Dinâmica do fósforo em solos derivados de materiais vulcânicos
– Versão Definitiva –
Dissertação de Mestrado em Engenharia Agronómica
Rui Nelson Bajouco da Silva Lopes
Orientador: Professor Doutor João Filipe Coutinho Mendes
The present results were partially used for the following communications:
Bajouco, R., Fraga, I. and Coutinho, J., 2015. Vertical distribution of acid phosphatases activity in Andosols. I Jornadas de Engenharia Agronómica, 21 de Outubro, p.26, UTAD, Vila Real, Portugal. Bajouco, R., Fraga, I., Pinheiro, J. and Coutinho, J., 2016. Effects of Al and Fe on the β-glucosidase potential activity in soils derived from volcanic material. VII Congresso Ibérico das Ciências do Solo e VI Congresso Nacional de Rega e Drenagem, 13-15 de Setembro, p.131-134, IPBeja, Beja, Portugal.
Bajouco, R., Fraga, I., Pinheiro, J. and Coutinho, J., 2016. Phosphorus acquisition effort in Andosols of Pico Island. X Jornadas de Biologia, 12-13 de Outubro, p.28, UTAD, Vila Real, Portugal.
“Have the courage to follow your heart and intuition. They somehow already know what you truly want to become. Everything else is secondary”.
xi
A
CKNOWLEDGMENTSGratitude and appreciation for the help and support are extended to the following persons that have contributed for making this study possible.
Professor João Coutinho, for the excellent guidance, caring, patience, support and friendship. For allowing me the opportunity to learn and work with him, providing me the atmosphere for this research. For his time and tolerance on this journey, teaching me every day how to dream and reach for more. Thanks so much for the knowledge transmitted, it wouldn’t be possible without the trust that he has placed in me.
Professor Jorge Pinheiro, for the excellent guidance, time spent, patience and support. For allowing me the opportunity to learn from him, thanks for the knowledge transmitted along this journey.
My collegue Irene Fraga, thanks so much for the knowledge shared along this journey. It wouldn’t be possible without her patience, support and friendship.
Agronomists Benilde Pereira and Rita Ferreira for the support collecting the soil samples.
Soil and Plants Laboratory technicians for the patience, support and friendship. For allowing me the opportunity to learn and work with them, providing me the atmosphere for this research, who provided me self-confidence with the lab work and for all the advices and precious words.
My sincere thanks to my friends for the affection and support, and for never stop to cheer me up with a good advice and for believing in me, even when I did not.
Finally, my parents, Mileta Bajouco and Rui Lopes, and my sister Liliana Lopes, that always have been there for me. For dreaming my dreams and for supporting my decisions, and for making this step of my life possible with all support that I needed. I’m proud to say that I am the reflection of the values, love, affection and guidance that they generously gave me.
xiii
R
ESUMO ALARGADOOs solos derivados de materiais vulcânicos, representando 0,84% da área de solos no mundo, são caracterizados por acumularem grandes quantidades de matéria orgânica (MO), cerca de 5,0 % de carbono global, e pelo elevado teor de fósforo (P) retido pelos abundantes compostos de alumínio (Al) e ferro (Fe), existentes nestes solos. A retenção de P referida é a principal limitação à absorção de P pelas plantas e ao rendimento das culturas, devido ao baixo teor biodisponível de P na solução do solo. Assim, torna-se necessário o recurso à adubação fosfatada, por forma a suprir as necessidades das culturas. Na ilha do Pico, arquipélago dos Açores, cerca de 40 % dos solos agrícolas são prados de pastagem, sendo praticada, portanto, a adubação P sem incorporação no solo, incluindo os solos de encostas mais declivosas.
O trabalho agora apresentado, articulado em dois estudos e dedicado a solos derivados de materiais vulcânicos e recolhidos na ilha do Pico, teve os seguintes objetivos: (i) a avaliação do risco potencial de perdas de P para águas, (ii) o relacionamento das propriedades de Andossolos, nomeadamente os teores de Al e Fe extraíveis por oxalato de amónio (Alox e Feox) e das frações de P, com a atividade das exoenzimas
fosfatase ácida (AP) e β-glucosidase (βG), bem como (iii) a avaliação do esforço microbiano na aquisição de P.
A adubação mineral e a deposição de urinas e fezes dos animais em pastoreio são os responsáveis pela acumulação de P nas camadas superficiais dos solos, tendo-se observado, neste estudo, um teor médio de 3,7 g Pt kg-1, valor que pode ser considerado muito elevado e que sugere situações de sobrefertilização.
Simultaneamente, a eutrofização de águas de superfície, devido a poluição difusa, é um problema real no arquipélago dos Açores, sendo os solos agrícolas a sua causa mais óbvia. Por outro lado, os compostos de Al e Fe, responsáveis pela intensa retenção de P, parecem estar também envolvidos na elevada acumulação de matéria orgânica nas camadas superficiais destes solos, reprimindo a atividade enzimática e microbiana, e/ou protegendo a matéria orgânica da mineralização.
Os Andossolos Háplicos e Plácicos apresentaram teores de carbono orgânico (OC), Alox e Feox mais
elevados que os Andossolos Vítricos. Em oposição ao OC e às diferentes frações de P, os teores de Alox e
Feox aumentam com a profundidade dos solos. O P orgânico (Po) e inorgânico (Pi) representam 54 e 46 %
do P total (Pt), observando-se um maior contributo de Po nos Andossolos Háplicos e Plácicos e de Pi nos
Andossolos Vítricos.
Os teores de Pi-Olsen, P-AL e P-CaCl2 encontram-se acima dos valores críticos agronómicos
referidos na literatura. Contudo, o índice de saturação de fósforo (ISP) nos solos estudados são inferiores a 25 %, valor acima do qual os solos se encontram em risco de desadsorção de P. Em média, o grupo de três Andossolos Vítricos apresentaram valores de ISP mais elevados (14,1 %) que os restantes três Andossolos Háplicos e Plácicos (6,6 %). Em condições laboratoriais que pretendem antever os riscos de P relativos à drenagem interna de solos (1: 100), escorrimento superficial (1: 1000) e sedimentos sob massas de água
xiv
estáticas (1: 10000), usando o 0,01 M CaCl2 como eletrólito, observa-se que os teores de P desadsorvido se
encontravam abaixo dos limites críticos ambientais, respetivamente de 0,1, 0,05 e 0,02-0,03 mg P L-1.
Deste modo, a eutrofização das massas de água, não deverá ter como fonte difusa o P desadsorvido dos Andossolos estudados. Uma vez que os adubos fosfatados são aplicados à superfície dos solos, sem incorporação, deve ser considerada a hipótese do direto escorrimento superficial dos mesmos durante episódios de precipitação, antes mesmo de ocorrer a completa solubilização do P aplicado e sua e interação com a matriz do solo. Assim, a aplicação localizada de fertilizantes, em profundidade com menor frequência, deverá ser considerada como uma alternativa à interditação do uso de adubos, mesmo nas áreas mais sensíveis e montanhosas das bacias hidrográficas das ilhas do Arquipélago dos Açores.
Considerando a influência do material originário dos solos e o efeito da sua gestão no teor e distribuição vertical da MO e da atividade enzimática, verificou-se a acumulação de OC e de diferentes formas de Po nas camadas mais superficiais. Em simultâneo, ambos os parâmetros apresentam-se altamente
correlacionados com os teores de Alox e Feox, em particular com Feox, e com o gradiente em profundidade
da AP e βG. Ao contrário do referido na literatura, o elevado teor de Pi nos solos, resultado da fertilização
mineral fosfatada, não parece promover, só por si, a inibição da atividade da AP. Considerando as 30 amostras de solo estudadas, observaram-se correlações significativas e diretamente proporcionais entre a AP e as diferentes formas de Pi, nomeadamente Pi total, Pi-Olsen e P-CaCl2.
Observaram-se elevadas razões AP: βG, quando se avaliou a relação estequiométrica entre ambas as exoenzimas. Estes resultados indicam que a biodisponibilidade real de P é mais reduzida do que a disponibilidade sugerida pelos métodos de avaliação laboratorial que utilizam soluções químicas extrativas. Se na matriz do solo, o teor de Pi existir em quantidade suficiente para suprimir as necessidades microbianas
para a manutenção da homeostasia e crescimento, a alocação energética para a produção de AP deverá diminuir. Contudo, devido à elevada retenção de Pi nos constituintes de Al e Fe, deverá ocorrer uma alocação
energética suplementar para a produção da AP, no sentido da mineralização de Po, por forma a assegurarem
a aquisição de P para a manutenção da homeostasia. No caso dos solos com elevados teores de Alox e Feox,
os Andossolos Háplicos e Plácicos, a razão AP: βG variou entre 10,7 e 14,7, em oposição, os Andossolos Vítricos, com menores teores de Alox e Feox, que apresentaram uma razão AP: βG média de 5,6.
