases with an inflam-matory component such as acute myocardial infarction, diabetesmellitus,multiplesclerosis,asthmaand thyrotoxico-sis,waslowerintheEskimoscomparedtoWesterncountries populations.1
Fattyacidsoftheomega-3family[mainlythe␣-linolenic acid, eicosapentaenoic (EPA) and docosahexaenoic (DHA)], aswellasthoseoftheomega-6family[representedmainly bylinoleicacid andarachidonicacid (AA)]areessential for the synthesis of eicosanoids, prostaglandins, leukotrienes, thromboxanesandotheroxidativefactors,majormediators andregulatorsofinflammation.2,3 Studies haveshownthat omega-3 fatty acids control inflammation by reducing C-reactiveprotein(CRP),eicosanoidproinflammatorycytokines, chemokinesand other inflammatorymediators.4–7 Further-more,theypresent beneficialeffects inthepreventionand
controlofcardiovasculardiseases,dyslipidemiaanddiabetes mellitus.8–13
Systemic lupuserythematosus (SLE) isaninflammatory autoimmunediseasecharacterizedbylossofcellularimmune regulationbalanceandincreasedlevelsofcirculating inflam-matory mediators.14 Thus,omega-3supplementationcould representadditionaltherapyforindividualswithSLE. How-ever,littleisknownontheroleofthesefattyacidsinpatients withSLE,includingtheeffectsoninflammatorycytokine con-centrationsandondiseaseactivity.
The aim ofthis study was to investigate the effects of omega-3 fatty acids on circulating levels of inflammatory mediatorsandbiochemicalmarkersinwomenwithSLE.
Patients
and
methods
CommitteeofUFMGapprovedthestudy.Allpatientsprovided awritteninformedconsent.
Studyparticipants
Female patients who met the revised American
Col-lege of Rheumatology (ACR) classification criteria for SLE (1982/1997),15agedover18yearsoldandbelow60yearsold, takingstabledosesofmedicationsforSLEtreatmentinthe lastthreemonthswereincluded.Exclusioncriteriawerethe following:pregnancy,diseasedurationoflessthanoneyear, allergytofish,fishoiloranyomega-3product,omega-3use within the previous six monthsand diagnosis ofdiabetes
mellitus,liverdisease,activenephritis,chronicrenalfailure, any type ofinfection at enrollment and/orthroughout the study.
A total of153 patients were screened for the trial and 66patientswereincluded,with33randomizedtoeacharm. Twenty-twowomeninthestudygroupand27inthecontrol groupcompletedthewholeprotocolandhadboththeirfirst and12-weekvisitassessments(Fig.1).
Studydesign
A 12weekclinical trial ofomega-3fatty acids supplemen-tationwasconducted.Participantswereseenatbaseline(T0)
Patients screened assessed for eligibility
(n=153)
Patients randomized (n=66)
Allocated to omega-3 group (n=33)
Completed study (n=22) Discontinued from study (n=11)
2 adverse events 2 disease activation 1 change in SLE medication dosage 1 lack of adherence to supplementation
5 loss of serum samples
Allocated to control group (n=33)
Completed study (n=27)
Discontinued from study (n=6) 2 disease activation 1 change in SLE medication dosage
3 loss of serum samples Not included (n=87)
42 diabetes mellitus 30 presence of infection
15 active nephritis
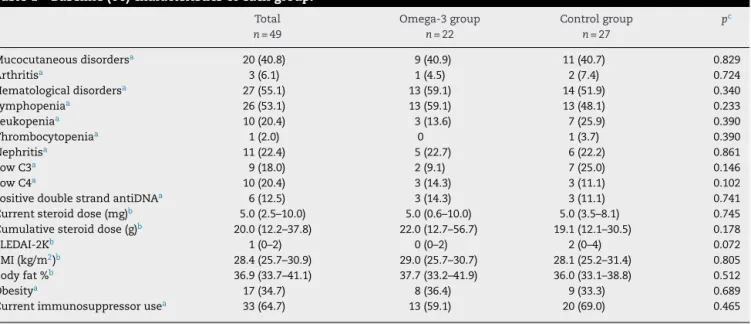
Table1–Baseline(T0)characteristicsofeachgroup.
