Struture and Property Relationships of Amorphous
CN
x
: A Joint Experimental and Theoretial Study
M.C. dos Santos and F. Alvarez
Institutode Fsia\GlebWataghin",UniversidadeEstadual deCampinas
CaixaPostal6165,13083-970, Campinas,S~aoPaulo,Brazil
Reeived30November,2000
AmorphousCN
x
and CN
x
:H have beenprepared by the ion beamassisteddeposition tehnique.
SampleswereharaterizedthroughX-rayandUVphotoemission,IRabsorptionandRaman
spe-trosopies. These spetra have been interpreted with the aid of quantum hemial alulations
basedupontheHartree-Foktheoryonseveralmoleular models. Theunderstandingofthe
ele-troni and strutural properties of the amorphous alloy as a funtion of nitrogen ontent ould
helpinthetaskofsynthesizingthemetastablesilion-nitridelike-phase-C
3 N
4
,asolidwhihhas
beenpredited tobeas hardasdiamond. Thephysial pitureemergingfromthe present study
helpsto larify the diÆulties inobtaining the rystallinephase of the material,suggesting new
experimentaldiretionsforsyntheses.
I Introdution
The intereston arbon nitridematerials wasraisedin
early 1920's [1℄, when it wassuggested that the solid
C
3 N
4
might exist in a graphiti form. However, the
searhfor thismaterialstarted muh later, attheend
of the 1980's, after Liu and Cohen [2℄ predited the
existene ofametastablefully ovalentarbonnitride
(-C
3 N
4
)havingthesamestrutureoftheknown
om-pound Si
3 N
4
. This theoretial predition was based
on ab initio alulationswhih suggestedfor the rst
time the possible existene of a material as hard, or
even harder, than diamond. In addition, the authors
demonstrated in this artile the powerfulness of
mod-ern eletroni struture tehniques in prediting new
struturesandpropertiesofsolids.
Considerable interest was devoted to
arbon-nitrogen alloys sine then. Although serious
prob-lemshaveemergedtoproduethepreditedrystalline
phase,amorphousCN
x
lmsexhibitusefuloptialand
mehanial properties [3℄. Reent experiments show
that CN
x
alloys undergo profound strutural hanges
upon the variationof the nitrogen onentration.
In-reasing the amount of N up to 20 at. % notably
augmentthehardnessandelastiityofthematerial[4℄.
Reently,asuperhardC-Nalloywithhardnessashigh
as 40-60 Gpa wasreported [5,6℄. Besides the obvious
tehnologialpotentiality,thealloyisaninteresting
sys-tem per sefor basi studies. For instane, theoretial
struturessuhasfullerene-likeandtubulararbon
ni-trides[7℄. Indeed,nanotubesformedinsomenon-
stoi-hiometriC-Nalloyswereattributedtobetheauseof
thesurprising hardnessofthematerial[5℄. Tehniques
toproduearbonnitridetubulitesareurrentlybeing
developed[8,9℄.
Inspiteofthelargeamountofstudiesalready
per-formed, the omplexity of the struture of
arbon-nitrogen materials has prevented a lear
understand-ingof several basiaspets of thealloy. In this paper
wepresenttheoretialalulationsandexperimental
re-sults regarding the study of this interesting material.
Carbonnitride thin lms were preparedwith varying
N ontent. Theloal struture and thetopof the
va-lene band as funtion of the nitrogen onentration
is reported. The role of N bending and bukling the
struture towardsthe formation of moleular forms is
disussed and the inuene of hydrogen in the
prop-erties of arbon-nitrogen alloy is also reported. The
vibrationalspetrumof the amorphousmaterial is
in-vestigatedthroughRamanandinfra-redspetrosopies
in ombination with isotopi 15
N and D substitution.
Moleularmodels were adopted to theoretially
inves-tigatetherolesofnitrogenbindingtoarbonaswellas
theisotopi shifton thevibrational spetra. This
pa-peris organizedasfollows: abriefpresentation ofthe
theoryadoptedismadeinsetionII,togetherwiththe
andtheinterpretationmadewiththeaidoftheoretial
models is presented in setion IV. The last setion is
devotedtoonlusions.
II Theory
Thetheoretialapproahrelies onalulationsof
den-sityofstates(DOS) andorelevelbindingenergiesof
modelmoleulesontainingCandN.Thestartingpoint
of all alulations is the determination of the ground
statemoleularonformation. Geometryoptimizations
of themoleular strutures and thevibrational
analy-siswereperformedbasedonthesemi-empirialAustin
Method 1(AM1) and ParametriModel3 (PM3)[10℄
quantumhemialtehniques. Onlythelustersrelated
to C
3 N
4
, taken from the predited rystal struture,
were notoptimized.