Relativamente ao potencial de aquisição C:P (ln(βG)/ln(AP)), os Andossolos Háplicos e Plácicos apresentaram um valor médio de 0.78 e os Andossolos Vítricos 0.85. Sendo o valor de referência global 0.95, os potenciais de aquisição C:P determinados sugerem, mais uma vez, a reduzida biodisponibilidade de P nos solos estudados. Em ambos os casos, observa-se que o esforço para a aquisição de P e o potencial de aquisição C:P são afetados pela dimensão das diferentes frações de Pi e teores de Al e Fe presentes no
solo.
Por outro lado, o valor da razão molar C:P média dos solos estudados é de 115. Sendo 166 o valor de referência para solos de pastagem, valor acima do qual a literatura indica que se deverão verificar situações de carência de P, os resultados obtidos parecem indicar que este valor crítico deverá ser ajustado no caso dos Andossolos.
xv
Por último, os resultados obtidos não nos permitem avaliar, de um modo inequívoco, a razão sobre os elevados teores de OC nos solos derivados de materiais vulcânicos. Contudo, fica evidente o papel central e determinante dos minerais ativos de Al e Fe nessa acumulação, em virtude: (i) da capacidade dos minerais ativos de Al e Fe em se ligarem e protegerem as moléculas orgânicas da mineralização; (ii) da reduzida atividade da βG, atividade que parece ser reprimida pelos compostos de Al e Fe, afetando a taxa de mineralização de OC; e (iii) da alocação energética para a aquisição de P devido à retenção de Pi nestes
solos, alterando as reações metabólicas celulares e limitando o crescimento do compartimento da biomassa microbiana do solo, responsável pela mineralização da matéria orgânica.
Palavras chave: Eutrofização; Índice de saturação de fósforo; Andossolos, Matéria orgânica; Fósforo
xvii
E
XTENDED ABSTRACTThe disproportionate amount of organic matter, nearly 5,0 % of global carbon, and the high Phosphorus (P) content sorbed by aluminium (Al) and iron (Fe) soil components are characteristic of soils derived from volcanic materials. Simultaneously, the reported P retention it is a main limitation for crop P uptake and production yield, due to its limited bioavailability. To overcome this major constraint, P fertilization is needed to supply production yield needs. In Pico island nearly 40 % of the agricultural land is used for grassland pastures and superficial P fertilization without incorporation is the main practice, even in situations with high slopes.
Working with soils derived from volcanic materials collected in Pico Island, Azores archipelago, this combined study aimed to assess the potential risk of P loss into receiving waters, and relate soil andic properties and P characteristics to the extra cellular enzyme acid phosphatase (AP) and β-glucosidase (βG) activities.
Fertilization is responsible for P accumulation at the soil surface, with values that can be classified as too high, such as the average value of 3.7 g Pt kg-1 observed in the present study, which suggest situations of P over-fertilization. Simultaneously, eutrophication of waterbodies is an actual problem in the Azorean archipelago and soils are the obvious source of this non-point pollution. On the other hand, the Al and Fe soil compounds seem to be involved in the high organic matter content retained in the superficial layers of these soils, repressing microbial and enzyme activity and/or protecting organic matter from mineralization.
Haplic and Placic Andosols presented higher organic carbon (OC), Alox and Feox (ammonium oxalate extractable) contents than the Vitric Andosols, and Alox and Feox values show an increase along the soils depth, opposite to the different P pools. The organic (Po) andinorganic (Pi) P contents represented nearly 54 and 46 % of total P (Pt), respectively. Haplic and Placic
Andosols had higher Po contribution, and Vitric Andosols a higher contribution of Pi.
Despite Pi-Olsen, P-AL and P-CaCl2 contents were above the agronomical threshold values, none of the studied soils had DPS values above the conservative critical value of 25 %. On average,
Vitric Andosols presented higher values (14.1 %) than Haplic and Placic Andosols (6.6 %). When
assessing the desorbed P with 0.01M CaCl2 as the electrolyte solution for mimicking conditions for P in drainage (1: 100), runoff (1: 1000) and static waterbodies (1: 10000), the desorbed P contents in all the soils were below the environmental critical limits, respectively 0.1, 0.05 and 0.02-3 mg P L-1.
The results indicate that soil Pi is still below the environmental thresholds, although above the agronomical threshold. Therefore, P desorption from the studied Andosols do not seem to be
xviii the main source for waterbodies eutrophication. Since inorganic fertilizers are superficially applied, it must be considered the hypothesis of their direct runoff during rainfall events, before complete dissolution and P interaction with soil matrix. Therefore, the fertilizer application with deep-banding systems in longer periods of time needs to be considered as an alternative to the interdiction of the use of fertilizers on the most sensitive and hilly areas of the watersheds.
Regarding the effect of the management on the size and vertical distribution of organic molecules and enzyme activity, it was observed a superficial accumulation of organic carbon (OC) and Po pool, which was highly correlated with Alox and Feox contents, particularly with Feox, and with the stratified gradient of the evaluated enzymes activities. Soil P richness, as the result of inorganic P fertilization, did not show an inhibition effect on AP activity. In fact, when considering all the 30 soil samples under study, AP activity showed a linear and significant relationships with the different Pi pools, namely with total Pi, Pi-Olsen and P-CaCl2.
When assessing the stoichiometric relationship between both enzymes, high values for the AP: βG ratio were obtained. These values suggest that microbial communities allocate high energy effort to ensure P acquisition for homeostasis maintenance. In Haplic and Placic Andosols, with higher Alox and Feox contents, the AP: βG ranged from 10.7 to 14.7, contrasting with the average value of 5.6 presented by the Vitric Andosols. Concerning the potential C:P acquisition (ln(βG): ln(AP)), Haplic and Placic Andosols showed an average of 0.78 and Vitric Andosols an average of 0.85, values which are below the global threshold limit of 0.95, above which soils are supposed to have an adequate P availability.
The effort for P acquisition and the potential of C:P acquisition indices are both affected by the size of the Pi pools and by Alox + Feox contents. If available Pi was present at levels able to supply growth requirements and ensure homeostasis condition, the energy allocation to produce AP enzymes would decrease. Since Pi retention is high in Andosols, due to high Al and Fe minerals content, energy allocation shifts to the production of AP enzymes to mineralize soil Po. Moreover, the average C:P molar ratio of the six soils (115) is below the threshold value proposed for pasture soils (166), above which soils may present low P availability, which means that threshold value needs to be adjusted for Andosols.
Despite the central and determinant role of the active Al and Fe, the present results do not allow a clear justification for the high OC contents on the soils derived from volcanic materials, since Al and Fe compounds are simultaneously related with different soil parameters. Therefore, some hypothesis may be considered for that accumulation: (i) the ability of Al and Fe compounds to bound and protect organic molecules from mineralization; (ii) the low βG activity, which seems to be repressed by Al and Fe compounds, affecting the rate of the OC mineralization; (iii) the high demand of energy for P acquisition due to the intense Pi retention on the Al and Fe compounds,
xix which shifts the allocation of cell resources in detriment of microbe growth, limiting the size of the soil microbial biomass compartment.
Key words: Eutrophication; Degree of Phosphorus Saturation; Andosols, Soil Organic Matter; Organic
Phosphorus; Acid phosphatase; β-Glucosidase; P acquisition effort.