Total n=49
Omega-3group n=22
Controlgroup n=27
pc
Mucocutaneousdisordersa 20(40.8) 9(40.9) 11(40.7) 0.829
Arthritisa 3(6.1) 1(4.5) 2(7.4) 0.724
Hematologicaldisordersa 27(55.1) 13(59.1) 14(51.9) 0.340
Lymphopeniaa 26(53.1) 13(59.1) 13(48.1) 0.233
Leukopeniaa 10(20.4) 3(13.6) 7(25.9) 0.390
Thrombocytopeniaa 1(2.0) 0 1(3.7) 0.390
Nephritisa 11(22.4) 5(22.7) 6(22.2) 0.861
LowC3a 9(18.0) 2(9.1) 7(25.0) 0.146
LowC4a 10(20.4) 3(14.3) 3(11.1) 0.102
PositivedoublestrandantiDNAa 6(12.5) 3(14.3) 3(11.1) 0.741
Currentsteroiddose(mg)b 5.0(2.5–10.0) 5.0(0.6–10.0) 5.0(3.5–8.1) 0.745 Cumulativesteroiddose(g)b 20.0(12.2–37.8) 22.0(12.7–56.7) 19.1(12.1–30.5) 0.178
SLEDAI-2Kb 1(0–2) 0(0–2) 2(0–4) 0.072
BMI(kg/m2)b 28.4(25.7–30.9) 29.0(25.7–30.7) 28.1(25.2–31.4) 0.805 Bodyfat%b 36.9(33.7–41.1) 37.7(33.2–41.9) 36.0(33.1–38.8) 0.512
Obesitya 17(34.7) 8(36.4) 9(33.3) 0.689
Currentimmunosuppressorusea 33(64.7) 13(59.1) 20(69.0) 0.465
BMI,bodymassindex.
a N(%).
b Median(interquartilrange).
c Pearson’sChi-square,Fisher’sexactorU-Mann–Whitneytest.
and atweek12 (T1) forclinical, laboratoryand nutritional assessment.Participantswerealsocontactedbytelephoneat week6tocheckoncomplianceandanyadverseevents.The patientswererandomizedintooneoftwogroupsina1:1ratio. Patientsinthestudygroupreceived,throughout12weeks,two tabletstakenorallyoncedailyofomega-3fattyacids(540mgof EPAand100mgofDHA;Hiomega-3supplementofNaturalis® company-registeredintheNationalHealthDepartment num-ber4.1480.0006.001-4). Patientsinthecontrolgroupdidnot receivethenutrientnoranykindofplacebo.Allparticipants were instructednot totake omega-3richfoods duringthe studyperiod.Theresearcher(FMMS)whodidclinical assess-mentandtheinflammatoryandbiochemicaldataassessment was blindto randomizationand intervention. Any adverse eventsobservedduringthestudywererecordedandmanaged accordingtolocalclinicalpractice.
Variablesmeasuredateach visitincluded:disease activ-ityindex, using theSystemic LupusDiseaseActivity Index (SLEDAI-2k)16; damageindex (SystemicLupusInternational Collaboration Clinics/American College of Rheumatology damageindex-SLICC/ACR)17;fastinglipidandglucose pro-file;standard laboratorytests toassess SLE(redand white bloodcount,plateletcount,creatinine,urinalysis,urine pro-tein/creatinineratio,anti-dsDNA,anticardiolipin,C3andC4 levels);cytokines(IL-6,IL-10),adipokines(leptin,adiponectin) C-reactiveprotein(CRP),nutritionalassessment,and medica-tionsbeingused.
Nutritional status was assessed by body mass index (BMI) and patients were classified as malnourished (BMI≤ 18.5kg/m2), normal weight (BMI=18.6–24.9kg/m2), over-weight (BMI=25–29.9kg/m2) and obese (BMI≥30kg/m2).18 Body composition assessment was performed by using bioimpedance(RJLQuantumX®)andpatientswereclassified inaccordancetoGallagheretal.19 asnormalorabovethe recommendedpercentagebodyfataccordingtosexandage.
The IL-6 and IL-10 levels were assessed by ultrasen-sitive flow cytometry (Cytometric Bead Array), leptin and adiponectinlevelsbyELISA.
Outcomevariables
Theprimaryoutcomesweremedian(interquartilerange–IQR) variations[V=pre-treatment(T0)−post-treatment(T1) con-centrations],betweengroups,ofserumcytokines,adipokines, C-reactive protein and biochemical markers (glucose and lipids)aftera12weektreatment.
Statisticalanalysis
TheStatisticalPackageofSocialSciencesSoftware(SPSS) ver-sion19.0(SPSSInc.,Chicago,IL,USA)wasused.Comparison ofgroupsatbaseline(withversuswithoutomega-3,with ver-sus without excessweight andwith adequate versusabove recommendedpercentagebodyfat)wereperformedby non-parametric U-Mann–Whitney test for continuous variables, andPearson’schi-squareorFisher’sexacttestforcategorical variables.Themedian(IQR)ofinflammatoryandbiochemical markersatT0andT1inomega-3andincontrolgroupwere comparedusingnonparametricWilcoxontest.