II.1. Core-level binding energy
Smallmoleulesrepresentativeofthehemialbond
in C-N alloys are hosen for evaluation of the
hemi-alshiftsontheN1seletronbindingenergy[11℄. The
eletronistruturesoftheoptimizedmoleular
onfor-mationswasobtainedthroughabinitio 6-31G
alu-lations[12℄. Themoleulesinludesp,sp 2
andsp 3
ar-bonand nitrogenhybridizations and fragmentsof the
theoretiallypredited and arbon-nitride(C
3 N
4 )
ompound [2,13℄. The numerial valuesassoiated to
eahstruturearegroupedasfollows: a)sp 3
Nbonded
to sp 3
C in \open"strutures; b) sp 2
N substituting
sp 2
arbon in aromati strutures; ) sp N in nitrile
ompounds andsp 2
Ninpyridine;d)sp 3
Nbondedto
sp 3
Cin alosed struturein whih C-N-Cbondsare
stressed. These alulationsfound four typial values
of binding energies: (a) 398.3-398.6eV forthreefold
oordinated N bonded to fourfold oordinated C, (b)
401.9-401.0eV for substitutional sp 2
N in
graphite-likestrutures;() 399.2eVforsp 2
N(pyridine-like)
or399.4eVforspN;and(d)399.0eVforastressed
strutureontainingsp 3
N.
II.2. Valene-band eletroni struture
Largelusters inluding up to 96 arbon atoms in
the graphitesymmetry were adopted to study the
va-lene band (VB) eletroni struture by randomly
re-plaing C with N atoms [11℄. The eletroni
stru-ture was obtained by the Valene Eetive
Hamilto-nian(VEH)method[14℄. Bondsat theboundarywere
saturated with hydrogen. Systems ontaining [N℄/[C℄
=0,7%,14%,20%,23%,37%and 100%were studied.
Two main sets were optimized: 1) one set imposing
C symmetry to maintain planar geometry; and 2) a
seond set allowing fullrelaxation ofthe atomi
oor-dinates. Asbefore, theeletronistruturesof the
op-timized moleuleswereobtainedbytheVEHMethod.
Alusterrepresenting-C
3 N
4
hasbeentakenfromthe
predited rystalstruture, ontaining36 C and48 N
atoms, with end bonds saturated with hydrogen, and
theDOSwasalulated. Foromparisonpurposes,the
Fermienergyofthepuregraphitelusterwasshiftedto
zero. Histogramswere obtainedby theusualounting
ofstatesproedure. TheDOSwerenormalizedfor
om-parisonpurposes,sinethelustersmayhavedierent
numberofatoms[7,11℄.
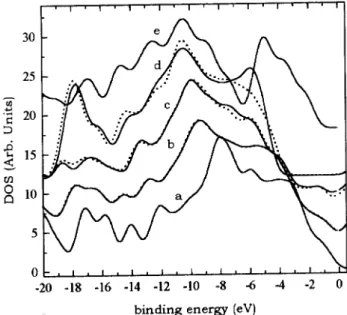
Figure 1. Simulateddensities of statesof arbon-nitrogen
lusters from VEH alulations, for a graphite, b [N℄/[C℄
= 14%, [N℄/[C℄ = 37%, d[N℄/[C℄ =100%, and e C3N4
luster. Plotswereshiftedupwardforbettervisualization.
Fig. 1displays thesimulated densitiesof statesof
graphite (urvea), N-substituted graphite atthe
on-entrations [N℄/[C℄= 14%(urvesb), 37%(urves),
and100%(urvesd),andtheDOSofthe-C
3 N
4
lus-ter(urvee). Theplotsareshiftedupwardforbetter
vi-sualization. Theurvesb,,anddareplottedinpairs,
representingtheDOSofplanar(dotted)andfully
opti-mized(ontinuous)N-substitutedgraphite lusters. In
athewidefeatureat3.0eV,duetoeletronsoming
fromthebonds,andat8.0eV,duetobonds,are
learly seen. These peaks are shifted to higher
bind-ing energiesasmoreandmorenitrogen atoms replae
arbons in the luster. At [N℄/[C℄ = 14%, urves b,
dierenes between the DOS are negligible sine the
fully optimizedlusterremainsplanar. Dierenes are
fully developedat [N℄/[C℄ =100%: whilethe
graphite-like system presents a DOS prole similar to that of
graphite, shiftedto higherbindingenergies, the
stru-tureof thefullyoptimizedluster is distortedand the
buklingresultsintwowelldenedpeaksinthe
originating from nitrogen lone-pairs. The last DOS,
urvee,presentstwofeaturesloatedat11.0eVand
5.0 eV and are due to eletrons oming from C-N
bonds and N lone-pairs, respetively, of the -C
3 N
4
struture.
II.3. Vibrational struture
We have performed alulations of the frequenies
andintensitiesoftheinfra-redativevibrationsof
sev-eral organi moleules. These alulations are aimed
at investigatingtherolesof symmetrybreakingeets
of substitutional N on graphite struture and isotopi
shifts due to 15
N. Firstly, we obtained the spetra of
the small moleules shematially shown in Fig. 2.