xxi
T
ABLE OF CONTENTSACKNOWLEDGMENTS xi
RESUMO ALARGADO xiii
EXTENDED ABSTRACT xvii
TABLE OF CONTENTS xxi
LIST OF FIGURES xxv
LIST OF TABLES xxvii
ABBREVIATIONS xxix Chapter 1 - Introduction 1 1 - Introduction 2 1.1 - Phosphorus (P) 2 1.2 - Soil P Cycle 3 1.2.1 - Soil P forms 5
1.2.1.1 - Inorganic P and chemical/physical processes 5
Primary P minerals 5
Secondary P minerals 6
Sorbed P 6
Pi in soil solution 7
1.2.1.2 - Organic P and biological processes 7
Organic P forms 8
Phosphorus immobilization/mineralization 9
1.3 - Enzymes 10
1.3.1 - β-Glucosidase 11
1.3.2 - Phosphatases 11
xxii
1.5 - Phosphorus Inputs/Outputs 14
1.6 - Phosphorus environmental impacts 16
1.7 - Volcanic ash soils 17
1.8 - The Azores archipelago, the Pico island case 18
Objectives 19
1.9 - References 20
Chapter 2 - Vertical distribution and loss risks of phosphorus in soils derived from volcanic
materials and altered by fertilized pastures. 27
2.1 - Abstract 28
2.2 - Introduction 29
2.3 - Materials and methods 33
2.3.1 - Soil classification and land management 33
2.3.2 - Soil Sampling 33
2.3.3 - Soil Analysis 34
2.3.4 - P desorption 35
2.3.5 - Statistical analysis 35
2.4 Results and Discussion 36
2.4.1 - Soil P status 36
2.4.2 - Extractable Alox and Feox 42
2.4.3 - Risk assessment indices 43
2.4.4 - Desorption risk assessment 46
2.5 - Conclusions 49
2.6 - References 50
Chapter 3 - Acid phosphatase and β-glucosidase activities in fertilized pastures over soils derived
xxiii
3.1 - Abstract 55
3.2 - Introduction 56
3.3 - Materials and methods 60
3.3.1 - Soil classification and land management 60
3.3.2 - Soil Sampling 60
3.3.3 - Soil Analysis 60
3.3.4 - P characterization 60
3.3.5 - Enzymatic activity 61
3.3.6 - Statistical analysis 61
3.4 - Results and Discussion 62
3.4.1 - Soil properties 62
3.4.2 - Soil enzyme activities 66
3.4.3 - P acquisition effort 72
3.5 - Conclusions 77
3.6 - References 79
Chapter 4 - General Conclusions 84
xxv
L
IST OF FIGURESChapter 1 - Introduction
Figure 1.1 - Soil phosphorus cycle synthesis. Major chemical and biological processes involved in P
bioavailability in soil ecosystem and pathways of P loss into superficial freshwaters and subterranean
waters, adapted from Pierzynski et al. (2005). 4
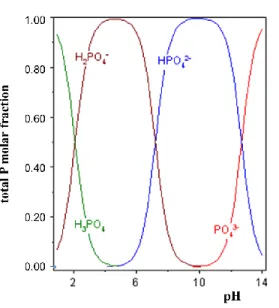
Figure 1.2 - Soil solution pH effect on the phosphate ions proportions (adapted from Hinsinger, 2001).7
Figure 1.3 - Soil organic P dynamic (adapted from Condron et al., 2005). 9
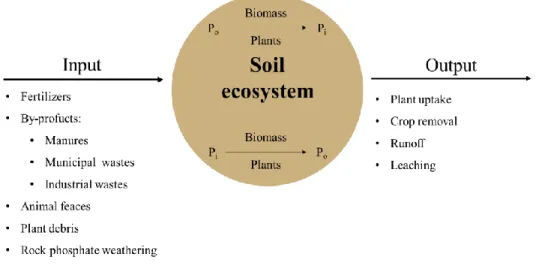
Figure 1.4 - Scheme synthetizing the soil P Input and Outputs. 14
Chapter 2 - Vertical distribution and loss risks of phosphorus in soils derived from volcanic materials and altered by fertilized pastures.
Figure 2.1 - Profile stratification of Pi-Olsen (mg P kg-1) in the top 15 cm layer of the six soils studied;
weighted average in solid line and agronomical threshold level in dashed line. 38
Figure 2.2 - Profile stratification of P-AL (mg P kg-1) in the top 15 cm layer of the six soils studied;
weighted average in solid line and agronomical threshold level in dashed line. 39
Figure 2.3 - Relationship between soil solution soluble P and extractable P: (a) Pi-Olsen versus P-AL
(p < 0.001, n = 30); (b) P-CaCl2 versus Pi-Olsen (p < 0.001, n = 20); (c) P-CaCl2 versus P-AL (p <
0.001, n = 20). 41
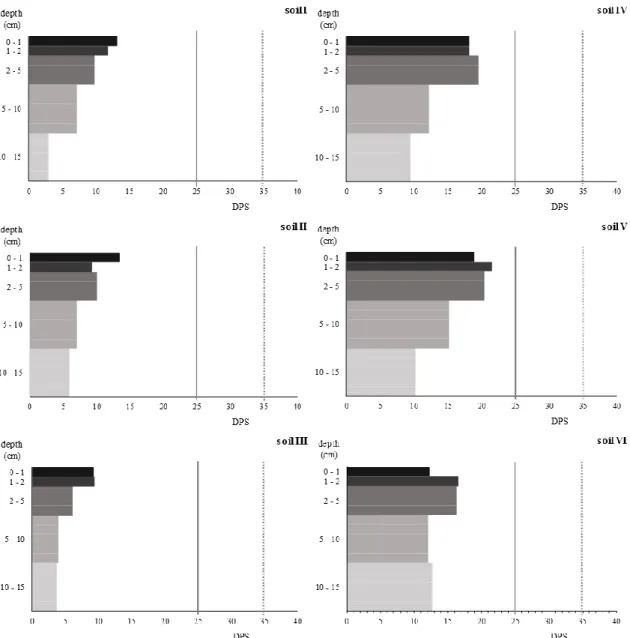
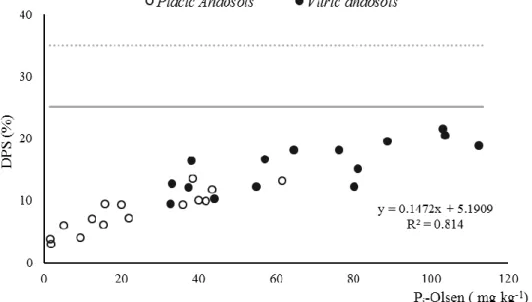
Figure 2.4 - Degree of Phosphorus saturation (DPS) profile stratification of the top 15 cm layer of the
six soils studied; solid line 25 % conservative critical value (van der Zee et al., 1988) and in dashed line the 35 % critical value for acid soils used by De Bolle et al. (2013). 44
Figure 2.5 - Correlation between Pi-Olsen versus Degree of P Saturation (DPS), in solid line the critical
DPS limit of 25 % (van der Zee et al., 1988) and in dashed line 35 % used by De Bolle et al. (2013). 45
Chapter 3 - Acid phosphatase and β-glucosidase activities in fertilized pastures over soils derived from volcanic material: distribution profile and P acquisition effort
Figure 3.1 - Soil profile distribution of a) organic carbon (OC) and b) organic Phosphorus (Po)
contents. 63
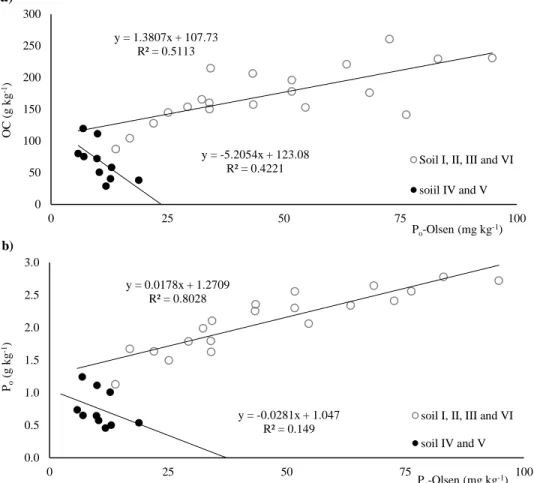
Figure 3.2 - Linear regression, with soil IV and V discrimination, of a) organic carbon (OC) versus
Olsen extractable organic phosphorus (Po-Olsen), b) organic phosphorus (Po) versus extractable
xxvi Figure 3.3 - Studied soils (a) β-glucosidase (βG) and (b) acid phosphatase (AP) activities, in the 15 cm
stratified assessed profile. 68
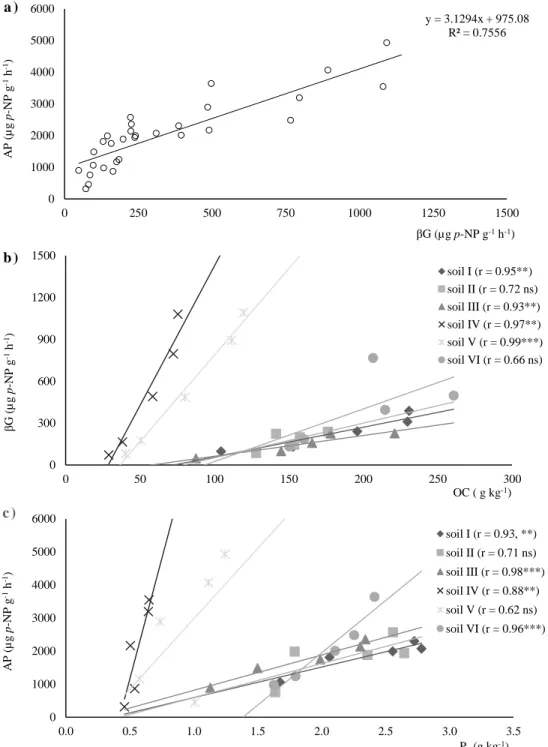
Figure 3.4 - Linear regression representation of: (a) acid phosphatase (AP) versus β-glucosidase (βG);
(b) β-glucosidase (βG) activity versus organic carbon (OC); and (c) acid phosphatase (AP) versus
organic phosphorus (Po) of the six studied soils. 70
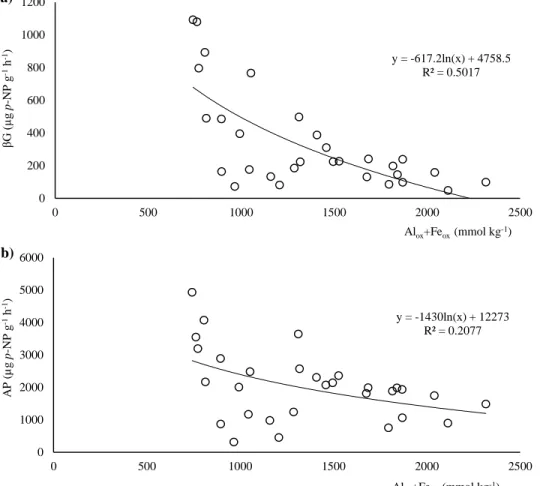
Figure 3.5 - Logarithmical regression of the enzymatic activities versus Alox and Feox content: a)