To investigate the effect of omega-3 on inflammatory mediatorsandbiochemicalmarkersthevariationsof labora-toryvariables[V=pre-treatment(T0)−post-treatment(T1) concentrations] betweenomega-3and control groupswere analyzedusingU-Mann–Whitneytest.Allanalyseswere con-sideredsignificantatalevelof2-sided5%(p<0.05).
Results
Table2–Serumconcentrationsofcytokines,adipokinesandbiochemicalmarkersofSLEpatientsatbaseline(T0)by treatmentgroups.
Omega-3group n=22 Median(IQR)
Controlgroup n=27 Median(IQR)
pe
IL-6(pg/mL) 0.57(0.40–2.90)a 1.09(0.52–1.98)b 0.692
IL-10(pg/mL) 19.05(9.88–40.87)c 21.41(6.72–51.64)d 0.699
Leptin(ng/mL) 80.03(63.21–129.40) 58.12(36.65–109.20) 0.067
Adiponectin(g/mL) 42.30(24.88–58.01) 40.08(27.69–59.47) 0.817
Glucose(mg/dL) 77.5(75.2–82.8) 78.0(71.0–86.0) 0.958
Cholesterol(mg/dL) 168.0(151.0–194.0) 182.0(155.5–192.2) 0.899
LDL-c(mg/dL) 95.0(80.0–116.0) 100.0(84.5–111.8) 0.926
HDL-c(mg/dL) 52.0(38.0–57.0) 53.0(37.8–63.2) 0.498
Triglycerides(mg/dL) 88.0(64.0–124.0) 79.5(59.5–114.0) 0.311
CRP(mg/dL) 5.0(4.9–8.1) 6.4(4.9–11.6) 0.370
IL,interleukin;IQR,interquartilerange;CRP,C-reactiveprotein.
a n=21.
b n=26.
c n=14.
dn=21.
e U-Mann–Whitneytest.
toadverseevents(one patientwithdiarrheaand theother reportingfish aftertaste).Both patientswherediscontinued fromthetrial.Thefinalsampleofthispilotstudyconsisted of 49 patients. Cumulative revised ACR classification indi-catedmucocutaneousmanifestationsin86.3%ofthepatients, hematologicaldisordersin80.0%,immunologicaldisordersin 77.6%,arthritisin66.7%,nephritisin56.9%,serositisin16.0% andneuropsychiatricdisordersin11.8%.
Baselineanalysis
Themedian(IQR)ofagewas37(29–48)yearsold,ofdisease durationwas7(4–13)years,ofdisease activityindexwas1 (0–2)andofdamageindexwas0(0–1).
Baseline (T0) clinical, laboratory and disease treatment characteristics,disease activityindexand nutritionalstatus ofparticipantspertreatmentgroupswerenotstatistically dif-ferent(Table1).
Nutritionalstatusof49patients,accordingtoBMI,showed that13patients(26.5%)hadnormalweight,19(38.8%)were overweightand17(34.7%)wereobese.Thisdistributionwas similar in both groups (p=0.875). Bioelectrical impedance analysis indicated that 29 (59.2%) patients had percentage body fat above recommended: 12 patients (54.5%) in the omega-3groupand17(63.0%)inthecontrolgroup(p=0.574).
Serumconcentrationsofcytokinesweresimilarinnormal weight and excess weightparticipants(BMI≥25kg/m2)[
IL-6:1.38(0.48–3.13)pg/mLversus0.92(0.40–1.95)pg/mL;p=0.429; IL-10:19.30(7.85–53.35)pg/mLversus21.42(9.40–51.16)pg/mL; p=0.956]. IL-10 levels were similar in both study groups consideringpatientswithadequateandaboverecommended percentage body fat [16.26 (5.51–22.25)pg/mL versus 22.53 (9.78–55.79)pg/mL;p=0.192].However,serumconcentrations of IL-6 were higher in above recommended percentage bodyfatpatients,showingatrendtowardsignificance[0.48 (0.19–1.04)pg/mLversus1.22(0.47–2.38)pg/mL;p=0.053].
Table3–Serumconcentrationsofcytokines,adipokinesandbiochemicalmarkersatT0andT1inbothgroups.