Moleule M1 is an aromatimoleular radial having
D
3h
symmetry. Substitutionoftheentralarbonatom
by N, as in moleule M2, is intended to investigate
the eets due to harge rearrangement while in M3
moleulethesubstitutionsitereduesthesymmetryto
C
2v .
Figure2. MoleularmodelsadoptedinPM3alulations
ofIRspetra.
Figure 3. Simulated PM3 IR-ative vibration spetra of
themodelmoleulesM1(ontinuousline,saledby10),M2
(dotted)andM3(dash-dotted). Theurvesweretranslated
upwardforlarity.
The moleular onformations were optimized and
the vibration analysis was performed. Fig. 3 depits
thesimulatedspetraintheregion800-1600m 1
eval-uatedasgaussianonvolutionsofthealulated
inten-sities. Thegaussian broadeningwasarbitrarily taken
as20m 1
.
MoleuleM1 isveryhomogeneousin harge
distri-butionand in C-Cbond lengths, resultinginverylow
irintensitiesinthestrething region,asexpeted.
No-tiethat the urvein Fig. 2orresponding toM1 has
beensaledbyafatorof10toallowomparisonswith
theotherspetra. ReplaementoftheentralC atom
by N brings a new harge distribution in whih
se-ond neighbors arbonsaround N get negativeharges
throughthe system. Theonsequeneis the
ativa-tionofC-Cstrething vibrations,asseenin Fig. 3,in
theregion1200-1600m 1
. Theseolletivevibrations
alsoinlude theC-N strething, whih shiftsthe
spe-trum tohigher frequenies,from 1500m 1
to 1600
m 1
. TherearedierenesbetweenthespetraofM2
and M3 due to the their partiular symmetry group,
buttheintensitiesareofthesameorderofmagnitude.
This means that the most relevanteet of N
substi-tutionin theirspetrumofthearomatisystemisthe
promotionofbonddipoles.
This result holds for largersystems provided that
thearomatiityispreserved. Wehaveperformed
simi-laralulationsonlargegraphitilustersontainingup
to 96arbonatoms, asdesribedin theprevious
sub-setion. Fig. 4depits the simulated spetra for
ar-bonlusterssaturatedwithhydrogenattheendbonds.
Two of them were randomly substituted by 6 and 26
nitrogenatoms, respetively. The latterluster is not
planar(seeRef.7). Theplotorresponding toC
96 has
beensaledbyafatorof10and suessiveplotshave
beenshiftedupwardforbettervisualization.
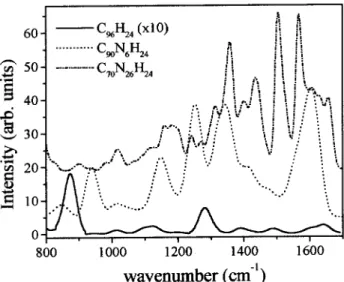
Figure4. SimulatedIRspetraoflargearbonlustersfrom
PM3alulations. Theurveswere translatedupwardfor
larity. C96H24 (ontinuous line, saledby 10),C96N6H24
These simulated spetra show the same features
seeninFig. 3,namely,weakirativityofthegraphene
lusterandthegrowingofstruturesintherange
1200-1600m 1
asnitrogenatomsareinorporatedintothe
aromatiframework. These features are assoiated to
olletiveC-CandC-Nvibrations. Theheavilydoped
luster C 96 N 26 H 24
is notgraphiti. In this disordered
systemthevibrationsareloalizedinsub-lusters,
giv-ing riseto amore intriate spetrum. The vibrations
above 1600 m 1
are due to the strething of C=C
bondsatthelusteredges.
Similar alulations were arried out to ev
alu-ated the isotopi eets of 15
N. We onsidered
sim-ple systems as the aromati moleules M2 and M3,
in whihsp 2
hybridized Natoms partiipatein
exten-sive bonds; sp and sp 2
N in H
3
C-CN and H
3 C-N=C(CH 3 ) 2
andthelargelusterC
96 N 26 H 24 , ontain-ing sp 3
N. The isotopi shifts of sp and sp 2
N
om-poundsare27m 1
and22m 1
,respetively. Inthe
aromati systems the frequeny shift is as small as 9
m 1
,beausethevibrationsinwhihNatoms
parti-ipateare olletive,thusminimizing theisotopeeet.
In the large luster ontainingsp 3
N several
frequen-ies are shifted by an amountranging from 2 to 15
m 1
, but onthe average theshift is below 10 m 1
.
Theonlusionisthat 15
Nisotopieetislearlyseen
whentheassoiatedvibrationisloalized. Forinstane,
-CNspeiesthatappearinamorphousarbonnitride
are expeted to at as end groups and the assoiated
vibrationsare loalized. In this asethe C-N
streth-ingvibrationshouldbeshiftedby30m 1
upon 15
N
substitution. Inolletivevibrations,theisotopi shift
ouldbemorediÆulttobeobservedsineothereets
mightproduesimilarmodiationsintheirspetrum.