β-glucosidase (βG) versus Alox + Feox; and b) acid phosphatase (AP) versus Alox + Feox. 71
Figure 3.6 - Linear regression of acid phosphatase (AP) versus inorganic P (Pi) for all samples (n = 30)
and for the weighted average of each soil (n = 6). 72
Figure 3.7 - Logaritnmical (ln) regression of acid phosphatase (AP) to β-glucosidase (βG) ratio (P
acquisition effort) against: a) inorganic P (Pi); b) extractable inorganic P (Pi-Olsen); c) soil solution
inorganic P (P-CaCl2); and d) linear regression of acid phosphatase (AP) to β-glucosidase (βG) ratio
versus Alox+Feox content. 75
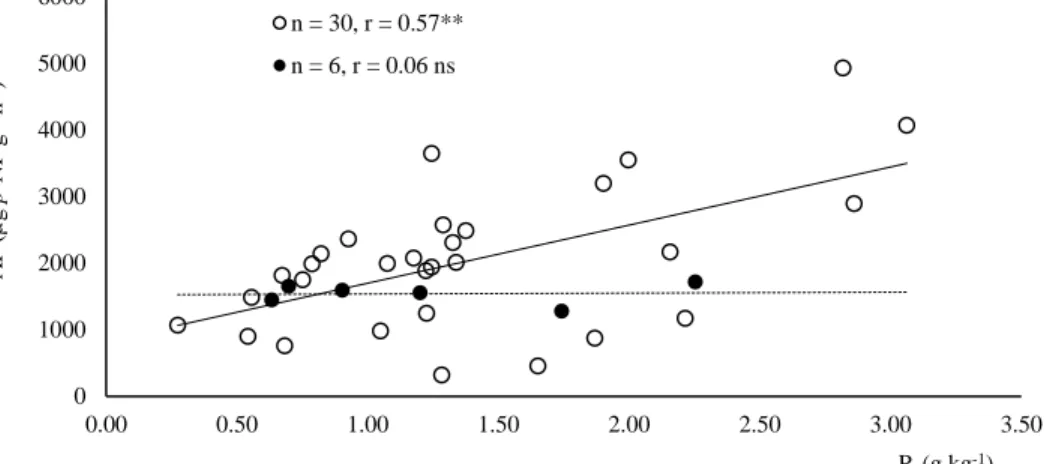
Figure 3.8 - Linear regression of potential of C:P acquisition (ln(βG):ln(AP)) versus Alox + Feox
xxvii
L
IST OF TABLESChapter 2 - Vertical distribution and loss risks of phosphorus in soils derived from volcanic materials and altered by fertilized pastures.
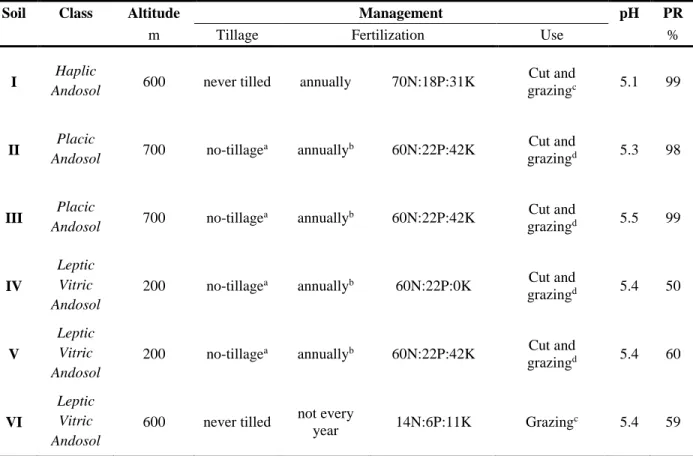
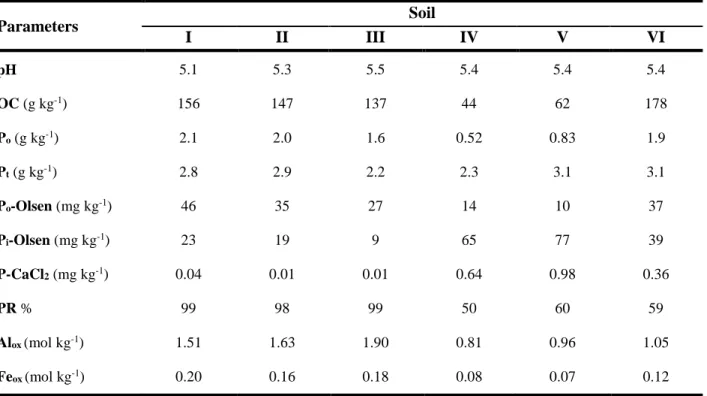
Table 2.1 - Soils Class (WRB, 2006), management practices, pH and Phosphorus Retention index (PR)
of the soils under study. 33
Table 2.2 - Extraction methods used for P determinations. 34
Table 2.3 - Soil P parameters obtained from the extracted P-CaCl2, total and mineral Phosphorus (Pt
and Pi) content and ammonium oxalate extractable Phosphorus (Pox),
aluminum (Alox) and iron (Feox). 37
Table 2.4 - Desorption experiment P values in 0.01M CaCl2 (soil:solution ratio 1: 100, 1: 1000 and 1:
10000 represent the risk of P losses through water drainage , runoff and waterbodies, respectively). 47
Chapter 3 - Acid phosphatase and β-glucosidase activities in fertilized pastures over soils derived from volcanic material: distribution profile and P acquisition effort
Table 3.1 - Chemical properties of the studied soils (weighed means of the five assessed layers). 62
xxix
A
BBREVIATIONSAL, ammonium lactate
Alox, ammonium oxalate extractable aluminum
AP, Acid phosphatase enzyme
AP: βG, effort for phosphorus acquisition (AP: βG activity ratio) ATP, Adenosine triphosphate
C:P, (carbon: phosphorus) molar ratio DNA, deoxyribonucleic acid
DPS, Degree of phosphorus saturation Eq., equation
Feox, ammonium oxalate extractable iron ln(AP), potential P acquisition
ln(βG) : ln(AP), potential C:P acquisition ln(βG), potential C acquisition
min, minutes
mRNA, messenger ribonucleic acid
NADPH, Nicotinamide adenine dinucleotide phosphate OC, Organic Carbon
P-AL, Extractable inorganic phosphorus with by the Ammonium Lactate method P-CaCl2, Extractable phosphorus with 0.01 M CaCl2
PHO, Repressible phosphatase gene Pi, inorganic phosphorus
Pi-Olsen, Extractable inorganic phosphorus with by the Olsen method Po, organic phosphorus
Po-Olsen, Extractable organic phosphorus with by the Olsen method Pox, ammonium oxalate extractable phosphorus
PR, phosphorus retention
PSC, Phosphorus sorption capacity Pt, total phosphorus
xxx RNA, ribonucleic acid
SOM, Soil organic Matter SPT, soil phosphorus test βG, β-glucosidase enzyme
2
1 - Introduction
The exponential growth of the world population observed in the last century led to a progressive need of food and fibers, which has been tentatively supported mainly by the increasing of crop yields per hectare (FAO, 2009; van Bavel, 2013), since agricultural areas are becoming scarce and poor, due to inadequate management practices. Nevertheless, the inappropriate use of the agricultural soils and overuse of the production factors, such as chemical fertilizers, animal manure and pesticides (Quansah, 2010), have led to environmental problems, namely soil and water quality depletion, and air contamination (Savci, 2012).
The nutrient dynamics in the agro-ecosystems is a function of integrative effects of soil nutrients transformations, availability, soil management, rhizosphere, microorganisms and plant biological processes (Sinsabaugh, 1994). Over the history, mankind learned to maximize agronomical yields through the use of animal manures and other natural residues, without relying only on the native properties and quality of the soil. From the beginning of last century, the use of chemical fertilizers became the prime resource of nutrients such as nitrogen (N), phosphorus (P) and potassium (K) in the form of salts, since they could give a greater and faster response to the requirements of the progressively more productive crop systems (Savci, 2012). Nevertheless, this policy has induced to the misuse of the synthetic fertilizers, most often without any technical and economic justifications, leading to the severe environmental problems detected during the final of the century and leading to the present societal concerns (Aggani, 2013). Therefore, it is clear the importance of the knowledge and implementation of agriculture management practices without causing damage on other ecosystems (Önder et al., 2011) and how to recover from the abuse of production factors from the past (Giovannucci et al., 2012).