Variable Omega3groupN=22 ControlgroupN=27
T0 Median(IQR)
T1 Median(IQR)
pa T0
Median(IQR)
T1 Median(IQR)
pa
IL-6(pg/mL)b 0.57(0.40–2.90) 1.10(0.60–2.80) 0.821 1.09(0.52–1.98) 0.88(0.33–2.08) 0.946 IL-10(pg/mL)b 19.05(9.88–40.87) 29.90(9.80–56.30) 0.363 21.41(6.72–51.64) 26.08(11.38–47.54) 0.332 Leptin(ng/mL) 80.03(63.21–129.40) 93.20(54.80–153.40) 0.506 58.12(36.65–109.20) 77.20(50.00–103.00) 0.416 Adiponectin(g/mL) 42.30(24.88–58.01) 44.9(23.90–57.20) 0.465 40.08(27.69–59.47) 44.50(20.00–59.00) 0.462 Glucose(mg/dL) 77.5(75.2–82.8) 83.0(75.0–87.0) 0.043 78.0(71.0–86.0) 77.5(72.2–85.0) 0.354 Cholesterol(mg/dL) 168.0(151.0–194.0) 188.0(162.0–214.5) 0.012 182.0(155.5–192.2) 176.0(152.0–199.8) 0.067 LDL-c(mg/dL) 95.0(80.0–116.0) 115.5(90.0–129.2) 0.003 100.0(84.5–111.8) 98.0(76.0–125.0) 0.019 HDL-c(mg/dL) 52.0(38.0–57.0) 53.0(47.0–67.0) 0.537 53.0(37.8–63.2) 53.5(45.5–59.0) 0.857 Triglycerides(mg/dL) 88.0(64.0–124.0) 70.0(57.0–98.5) 0.520 79.5(59.5–114.0) 87.0(63.2–128.0) 0.657 CRP(mg/dL) 5.0(4.9–8.1) 4.9(4.9–7.2) 0.230 6.4(4.9–11.6) 5.0(4.9–11.6) 0.009
IL,interleukin;CRP,C-reactiveprotein.
a NonparametricpairedWilcoxon.
Table4–Variation(V)ofserumcytokinesandbiochemicalmarkersconsideringtheend(T1)andthebeginning(T0)of thestudybytreatmentgroups.
Omega-3group Median(IQR)
Controlgroup Median(IQR)
pe
VIL-6(pg/mL) 0.12(−1.19to1.45)a −0.05(−0.57to0.58)b 0.915 VIL-10(pg/mL) 1.32(−8.94to18.80)c 1.04(−7.17to12.19)d 0.920 VAdiponectin(g/mL) 0.6(−3.2to11.8) −3.4(−6.6to5.8) 0.171
VLeptin(ng/mL) 3.4(−18.2to22.6) 0.0(−16.3to28.0) 0.924
VCRP(mg/dL) 0.0(−1.5to0.0) 0.0(0.0to1.5) 0.008
Glucose(mg/dL) −4.0(−8.0to0.0) −1.0(−8.0to4.0) 0.496
Cholesterol(mg/mL) 14.0(−2.5to27.2) 4.5(−8.5to23.8) 0.477
LDL-c(mg/dL) 17.0(3.0to27.0) 10.5(−2.5to20.8) 0.288
HDL-c(mg/dL) 0.0(−4.5to12.5) −0.5(−7.0to5.0) 0.536
Triglycerides(mg/dL) −1.0(−39.2to22.8) −4.5(−17.8to16.8) 0.867
IL,interleukin;IQR,interquartilrange;CRP,C-reactiveprotein.
a n=21.
b n=26.
c n=14.
d n=21.
e U-Mann–Whitneytest.
Serum leptin concentrations were higher in excess weight patients comparing to normal weight ones [84.0 (52.9–12.3)ng/mLversus47.6(33.5–73.5)ng/mL;p=0.033],and inindividualswithaboverecommendedpercentagebodyfat compared to those with normal percentage body fat [93.8 (56.5–143.6)ng/mLversus45.8(33.5–63.6)ng/mL;p=0.002].In contrast, adiponectin levels did not differ between groups [normalweight:46.4(34.6–61.0)g/mLversusexcessweight: 42.5(24.7–58.0)g/mL;p=0.571;andnormalpercentagebody fat:46.4(35.1–59.4)g/mLversusaboverecommended percent-agebodyfat:42.9(24.3–59.6)g/mL;p=0.365].
SerumlevelsofIL-6,IL-10andadipokines,serumfasting glucose,lipidprofileandC-reactiveproteinatbaselinewere similarinomega-3andcontrolgroups(Table2).
TheserumlevelsofIL-6andIL-10,leptinandadiponectin didnotchangeaftera12weektreatment.Theconcentrations offastingbloodglucose,totalcholesterolandLDL-cholesterol increased in the omega-3 group, and of LDL-cholesterol increasedincontrolgroup, althoughtheyremained within normallimits(Table3).
Comparisonofvariations(V)betweenthetwogroups
The median (IQR) variations (V=T1-T0) of cytokines, adipokines,fasting glucose and lipids concentrations were similarforthetwogroups(Table4).Themedian(IQR)inCRP levelsvariationbetweenthetwogroupsisrepresentedinFig.2, showingadecreaseintheomega-3groupwhiletherewasan increaseinthecontrolgroup(p=0.008).