III Experimental
Two types of samples were prepared: amorphous
arbon-nitrogen thin lms and graphite powder
on-tainingnitrogen. Thepowderwasproduedin similar
onditions of those used to obtain fullerene [15℄. In
general,fullerenes aredeposited in aonventional bell
jar where two high purity graphite eletrodes are
va-porized by an ar dishargebetweenthem. The base
pressure of the hamber was 10 4
Pa. In fullerene
prodution, thenuleation ispromoted byHe [16℄. In
orderto quenh thereation,asuitableatmosphereof
N
2
andHeat 100-200mbwasused. Theresidualsoot
onthe hamberwasolletedand analyzedex situ by
massspetrometry. Thea-CN
x
:Hlms were prepared
in an Ion Beam AssistedDeposition (IBAD) attahed
to an Ultra High Vauum hamber for Photoeletron
SpetrosopyAnalysis(PES).InIBADmethod,ahigh
puritygraphitetarget(99.99at. %)wassputteredbya
N ionsbeam. Simultaneouslyasuitablemixtureof N,
relativepartial pressures,energiesandurrentdensity
of the sputtering and assisting beams allow the
vari-ation of the deposition onditions. In the attempt to
eluidate the origin of the IR absorption bands,
sam-plesdepositedusingtheisotope 15 N 2 andregular 14 N 2
weregrownin nominally idential onditions. To
sep-arate the inuene ofhydrogen andnitrogen, wehave
studied alloyswithand withouthydrogenoveringthe
range below and above 20 at. % nitrogen ontent.
ThesamplesweredepositedonpolishedSiwafers(100)
and Corningglass a substratemaintainedat onstant
temperature. The base pressure of the hamber was
210 5
Pa. Immediatelyafter deposition thelms
were transferred to the UHV hamber (< 2:010 9
mbar)andmeasuredwithoutfurther treatment. Some
sampleswerepreparedexsitubyradiofrequeny
sput-tering of a high purity graphite target in aontrolled
gaseous mixture of Ar and N
2
[11,18℄. No important
dierenes were found between these lms and those
grown by IBAD. On the other hand, hydrogenated
samples show profound dierenes ompared to
non-hydrogenated samples. Asweshall see bellow,
hydro-genatedsamplesdrastiallyevolvewhenexposedtothe
atmosphere.
Theevolutionof the binding energies of orelevel
eletronsand the topof thevalene band onnitrogen
inorporationinthesampleswereanalyzedbyXPSand
UPS,respetively. FortheXPSanalysistheAlKline
wasused (h = 1486.6eV, width 0.85 eV). For UPS
theHeI(21.3eV)andHeII(40.8eV)linesfroma
reso-nantdishargelampwereused. Whenappropriate,the
inelasti bakgroundof the spetrawassubtrated by
standardsmethods[19℄. Thetotalspetrometer
resolu-tionwas 0.3eV.AftertheXPSandUPSexperiments,
the samples were further analyzed by infra-red (IR),
Raman,andvisible spetrosopy. Thethiknessofthe
samples was measured using a prolometer. The IR
and Raman spetra were obtained ex situ in a FTIR
NICOLET 850 spetrometer and in a Miro-Raman
(exitation lines: 520.8 nm and 488 nm from Krand
Ar lasers),respetively. Whenneessary,nulear
teh-niques were used to measure hydrogen onentrations
[20℄. Measurements of stress, ondutivity and
hard-nesswerealsoperformed.
IV Results
IV.1 Core levels
Fig. 5showstheexperimentalevolutionoftheN1s
orelevelspetraonNonentration. Theurvesshow
twowell-resolvedstruturesat398.2and400.5eV(from
nowonpeaks P
1 and P
2
) assoiatedwith N atoms in
two quite dierent loal ongurations [11℄. Peak P
1
beomes dominant in the material ontaining high N
strutureisfavored(urvea,Fig. 5). Theabinitio
sim-ulation shows that the N1s spetra of ongurations
ontaining N bonded to C sp 3
(i.e., group a, setion
II.2) and substitutional N sp 2
(i.e., group b, setion
II.2)areonsistentlynear tothepositions ofpeaksP
1
and P
2
, respetively. Therefore, these results suggest
thattheoordinationofNgoesfromaplanarstruture
to athree-dimensionalstruture.
Figura5. PhotoemissionspetraoftheN1sore-levelof
thea-CNx alloy.