1.1 - Phosphorus (P)
Phosphorus (P) is an essential element for crop production, since it is a component of several fundamental biochemical molecules such as DNA, RNA, ATP, NADPH and phospholipids (Santos, 2015). Moreover, research attention has been given to P, since it is a nonrenewable resource (Cordell et al., 2009). In nature, P as its origin through the weathering process of soil minerals, such as apatite and other geologically stable materials (Lindsay et al., 1989). Phosphorus is the 11th most abundant element in the earth’s crust and it is at the center of serious environmental
3 issues as the depletion of the available reserves and the decline of fresh water quality. This non-renewable resource is confined to small areas in the planet, and although new reserves have been discovered, is still lacking the evaluation of its quality and feasibility as raw material for the fertilizer industry (Sharpley et al., 2001).
Efficient P absorption and acquisition by crop is essential to achieve a desired crop yield. So, maintaining a proper P content in soils enhances plant P efficient uptake from the rhizosphere by an integrative morphological and physiological root adaptive strategies (Cabrera, 2005). The amount of P required to be uptaken by plants may not be are immediately available on the soil. Therefore, the low soil P availability is a limiting factor for crop production in many of the soils, which is aggravated by the P fixation into soils components, which leads to slow diffusion rates in soil solution. Mineral P fertilizers and animal manure application improve P availability and crop yield (Schröder et al., 2010), but may lead to environmental consequences in several ecosystems, such has freshwater eutrophication (Daniel et al., 1998). Consequently, maintaining the concentration of P in the soil solution in an adequate range for plant uptake, often cited as > 0.2 mg L-1, while restricting P content in surface waters below 0.03 mg L-1, is a challenge faced by those involved in soil and crop nutrition management (Pierzynski et al., 2005).
1.2 - Soil P Cycle
Several inorganic and biological processes are involved in P cycling, as it is shown in Figure 1.1. Orthophosphate (PO43-) is the major form of P in soils, and is present on a content ranging from 100 to 3000 mg P kg-1 (Frossard et al., 1995). Generally, the bioavailable orthophosphate ions are present either as HPO42- (pH > 7.2) and H2PO4- (pH 4.0 - 7.2) (Pierzynski et al., 2005). Aiming to reduce the consumption of chemical P fertilizer and their environmental impacts, several practices are needed to be thoughtful implemented, such as maximizing exploitation of the biological potential of root/rhizosphere processes for efficient mobilization and acquisition of soil P by plants, as well the P recycling from manures and other residues (Sharpley and Beegle, 2001). Therefore, a holistic understanding of P dynamics in soil is necessary for a better P management and maximization on the P-use efficiency.
4 Figure 1.1 - Soil phosphorus cycle synthesis. Major chemical and biological processes involved in P bioavailability in soil ecosystem and pathways of P loss into superficial freshwaters and subterranean waters, adapted from Pierzynski et al. (2005).
Inorganic processes of the P cycle involve several physical and chemical phenomenas like precipitation/dissolution and sorption/desorption. Biological processes involve primarily uptake by higher plant and microorganisms of dissolved P released for soil solution during the weathering fenomenon of primary or secondary minerals. The inorganical P (Pi) forms are cycled through several processes in a complex food chain via a serie of mineralization and immobilization reactions (Frossard et al., 1995). The proportion of organic P (Po) varys between 30 to 65% of the soil total P (Pt), but in grassland pastures the proportion is normaly higher than 50% (Nash et al., 2014) and in soils rich organic matter (SOM), the Po contribution may reach up to 90% (Harrison, 1987). Plant P uptake is the primary pathway through which P is removed from soil, although surface leaching, runoff and erosion are also pathways for P losses into other environmental compartments, while gaseous losses do not occurs (Pierzynski et al., 2005).
5
1.2.1 - Soil P forms
As referred, soil P is usually distinguished between inorganic and organic forms (Santos, 2015). Soil Pi forms are governed by precipitation, dissolution and sorption/desorption processes and chemical equilibriums. Generally, Pi has low solubility, is easily fixed and, consequently, it is relatively immobile in soil. Its molecular chemistry is determined by the ionic bonds to the surrounding atoms and it is the relative strength of these ionic bonds that explains how P behaves in soil (Doolette and Smernik, 2011). Soil Po may be also affected by precipitation, dissolution and sorption. Po forms have one or more covalent bonds to a C atom, generally via an ester linkage (through an oxygen atom). Therefore, the majority of Po transformations, in particular their conversion to Pi, requires the covalent bond breaking (Berg and Joern, 2006).
The differentiation between Pi and Po forms is easy to make using long-established chemical techniques. Nevertheless, this differentiation is only a simplification of soil P speciation, because each of these groups comprise a huge range of chemical P forms. Pi is traditionally detected spectrophotometrically by molecular absorption as a blue-colored phosphomolybdenum complex, formed when free phosphate anions react with an acidified molybdate reagent (Murphy and Riley method). Because Po does not form a colored complex with that reagent, it can be only determined as the difference between the total P (measured as Pi after a soil extract digestion) and the readily Pi (Doolette and Smernik, 2011).
1.2.1.1 - Inorganic P and chemical/physical processes
Pi exists as primary and secondary solid P phases or adsorbed onto soil minerals, being well established its propensity to be precipitated or to be bounded on existing solid phases, according to the several chemical equilibriums that are established in soil solution (Lindsay, 1979). The majority of soil P minerals, which are divided in two groups, exist in the form of amorphous co-precipitates, with different degrees of substitution.
Primary P minerals
The group of stoichiometric apatites, Ca10(X)(PO4)6, where X represents F-, Cl-, OH-, or CO32-, is the most common and abundant primary P mineral on earth crust, and its weathering is the first step of P cycling (Frossard et al., 1995). There are also some forms of apatites in soils
6 known as non-stoichiometric, where part of the Ca is substituted Na+, K+, Mg2+, Sr2+, Ba2+, Fe3+ or PO43- is substituted by HPO42-, CO32-, AsO32- or VO43-, which present higher solubility products than stoichiometric apatites (Bengtsson et al., 2009). In calcareous environments apatites are very stable, requiring a H+ source to supply Ca and P for soil solution. For that reason, in soils with pH above 6.2, rock phosphate have no agronomical usefulness due to its reduced P availability for crop uptake (Daniel et al., 1998 and Fardeau et al., 1988).
Secondary P minerals
These minerals are the reactions products between soluble P and Al, Fe or Ca. In calcareous soils, after an initial absorption of P onto the surface of calcite, calcium phosphates can be formed by precipitation, in a chain of composts, depending on the abundance of Ca. Monocalcium phosphate precipitates, and then transforms to dicalcium phosphate, to octocalcium (Ca8H2(PO4)6.5H2O) and finally to hydroxyapatite, and the mineral having the lowest solubility should ultimately control the concentration of P in the soil solution, while the intermediate precipitates are unlikely to persist in soils (Frossard et al., 1995). With the exception of the mono and dicalcium phosphates, all the others have low solubility and reduced P availability for plant uptake (Pierzynsk et al., 2005; Santos 2015).
In acid and well drained soils, the presence of Al and Fe cations, leads to the P precipitation in the form of Al or Fe-phosphates. In these conditions, variscite (AlPO4.2H2O) and stregite (FePO4.2H2O) are the main phosphate forms, but vivianite (Fe3(PO4)2.8H2O) is dominant in hydromorphic soils (Morel, 1996). Nevertheless, the abundant presence of amorphous oxides-Al or Fe can lead to occlusion, becoming difficult to identify the P-Al and P-Fe minerals (Pierzynsk et al., 2005).
Sorbed P
The behavior phosphate anions in soil is mediated by soil colloids that play a major role in its availability to crop uptake (Guppy et al., 2005). Adsorption occurs when P accumulates and bonds onto the surface of soil solid constituents, and desorption is the diffusion of sorbed P into the soil solution (Sposito, 1986). Regarding sorption, the process is considered as a continuous sequence of adsorption and precipitation and consists of two processes: (i) a reversible adsorption,
7 which is relatively fast and (ii) a relatively slow, practically irreversible precipitation-like process being very difficult to distinguish between the two of them (van der Zee and van Riemsdijk, 1988). By replacing H2O or OH-, P is sorbed onto Al and Fe hydrous oxides, clay minerals and carbonates surfaces and edges (Corey, 1981) and sorption and desorption reactions tend to equilibrate with soil P solution. On a P-rich environment, when the solubility product constants are exceeded due to over fertilizations, sorption can evolve into precipitation as secondary P solid phases previously referred (Pierzynsk et al., 2005).
Pi in soil solution
The proportion and form of Pi is governed by soil pH (Figure 1.2). In acidic conditions, the H2PO4- anion is the form dominant and when the pH arises, HPO42- anion form is dominant (Santos, 2015). Due to the prevailing pH values of agricultural soils, P in the forms of PO43- anion or H3PO4 molecule are not expected to exist in soil solution.
Figure 1.2 - Soil solution pH effect on the phosphate ions proportions (adapted from Hinsinger, 2001).
1.2.1.2 - Organic P and biological processes
Defined as a P bonded to a C, it may represent nearly 90 % of total P in soils very rich in SOM (Harrison, 1987). Organic P transformations in soil are important for P availability, determining the productivity of ecosystems (Condron et al., 2005). P is absorbed from soil solution by plant and microorganism, and bonds to C through phosphorylation by different biochemical
8 pathways. Therefore, Po has its primary origin in animal and plant residues, but it is also synthesized by microbes and released to the soil after its death. In nature, Po remains at its highest oxidation state, forming always covalent bonds (Goldwhite, 1981).