Discussion
Supplementation with omega-3 (2g: 1080mg of EPA and 200mgofDHA)for12weekshadnoimpactonserum concen-trationsofIL-6andIL-10cytokinesaswellasonadipokines (leptinandadiponectin)in49womenwithSLEandlow dis-easeactivity.Belloet al.20 reportedsimilarresultsstudying 85SLEpatientswhopresentednoreductionininflammatory
–4.00
No Yes
Omega-3 use
CRP variation (mg/dL)
–3.50 –3.00
–2.50 30 –2.00
–1.50 –1.00 –0.50 0.00 0.50 1.00 1.50 2.00
Fig.2–Box-plotofmedian(range)ofC-reactiveprotein levelsvariations(V)betweenT0andT1intreatmentand controlgroups.
mediatorslevels(sICAM-1,sVCAM-1andIL-6)aftertheuseof higherdosesofthisfattyacid(3gofomega-3:1800mgofEPA and1200mgofDHA)for12weeks.Incontrast,inhealthy sub-jects,cellculturestudiesdemonstratedthatEPAandDHAcan inhibittheproductionofIL-6,TNF-␣,IL-1andIL-1.7,8,21,22In areviewstudy,wefoundconflictingresultsoftheeffectsof omega-3ondiseaseactivity,oncytokinesandonbiochemical markerslevels,duetothemanydifferentmethodsused.23
withserumCRP.Noinformationcouldbefoundinthe litera-tureabouttheeffectsofomega-3onCRPlevelsinSLEpatients. Thehigher serum concentrations ofleptin described in thisstudyinoverweightindividualsandinthosewithabove recommended percentage body fat were also observed by otherresearchers.31–34StudiesevaluatingleptinlevelsinSLE patientsare consistent and indicate higherlevels ofleptin comparedto control subjects, even afterstatistical adjust-mentforbodymassindex(BMI),hypertension,hyperlipidemia anddiabetes.35–38 Thisfinding suggests thatadipose tissue couldhaveasignificantroleinSLEinflammatoryresponse.In contrasttoobesityandmetabolicdiseases,elevatedsystemic andlocallevelsofadiponectinare presentinpatientswith inflammatoryandimmune-mediated conditions,likeSLE.31 Sinceadiponectinhasbeen foundtopresent bothproand anti-inflammatoryactivities,controversialfindingshavebeen observedontheroleoftotaladiponectininsystemic autoim-muneandinflammatorydiseases.32
To our knowledge, the analysis of serum leptin and adiponectinlevelsafteromega-3fattyacidsupplementation inSLEpatientsisoriginaldataofourstudy.Inhealthy sub-jectstheresultsareconflicting.Rameletal.39foundthatdaily consumptionof1.3gEPA+DHAcausedasignificantreduction inserumlevelsofleptin,notingthattherewasconcomitant weightlossofabout1kgintheseindividuals,whichcouldbias theseresults.Itohetal.40reportedthataftertreatmentwith dailydosesof1.8gofEPAtherewasasignificantincreasein adiponectinproductioninobeserodentsandhumans. Stud-ies evaluatingomega-3 andomega-6 concentrationsinred cellmembranesdemonstratedpositiveassociationbetween omega-3 with increased adiponectin and decreased leptin serumlevels,indicatingapotentialeffectofthisfattyacidin controllinginflammation.41,42However,otherauthorsshowed norelationshipbetweentheconsumptionofthisnutrientand serumconcentrationsofthoseadipokines.43,44
Anincreaseoftotalserumcholesterol(p=0.012)and LDL-c (p=0.003) in patients receiving omega-3 fatty acid was seen in our study, as well as an increase in serum LDL-c (p=0.019) in control group individuals. Nonetheless, the medianserumconcentrationsbetweenT1andT0remained withinthelaboratorynormallevels.Inaccordancewithour findings,Belloetal.20 andWrightetal.44 alsodescribed an increaseintotalcholesterolandinLDL-cinSLEpatientswho receivedomega-3. Studies innonSLE subjectswith hyper-triglyceridemiademonstratedincreasedserumlevelsofLDL-c aftersupplementationwiththisfattyacid.45,46Thisisa clin-icallyimportantfinding,sinceSLEpatientsareatincreased riskofatherosclerotic cardiovascular disease,whichis one oftheleadingcauses ofmortalityintheseindividuals.47–50 Interestingly,intwometa-analysisofpopulationwithhigh riskofcardiovascularandcerebrovasculardiseasestherewas noreductioninthefrequencyofcardiovascularevents, coro-naryandcerebrovascularaswellasoverallmortalitywiththis supplementation.51,52
In the present study, the low levels ofinflammation of the SLE patients may have contributed to the absence of changesinserumcytokinelevelsafteromega-3 supplemen-tation. Therefore, it is notpossible to rule out a potential reductioninserum concentrationsinpatientswith moder-atetohighinflammatoryactivityindexes.Longerperiodsof
supplementationwouldyielddifferentresults?Largerdoses couldbebeneficialwithnoriskstothepatients?These ques-tionscanonlybeansweredbylongtermrandomizedstudiesin whichcompliancemustbecontrolledbyomega-3celluptake, whichwedidnotperform.