IV.2. Valene-band results
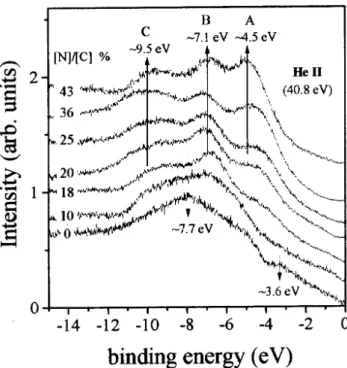
Fig. 6showsthe UPSspetraof the studied
sam-ples. The spetrum of pure a-C shows twobands
lo-atedatbindingenergies7.7eVand3.6eV,
respe-tively. These bands ome from and bondsdue to
C2peletrons. OninreasingN ontentthreenew
fea-turesemergeatenergiesnearto9.5eV,7.1eV,and
4-5 eV,labeled aspeaks C,B,and Ain Fig. 6. For
N/Cratioslargerthat20%,thebandsat7.7eVand3.6
eVpresentinpurearbondisappearompletely. Also,
the band at 7.1 eV dominates the spetrum for lms
withintermediateNontent(10%<N/C<25%). The
bands at9.5eVand 4-5eV arewiderandinrease
slowlyforlargerN/Cratios(>25%). Thesebands
dom-inate thespetraforthehighestnitrogenatedsamples.
Finally,theleadingedge oftheVBreedeson
inreas-Figura6. Valenebandphotoeletronspetraofa-CNx
samples.
A omparison of the experimental urveswith the
theoretial simulations suggests that peaks A, B and
C are due to N lone-pair eletrons, eletrons of
C-N bonds, and eletrons of C-N bonds, respetively.
Moreover,inordertoreproduethetotalDOSof
sam-pleswith N/C >20%ratio, it is neessaryto assume
apartialontribution ofDOS due toN lone-pair
ele-tronsasinthe-C
3 N
4
phase(seeFigs. 1and6). Below
N/C18-20%ratio,the lakofstrutureat 4-5eV
in theexperimental urvessuggeststhat N is
oupy-ingsites ingraphite-likestrutures. Conluding,below
the N/C 18-20% ratio, N is oupying a C site in
graphite-likestrutures. Above N/C 18-20% ratio,
the alloy tends to form a three-dimensional struture
withCandNatomsfourfoldandthreefoldoordinated,
respetively.
IV.3. Vibrational spetra
MostoftheinterpretationoftheRamanand
infra-red(IR)spetraofarbon-nitrogenalloys(a-CN
x )
re-liesonthepioneeringworkofKaufmanetal.[21℄. These
authorsreported nosignianthanges in the Raman
spetra for hydrogenated samples ontaining nitrogen
between 0 and 20 at. %. On the ontrary, the IR
spetra showprofound hanges asafuntion of
nitro-gen ontent. The onlusion of this work is that the
inreasingIRativitystemsfromthefatthatnitrogen
breaksthesymmetryofthearomatiand/oraetylene
groupsthatonstitutethematerial. Neverthelessthere
First,theinlusionofhydrogenprofoundlymodiesthe
struture of the alloy [17,20,22℄. Seond, above 20
at. %nitrogenontentthealloyundergoesastrutural
hange [7,11,23℄. Theformerresultis partiularly
im-portantsinein Ref. 21thehangesinthe IRspetra
areexlusivelyasribedtonitrogen. Inapreviouswork,
we haveshown that hydrogenmodies theIR spetra
in samplesontainingsimilaramountsofnitrogenand
dierenthydrogenontents. Inregardtothestrutural
hangesintroduedbynitrogen,theitedexperimental
ndingspreventtoextendaprioritheresultsof
Kauf-manetal. toalloyswithnitrogenonentrationsabove
20 at. %. Indeed, below this onentration,
nitro-gensubstitution ofarbonmaintainstheplanar
geom-etryofgraphite,produingastressedandhardmaterial
[23,24℄. Above20at. %nitrogenontentthegraphite
sheetsurlandabandofloalizednitrogenlonepairis
formed[7℄. Thematerialbeomeslessdenseand-CN
speiesarepromoted. Therefore,anystudyshouldtake
in onsiderationthesestruturaldierenes.
Figure7. Raman(a)andinfrared(b)spetraof
non-hydrogenated samples.
Fig. 7showsthenormalizedRaman(a)andIR(b)
spetraof the studied non-hydrogenated samples. As
observed, only minor dierenes are found in the
Ra-man spetra. Although less dramati than hydrogen
[24℄, nitrogen also inreases the I(G) / I(D) intensity
ratioof the IR spetra. Here, \G"identiesthe band
at 1570 m 1
(\graphiti" omponent) and \D" the
band at 1360 m 1
(\disordered" omponent) [25℄.
Oninreasingamountofnitrogenthesimilitudeofthe
IR and Raman spetra is evident. Wehave remarked
abovethattheinreasingIRativityisassoiatedwith
the symmetry breaking of aromati and/or aetylene
groupson nitrogen inorporation. This onlusion
re-m 1
region of theIR spetraby substitutional 15N2
in hydrogenatedsamplesontaininguptoat. 20at.
% nitrogen. Inthe attemptto separatetheroleof
hy-drogen in theresults,wehavestudiedthe inueneof
15
N
2
on the IR spetra of non-hydrogenated samples
ontaining25-26 at. % nitrogen. Thehoie ofthis
onentrationisduetothestruturalhangesourring
in thematerialabove20at. % nitrogenontent.