Several studies have demonstrated that Po forms contribute significantly to plant P uptake. Oehl et al. (2001), in Swiss arable soils, reported that Po mineralization rate was equivalent to soil
solution P, indicating that soil P mineralization is a significant process in delivering available Pi.
The amount, forms and dynamic of organic P are determined by the combination of biological,
chemical and physical factors, and influenced by environmental parameters and soils management, as represented in Figure 1.3 (Condron et al., 2005).
Organic P forms
Although representing only a small part of the total Po, organic phosphorus compounds in soils
can be divide in tree groups (Morel, 1996):
Inositol phosphates, widely resistant to mineralization, and may represent up to 50% of total organic P;
Phospholipids and glycerophospholipids, representing between 0.5 to 7% of total organic
P;
9 Figure 1.3 - Soil organic P dynamic (adapted from Condron et al., 2005).
The methods used to study these fraction are divided in two groups: one group measures
the total concentrations of Po; other group includes methods to identify the chemical forms Po, their
concentrations and behavior in soil, such as bonding and mineralization fluxes (Condron et al., 2005). In the latter methods, important advances have been made with the use of layer chromatography, anion exchange high performance chromatography, gas chromatography, mass
spectroscopy and 31P NMR spectroscopy (Turner and Mckelvie, 2002).
Phosphorus immobilization/mineralization
The immobilization/mineralization rate determine the P turnover in soils (Condron et al., 2005).
The conversion of Pi to Po is a biological process designated as immobilization, occurring via two
important pathways (Condron and Goh, 1989):
To supply microbial biomass requirements, Pi is removed from soil solution (Figure 1.3),
10 is expected to prevail over mineralization when soil C/P ratio is above 300 (Stevenson and Cole, 1999).
To supply crop requirements, Pi is removed from soil solution by plant uptake (Figure 1.3),
returning to the soil as Po in litter and root debris, or as animal manure and feaces. When
grassland pastures are subjected to fertilization, a net immobilization occurs, although an equilibrium is established between Po inputs and mineralization, leading to a decline of
organic P accumulation (Condron et al., 2005).
Regarding P mineralization, it can be defined as the inverse process of immobilization, since is the conversion of Po into Pi. Phosphorus is released from soil organic matter or during the decomposition process of microbial and plant residues (Condron et al., 2005). The Po mineralization is mediate by a large group of phosphatases, which are periplasmic or extracellular enzymes, also designated as exoenzymes or ecoenzymes (Frossard et al., 2000).
1.3 - Enzymes
Soil organic matter complex is both synthesized and degraded by soil microbial activity (Burns et al., 2013). The balance between these two processes determinates several soil properties, such as: (i) carbon sequestration; (ii) soil aggregation structure and stability; (ii) plant nutrient availability and; (iii) water retention. Microbial diversity and activity host the enzyme production that also determine soil fertility and crop productivity (Burns et al., 2013).
The enzymes produced and exuded by plant roots, mycorrhizae, and microorganisms occurs according to the nutrient demand and nutrient availability in soils (Sinsabaugh, 1994). Being considered the proximate agents of soil organic matter decomposition, enzymes disrupts plant and microbial cell walls and reduce macromolecules to soluble substrates for posterior uptake and assimilation (Dick et al., 1988).
In nature, natural selection leads to enzyme production strategies that minimize costs to the cell and maximize the associated benefits. The costs to the cells are the carbon and nutrients required for energy (i.e. ATP), enzyme synthesis and secretion, formation and organization of membrane transporters for the uptake of the products, and signal production and detection of potential substrates. Therefore, exoenzyme activities are the result of cellular economics:
11 expending resources to produce enzymes versus the benefit of increases on low molecular mass organics molecules, with consequences in terms of availability of assimilable mineral nutrients and energy sources (Burns et al., 2013).
1.3.1 - β-Glucosidase
Glucosidases, or glucoside hydrolases, is the general designation used to describe a group of enzymes that catalyze the hydrolysis of different glucosides. Widely distributed in nature, they are detected in microorganisms, animals and plants (Eivazi and Tabatabai, 1988), being also reported their free existence in soils (Skujins, 1976).
Considerable concern has been given to the synthesis, role, and properties of these enzymes in microorganisms and plants. Nevertheless, only more recently the attention has been also devoted to studies regarding their role in soils. Glucosidase reaction products are important energy sources for soil microorganism, playing a major role in the degradation of carbohydrates (Eivazi and Tabatabai, 1988). Its synthesis is induced by the products of cellulose breakdown, including cellobiose, glucose and their metabolites (Stewart and Leatherwood, 1976), and its activity has been referred as the rate-limiting step in cellulose degradation (Alef and Nannipieri, 1995). Objectively, glucosidases catalyze the cleavage of one molecule of cellobiose in two molecules of glucose, regulating the energy supply for microorganisms, which are unable to take up cellobiose directly.
β-glucosidase (βG) is the most abundant and easily detectable of the three enzymes (endo-1,4-glucanase, exo-1,4-glucanase and β-D-glucosidase) involved in cellulose degradation in soil. Rarely substrate limited, it is considered ideal to examine the importance of physico-chemical controls on the turnover of soil organic matter (Turner et al., 2002). Therefore, βG activity has been used to closely monitor rapid changes in soil organic C associated with soil management effects (Bandick and Dick, 1999).
1.3.2 - Phosphatases
Mineralization of Po may be enzymatic (Figure1.3), and this has stimulated much of the attention in phosphatases activity in soils, microorganisms, and plant roots. Phosphatases are a
12 broad group of enzymes that catalyze the hydrolysis of both esters and anhydrides of phosphoric acid (Schmidt and Laskowski, 1961).
Like other exoenzyme molecule, phosphatases diffuse poorly into the soil matrix (Lefebvre et al., 1990) and their activity are dependent of different parameters such as soil characteristics, clay and humus adsorption, microorganisms, plant cover, inputs and the presence of inhibitors or activators (Speir and Ross, 1978). There are three types of phosphatases in soils, all of them acting on ester bonds (Deng and Tabatabai, 1997):
Phosphomonoesterases, a group that includes acid phosphatases and alkaline phosphatases; both of them are phosphoric monoester hydrolases with optimal activities in acid and alkaline environments. These enzymes catalyze the break of the ester bond of the orthophosphate group to a C. Phytic acid, mononucleotides such as adenosine-5’-phosphate, or sugars such as glucose-6-adenosine-5’-phosphate, are some of the molecules which Po is hydrolyzed by these groups of enzymes.
Phosphodiesterases are phosphoric diester hydrolases. These enzymes catalyze the break of a diester bond, orthophosphate group to two C. Ribonucleic and deoxyribonucleic acids or phospholipids, such as phosphatidyl inositol, are some of the molecules which Po is hydrolyzed by these enzymes
Phosphotriesterases are phosphoric triester hydrolases. These enzymes catalyze the break of a triester bond to an orthophosphate. Phosphotriesters have not been found in nature (Saxena, 2008) and they are a class of highly toxic synthetic compounds known as organophosphates, widely used as insecticides and herbicides (Bigley and Raushel, 2013).
The activity of these enzyme is determinant in the soil P cycling and on the microbial and plant nutrition (Tabatabai and Dick, 2002), by catalyzing mineralization of Po and contributing to soil P bioavailability.
1.4 - Resource allocation for P acquisition
Once released from cells, enzyme activity is mediated by environmental factors (Allison, 2006), although their expression is regulated by environmental signals (Dick et al., 2011), which in soils are related with the nutrient availability and net resources (Moro et al., 2015). The
13 extracellular enzyme degradation of organic matter can be seen as a rate-controlling step of Carbon cycle, which requires a large number of hydrolytic and oxidative reactions, stoichiometric-linked to the composition and oxidation state of organic matter. Enzyme activities can relate microbial biomass and organic matter to the nutrient assimilation and growth stoichiometry (Sinsabaugh and Moorhead, 1994).
β-Glucosidase and other cellulolytic enzymes activities are correlated with microbial metabolism and the rate of mass loss from plant litter (Sinsabaugh and Moorhead, 1994). Although their magnitude and correlation vary widely in the terrestrial ecosystems when relating to organic matter and available Pi, phosphomonoesterases activity presents an inverse relationship with available Pi, being one of the most studied extracellular enzyme (Sinsabaugh and Shah, 2012). Describing different enzymatic activities, such as βG and acid phosphatase (AP), in relation to edaphoclimatic factors, Sinsabaugh et al. (2008) reported that enzymes activities were related to soil pH and that hydrolytic activities reflected nutrient availability trends.