Inconclusion,inthis12weeksstudyinlowdiseaseactivity lupuspatients,thesupplementationwithomega-3fattyacids wasnotassociatedwithchangesinserumlevelsofIL-6,IL-10, leptinandadiponectin,althoughasignificantdecreaseofCRP concentrationswasobserved.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
TheauthorsthankFundac¸ãodeAmparoàPesquisadoEstado deMinasGerais(FAPEMIG)fortheresearchgrant(CDS- APQ-02095-08).
r
e
f
e
r
e
n
c
e
s
1.KronmannN,GreenA.Epidemiologicalstudiesinthe Upernavikdistrict,Greenland:incidenceofsomechronic diseases1950–1974.ActaMedScand.1980;208:401–6. 2.BhangleS,KolasinskiSL.Fishoilinrheumaticdiseases.
RheumDisClinNorthAm.2011;37:77–84.
3.LiK,HuangT,ZhengJ,WuK,LiD.Effectofmarine-derived n-3polyunsaturatedfattyacidsonC-reactiveprotein, interleukin6andtumornecrosisfactoralfa:ameta-analysis. PLOSONE.2014;9:e88103.
4.SchwabJM,SerhanCN.Lipoxinsandnewlipidmediatorsin theresolutionofinflammation.CurrOpinPharmacol. 2006;6:414–20.
5.ThiesF,MilesEA,Nebe-von-CaronG,PowellJR,HurstTL, NewsholmeEA,etal.Influenceofdietarysupplementation withlong-chainn-3orn-6polyunsaturatedfattyacidson bloodinflammatorycellpopulationsandfunctionsandon plasmasolubleadhesionmoleculesinhealthyadults.Lipids. 2001;36:1183–93.
6.TrebbleT,ArdenNK,StroudMA,WoottonSA,BurdgeGC, MilesEA,etal.Inhibitionoftumornecrosisfactor-␣and interleukin-6productionbymononuclearcellsfollowing dietaryfish-oilsupplementationinhealthymenandresponse toantioxidantco-supplementation.BrJNutr.2003;90:405–12. 7.WallaceFA,MilesEA,CalderPC.Comparisonoftheeffectsof linseedoilanddifferentdosesoffishoilonmononuclearcell functioninhealthyhumansubjects.BrJNutr.2003;89:679–89. 8.CawoodAL,DingR,NapperFL,YoungRH,WilliamsJA,Ward
MJ,etal.Eicosapentaenoicacid(EPA)fromhighly
concentratedn-3fattyacidethylestersisincorporatedinto advancedatheroscleroticplaquesandhigherplaqueEPAis associatedwithdecreasedplaqueinflammationand increasedstability.Atherosclerosis.2010;20:252–9.
9.Skulas-RayAC,Kris-EthertonPM,HarrisWS,VandenHeuvel JP,WagnerPR,WestSG.Dose-responseeffectsofomega-3 fattyacidsontriglycerides,inflammation,andendothelial functioninhealthypersonswithmoderate
hypertriglyceridemia.AmJClinNutr.2011;93:243.
sensitivityintype2diabetesmellitus.JClinDiagnRes. 2012;6:1469–73.
11.BrowningLM,KrebsJD,MooreCS,MishraGD,O’ConnellMA, JebbSA.Theimpactoflongchainn-3polyunsaturatedfatty acidsupplementationoninflammation,insulinsensitivity andCVDriskinagroupofoverweightwomanwithan inflammatoryphenotype.DiabetesObesMetab.2007;9:70–80. 12.HartwegJ,FarmerAJ,HolmanRR,NeilA.Potentialimpactof
omega-3treatmentoncardiovasculardiseaseintype2 diabetes.CurrOpinLipidol.2009;20:30–8.
13.HeK,LiuK,DaviglusML,JennyNS,Mayer-DavisE,JiangR, etal.Associationsofdietarylong-chainn-3polyunsaturated fattyacidsandfishwithbiomarkersofinflammationand endothelialactivation(fromtheMulti-EthnicStudyof Atherosclerosis[MESA]).AmJCardiol.2009;103:1238–43. 14.HahnBH,EblingF,SinghRR,SinghRP,KarpouzasG,LaCava
A.Cellularandmolecularmechanismsofregulationof autoantibodyproductioninlupus.AnnNYAcadSci. 2005;1051:433.