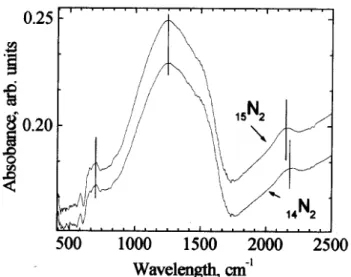
Fig. 8showstheirspetraof twosamples
ontain-ing, 14
N
2
(26.00.5)at. % and 15
N
2
(25.0 0.5)at.
%. Theband assoiatedto-CN(2150m 1
)shifts
!35m 1
,anamountompatiblewiththe
inreas-ing redued atomi mass and by assuming aonstant
osillatorstrength.
Figure 8. Infrared spetra of the a-C 15
Nx and a-C 14
Nx
sampleswith25-26at. %nitrogenontent. For thesake
oflarity,theurvesareshiftedbyaonstantamount.
Theregionaround1000-1600m 1
ismore
ompli-atedtoanalyzesineonlysmallhangesareobserved.
Thisis duetothefatthat theregionontainsalarge
ontributionofvibrationmodesstemmingfromthesp 2
arbon skeletal struture. In theattempt to observed
isotopieetswehavestudiedthedierenespetrum
of the samples (non-shown). The residualarea under
themainbandsoftheIRspetraistoosmallwhen
om-paredwiththeareaoftheoriginalbands. Furthermore,
wehavefoundthatthespetraoftwosamples
ontain-ing(26.00.5)at. %and(24.00.5)at. %respetively
of 14
N
2
an aountfor the residual areaobtained in
thedierenespetrum.
V Disussion
Theabovedataallowedustodrawaonsistentpiture
ofthestrutureofamorphousarbonnitridealloys
pre-paredbyIBAD.Additional informationobtainedfrom
Inthe theoretial study ofrandom a-C1-xNx
lus-ters, we alulated the enthalpy of formation (H
f )
of the alloyfor dierent nitrogen onentrations. Fig.
9 (bottom) shows the quantity [H
f (C
n x N
x )
H
f (C
n
)℄=x as a funtion of the N onentration.
Here, n is the total number of nitrogen and arbon
atoms. H
f
represents the average energy neessary
to inorporate onenitrogen atom either in the planar
(urvea)orintheorrugatedstrutures(urveb). The
optimizedgeometryofbothsetsoflustersisvery
sim-ilarforlownitrogenonentrations,i.e.,evenfull
relax-ationalulationresultsinaplanargeometry. Bukling
of thestruture developsonly above[N℄/[C+N℄20%.
Thesealulationsshowtwoimportanttrends: 1)Both
theoretialurvesshowabarrierfornitrogen
inorpora-tion at [N℄/[N+C℄20%; and 2) above this threshold,
the orrugated struture ollapses to a state of muh
smallerenergythantheplanarstruture.
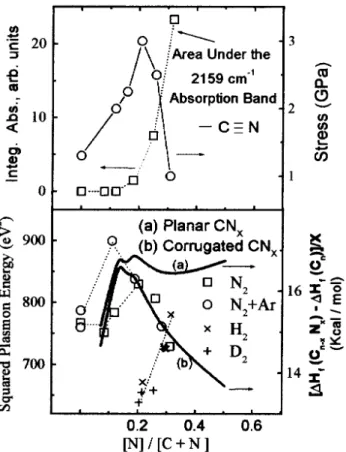
Figura9.Bottom: plasmonenergiesforthestudiedsamples
vs. N onentration. The theoretialurves(solid)
repre-sentthediereneintotalenthalpiesofformationbetween
Nsubstitutedandnon-substitutedClustersforplanar(a)
andorrugated (b)strutures. Top: integratedintensityof
the absorptionbandassoiated with-CN(yanogroups)
vs. N.Thegasesusedduringthedepositionare indiated.
Thedashedlineisaguidetotheeye.
Inorderto understand thisbehavior,wenotethat
substitutional N in graphene sheets ontributes two
eletrons to the graphite ondution band, whih has
an anti-bonding harater. Therefore, the inrease in
mentedeletronienergy,distortingthesystemloally.
Due to this, the hybridization of arbon and
nitro-gen hange the harater from sp2 to a sp3,
loaliz-ing eletrons in nitrogen lone-pairs. This is
onsis-tent with the fat that above 20% [N℄/[C℄ a band
assoiated with loalize lone-pair eletrons emerge at
thetopof thevaleneband [11℄. Theplasmon energy
(h!
m )
2
assoiated to theC1s eletronis ameasure of
thedensityofthematerial[26℄. InFig. 9(bottom)we
haveplotted (h!
m )
2
obtainedfrom XPSspetra. [23℄.
Theplasmonenergyofthehydrogenfreesamplesgoes
througha maximum around [N℄/[C+N℄20%. Above
[N℄/[C+N℄20%theexperimentaldatareasonably
fol-lowthe \orrugated"theoretialenthalpyurve. This
resultsuggestsaspontaneousbuklingofthestruture,
probablywiththereationofdanglingbondsandvoids.