Sinsabaugh et al. (2010) proposed that the exoenzymes activities are consequence of stoichiometry and metabolism aspects, as result of the equilibrium among microbial biomass, organic matter, efficiency of nutrient assimilation and growth. Latter, the same authors (Sinsabaugh and Shah, 2011) presented a conceptual model linking organic matter to exoenzime activities stoichiometry. Moreover, enzymatic activities can be related among them, since they are stoichiometric-linked to the nutrient availability and acquisition by microbial biomass. So, enzyme activity ratios in soils can be understood as a complemental index of potential nutrient limitation for growth (Moro et al., 2015), since energy resources are allocated to supplemental exoenzyme production only if labile forms of substrate (P or N) are in short supply (Allison et al., 2007), leading to a shift in energy consumption from cell growth towards nutrient acquisition .
Moreover, Sinsabaugh and Moorhead (1994) and Sinsabaugh and Shah (2012) proposed a resource allocation model for extracellular enzymes in relation to nutrient availability, in which the ratio of a certain nutrient acquiring enzyme activity to C acquiring enzyme activity (βG) would become an indicator of the availability of such nutrient. If related with crops, this proposal may become a complement to the conventional laboratorial approaches to assess nutrients availabilities in soils.
14
1.5 - Phosphorus Inputs/Outputs
The P cycle has been described, in Ecology, as a 'tight cycle', since the P amount present within an ecosystem itself (in the soil and microbial and vegetal biomass) is much larger when compared to the gains and losses of the ecosystem (Newman, 1995). In agricultural systems, the P input has begun with the use of organic materials (Figure 1.4), as referred previously. However, after the middle of 1800, with the advances in plant nutrition by Justus von Liebig, and after the late 1800, with the advent of the fertilizer industry, precisely with the calcium superphosphate manufacturing, the inputs of Pi in the agricultural systems increased continuously till nowadays.
Fertilization affects soil biochemical and biological properties (Liang et al., 2014), and the influence of external nutrient inputs on soil microbial ecology has been frequently emphasized (Ai et al., 2012). As an example, PO43- anions are a competitive inhibitor of both phosphatases in soils (Juma and Tabatabai, 1978), although the absence of response of phosphatase activities to P addition has been also reported (Schneider et al., 2001). On the other hand, N fertilization generally increases the activity of phosphatases in soils (Marklein and Houlton, 2012).
Figure 1.4 - Scheme synthetizing the soil P Input and Outputs.
Due to its low mobility, solubility and concentration in soil solution, P leads to limit the net production in a continuous agriculture land use (Frossard et al., 1995). Therefore, the application of fertilizers and by-products containing P to soils has the main purpose to increase the bioavailable phosphorus in soil solution, to supply plants requirements (Hedley and McLaughlin, 2005), and
15 remove P crop starvation. To be effective, the fertilizer P need to be more soluble than the existent soils phosphorus compounds (Hedley and McLaughlin, 2005).
Animal manures, crop residues, municipal and industrial wastes and other residues are often called by-products, since they converge diverse mixtures that includes Pi and Po at different stages of decomposition, potentially acting in a similar manner as mineral P fertilizers (Hedley and McLaughlin, 2005). Although by-products efficiency is also affected by soil components, such as Al, Fe and Ca, their fate is usually a function of the microbial decomposition rate (Hedley and McLaughlin, 2005).
The rate of organic matter accumulation in terrestrial systems is directly related to their net primary productivity and subsequent rate of organic matter return to soil (Grace et al., 1998). Relationships are found between soil organic matter accumulation and P fertilizer use when these inputs regulate the net primary productivity of the system (Simpson et al., 2011). Accumulation of Po in the range of 1.7 to 4 kg ha-1 yr-1 were recorded for pastures on low P available soils (Kohn et al., 1977).
The accumulation of P in soil it is not inevitable, and is extremely influenced by the farming system and management practices (Simpson et al., 2011). Agricultural P applications may be adjusted in order to maintain soil bioavailability in a steady state or the returning rate of Po to the soil may be stabilize provide that primary productivity is stabilized. Nevertheless, in many of the agricultural systems, bioavailable P exceeds the agronomical threshold value for plant growth as a result of over fertilization practices. In other situations, organic matter and Po continues to accumulate in soils, either because the capacity of the soil to retain organic matter is not yet achieved, or a balance between theinput of plant residues and the rate of mineralization was not reached (Baldock et al., 2007).
Another important part of the P cycle (Figure 1.1) is the transfer of P from soils into watersheds. When not enhanced by the misuse of fertilizers, the runoff flux needs to be considered as a natural process. Similarly, erosion is a natural process in the watersheds, but its intensity is also enhanced by inadequate soil management practices. In both situations, the increase of P enrichment on the receiving waters contributes to the decrease of its quality (Sharpley et al., 2003).
16
1.6 - Phosphorus environmental impacts
Being an essential element for plants and animals and required to achieve profitable yield of crop and livestock production, P increases also the biological productivity of surface waters. As a result, escalation on water eutrophication accelerates the natural aging of lakes and streams (Sharpley and Beegle, 2001).
Water eutrophication is the biological enrichment of surface waters due to anthropogenic inputs of nutrients (Kleinman et al., 2009). Since P is the one of the limiting factor for algal growth, the increase on agricultural P inputs has been associated with the eutrophication of freshwaters systems (Sharpley and Menzel, 1988). The P transfer to surface waters is defined as a nonpoint-source pollution of lakes and streams and promotes eutrophication at concentration levels between 0.02 and 0.035 mg P L-1 (Daniel et al., 1998). The transfer intensity from soil to waters depends on
the P desorption from soil constituents. Therefore, sorption by hydroxides and oxides of Al and Fe in surface horizons and subsoils or precipitation with Ca can maintain runoff and leached P levels below the eutrophication thresholds values (Elliott et al., 1999).
Nevertheless, the growing concerns about water eutrophication and the misuse of P fertilizers lead to the urgent need for restrictions in P losses from agricultural soils to water and to the development of decision support systems to assess and anticipate the risks of those P transfers (Daniel et al., 1998).
Van der Zee et al. (1988) proposed a parameter named Degree of P Saturation (DPS), expressed as the ratio between the total amount of sorbed P and the P Sorption Capacity (PSC) of the soil. Under these concepts, the PSC is a measure of the potential P sorption by the major P-sorbing components in acid soils matrix: the organic Al and Fe complexes and the poorly crystalline Al and Fe hydroxides and oxides (Freese et al., 1992):
𝐷𝑃𝑆 = 𝑃𝑜𝑥
𝑃𝑆𝐶 × 100 Eq. 1
𝑃𝑆𝐶 = 𝛼 (𝐴𝑙𝑜𝑥+ 𝐹𝑒𝑜𝑥 ) Eq. 2
17 DPS relates the P sorbed to PSC, and the increase of P content in a specific soil leads to the increase of DPS. Therefore, P enrichment forwards to the loss of soil ability to remove extra P from soil solution due to the buildup equilibrium of P sorbed relative to PSC, driving to situations where P can be readily loss by superficial runoff and internal leaching (Kleinman et al., 1999). A DPS value of 25% is considered critical, above which P leaching is susceptible to occur (van der Zee et al., 1988). However, for acid sandy soils in Flanders, a critical limit of 35 % was set, and soils with values below 25% were classified as unsaturated (De Bolle et al., 2013).
Other methods of assessing the risk of P loss to water are based on standard soil P test (SPT), as Mehlich-3, Olsen, ammonium lactate methods, iron oxide–coated paper strips and P-water extraction (Pote et al., 1999). As an example, Horta and Torrent (2007) evaluated the ability of Olsen P method to predict the P release from Portuguese acid soils into waters, achieving an environmental threshold value of 50 mg P kg-1. Tentatively, an equivalent threshold value for P-ammonium lactate method may be set to 106 mg P kg-1 for the same type of soils (Horta et al, 2010). Both values may be considered as a change point, above which P losses to receiving waters became unacceptably high. Nevertheless, these values depend not only on the amount of extractable P, but also on the soil type and desorption situation (Maguire et al., 2005), drawbacks that may be overcame by the use of DPS index.
Torrent and Delgado (2001) proposed another P desorption approach, where different soil:solution rations mimic various scenarios of soil P desorption to water. According to those authors, 1:100, 1:1000 and 1:10000 soil: CaCl2 solution ratios would mimic respectively P desorption for drainage and runoff waters and P desorption from sediments to static waterbodies. Within this approach, critical values are (i) 0.1 mg P L-1 for drainage water (EU-WFD, 2000); (ii) 0.05 mg P L-1 for water runoff (Golterman and Oude, 1991) and; 0.02-0.03 mg P L-1 for static waterbodies (Correll, 1999).
1.7 - Volcanic ash soils
Soils derived from volcanic materials, also known as Andosols (WRB, 2006), are formed from volcanic ash and cinder deposits. These soils are very light and fluffy, with a high capacity to retain water, and are rich in amorphous minerals, such as allophones and imogolite that form strong
18 bonds with organic colloids and P (Shoji et al., 1993; Takahashi and Shoji, 2002). Andosols account for nearly 1% of the global land area, but they contain approximately 5% of the global soil organic carbon (Eswaran et al., 1993; Batjes, 1996), since these soils tend to accumulate OM in the superficial layers (Takahashi and Shoji, 2002; Dahlgren et al., 2004). On the other hand, cultivated
Andosols need to be fertilized periodically due to its high capacity to sorb P, in order to supply crop
requirements (Shoji et al., 1993).