15.HochbergMC.UpdatingtheAmericanCollegeof Rheumatologyrevisedcriteriafortheclassificationof systemiclupuserythematosus.ArthritisRheum. 1997;40:1725.
16.GladmanDD,IbanezD,UrowitzMB.Systemiclupus erythematosusdiseaseactivityindex2000.JRheumatol. 2001;29:288–91.
17.GladmanD,GoldsmithC,UrowitzMB.Thereliabilityofthe SystemicLupusInternationalCollaboratingClinics/American CollegeofRheumatologydamageindexforsystemiclupus erythematosus.ArthritisRheum.1997;40:809–13.
18.WHO(WorldHealthOrganization).Obesity:preventingand managingtheglobalepidemic.ReportofWHOconsultation. Geneva:WHO;1997,276p.
19.GallagherD,HeymsfieldSB,HeoM,JebbSA,MurgatroydPR, SakamotoY.Healthypercentagebodyfatranges:anapproach fordevelopingguidelinesbasedonbodymassindex.AmJ ClinNutr.2000;72:694–701.
20.BelloKJ,FangH,FazeliP,BoladW,CorrettiM,MagderLS,etal. Omega-3inSLE:adouble-blind,placebo-controlled
randomizedclinicaltrialofendothelialdysfunctionand diseaseactivityinsystemiclupuserythematosus.Rheumatol Int.2013;33:2789–96.
21.SchubertR,KitzR,BeermannC,RoseMA,BaerPC,ZielenS, etal.Influenceoflow-dosepolyunsaturatedfattyacids supplementationontheinflammatoryresponseofhealthy adults.Nutrition.2007;23:724–30.
22.FujiokaS,HamazakiK,ItomuraM,HuanM,NishizawaH, SawazakiS,etal.Theeffectsofeicosapentaenoic acid-fortifiedfoodoninflammatorymarkersinhealthy subjects–arandomized,placebo-controlled,double-blind study.JNutrSciVitaminol.2006;52:261–5.
23.BorgesMC,SantosFMM,TellesRW,CorreiaMITD,LannaCCD. Polyunsaturatedomega-3fattyacidsandsystemiclupus erythematosus:whatdoweknow?BrJRheumatol. 2014;54:459–66.
24.FerrucciL,CherubiniA,BandinelliS,BartaliB,CorsiA, LauretaniF,etal.Relationshipofplasmapolyunsaturated fattyacidstocirculatinginflammatorymarkers.JClin EndocrinolMetab.2006;91:439–46.
25.TsitourasPD,GucciardoF,SalbeAD,HewardC,HarmanSM. Highomega-3fatintakeimprovesinsulinsensitivityand reducesCRPandIL6,butdoesnotaffectotherendocrineaxes inhealthyolderadults.HormMetabRes.2008;40:199–205. 26.MicallefMA,MunroIA,GargML.Aninverserelationship
betweenplasman-3fattyacidsandC-reactiveproteinin healthyindividuals.EurJClinNutr.2009;63:1154–6.
27.Farzaneh-FarR,HarrisWS,GargS,NaB,WhooleyMA.Inverse associationoferythrocyten-3fattyacidlevelswith
inflammatorymediatorsinpatientswithstablecoronary arterydisease:theHeartandSoulStudy.Atherosclerosis. 2009;205:538–43.
28.KelleyDS,SiegelD,FedorDM,AdkinsY,MackeyBE.DHA supplementationdecreasesserumC-reactiveproteinand othermarkersofinflammationinhypertriglyceridemicmen. JNutr.2009;139:495–501.
29.LiK,HuangT,ZhengJ,WuK,LiD.Effectofmarine-derived n-3polyunsaturatedfattyacidsonC-reactiveprotein, interleukin6andtumornecrosisfactor␣:ameta-analysis. PLoSONE.2014;9:e88103.
30.JuliaC,TouvierM,MeunierN,PapetI,GalanP,HercbergS, etal.IntakesofPUFAswereinverselyassociatedwithplasma C-reactiveprotein12yearslaterinamiddle-agedpopulation withvitaminEintakeasaneffectmodifier.JNutr.
2013;143:1760–6.
31.TilgH,MoschenAR.Adipocytokines:mediatorslinking adiposetissue,inflammationandimmunity.NatRev Immunol.2006;6:772–83.
32.Al-SuhaimiEA,ShehzadA.Leptin,resistin,andvisfatin:the missinglinkbetweenendocrinemetabolicdisordersand immunity.EurJMedRes.2013;18:12.
33.KershawEE,FlierJS.Adiposetissueasanendocrineorgan.J ClinEndocrinolMetab.2004;89:2548–56.