Therefore, a dereasing density of the material is
ex-peted. Results from IR spetrosopyand stress
sup-port theseonlusions.
Theabrupthangeintheplasmonenergyisalso
a-ompaniedbytheemergeneofa-CequivNabsorption
bandin thetransmissionIR spetra(not shown). The
absorptionharateristiofthe-CNstrethingmode
(2159m 1
)isinipientat[N℄/[N+C℄20%
indiat-ingthatonlyabovethisonentrationyanogroupsare
formed(Fig. 9, top). Thisis probablyinduedbythe
existeneofdanglingbondsandvoids,aordingtothe
theoretialsimulation. Thestressofthelmsasa
fun-tionofthenitrogenontentshowsthesametrendthat
theplasmonenergy(Fig. 9,top). Forlownitrogen
on-entration, the inreasing stress is onsistent with an
inreasingdensityofthematerialonnitrogen
substitu-tionofarbon. Above[N℄/[N+C℄20%,thedereasing
stressisaresultofthebuklingofthestruture,i.e.,a
dereasingdensityofthematerial.
The plasmon energy of hydrogenated and
deuter-ated samples inreases monotonially in the range of
studiedompositions (rossand plus,Fig. 9,bottom).
This result suggests that hydrogen promotes the
for-mationof asoft,polymeri stress-freematerial witha
quite small relative density. Furthermore, deuterated
samplesshowtheexisteneofprimaryamines. Finally,
whenexposedtoair,thematerialinorporates
hydrox-ylsbyextensivehydrogen-bondformation[20℄.
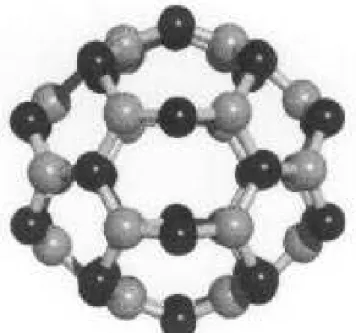
Thebuklingof theotherwiseplanararbonsheets
upon nitrogen inorporation has another interesting
onsequene, whih is the growing of losed
mole-ular strutures. These inlude heterofullerenes and
nitrogen-dopednanotubesaswellasothermoleulesyet
tobeharaterized. Wewereabletobuildandoptimize
amoleularage with omposition C
24 N
32
[7℄,a
om-pound belonging to the to the C
3 N
4
family, skethed
in Fig. 10. This moleulehas C
4h
symmetry and its
buildingbloksareeight-memberedonnetedrings
re-semblingthestrutureof-C
3 N
4
. Thenitrogenatoms
Thebondsaroundaonnetivity3nitrogenare1.47
A
to 1.48
A in lengthand arenotall in thesameplane.
Bond angles vary from 117 Æ
to 119 Æ
. Connetivity 2
atoms,ontheotherhand,showtypialaromatibonds
of 1.36
A and bond angle of 120 Æ
. All arbons are in
equivalent sites,withall thebonds in thesameplane.
Computeralulationsandexperimentalresultssuggest
that other strutures with dierent ompositions are
also possible. Preliminary results on soot prodution
in anitrogenatmosphereanalyzedthroughmass
spe-trosopymeasurementsindiatethepreseneofspeies
having mass/harge ratio (m/z) of 368, whih is half
themassofC
24 N
32
. Assumingsingleionization(z=1),
this peak at m/z =368 orrespond to a hypothetial
moleuleofmassequivalentto4x(C
3 N
4
). Ofourse,
as-sumingdouble ionization(z=2) this massorresponds
exatly to the mass of the ball displayed in Fig. 10.
Furthermore, a set of speies appear grouped around
the ratio m/z=523 (\shrink-wrapping"). Starting in
m/z=481andupto537,thepeaksaresequentially
ob-tainedby addingthe massofonenitrogen atom. The
sequenestartinginm/z=552isobtainedaddingtothe
537speie themasses of anitrogen and aproton,the
latestoneprobablystemmingfromresidualwaterinthe
deposition hamber. Higherfullerenes are notpresent
in the mass spetra suggesting that nitrogen
substi-tution energetially favors smaller fullerenes.
Experi-ments are urrently being performed to produe and
furtherharaterizetheseheterofullerenes.
Figura 10. Ball-and-stik model of the moleular age
(C
24 N
32
)=8x(C
3 N
4
). C(gray)andN(blak).
VI Conlusions
In summary, the eletroni struture and vibrational
spetra of CN lms were experimentally determined
byphotoemission, Ramanand IRspetrosopies. The
spetrawereomparedandinterpretedwithresults
the-oretially obtained on model moleules ontaining C
and N atoms. The inorporationof N up to 20 at.
% produes hard, dense and stressed material having
a planarstruture. Above this onentration, the
ex-tra eletronsintrodued by N destabilize thematerial
and the struture urls and bends. A band the
lo-alized states arises at the top of the valene band,
as in -Si
3 N
4
. The introdution of hydrogen
pro-duesahygrosopi,soft,andlessdensematerialwith
polymeriharateristis. Numerialsimulationsshow
that several losed moleular strutures are possible
in hydrogen-freealloys ontainingmorethan20 % at.