1.8 - The Azores archipelago, the Pico island case
According to WRB (2006), most of the soils in the Azores archipelago, including Pico island, have been classified as Andosols, showing unique properties as defined by their main physical, chemical and mineralogical characteristics (Pinheiro et al., 2001). Simultaneously, eutrophication has been reported in several lakes of the Azores, despite the adoption of several measures included on the European Union Water-Framework Directive (Cruz et al., 2015).
In S. Miguel Island, Sete-Cidades watershed, Simões (2001) reported values of DPS and
Pi-Olsen higher than the threshold values. Also on the same island, but in Furnas watershed, Fontes
and Pinheiro (2016) in studies being conducted presently, observed up to 0.19 mg P L-1 in runoff waters, above the environmental threshold value of 0.05 mg L-1.
In Pico island, about 40 % of the territory is dedicated to dairy livestock, and several watersheds have been identified as under environmental risk and P fertilization practices in grazed pastures are associated to those risks (SRAM, 2011). Some examples are the Lagoas Capitão,
Rosado and Peixinho, where the watersheds are under significant pressure due to the water low
19
Objectives
Taking into consideration many of the aspects referred in this introduction, a study was conducted, using six fertilized grassland soils collected in from commercial farms from Pico island. In each soil, the top 15 cm were sampled and divided in five layers. In terms of presentation, the work is divided in two parts, which represent the two main chapters of this dissertation.
In chapter 2, the main objectives were (i) to evaluate the vertical distribution of different Pi
pools and (ii) to assess the potential risk of P losses from the soils to the receiving waters, using the established chemical parameters referred previously: DPS and P desorption in CaCl2 suspensions with high soil:solution ratios.
In chapter 3, the main objectives were (i) to evaluate the vertical distribution of βG and AP activities in the 15 cm layer of the studied soils, (ii) to assess the stoichiometric enzymatic ratio and effort for P acquisition in soils derived from volcanic materials, and (iii) stablishing the relationship between these enzymes activities, the mineral P fertilization, SOM accumulation and the active Al and Fe extracted by ammonium oxalate.
20
1.9 - References
Aggani, S. L., 2013. Development of bio-fertilizers and its future perspective - Review article. Journal of Pharmacy, 2: 327-332.
Ai, C., Liang, G., Sun, J., Wang, X. and Zhou W., 2012. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma, 173-174: 330-338.
Alef, K. and Nannipieri, P., 1995. Methods in applied soil microbiology and biochemistry. Academic Press, London, 576 pp.
Allison, S. D., 2006. Soil minerals and humic acids alter enzyme stability: Implications for ecosystem processes. Biogeochemistry, 81: 361-373.
Allison, S. D., Hollandk, G. T., Weintraub, M. and Sinsabaugh, R. L., 2007. Soil enzymes: Linking proteomics and ecological process. In: Manual of Environmental Microbiology, pp. 704-711, ASM Press, Washington, DC, USA.
Baldock, J., Skjemstad, J. and Bolger, T., 2007. Managing the carbon cycle. In: Pasture systems, managing for a variable climate. Proc. 22nd Annual Conference Grassland Society of New South Wales, pp 5-9, Orange, N.S.W.,
AU.
Bandick, A. K. and Dick, R. P., 1999. Field management effects on soil enzyme activities. Soil Biology and Biochemistry, 31: 1471-1479.
Batjes, N. H., 1996. Total carbon and nitrogen in the soil of world. European Journal of Soil Science, 47: 151-163. Berg, A. S. and Joern, B. C., 2006. Sorption dynamics of organic and inorganic phosphorus compounds in soil. Journal
of Environment Quality, 35:1855-1862.
Bengtsson, Å., Shchukarev, A., Persson, P. and Sjöberg, S., 2009. A solubility and surface complexation study of a non-stoichiometric hydroxyapatite. Geochimica et Cosmochimica Acta, 73: 257-267.
Bigley, A. N. and Raushel, F. M., 2013. Catalytic mechanisms for phosphotriesterases. Biochimica et Biophysica Acta - Proteins and Proteomics, 1834: 443-453.
Burns, R. G., DeForest, J. L., Marxsen, J., Sinsabaugh, R. L., Stromberger, M. E., Wallenstein, M. D., Weintraub, M. N. and Zoppini, A., 2013. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biology and Biochemistry, 58: 216-234.
Cabrera, M. L., 2005. Phosphorus: Plant nutrition and crop management. In: Phosphorus agriculture and the environment. (Eds.), Sims J. Th. and Sharpley, A. N., pp. 353-378, ASA-CSSA-SSSA. Madison, Wisconsin, WI, USA.
Condron, L. M. and Goh, K. M., 1989. Effects of long-term phosphoric fertilizer applications on amounts and forms of phosphorus in soils under irrigated pasture in New Zealand. Journal of Soil Science, 40: 383-395.
Condron, L. M., Turner, B. L. and Cade-Menun, B. J., 2005. Chemistry and dynamics of soil organic phosphorus. In: Phosphorus, agriculture and the environment. (Eds.), Sims, J. Th. and Sharpley, A. N., pp. 87-122 ASA-CSSA-SSSA. Madison, Wisconsin, WI, USA.
Cordell, D., Drangert, J. O. and White, S., 2009. The story of phosphorus: Global food security and food for thought. Global Environmental Change, 19: 292-305.
21 Corey, R. C., 1981. Adsorption vs precipitation. In: Adsorption of inorganics at solid-liquid interfaces. (Eds.),
Anderson, M. A. and Rubin, A. J., pp.162-182, Ann Arbor, Michigan, MI, USA.
Correll, D L., 1999. Phosphorus: A rate limiting nutrient in surface waters. Poultry Science, 78: 674-682.
Cruz, J. V., Pacheco, D., Porteiro, J., Cymbron, R., Mendes, S., Malcata, A. and Andrade, C., 2015. Sete cidades and furnas lake eutrophication (São Miguel, Azores): Analysis of long-term monitoring data and remediation measures. Science of the Total Environment, 520: 168-186.
Dahlgren, R. A., Saigusa, M. and Ugolini, F. C., 2004. The nature, properties and management of volcanic soils. Advances in Agronomy, 82: 113-182.
Daniel, T. C., Sharpley, A. N. and Lemunyon, J. L., 1998. Agricultural phosphorus and eutrophication: A symposium overview. Journal of Environment Quality, 27: 251-257.
De Bolle, S., De Neve, S. and Hofman, G., 2013. Rapid redistribution of P to deeper soil layers in P saturated acid sandy soils. Soil Use and Management, 29: 76-82.
Deng, S. P. and Tabatabai, M. A., 1997. Effect of tillage and residue management on enzyme activities in soils: III. Phosphatases and arylsulfatase. Biology and Fertility of Soils, 24: 141-146.
Dick, C. F., Dos-Santos, A. L. A. and Meyer-Fernandes, J. R., 2011. Inorganic phosphate as an important regulator of phosphatases. Enzyme Research, 2011: 1-7.
Dick, R. P., Rasmussen, P. E. and Kerle, E. A., 1988. Influence of long-term residue management on soil enzyme activities in relation to soil chemical properties of a wheat-fallow system. Biology and Fertility of Soils, 6: 159-164.
Doolette, A. L. and Smernik, R. J., 2011. Soil organic phosphorus speciation using spectroscopic techniques. In: Phosphorus in action: Biological processes in soil phosphorus cycling. (Eds.), Bünemann, E. K., Oberson, A. and Frossard, E., pp. 3-36, Springer, Berlin, Heidelberg, D.
Eivazi, F. and Tabatabai, M. A., 1988. Glucosidases and galactosidases in soils. Soil Biology and Biochemistry, 20: 601-606.
Elliott, H. A., O’Connor, G A. and Brinton, S., 1999. Phosphorus leaching from biosolids-amended sandy soils. Journal of Environmental Quality, 31: 681-689.
Eswaran, H., Berg, E. V. and Reich, P., 1993. Organic carbon in soils of the world. Soil Science Society of America Journal, 57: 192-194.
FAO. 2009. Global Agriculture towards 2050. High Level Expert Forum-How to Feed the World 2050, 12-13 October, pp. 1-4, Rome, I.
Fardeau, J. C., Morel, C. and Jahiel, J. M., 1988. Does long term contact with the soil improve the efficiency of rock phosphate - results of isotopic studies. Fertilizer Research, 17: 3-19.
Fontes, J. C. and Pinheiro, J. A., 2016. Balanço hídrico para a bacia hidrográfica da Lagoa das Furnas, S. Miguel Açores. VII Congresso Ibérico das Ciências do Solo/VI Congresso Nacional de Rega e Drenagem, 13-15 setembro, pp. 33-36, Beja, Portugal.
Freese, D., Van Der Zee, S. E. A. T. M. and Van Riemsdijk, W. H., 1992. Comparison of different models for phosphate sorption as a function of the iron and aluminum-oxides of soils. Journal of Soil Science, 43: 729-738.