34.VanHarmelenV,ReynisdottirS,ErikssonP,ThorneA, HoffstedtJ,LonnqvistF,etal.Leptinsecretionfrom subcutaneousandvisceraladiposetissueinwomen. Diabetes.1998;47:913–7.
35.ChungCP,LongAG,SolusJF,RhoYH,OeserA,RaggiP,etal. Adipocytokinesinsystemiclupuserythematosus:
relationshiptoinflammation,insulinresistanceandcoronary atherosclerosis.Lupus.2009;18:799–806.
36.Garcia-GonzalezA,Gonzalez-LopezL,Valera-GonzalezIC, Cardona-Mu ˜nozEG,Salazar-ParamoM,González-OrtizM, etal.Serumleptinlevelsinwomenwithsystemiclupus erythematosus.RheumatolInt.2002;22:138–41.
37.VadaccaM,MargiottaD,RigonA,CacciapagliaF,CoppolinoG, AmorosoA,etal.Adipokinesandsystemiclupus
erythematosus:relationshipwithmetabolicsyndromeand cardiovasculardiseaseriskfactors.JRheumatol.
2009;36:295–7.
38.KimHA,ChoiGS,JeonJY,YoonJM,SungJM,SuhCH.Leptin andghrelininKoreansystemiclupuserythematosus.Lupus. 2010;19:170–4.
39.RamelA,ParraD,MartinézJA,KielyM,ThorsdottirI.Effects ofseafoodconsumptionandweightlossonfastingleptinand ghrelinconcentrationsinoverweightandobeseEuropean youngadults.EurJNutr.2009;48:107–14.
40.ItohM,SuganamiT,SatohN,Tanimoto-KoyamaK,YuanX, TanakaM,etal.Increasedadiponectinsecretionbyhighly purifiedeicosapentaenoicacidinrodentmodelsofobesity andhumanobesesubjects.ArteriosclerThrombVascBiol. 2007;27:1918–25.
41.AnWS,SonYK,KimSE,KimKH,BaeHR,LeeS,etal. Associationofadiponectinandleptinwithserumlipidsand erythrocyteomega-3andomega-6fattyacidsindialysis patients.ClinNephrol.2011;75:195–203.
42.MinY,LowyC,IslamS,KhanFS,SwaminathanR. Relationshipbetweenredcellmembranefattyacidsand adipokinesinindividualswithvaryinginsulinsensitivity.Eur JClinNutr.2011;65:690–5.
43.OlzaJ,MesaMD,AguileraCM,Moreno-TorresR,JiménezA, PérezdelaCruzA,etal.Influenceofaneicosapentaenoicand docosahexaenoicacid-enrichedenteralnutritionformulaon plasmafattyacidcompositionandmediatorsofinsulin resistanceintheelderly.ClinNutr.2010;29:31–7.
polyunsaturatedacidsonendothelialfunctionanddisease activityinsystemiclupuserythematosus.AnnRheumDis. 2008;67:841–8.
45.PownallJH,BrauchiD,KilincC,OsmundsenK,PaoQ, Payton-RossC,etal.Correlationofserumtriglycerideandits reductionbyomega-3fattyacidswithlipidtransferactivity andtheneutrallipidcompositionsofhigh-densityand low-densitylipoproteins.Arteriosclerosis.1999;143:285–97. 46.HarrisWS,GinsbergHN,ArunakulN,ShachterNS,Windsor
SL,AdamsM,etal.SafetyandefficacyofOmacorinsevere hypertriglyceridemia.JCardiovascRisk.1997;4:385–91. 47.UrowitzMB,BookmanAA,KoehlerBE,GordonDA,Smythe
HA,OgryzloMA.Thebimodalmortalitypatternofsystemic lupuserythematosus.AmJMed.1976;60:221–5.
48.ManziS,MeilahnEN,RairieJE,ConteCG,MedsgerTAJr, Jansen-McWilliamsL,etal.Age-specificincidenceratesof myocardialinfarctionandanginainwomenwithsystemic
lupuserythematosus:comparisonwiththeFramingham Study.AmJEpidemiol.1997;145:408–15.
49.TellesRW,LannaCC,SousaAJ,NavarroTP,SouzaFL, RodriguesA,etal.Progressionofcarotidatherosclerosisin patientswithsystemiclupuserythematosus.ClinRheumatol. 2013;32:1293–300.
50.TellesRW,LannaCC,SouzaFL,RodriguesLA,ReisRC,Ribeiro AL.CausesandpredictorsofdeathinBrazilianlupus patients.RheumatolInt.2013;33:467–73.
51.ChowdhuryR,StevensS,GormanD,PanA,WarnakulaS, ChowdhuryS,etal.Associationbetweenfishconsumption, longchainomega3fattyacids,andriskofcerebrovascular disease:systematicreviewandmeta-analysis.BMJ. 2012;345:e6698.