N. In partiular, alosed ballhavingthe 8x(C
3 N
4 ) =
C
24 N
32
stoihiometry was theoretially predited [7℄.
In order to test this possibility, the soot obtained
va-porizingarboninaardishargewasstudiedbymass
spetrometry. Themassspetraindiate,amongother
things, the preseneof speies havingamass
ompat-ible with the 4xC
3 N
4
stoihiometry (i.e., half of the
atomi mass obtained by the numerial results). Of
ourse, other ombinations giving the samemass and
dierent stoihimetry arealso possibleand morework
isneessarytolearlyidentifythespeiespresentinthe
soot. Finally,attemptstoonentratethehypothetial
4xC
3 N
4
phaseareunder the waywith thegoal to use
it aspreursor forsynthesizing thebulk rystalline
-C
3 N
4 phase.
Aknowledgements
Theauthorsareindebtedtoallthemembersofthe
Photovoltaigroupand theLaboratoryof Alternative
Fuels, Uniamp, and to the Mass Spetrosopy
Labo-ratory,Uniamp,forthemassspetra. This work was
partiallysponsoredbyFapesp. TheauthorsareCNPq
fellows.
Referenes
[1℄ ForareentreviewseeE.G.Wang,Prog.Mat.Si.41,
241(1997).
[2℄ A.LiuandL.Cohen,Siene245,841(1989).
[3℄ D. Marton, K. J. Boyd and J. W.Rabalais, Int.J. of
ModernPhys.B,9,3527(1995),andreferenestherein.
[4℄ P.Hammer,N.M.Vitoria,andF.Alvarez,submitted,
J.Va.Tehnol.A(1999).
[5℄ H.Sjostrom, S.Stasfsrom,M. Boman,andJ.-E.
Sund-gren,Phys.Rev.B,75,1336 (1995).
[6℄ N.Hellgren,M.P.Johansson,E.Broitman,L.Hultman,
andJ.E.Sundgren,Phys.Rev.B,59,5162(1999).
[7℄ M. C. dos Santos and F. Alvarez, Phys. Rev. B, 58,
13918(1998).
[8℄ K.Suenagaetal.,Chem.Phys.Lett.300,695(1999).
[10℄ M.J.S. Dewar, E.G.Zoebish, E.F.Healy, J.J.P.
Stew-art,J.Am.Chem.So.107,3902(1985).
[11℄ S.Souto,M.Pikholz,M.C.dosSantosandF.Alvarez,
Phys.Rev.B,57,2536(1998).
[12℄ For basis set denitions in Hartree-Fok alulations
see, for instane, I.N. Levine in Quantum Chemistry,
3 rd
Edition,AllynandBaon,1986.
[13℄ Y.GuoandW.A.GoddardIII,Chem.Phys.Lett.237,
72(1995).
[14℄ R. Lazzaroni,N. Sato,W.R.Salanek,M.C. dos
San-tos,J.L.Bredas,B.Tooze,andD.T.Clark,Chem.Phys.
Lett.175,175(1990),andreferenestherein.
[15℄ F.AlvarezandM.C.dosSantos,J.Non-Cryst.Solids,
inpress.
[16℄ See,forinstane,D.H.Parker,K.Chaterjjee,P.Wurz,
K. R. Likke, M. J. Pellin, and L. M. Stok, in The
Fullerene, Edited by: H. W. Kroto, J. E. Fisher, and
D.E.Cox,PergamonPress,Oxford,England,1993.
[17℄ P. Hammer, N. M. Vitoria, and F. Alvarez, J.Va.
Tehnol.A16,2941(1998).
[18℄ S.Souto and F. Alvarez, Appl. Phys. Lett. 70, 1539
(1997).
[19℄ D.A.Shirley,Phys.Rev.B5,4709 (1972).
[20℄ F.Alvarez, N. M. Vitoria, P. Hammer, F.L. Freire
Jr., andM. C. dosSantos, Appl. Phys.Lett. 73,1065
(1998).
[21℄ J.H.Kaufman,S.Metin, and D.D. Saperstein, Phys.
Rev.B39,13053 (1989).
[22℄ S.Souto and F. Alvarez, Appl. Phys. Lett. 70, 1539
(1997).
[23℄ F.Alvarez, M.C. dos Santos, and P.Hammer, Appl.
Phys.Lett.73,3521(1998).
[24℄ N.M. Vitoria, P.Hammer, M.C.dosSantos, and F.
Alvarez,Phys.Rev.B61,inpress.
[25℄ J.Robertson,Prog.SolidSt.Chem.21,199(1991)
[26℄ See,forinstane,L.LeyinTopis inAppliedPhysis,
Vol56: ThephysisofHydrogenatedAmorphousSilion
II.Editor: J.D.JonapoulosandG.Luovsky,